Abstract
Nicotine withdrawal-related disruption of cognitive control may contribute to the reinforcement of tobacco use. Identification of gene variants that predict this withdrawal phenotype may lead to tailored pharmacotherapy for smoking cessation. Variation on the cannabinoid receptor 1 gene (CNR1) has been related to nicotine dependence, and CNR1 antagonists may increase attention and memory functioning. We targeted CNR1 variants as moderators of a validated neural marker of nicotine withdrawal-related cognitive disruption. CNR1 polymorphisms comprising the “TAG” haplotype (rs806379, rs1535255, and rs2023239) were tested independently, as no participants in this sample possessed this haplotype. Nicotine withdrawal-related cognitive disruption was indexed as increased resting electroencephalogram (EEG) alpha-1 power density across 17 electrodes. 73 Caucasian Non-Hispanic smokers (≥ 15 cigarettes per day) visited the laboratory on two occasions following overnight smoking/nicotine deprivation. Either two nicotine or two placebo cigarettes were smoked prior to collecting EEG data at each session. Analyses showed that rs806379 moderated the effects of nicotine deprivation increasing slow wave EEG (p = .004). Smokers homozygous for the major allele exhibited greater nicotine withdrawal-related cognitive disruption. The current findings suggest potential efficacy of cannabinoid RECEPTOR antagonism as a pharmacotherapy approach for smoking cessation among individuals who exhibit greater nicotine withdrawal-related cognitive disruption.
Keywords: alpha-1, EEG, ERP, cannabinoid, CNR1, cognitive control, genetics, nicotine withdrawal, smoking
Introduction
Cognitive control refers to a broad array of attention and memory-related processes relevant to the performance of effortful daily activities (Pontifex et al., 2011). Nicotine withdrawal disrupts various aspects of cognitive control, and this has been implicated as a source of tobacco reinforcement (Ashare et al., 2014). That is, smokers who exhibit chronically poor cognitive control may experience greater cognitive disruption during nicotine withdrawal (Evans and Drobes, 2009; Evans et al., 2013; Evans et al., 2015) and this may serve to increase smoking motivation in order to increase (or restore) functional levels of cognitive control. Identifying genetic variations in neurochemical systems contributing to withdrawal-related cognitive disruption may enable us to identify smokers who find smoking more reinforcing due to cognitive restoration. In turn, this may inform the development of behavioral and/or pharmaceutical treatments that target the needs of this subgroup of smokers (Evans and Drobes, 2009).
CNR1 gene variants as moderators of nicotine withdrawal-related cognitive disruption
The cannabinoid and nicotinic acetylcholine systems are highly interactive, with cannabinoid receptor 1 (CNR1) antagonists increasing acetylcholine (Degroot et al., 2006; Spivak et al., 2007). Further, both of these systems influence cognitive control, with nicotine agonists (Evans and Drobes, 2009) and CNR1 antagonists (Fujiwara and Egashira, 2004) improving cognitive control. The CNR1 antagonist SR141716A (i.e., Rimonabant) has been found to enhance cognitive control-related attention and memory processing in animals (Lichtman, 2000; Terranova et al., 1996). Furthermore, animal studies show that CNR1 antagonists reduce nicotine self-administration (Cohen et al., 2002). Integration of the above findings suggests that smoking may be more reinforcing when there is greater CNR1 activity, and that the influence of CNR1 may interact with nicotine withdrawal-related cognitive deficits. Finally, nicotinic acetylcholine and CNR1 receptors are both present on important cognitive control brain areas such as the anterior cingulate cortex (Gallagher, Mohlberg, Zillies, & Vogt, 2008; Zavitsanou, Garrick, & Huang, 2004) and the dorsolateral prefrontal cortex (Eggan, Mizoguchi, Stoyak, & Lewis, 2010; Yang et al., 2013). Activity among these receptors at common brain sites may determine how these neurotransmitters interact in the context of nicotine withdrawal and cognition smoking behavior.
The “TAG” haplotype of the CNR1 gene (CNR1) has been associated with substance abuse Zhang et al., 2004), In addition, CNR1 variants have been associated with smoking cessation (Fernandez and Allison, 2004). Taken together, the associations between CNR1 in potentially influencing both nicotine self-administration and cognitive control processing suggests that CNR1 activity may impact nicotine withdrawal-related cognitive disruption. CNR1 variants are not functionally well characterized. However, analysis of the “TAG” haplotype (i.e., base “T” at rs806379, “A” at rs1535255, and “G” at rs2023239) on CNR1 predicts substantially reduced mRNA expression in postmortem analyses of cerebral cortices and midbrain (Zhang et al., 2004), thereby suggesting that the alleles comprising this haplotype may be predictive of less withdrawal-related cognitive disruption. Reduced mRNA expression is consistent with the above findings linking CNR1 antagonists with reduced smoking and enhanced cognition.
This study examines the effects of cannabinoid receptor 1 gene (CNR1) variants on a neural phenotype of nicotine withdrawal-related cognitive disruption discussed in the next section. The three polymorphisms comprising this haplotype were selected as potential moderators of nicotine deprivation-induced reduction of cognitive control. The haplotype itself could not be tested because there were no participants in this smaller sample possessing the haplotype. Thus, the polymorphisms were independently examined as predictors of withdrawal-related cognitive disruption.
Neural endophenotypes of withdrawal-related deficits in cognitive control
Intermediate phenotypes with well-defined biological underpinnings (i.e., endophenotypes) may advance pharmacogenomics research. For example, genes that provide the instructions for building specific neurotransmitter receptors may yield greater effect sizes in predicting endophenotypes relative to broader phenotypes (e.g., cigarettes per day) that involve a wider range of genetic contributors (National Cancer Institute, 2009). Relatively smaller samples might thus be able to capture these larger effects. This approach has been previously used to study the genetics of nicotine withdrawal-related cognitive disruption (e.g., Evans et al. 2009; 2014).
Reduced amplitude of the target P300 (P3b) component of the event-related brain potential (ERP) waveform is an established endophenotype of nicotine withdrawal-related cognitive disruption (Evans et al. 2013; Evans et al., 2014). Hypothesis driven testing of polymorphisms on the nicotinic acetylcholine alpha-5 receptor subunit gene (CHRNA5) were found to moderate the influence of nicotine deprivation on this P3b endophenotype of withdrawal-related cognitive disruption (Evans et al., 2014). Unpublished exploratory data from our lab found preliminary support for variation of the rs806379 SNP (i.e., from the CNR1 TAG haplotype) as a moderator of the influence of nicotine deprivation on P3b amplitude. This effect was nominally significant, but did not survive statistical correction for multiple (384 SNPs) tests in a small sample (N = 71). Nevertheless the pattern of findings was consistent with what one might be expect. That is, the T allele on rs806379 may be indicative of reduced mRNA expression, which may also be predictive of less nicotine withdrawal-related cognitive disruption. This tentative conclusion is based on the knowledge that the T allele as part of the TAG haplotype showed robust levels of reduced CNR1 mRNA expression (Zhang et al., 2004). Participants possessing at least one T allele exhibited reduced nicotine deprivation-induced reductions in P300 amplitude. This preliminary finding coupled with the aforementioned links between nicotinic acetylcholine and CNR1 motivated us to conduct the present targeted examination of the moderating effect of CNR1 SNPs on another marker associated with nicotine withdrawal-related cognitive disruption: resting electroencephalogram (EEG) increases in slow wave alpha-1 power.
Resting EEG Alpha-1 enhancement as a marker of cognitive deficits
Distinct patterns of EEG activity obtained while sitting quietly have been shown to be neural correlates of cognitive control. Individuals with reduced cognitive control show higher power density values within the slower EEG bands (4–13 Hz) relative to faster wave activity (e.g., 14–30 Hz) while sitting upright and not performing any cognitive task (Hermens et al. 2005; Lansbergen et al. 2011). For example, greater slow wave compared to fast wave (e.g., beta. 14–30 Hz) ratio was found to be negatively correlated with both response inhibition on a go/nogo task in the laboratory and self-reported attentional control (Putman et al. 2010). Stimulant drugs such as methylphenidate produce the same pattern of resting EEG effects (Clarke et al. 2002; Loo et al. 1999), and nicotine appears to have a stimulant-like effect on resting EEG (Fisher et al. 2012; Knott and Fisher 2007; Knott et al. 2005), which may account for nicotine having stimulant-like reductions of attentional deficits (e.g., Potter 2008).
Alpha-1 enhancement as an endophenotype of nicotine withdrawal-related cognitive disruption
Regarding nicotine and slow wave resting EEG, a recent study of 124 smokers found that nicotine deprived smokers show markedly greater alpha-1 (8–10 Hz) resting EEG compared to when the same smokers were satiated (Evans et al. 2015). Nicotine patches given to nonsmokers show a similar effect, with nicotine reducing alpha-1 power density values (Sutton et al., 2016). Additionally, it was shown that nonsmokers who reported lower cognitive control showed greater nicotine-induced reductions in alpha-1 power density than individuals who are higher in cognitive control, thereby additionally linking this nicotine-related EEG effect to cognitive control (Evans et al. 2016). Altogether these effects are consistent with reduced alpha-1 power following nicotine administration indicating reduced cognitive control.
Exploratory data was suggestive of a CNR1 SNP moderating the influence of nicotine deprivation-induced reductions of P3b amplitude. The direction of effect made theoretical sense with respect to interactions between acetylcholine and CNR1 systems in the context of nicotine and cognitive control. We therefore expected that CNR1 SNPs would moderate nicotine deprivation-induced reductions of cognitive control, with SNPs suggestive of more CNR1 activity predicting greater nicotine deprivation-induced reductions in cognitive control. We tested this association using another established neural marker of nicotine deprivation-induced reduction of cognitive control: increases in resting EEG alpha-1 power.
Materials and Methods
Participants
Participants were recruited via internet and newspaper ads and from our existing data base at the Tobacco Research and Intervention Program. 73 Caucasian Non-Hispanic participants (19–60 years of age) from the parent resting EEG study (Evans et al., 2015) provided data for analyses. Inclusion was based on availability of valid resting EEG data and at least one valid genotype. There were 58 men (mean age = 36.88, SD = 10.90) and 15 women (mean age = 33.33, SD = 13.05). Education level ranged from 6 to 18 years (mean = 12.73, SD = 1.85). Mean score on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was 5.62 (SD = 1.78), which indicates a moderate to high level of nicotine dependence. Eight participants were missing data for the rs806379 SNP.
Eligible participants reported smoking at least 15 cigarettes per day for the past two years, and reported that they were not actively attempting to quit smoking. Smoking status was verified biochemically by carbon monoxide and cotinine testing (see parent studies, Evans et al., 2013; Evans et al., 2015). Inclusion in this genetic analyses was limited to Caucasian Non-Hispanic participants to avoid confounds produced by differential frequency of CNR1 variants across race/ethnicity. Participants were excluded for recent use of nicotine products other than cigarettes, neurological conditions, other serious medical conditions (e.g., cardiopulmonary problems or respiratory-related illness exacerbated by smoking), current use of psychoactive substances (as assessed by a urine drug test), vision problems, pregnancy or breast feeding, current psychosis, mood disorders, or non-nicotine substance dependence disorders . The Structured Clinical Interview for DSM disorders (SCID; First et al, 1994) was used to assess for psychopathology-related exclusionary criteria. A more detailed description of the inclusion/exclusion criteria are reported in Evans et al. (2015).
Procedure
This study was approved by the University of South Florida internal review board. Informed consent was acquired at the beginning of an initial screening session, which was followed by confirmation of the study eligibility criteria. Participants next completed questionnaires, including the FTND as a measure of nicotine/smoking dependence and the Wisconsin Smoking Withdrawal Scale (WSWS). This version of the WSWS did not include sleep- and diet-related items because they were not appropriate for overnight deprivation. Buccal cells were collected for DNA extraction and subsequent genotyping. Participants were then scheduled to attend two 2.5-hour experimental sessions that followed biochemically confirmed overnight (12-hour) nicotine deprivation (Evans et al., 2013). Deprivation was confirmed biochemically by a carbon monoxide (CO) level that was either less than or equal to 10 parts per million (ppm) or less than or equal to half of the CO level obtained during the screening session. Four cigarettes were smoked (Quest, Vector Tobacco, Inc.) during each experimental session, with two of these cigarettes smoked prior to collection of resting EEG data and subsequent performance of the visual oddball task. Time elapsed between initiation of each cigarette was 40 minutes. These cigarettes contained moderate nicotine (.60 mg nicotine yield) at one session and very low nicotine (< .05 mg nicotine yield) at the other session. Nicotine content of cigarettes was administered in counterbalanced order and in a double-blind fashion across the sessions. Resting EEG data was collected during 3 minutes of eyes open while participants relaxed (Evans et al., 2015).
Genotyping
Samples of buccal cells were used for Genomic DNA extraction (Gentra Puregene tissue kit; Valencia, CA) according to the manufacturer’s instructions. Samples were genotyped using the Illumina GoldenGate™ assay at the Molecular Genomic Core at the Moffitt Cancer Center. The BeadStudio algorithm was used to call genotypes. Four CNR1 SNPs were genotyped, including the three SNPs in the TAG haplotype (rs806379, rs1535255, and rs2023239) and an additional SNP (rs6928499) that was investigated in a previous study (Herman et al., 2006). However, the number of valid SNPs to be independently evaluated was reduced to two because Linkage disequilibrium (LD) values between rs2023239 and rs1535255, and rs806379 and rs6928499 were 1.0, indicating perfect correlation. The very high LD between rs1535255 and rs2023239 in this sample is consistent with the high LD (but less than 1.0) shown in the LD plot in the Zhang et al. (2004) CNR1 TAG study. Note that we only refer to rs2023239 and rs806379 from this point forward. However, keep in mind that reference to rs2023239 also includes rs1535255 and that rs806379 also includes rs6928499. Table 1 presents SNP characteristics, including minor/major allele frequencies and number of participants per genotype. R2 as a measure of LD between the two SNPs included in subsequent analyses was modest (.31). Both SNPs followed the Hardy-Weinberg equilibrium (p’s > .35).
Table 1: SNP characteristics and distribution.
| SNP | Allele (minor/major) |
Chr. | Location on gene |
MAF | Genotype (n=73) WT/Het/HoP |
Genotype WT/Carrier |
|---|---|---|---|---|---|---|
| rs806379 | T/A | 6 | Intron 2 | .41 | 21/35/9 | 21/44 |
| rs2023239 | G/A | 6 | Intron 2 | .18 | 50/20/3 | 50/23 |
Het, heterozygous; MAF: minor allele frequency; WT: wild type; HoP: homozygous polymorphic.
EEG Data processing
Resting EEG data was processed as described in the parent study (Evans et al., 2015). As with the parent studies, the current study focused on 7 homologous pairs (Fp1/2, AF3/4, F3/4, F7/8, C3/4, T7/8, P3/4) and 3 midline sites (Fz, Cz, & Pz) for the EEG power density analyses. For the 73 participants providing sufficient resting EEG data, there was a small amount of missing data from a variety of EEG sites (e.g., due to faulty electrode). The number of available observations for analyses ranged from 89.7% (Fp1/Fp2 & AF3/AF4) to 99.7% (C3/C4). In order to assess the effect of nicotine deprivation on resting alpha-1 power density values across the entire cortex, a single measure of alpha-1 power density values was computed. The highly correlated power density values were first standardized at each site because the distribution of alpha-1 varies by site. Standardized values across the 17 sites within the deprivation and the satiation condition were averaged to derive a single value to represent alpha-1 power density values across the entire cortex.
Statistical analyses
The primary focus of this study was to assess the influence of CNR1 SNPs as moderators of an established neural marker of withdrawal-related disruption of cognitive control. As described below, two of the SNPs were redundant (1.0 linkage disequilibrium), resulting in analysis of only two SNPs. Therefore, alpha was set at .025 to adjust for the parallel analyses for 2 SNPs. Mixed modeling was used to parallel the analyses revealing the main effect of deprivation versus satiation reported in Evans et al. (2015). Mixed modeling was also used to test the genotype x nicotine deprivation interaction. Genotypes for each analysis contrasted minor allele carriers versus noncarriers (major homozygote) in order to maximize the number of participants per group. Although not the main purpose of this study, we also report on self-reported withdrawal and SNP moderation thereof.
Chi-square and t-tests were used to test if genotypes at the 2 SNPs were differentially associated with gender, age, and smoking/nicotine dependence (FTND) as these variables may influence EEG measures. There were no significant differences. Some correlations between age and resting alpha-1 power under the deprivation or satiation condition were significant (r’s from −.01 to −.32, p’s from .006 to .915). Therefore, age was included as a covariate in models testing genetic moderation of nicotine withdrawal-related cognitive disruption.
Results
Smoking/nicotine deprivation
Mean breath carbon monoxide (CO) was 29.28 (SD = 13.56) ppm at the baseline session. Relative to baseline, there were significant reductions in CO following overnight deprivation at the start of both the nicotine deprivation (M = 10.29 ppm, SD = 4.64) and nicotine satiation (M = 10.14, SD = 4.59) sessions: F (1,72) = 215.67 and F(1,72) = 220.73, p’s < .0001, respectively, with no differences at the start of these sessions.
Self-reported smoking/nicotine withdrawal
Pre- versus post-smoking WSWS scores across sessions supported that smoking reduced withdrawal, F(1,72) = 48.15, p < .001. However, the smoking x nicotine deprivation interaction was not significant as a predictor of WSWS (p = .19). Nevertheless, the concentration difficulties subscale of the WSWS, which is more relevant to the aspect of withdrawal being examined in this paper, did show a significant smoking x nicotine deprivation interaction, F(1,72) = 4.04, p = .048. Smoking placebo cigarettes (pre-smoking M = 1.58, SD = .0.92 and post-smoking M = 1.52, SD = .0.87) resulted in greater concentration difficulties relative to nicotine cigarettes (pre-smoking M = 1.53, SD = .81 and post-smoking M = 1.26, SD = .79) following overnight deprivation. Neither rs806379 nor rs2023239 moderated the effect of nicotine deprivation on concentration difficulties (ps > .17 ).
Correlations among neural measures of cognitive disruption
Nicotine deprivation-induced reductions in P3b were not significantly correlated with nicotine deprivation-induced changes in alpha-1 resting EEG across all scalp sites. This is important to note because analysis of the ERP data was the basis for predicting genetic moderation of resting EEG alpha-1 power.
Resting EEG
An initial model assessed condition (deprivation versus satiation), session (first versus second), and their interaction with age included as a covariate. Replicating Evans et al. (2015) with a smaller sample, the measure of average standardized alpha-1 across the 17 electrodes was significantly greater during deprivation than during the satiation (p=.005). There were no other significant predictors in this model.
Our primary prediction focused on rs806379 (n=65) and rs2023239 (n=73) as a prospective moderators of the deprivation effect. These models added the SNP, and the interaction of the SNP with condition and with session to the initial model. Supporting the moderation prediction, the rs806379 × condition interaction was significant (p=.004) with a greater difference in those with the major allele homozygote. The interaction term in the parallel model assessing rs2023239 was not significant (p=.64).
To further understand the moderation of the deprivation-satiation difference, alpha-1 power density values were evaluated for each of the 7 homologous electrode pairs and the set of 3 midline sites. Figure 1 presents the difference in alpha-1 between the deprivation and satiation condition for each of the 8 electrode sets. Those with the major allele homozygote for rs806379 consistently exhibited a bigger difference (i.e., relatively greater alpha-1 during deprivation). As highlighted in the figure, the rs806379 × condition interaction was significant (p’s < .018) for 5 of the 8 cortical regions.
Figure 1. Alpha-1 power density value differences for rs806379.
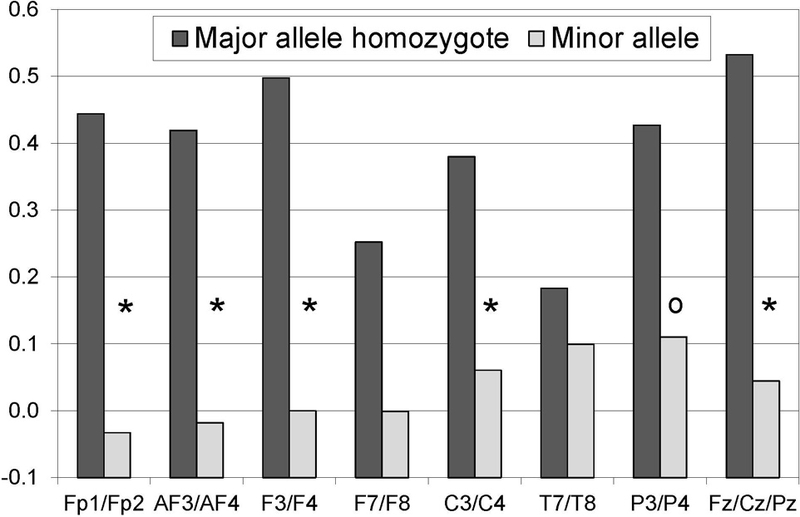
Each bar represents the difference in estimated alpha-1 power density value means for the deprivation and satiation conditions as generated by a mixed model analysis. Each analysis included condition, rs806379, and their interaction along with age, session, hemisphere, session x rs806379, hemisphere x rs806379, and condition × hemisphere × rs806379. Those with the major allele homozygote showed a significantly greater difference between deprivation and satiation than did those with the minor allele for 5 of the 8 cortical regions (*, p<.025). One other cortical region exhibited a marginally significant difference (o, p=.030).
Discussion
This study examined CNR1 variants as moderators of an established marker of nicotine deprivation-induced changes in cognitive functioning. Consistent with our prediction, it was found that major allele homozygotes in rs806379 experienced significantly greater nicotine deprivation-induced reductions in cognitive control as indicated by enhanced alpha-1 resting EEG power. This finding makes theoretical sense in that analysis of the “TAG” haplotype on CNR1 predicts reduced mRNA expression in postmortem analyses of the brain (Zhang et al., 2004), which suggest that the alleles comprising this haplotype may be predictive of less withdrawal-related cognitive disruption. Carriers of the minor allele (T) at rs806379 would thus be expected to have less CNR1 mRNA activity, which in turn should result in fewer CNR1 receptors and hence less CNR1 activity. As noted, CNR1 agonists may reduce cognitive control, and antagonists have been associated with improved cognitive control (e.g., Lichtman, 2000) and increased smoking cessation efficacy (Fernandez and Allison, 2004). The rs806379 findings suggest that CNR1 antagonism may mitigate the influence of nicotine deprivation on cognitive disruption, especially among individuals who experience greater disruption and/or possess genotypes indicative of greater CNR1 activity. The lack of effect from SNP rs2023239 may have possibly been the result of less power amid this small sample, as the minor allele frequency is much smaller (.15) than was the case for rs806379 (.41).
A potential limitation is that the functionality of these specific SNPs is not firmly established. Our rationale for selecting CNR1 SNPs was based on including SNPs from a previously examined haplotype that suggests the direction of influence of variants at these sites. That is, the TAG haplotype predicted reduced mRNA expression, thereby suggesting that the SNPs comprising this haplotype independently predict reduced mRNA expression (Zhang et al., 2004). However, the effect of rs806379 on mRNA expression in the brain without consideration of the more inclusive haplotype has not been reported. As little is known about the functional impact of CNR1 variants, these SNPs serve as our best candidates at this point in time.
A second potential limitation is that minor allele carriers were compared with major allele homozygotes for all genetic analyses in order to maximize statistical power by increasing the number of participants per genotype group. Future studies should be sufficiently powered to examine all three genotypes (major homozygote, heterozygote, and minor homozygote) for each SNP, as well as additional SNPs and haplotype combinations (e.g., the TAG).
Some of the measures may also serve as limitations. EEG was measured during a single period of 3-minutes of eyes open. Future research should use the more optimal approach of alternating eyes open and closed across 1 minute epochs. The CO criteria for 12-hour nicotine deprivation may be more liberal than used by some researchers, and has not been empirically established. However, the mean difference between deprivation (M ~ 10 ppm) and baseline satiation (M~ 30 ppm) were substantial, and were clearly indicative of greater deprivation.
Additional research is needed to determine the influence of centrally acting CNR1 antagonists on nicotine withdrawal-related cognitive disruption, including a better understanding of the potential brain mechanisms that might account for these effects. The influence of such drugs on cognitive disruption may subsequently be tested as adjunct smoking cessation pharmacotherapy. CNR1 antagonism may be more efficacious among smokers who find smoking more reinforcing for cognitive reasons (e.g., smokers with premorbid cognitive deficits and/or individuals exhibiting greater withdrawal-related cognitive deficits), or among individuals with specific CNR1 genotypes indicative of reduced mRNA expression in the brain. CNR1 antagonism may offer a unique pharmacologic pathway that improves the probability of smoking cessation among subgroups of smokers.
Acknowledgements
This study was funded by NIH grants R21 DA027001 (David Evans) and R21 DA024226 (David Drobes). The authors would like to thank Renee Ornduff and Natasha Garcia for their work on the project.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest (financial or other) regarding information reported herein. David Drobes has served as an expert witness in litigation against tobacco companies.
References
- Ashare RL, Falcone M & Lerman C (2014) Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology 76, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M (2002) Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology 164, 277–284. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R & Soubrie P (2002) SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol 13, 451–463. [DOI] [PubMed] [Google Scholar]
- Degroot A, Kofalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, Cunha RA & Nomikos GG (2006) CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol 70, 1236–1245. [DOI] [PubMed] [Google Scholar]
- Dien J (2010) Evaluating two‐step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology 47, 170–183. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Mizoguchi Y, Sovak SR, & Lewis DA (2010). Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex 20, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE & Drobes DJ (2009) Nicotine self-medication of cognitive-attentional processing. Addict Biol 14, 32–42. [DOI] [PubMed] [Google Scholar]
- Evans DE, Jentink KG, Sutton SK, Van Rensburg KJ & Drobes DJ (2014) 7 mg nicotine patch fails to enhance P300 neural indices of cognitive control among nonsmokers. Pharmacol Biochem Behav 126, 77–82. [DOI] [PubMed] [Google Scholar]
- Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG & Drobes DJ( 2013) Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology 38, 2525–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Sutton SK, Oliver JA & Drobes DJ( 2015) Cortical activity differs during nicotine deprivation versus satiation in heavy smokers. Psychopharmacology (Berl) , 232, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JR & Allison DB (2004) Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs , 5, 430–435. [PubMed] [Google Scholar]
- First MB, Spitzer R,L, Gibbon M , &Williams JBW (1994) Structured Clinical Interview for Axis IDSM-IV Disorders—Patient Edition (SCID-I/P, version 2.0) Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fujiwara M & Egashira N (2004) New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J Pharmacol Sci , 96, 362–366. [DOI] [PubMed] [Google Scholar]
- Gallagher NP, Mohlberg H, Zillies K, & Vogt BA (2008). Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol , 508, 906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen GF, Gatherwright JR, Lopez BA & Polich J (2006) P3a from visual stimuli: task difficulty effects. Int J Psychophysiol , 59, 8–14. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC & Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Herman AI, Kranzler HR, Cubells JF, Gelernter J & Covault J (2006) Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet , 141B, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Soei EX, Clarke SD, Kohn MR, Gordon E, Williams LM (2005) Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr Neurol 32, 248–256. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Arns M, Van Dongen-Boomsma M, Spronk D & Buitelaar JK (2011) The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog Neuropsychopharmacol Biol Psychiatry 35, 47–52. [DOI] [PubMed] [Google Scholar]
- Lichtman AH(2000) SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 404, 175–179. [DOI] [PubMed] [Google Scholar]
- Loo SK, Teale PD, Reite ML (1999) EEG correlates of methylphenidate response among children with ADHD: a preliminary report. Biol Psychiat 45, 1657–1660. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (2009). Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence NIH, Bethesda. [Google Scholar]
- Polich J (2007). Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF & Hillman CH (2011) Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J. Cognitive Neurosci 23, 1332–1345. [DOI] [PubMed] [Google Scholar]
- Putman P, van Peer J, Maimari I, van der Werff S (2010) EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol Psychol 83, 73–78. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR & Oz M (2007) The endocannabinoid anandamide inhibits the function of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol 72, 1024–1032. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliövaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nöthen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI & Beirut LJ (2010) Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet , 6, pii e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Janse Van Rensburg K, Jentink KG, & Drobes DJ, Evans DE (2016) Nicotine-induced cortical activation among nonsmokers with moderation by trait cognitive control. Psychopharmacology , DOI 10.1007/s00213-016-4276-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G & Soubrie P (1996) Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) , 126, 165–172. [DOI] [PubMed] [Google Scholar]
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Müller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Möller H-J, Gieger C, Wichmann H-E, Illig T, Dahmen N & Rujescu D (2010) Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet , 153B, 1448–1458. [DOI] [PubMed] [Google Scholar]
- Yang Y, Pspaalas CD, Jin LE, Picciotto MR, Arnsten FT, & Wang M (2013). Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. PNAS, 110, 12078–12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, & Huang XF (2004) Selective antagonist [3 H]SR141716A binding to CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry , 28, 355– 360. [DOI] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, Onaivi ES, Arinami T & Uhl GR (2004) Human cannabinoid receptor 1: 5' exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry , 9, 916–931. [DOI] [PubMed] [Google Scholar]


