Abstract
Septins belong to a family of conserved GTP-binding proteins found in majority of eukaryotic species except for higher plants. Septins form nonpolar complexes that further polymerize into filaments and associate with cell membranes, thus comprising newly acknowledged cytoskeletal system. Septins participate in a variety of cell processes and contribute to various pathophysiological states, including tumorigenesis and neurodegeneration. Here, we review the structural and functional properties of septins and the regulation of their dynamics with special emphasis on the role of septin filaments as a cytoskeletal system and its interaction with actin and microtubule cytoskeletons. We also discuss how septins compartmentalize the cell by forming local protein-anchoring scaffolds and by providing barriers for the lateral diffusion of the membrane proteins.
Keywords: septins, septin complexes, septin filaments, septin scaffold
INTRODUCTION
Septins are a family of conserved GTPases first identified in Saccharomyces cerevisiae cells [1], where they are present as 10-nm filaments in the bud neck. Based on this localization, as well as on the phenotypic presentation of their mutations, septins were described as proteins involved in the cytokinesis of budding yeasts [2–5]. At present, septins have been found in all eukaryotes except higher plants and have been implicated in many cell processes in addition to cytokinesis [6, 7].
Proteins of the septin family belong to a large superclass of P-loop GTPases [8]. Septins can interact with each other, which gives rise to hetero-oligomer complexes, which can in turn polymerize into filaments. These filaments are a functional form of septins. They interact with the cell membrane; serve as scaffolding for protein attachment; and, together with actin filaments and microtubules, act to maintain the shape of animal cells, and facilitate their migration [9, 10]. Furthermore, septins can form diffusion barriers that contribute to cell compartmentalization. Septins are also involved in the formation of flagella and cilia, in chromosome segregation, and in apoptosis, as well as in key processes of cell division, differentiation, and morphogenesis. Mutations and deletions of septin genes have been linked to different pathologic conditions, such as male sterility, ciliopathy, or neuromuscular disorders. Abnormal functioning of septins is observed in many types of tumors, Alzheimer’s disease, Parkinson’s disease, schizophrenia, and infectious diseases [11–14].
In this review, we describe the structural and functional properties of septins, as well as specific features of the regulation of their dynamics. In particular, we focus on the role of septins in cytoskeleton formation and discuss their interaction with the actin filament system and microtubules.
EVOLUTIONARY CONSERVATION OF SEPTINS
For a long time, it was assumed that septins were present in the cells of animals and fungi, but not in other eukaryotes. The recent discovery of septins in some photosynthesizing and brown algae, as well as in infusoria [15], has considerably changed the notions concerning the origin and early evolution of septins. It is currently believed that the primordial septin-encoding gene appeared in an ancient ancestor of eukaryotes and was later lost in the ancestor of plants, but multiplied in the ancestors of fungi and animals [15]. Interestingly, bacteria were found to possess septin-like proteins, paraseptins. Therefore, it was supposed that eukaryotes may have acquired septin genes from bacteria by horizontal transfer of the ancestor paraseptin gene [8]. It remains unclear why land plants completely lost septins and why the increase in the number of septin genes in different groups of animals and fungi has been so nonuniform.
The genome of the budding yeast S. cerevisiae comprises seven septin-encoding genes, i.e., cdc10, cdc3, cdc11, cdc12, shs1, spr3, and spr28, the same number as the fission yeast Schizosaccharomyces pombe. There are only two septin genes in the nematode Caenorhabditis elegans (unc-59 and unc-61), five in the fruit fly Drosophila melanogaster (pnut, sep1, sep2, sep4, and sep5), and thirteen in the mouse Mus musculus and the human Homo sapiens (SEPT1–SEPT12, and SEPT14). Furthermore, the diversity of mammalian septins is even higher due to the existence of transcript isoforms expressed from alternative promoters and variants produced by alternative splicing. At present, the highest level of diversity has been described for products of SEPT9 expression, i.e., 18 mRNAs and 15 polypeptides.
The currently most popular classification of metazoan septins was originally proposed by Kinoshita for human septins [16]. Subsequently, Cao et al. constructed a detailed map of septin evolution based on 78 primary septin sequences of various Metazoa [7]. In this work, metazoan septins were divided into four groups previously described by Kinoshita, i.e., the groups of septin 2 (SEPT2), septin 3 (SEPT3), septin 6 (SEPT6), and septin 7 (SEPT7). Examples of proteins that represent these groups in different organisms are given in the table. Unfortunately, the currently existing classification is inappropriate for describing the orthology relationships between septins of animals and fungi, which makes it considerably more difficult to compare yeast and animal model systems.
An important conclusion by Cao et al. is that septin homologs of all four groups are present even in deu-terostomatous invertebrates, i.e. the sea urchin Strongylocentrotus purpuratus and the ascidium Ciona intestinalis [7]. This shows that all four septin groups were formed before the division between vertebrates and invertebrates occurred, which means that the number of septin genes in vertebrates increased mainly by duplication of the already existing genes, and not by the genesis of novel groups of septins. Orthologs of most human septins (except for SEPT1 and SEPT14) are present in fish [7]. Thus, the principal repertoire of septin genes was formed before the division between fish and terrestrial vertebrates.
The multiplication of septin genes in vertebrates led to an increase in the number of processes involving septins and at the same time provided the possibility of functional substitution among different septins, which complicates the study of individual septin functions.
PHYSICAL PROPERTIES OF SEPTINS
Septins are GTPases of the P-loop NTPase super-class, which includes numerous nucleotide-binding proteins, in particular, the subfamily of Ras-like GTPases, translation factors, as well as myosin and kinesin ATPases. Septins possess a GTPase domain with conserved motifs G1, G3, G4, and G5 that are characteristic of GTP-binding proteins [8]. The G1 motif (GxxxxGK), known as Walker A, forms a flexible loop that interacts with the α-and β-phosphate groups of GTP. It is this phosphate-binding loop (P-loop) found in most nucleotide-binding proteins that the super-class owes its name to. The G3 motif (DxxG), also known as Walker B, participates in the binding of Mg2+ and the γ-phosphate of GTP. The G4 motif (xKxD) is responsible for the specific recognition of the guanine ring.
Usually, the GTPase domain occupies the central position in the protein sequence (Fig. 1a). In most septins, the GTPase domain is flanked by a polybasic fragment of ~20 amino acids, which binds to membrane phospholipids, at the N-terminus [17, 18] and by a sequence of 53 amino acid residues (septin unique element) at the C-terminus [19]. The role of this element is not known exactly; however, it is known that it is required for the interaction of septin monomers during the assembly of complexes and filaments [20]. Many septins feature a C-terminal α-helical domain that can form a double supercoil (coiled-coil domain). This domain participates in protein–protein interactions [6]. Septins of some fungi and algae lack a coiled-coil domain, but possess a hydrophobic domain, which has probably evolved as an alternative solution to enable protein–protein interactions and septin anchoring [15].
Fig. 1.
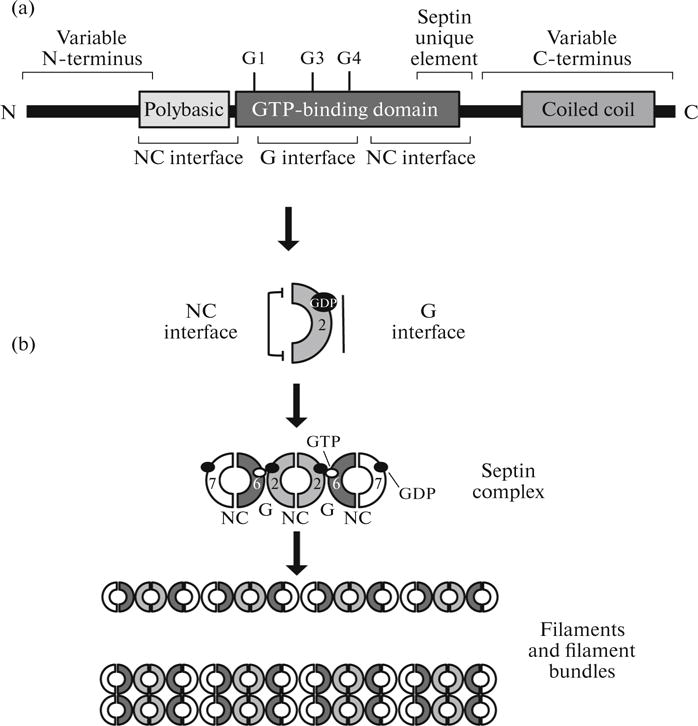
Structure of septin complexes and filaments. (a) Typical septin structure. Septins possess a GTP-binding domain with G1, G3, and G4 motifs. Between the N-terminus and the GTPase domain, there is a highly conserved polybasic region required for interactions with membranes. C-terminus of the protein frequently contains a coiled-coil domain involved in protein–protein interactions. In addition, septins include the septin unique element of 53 amino acids, whose functions are currently unknown. (b) Structure of septin complexes and filaments shown for human SEPT2/6/7 complex. G is interactions via the G interface; NC is interactions via the NC interface.
An important distinguishing feature of septins is their ability to interact with each other and to form heteromeric complexes that comprise two (C. elegans [21]), three (human [20], drosophila [22]), or four (S. cerevisiae [23]) different septins, each of them present in two copies. Usually, a complex is formed by septins of different classification groups (table). In some cases, proteins of the same group may be interchangeable [7, 16, 23–27]. Septin subunits are located symmetrically relative to the center of the complex; for example, a human septin hexamer has the following structure: SEPT7–SEPT6–SEPT2–SEPT2–SEPT6–SEPT7. In some cases, this complex may include SEPT9, which produces an octamer similar to the complex found in yeast [25, 26, 28]. The exact size and composition of all vertebrate septin complexes has not been described yet; however, SEPT2/6/7/9, SEPT2/6/7, SEPT7/9/11 [29], SEPT3/5/7, and SEPT5/7/11 complexes were isolated from cell cultures [25, 30, 31]. Noncanonical complexes composed of subunits of the same group, such as SEPT2/5/6/7, SEPT4/5/8, SEPT4/14, SEPT2/5, and SEPT1/5, have also been described [32–37], but their functions are currently unknown.
Table 1.
Classification of metazoan septins
| Septin group | Mammals | Fruit fly D. melanogaster | Nematode C. elegans | Ascidium C. intestinalis | Sea urchin S. purpuratus |
|---|---|---|---|---|---|
| SEPT2 | SEPT1, SEPT2, SEPT4, SEPT5 | Sep1, Sep4 | – | gi*:198428956-Ci | gi*:115951499-Sp |
| SEPT6 | SEPT6, SEPT8, SEPT10, SEPT11, SEPT14 | Sep2, Sep5 | Unc-61 | gi*:198432765-Ci | gi*:115770370-Sp |
| SEPT7 | SEPT7 | Pnut | Unc-59 | gi*:198436549-Ci | gi*:115720187-Sp |
| SEPT3** | SEPT3, SEPT9, SEPT12 | – | – | gi*:198417201-Ci | gi*:115715387-Sp |
gi: sequence number in the NCBI database.
In some sources, this group is termed SEPT9.
The best-studied septin complex is the human SEPT2/6/7; its structure has been determined using X-ray crystallography [20]. Subunit dimerization takes place via two interfaces, one of which includes the nucleotide-binding site (G-interface), while the other one involves the N- and the C-terminal helices that flank the nucleotide-binding site (NC interface). In a septin complex, these two interfaces occur intermittently. For instance, SEPT7–SEPT6 and SEPT2–SEPT2 interactions are mediated by the NC interface, and SEPT6–SEPT2 interactions are mediated by the G interface (Fig. 1b). The stability of interactions mediated by a G interface depends on the presence of a guanosine phosphate in the GTP-binding site [20, 38, 39], while NC interfaces are not affected by this.
The end-to-end interaction of septin complexes results in the formation of long filaments (Fig. 1b) [20, 21, 23]. Septin complexes are nonpolar, since the subunits are located symmetrically relative to the center. Therefore, filaments made of these complexes are also nonpolar, which makes them similar to intermediate filaments. In contrast, polar actin microfilaments and microtubules possess the plus and the minus end where their assembly and disassembly occur, respectively. Septin filaments may form more highly ordered structures, such as filament bundles and rings (Fig. 1b). Apparently, the lateral contact between filaments is in such cases mediated by C-terminal coiled-coil domains of septin subunits that are positioned perpendicular to the long axis of filaments [23, 30, 40].
Complexes and filaments are the functional form of septins. Many septins, if expressed individually, are poorly soluble and tend to aggregate [19, 41, 42]. In yeast, mutations that impair the formation of septin filaments are lethal [27].
REGULATION OF SEPTIN FILAMENT ASSEMBLY
Septins are GTPases, most of them can hydrolyze GTP and be bound either to GTP or to GDP [17, 22, 43–46], except for septins of the SEPT6 group, which have no catalytic activity; they are constitutively bound to GTP and cannot hydrolyze it. In the actin and microtubular cytoskeleton, the role of nucleotide hydrolysis (ATP for actin and GTP for tubulin) is to regulate the filament assembly and disassembly. Actin and tubulin monomers bound to ATP and GTP, respectively, can polymerize and form filaments. Nucleotide hydrolysis destabilizes contacts between monomers, facilitating filament disassembly [47, 48]. This mechanism enables the rapid reorganization of actin and microtubular cytoskeleton.
Septin polymerization represents a different case. The human septin complex SEPT7–SEPT6–SEPT2–SEPT2–SEPT6–SEPT7, which is currently the best studied, forms filaments due to the interaction between G interfaces of SEPT7 subunits of neighboring complexes (Fig. 1b) [20], and the presence of GDP in the GTPase domain of SEPT7 stabilizes the contact [49]. Thus, nucleotide hydrolysis may contribute to septin regulation; however, unlike actin filaments and microtubules, septin filaments are more stable in the GDP-bound form. The low rates of hydrolysis and nucleotide exchange typical for septins allow the existence of two relatively stable states: the GTP- and the GDP-bound one. This, in turn, may underlie the slower dynamics and higher stability of the septin cytoskeleton, in comparison to the actin and the microtubular cytoskeleton components [50, 51].
Along with GTP hydrolysis, dynamics of septin filaments in vivo may be regulated by various posttranslational modifications, such as phosphorylation, sumoylation, acetylation, or ubiquitination [52]. Most modifications were described in septins of yeasts and filamentous fungi [53–56]. Among the few modifications detected in metazoan septins, the most interesting phenomenon is the ubiquitination of human SEPT4 and SEPT5 [57], as well as of Sep4, the SEPT5 ortholog in drosophila [58]. This reaction is mediated by ubiquitin ligase PARK2 (Parkin in drosophila), and its mutations cause an autosomal recessive form of early Parkinson’s disease [59]. Both SEPT4 and SEPT5 are PARK2 substrates, and the enzyme is essential for their degradation. According to one of the existing hypotheses, a decrease in PARK2 levels leads to septin accumulation and contributes to the pathogenesis of Parkinson’s disease [60–62].
The assembly and disassembly of septin filaments may be regulated by their interaction with other proteins. Most septins possess a C-terminal coiled-coil domain, which participates in protein–protein interactions. X-ray studies showed that the C-ends of septin molecules within complexes are positioned perpendicular to the long axis (Fig. 2) and, thus, represent a convenient anchoring site for other proteins [20]. For example, in mammalian cells, septins interact with the Cdc42-binding Borg3 protein, the excessive production of which leads to the aggregation of septin filaments [63]. In yeast cells, Gic1 protein functionally homologous to Borg also binds to septin complexes and stimulates the formation of long filament bundles [64]. Cdc42-dependent aggregation of septin filaments helps to establish cell polarity (Fig. 3a). The assembly of the septin ring in yeast involves TRiC/CCT chaperones, which are also responsible for component folding in the actin and microtubular cytoskeleton [65]. Another example is the Orc6 protein in drosoph-ila, an ORC subunit required for DNA replication, which binds to septin complexes in the stoichiometric ratio of two Orc6 molecules per hexamer and stimulates polymerizaton of filaments (Fig. 2) [66, 67]. Orc6 remains bound to the polymerized filaments and apparently stabilizes them due to Orc6–Orc6 dimerization [67].
Fig. 2.
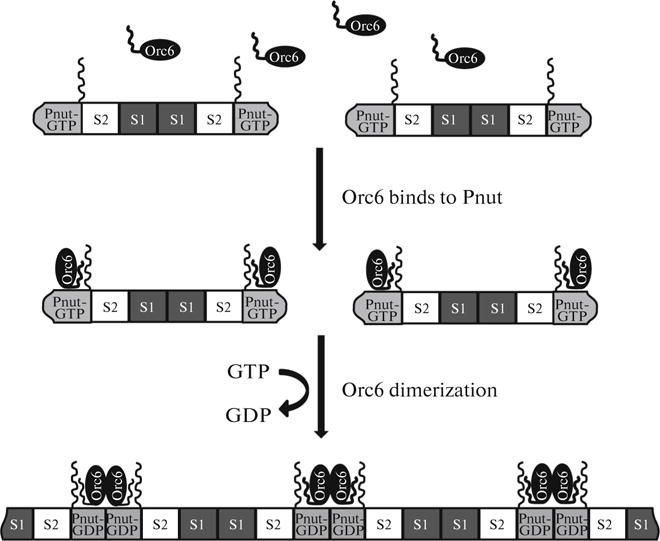
Orc6 and GTP promote the formation of septin filaments in drosophila. C-terminal domains of septins are located perpendicular to the long axis of the complex (shown only for Pnut). Orc6 molecules bind to Pnut septins located at both ends of the complex. Binding to Orc6 stimulates GTP hydrolysis by Pnut. As a consequence, contacts between Pnut subunits become tighter, which stabilizes the resulting filaments. S1, Sep1; S2, Sep2.
Fig. 3.
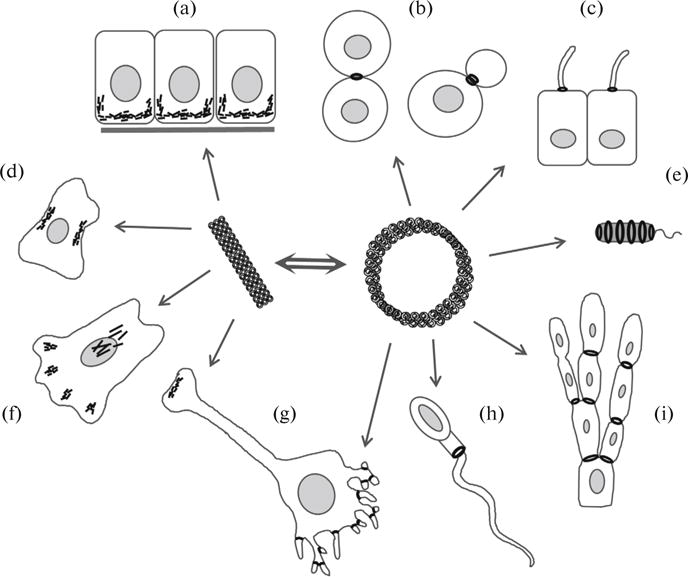
Septin functions in various biological processes: (a) polarization of epithelial cells; (b) cleavage furrow in an animal cell cytokinesis and in yeast budding; (c) diffusion barrier in cilia; (d) cell rigidity corset; (e) immobilization of bacteria within an infected cell; (f) cell migration; (g) mediator exocytosis in a neuronal synapse and dendtritic spine growth in a neuron; h, structural and diffusion barrier in the annulus of a mammalian spermatozoon; (i) growth and branching of hyphae in filamentous fungi.
SEPTINS AS PROTEIN SCAFFOLDS
Thanks to their ability to bind to the cell membrane and to form filaments, septins frequently act as scaffolding for a wide range of proteins from membrane-bound receptors to cytoskeletal proteins. The great variety of mammalian septin isoforms produced by alternative splicing serves to modulate the properties of septin complexes and, accordingly, septin filaments. The septin cytoskeleton represents a discrete system of scaffolds involved in various cell processes. At different stages of the cell cycle, the septin scaffolding may be either relatively stable or dynamically mobile, e.g., during cytokinesis or cell migration.
In the course of cytokinesis, septin filaments of budding yeasts form a ring in the bud neck (Fig. 3b), which serves as a platform for approximately 40 proteins [68, 69]. Some of them are structural proteins, such as chitin synthases Chs3 and Chs4, which are attached to the septin ring by means of Bni4 [69]; during the G1 phase, Bni4 binds to the septin ring on the maternal cell side and then passes through the ring to reappear on the daughter cell side [69]. The septin scaffolding is also employed by the proteins Bud3, Bud4, and Axl2/Bud10, which determine the direction of the division axis in a yeast cell, as well as by Bud5 and Bud2 factors, which regulate the accuracy of budding [69]. Furthermore, the septin ring also binds a regulatory module that controls cytokinesis and morphogenesis during budding. In S. cerevisiae, the central component of this module is protein kinase Swe1 (orthologous to Wee1 in S. pombe); in case of abnormal morphogenesis it phosphorylates cyclin-dependent kinase Cdc28, which results in arrest of the cell cycle. In the course of normal cytokinesis, components of the checkpoint system consecutively dock to the septin ring and phosphorylate Swe1, inducing its degradation [69].
In mammals, cytokinesis is also usually accompanied by the formation of a septin ring that serves as a platform for signaling effectors and some other proteins [70, 71]. In mammalian fibroblasts, mouse embryonic epithelial cells, or human HeLa cells, cyto-kinesis is impossible in the absence of septins. Different mammalian septins control different stages of cytokinesis; for instance, in HeLa cells, SEPT2, SEPT7, or SEPT11 deficiency impairs early stages of cytokinesis, while the lack of SEPT9 affects the last stage of cell division [72]. Some types of mammalian cells seem to possess compensatory mechanisms that allow them to complete mitosis in the absence of septins [73–75]. Hematopoietic cells exhibit the highest level of resistance to septin depletion: murine amoeboid T cells divide normally in the absence of the major ubiquitously expressed SEPT7. Human cells of the lines K562 and Jurkat are also capable of septin-independent division [25, 73–75]. Presumably, in complex tissues where cells are attached to the extracellular matrix or densely packed, cytokinesis is septin-dependent. In tissues of simpler structure with unattached cells, cytokinesis may occur without involving septins [75]. To obtain a complete picture of septin involvement in cytokinesis in multicellular organisms, a comprehensive investigation of cell division in different tissues is required.
In filamentous fungi Ashbya gossypii and Aspergillus nidulans, septins regulate the branching of hyphae (Fig. 3i). In A. gossypii, mitosis takes place primarily at the branching sites, which feature septin rings; accordingly, septins may regulate the growth of hyphae in response to nutrition gradients [76]. In a similar way, septin scaffolds regulate the growth of germ tubes and the branching of hyphae in multicellular fungi A. nidulans [77].
Metazoan septins serve as scaffolding not only during cell division. Septin cytoskeleton contributes considerably to the regulation of surface dynamics of different receptors and transporters by anchoring them and their partners at certain sites on the membrane. For example, in astrocytes of the mouse cerebellum, SEPT2 colocalizes with the GLAST receptor of glutamate, the most common excitatory neurotransmitter in the nervous system of vertebrates. The expression of SEPT2 with a mutated GTPase domain, which is critical for the formation of septin complexes, caused a decrease in the level of glutamate uptake in a primary culture of glial cells due to GLAST internalization [78]. Apparently, the mutation of the GTPase domain affected the structure of SEPT2 and interfered with the formation of the cortical septin cytoskeleton. As a result, the receptor was no longer anchored to the membrane, which led to its internalization and decreased the glutamate uptake [78].
The septin cytoskeleton also participates in the maintenance and regulation of the cell shape and motility [73, 79–82]. Membrane-associated septins interact with other components of the cytoskeleton and determine the rigidity of the cell (Fig. 3d) [79]. In the absence of SEPT2 or SEPT11, rigidity and cortical elasticity of HeLa cells decreased [83]. SEPT7 depletion in mouse amoeboid T cells damaged significantly their morphology (cells lost their rigidity, their membranes bubbled, and additional protrusions appeared), but did not alter the cell volume. Cells lacking the septin foundation can squeeze through narrow openings, which may facilitate the progression and metastasis of malignant tumors [73].
Many processes of individual development depend on collective cell migration. For example, during the development of gonadal anlage in drosophila embryo, germline cells must migrate from the posterior pole into the embryo. During migration, this group of cells divides in two, which, by passing through the primary gut, associate with mesenchymal cells to form two gonadal primordia. As such, cell motility depends on the dynamic rearrangement of the actin and microtubular cytoskeleton. Septin filaments with their slower dynamics can serve as a sort of scaffolding for microtubules and actin filaments, maintaining the cell shape and the direction of motion (Fig. 3f). Dolat et al. showed that SEPT9 promoted the motility of kidney epithelial cells by enhancing the crosslinking of lamellar actin stress fibers developing on the apical edge of migrating cells [84]. In Xenopus laevis, the knockdown of SEPT2 or SEPT7 impaired collective cell migration in embryogenesis [81]. The lack of septins hindered the correct axonal guidance of motor and sensory neurons in C. elegans [85], as well as cortical neuron migration in the mouse [35].
Septin scaffolding also participates in the transportation of cell vesicles. The process of protein secretion involves the delivery of protein-containing vesicles to the cell membrane, their docking, and the SNARE-mediated fusion of the vesicle membrane and the plas-malemma. Intracellular transportation of vesicles, both from the endoplasmic reticulum (ER) to the Golgi apparatus and from the Golgi apparatus to the cell membrane, is mediated by microtubules, while septins regulate the stability and growth of microtu-bules (see Interaction with Actin and Microtubules). In addition to the indirect effect mediated by microtubules, septins can directly participate in exocytosis. It was shown that mammalian septins are located at the sites of exocytosis and interact directly with components of the exocyst and with SNARE proteins, which guide the docking of vesicles and their fusion with the cell membrane [36, 86–90]. Downregulation of SEPT2 expression by RNA interference, or blocking the septin dynamics by forchlorfenuron, a septin-specific agent, leads to a decrease of neurotransmitter secretion in motor neurons of the mouse [90]. In these cases, late membrane-associated stages of exocytosis were affected, in particular the docking of vesicles and membrane fusion, which agrees with the fact that septins colocalize with the proteins involved in these processes. Interestingly, different septins may have different effects on exocytosis. For example, SEPT2 and SEPT4 stimulate exocytosis [90], SEPT5 suppresses it [89], while SEPT3 and SEPT6 do not influence this process [91, 92].
SEPTINS AS DIFFUSION BARRIERS
The functioning of septin filaments as diffusion barriers was first discovered in S. cerevisiae. Using the FRAP technique (fluorescent recovery after photobleaching), it was shown that the septin ring in the bud neck represents a physical barrier that prevents free diffusion of certain proteins. For instance, during budding, Ist2p protein of the internal membrane is only found in the daughter cell, but if the septin ring was destroyed, Ist2p spread into the maternal cell [93]. In a similar way, the septin ring of the bud neck limits the diffusion of Lte1 factor to the daughter cell. In cells with a septin gene mutation, Lte1 spread into the maternal cell and activated Tem1, triggering premature termination of division [69].
The role of septins as diffusion barriers is not limited to dividing cells. Septin filaments form the ring at the annulus, a special structure required to separate two membrane domains of a mammalian sperm tail and ensure the correct tail architecture (Fig. 3h). In mice lacking SEPT4, spermatozoa have no annulus, their tails look broken and are immobile; these animals are sterile [94]. A study of the structure and morphology of spermatozoa in men with astenozoospermia (reduced sperm motility) also revealed the abnormal structure of the annulus [95]. It was later shown that the role of septins in the annulus is not merely structural; they also act as a diffusion barrier that restricts the spread of basidin, a transmembrane glycoprotein [96]. An unexpected finding was the impaired fusion of mitochondria in SEPT4-knockout mice, which suggests possible involvement of septins in this process [94].
Septin diffusion barriers in cilia are worth special discussion. Membranes of these organelles contain a large number of receptors and sensors that perceive various extracellular signals, including mechanical stimuli, morphogenic agents, or light [97]. Cilia are involved in the motion of the intercellular fluid at different stages of ontogenesis, e.g., in the Hensen’s node in early mammalian embryogenesis, or in renal tubules. Although the ciliary membrane is a continuation of the cell membrane, it is nevertheless distinct from it, since FRAP data indicate that membrane proteins could circulate freely within the cilial membrane but not beyond [98]. A septin ring is a structure frequently present at the base of both primary (immotile) and motile cilia [81, 98–100] (Fig. 3c). SEPT2, SEPT7, or SEPT9 depletion in human retinal pigmented epithelial cells (RPE1), which represent a canonical model for study of primary cilia, resulted in shortening of cilia and decrease in their number [99]. SEPT2 depletion in kidney epithelial cells or in mouse embryonic fibroblasts leads to enhanced diffusion of membrane proteins between cilia and the remaining cell membrane, which demonstrates the importance of the septin diffusion barrier in ciliogenesis [100]. In zebrafish Danio rerio, sept7b knockout caused a range of abnormalities typical of defects of the ciliary structure and function, including impaired left–right asymmetry resulting in organ disposition, pericardial and yolk sac edema, body curvature, and hydrocephaly, as well as the reduction of fluid flow in renal tubules and formation of kidney cysts [101].
SEPTINS AND COMPARTMENTALIZATION OF THE CELL
An important example illustrating the involvement of septins in cell compartmentalization is asymmetrical division in the budding yeast S. cerevisiae, which allows the daughter cell to inherit only healthy and fully functional cell components, undergoing a sort of rejuvenation. This specific mode of component distribution is supported by coordinated activity of several factors that generate a diffusion barrier and by directed transportation of the corresponding components [102]. The septin ring, which has been formed by the beginning of division, recruits cell polarization proteins, including Bud1p and Bud5p proteins of the Ras family. These proteins, in turn, activate components of the Cdc42p signaling cascade; this induces the assem bly of actin filament bundles and accumulation of sphingolipids near the bud neck, which, together with the associated secondary proteins, prevent misfolded ER proteins from entering the daughter cell. Recently, it was shown that the importance of the septin ring in the maintenance of the diffusion barrier for ER proteins is even greater: Epo1p can bind both the ER protein Scs2p and septin Shs1p [103]. Mutations that impair the functioning or interactions of Shs1p, Scs2p, or Epo1p lead to free diffusion of membrane ER proteins between the maternal and the daughter cells. Thus, yeast septins play a double role in the establishment of cell polarity. First, they form a platform for recruiting polarization proteins in the beginning of the process, and secondly, they participate in the generation of diffusion barriers during bud growth. The coordinated functioning of the actin cytoskeleton responsible for the component transport, as well as of septins and sphingolipids that generate the diffusion barrier, enables asymmetrical segregation of proteins, mRNAs, and organelles.
Cell polarization can be considered an extreme form of compartmentalization in which the contents of a cell and its membrane are divided into functionally different domains, such the apical and the basolateral domains in epithelial cells. To a considerable extent, the development and maintenance of membrane domains rely on the association of septins with phospholipids and on vesicular transport. The conserved polybasic N-terminal region of a septin molecule (Fig. 1a) can bind membrane phospholipids. Yeast septins bind primarily to phosphatidylinositol 4-phosphate (PI4P) and phosphatidylinositol 5-phosphate (PI5P). Mammalian SEPT4 specifically binds to phosphatidylinositol 4,5-biphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3) [17, 18]. Cell polarization and shape maintenance depend on directed vesicle trafficking from the Golgi apparatus (trans-Golgi) to the corresponding domain of the cell membrane [104]. It was shown that in Madin-Darby canine kidney (MDCK) epithelial cells, SEPT2 colo-calizes with a subset of microtubule bundles and is required for the efficient transportation of vesicles from the Golgi apparatus to the cell membrane [104].
Neurons represent another impressive example of septin involvement in cell compartmentalization. Many septins are actively expressed in the central nervous system, and aberrant patterns of their expression correlate with a wide range of neurological disorders [10]. In mammals, septins form a ring at the base of dendritic spines (Fig. 3g), as well as in the area of dendrite branching in hippocampal neurons [10]. Knockdown of SEPT7 or SEPT11 decreases dendrite branching and the spine density, while ectopic SEPT7 expression has an opposite effect [105–108]. Den-dritic spines are small membrane protrusions from a dendrite that participate in the processing and storage of information. Each spine acts autonomously, and a neuron may possess dozens of thousands of them [10]. The septin ring at the base of each spine serves as a diffusion barrier ensuring that the composition of the spine membrane differs significantly from the composition of the neuron body membrane [107, 109]. For instance, only certain groups of spines possess AMPA glutamate receptors, one of the most common receptor types in the nervous system [110]. The functions of the septin ring in the morphogenesis of spines are not limited to generating the diffusion barrier, but also include interactions with actin, microtubules, and myosin II [111–113], although the mechanisms of their coordination have not yet been fully elucidated.
INTERACTIONS WITH ACTIN AND MICROTUBULES
There is no doubt that septin and actin cytoskeletons are closely interacting. Septins colocalize with different populations of actin filaments, such as the contractile ring, filopodia, and lamellopodia of motile cells, stress fibers, and cortical patches of actin bundles [50]. Initially, it was shown that septins’ interactions with actin involve adapter proteins, i.e., anillin [30] and myosin II motor protein [112], as well as proteins of the BORG family [114]. Anillin is an important component of the contractile ring in mitosis. It contains both actin- and septin-binding domains and serves as a platform for septins in the zone of cytokine-sis [30, 115, 116]. Since for the most part of the cell cycle anillin is located in the nucleus and is released into the cytosol only during cell division, it was supposed that the interphase adapter protein might be nonmuscular myosin II, a motor protein involved in the formation of actin bundles and in contraction of actin structures. SEPT2 serves as a support for phosphorylation of the light chain of myosin II by Rho-and citronkinases and thus participates in the contraction of the actomyosin contractile ring in cytokinesis [112]. Proteins of the BORG family can also act as adapters. BORG2 can bind both to actin and to septins; it is located between actin stress fibers and septin filaments and might serve as a sort of molecular glue that connects these two cytoskeletal systems to each other. It was also found that elevated BORG2 expression stimulated the formation of both septin and actin filaments, which suggests that these interactions stabilize both cytoskeletons [114].
In drosophila, Sep1/Sep2/Pnut complexes can interact directly with actin and bind actin filaments together into curved bundles, which is of critical importance for the organization and functioning of the contractile ring in early embryos [117]. Human SEPT2/SEPT6/SEPT7 complexes also have a similar effect on the bending of actin bundles in vitro, which shows that the septin–actin interaction is evolutionarily conserved. In MDCK cells, SEPT9 binds directly to actin and promotes its crosslinking, thus contributing to the stabilization of developing focal contacts and the motility of epithelial cells [84]. The association of SEPT9 with actin depends on the unique fragment of its N-terminal domain, which is absent in other septins [118]. The mechanism by which other septins, as well as septin complexes, interact with actin has not been established so far. It should be noted that the mentioned studies used actin of different origin with different isoform composition (actin of rabbit muscle or nonmuscular actin of human platelets), which could also have influenced the results.
The nature of interdependence between actin and septin cytoskeletons apparently varies considerably with the cell type and the particular actin cytoskeleton population. For instance, abnormal dynamics of cortical actin affect the dynamics of colocalized cortical septins, but not the other way around [50]. The situation with stress fibers is different. Depolymerization of actin disrupts septin–actin colocalization; as a result, septin rings and arch-shaped structures appear in the cytosol. On the other hand, disruption of septin filaments by RNA interference leads to the disappearance of stress fibers [30, 119].
The first data concerning the interaction between septins and microtubules were obtained in yeast [120, 121]. In mammals, SEPT2, 6, 7, and 9 colocalize with microtubules, and the extent of colocalization depends on septin isoform, cell line, and cell cycle phase. As a rule, colocalization is limited to a certain cell region, such as the area near the nuclear membrane or the cell periphery [122].
Septin deficiency or overexpression impairs the organization, dynamics, and posttranslational modifications of microtubules. As a result of the downregulation of SEPT7 expression in HeLa cells, microtubules acquired resistance to nocodazole, and the level of acetylated tubulin increased [123]. In MDCK cells, polyglutaminated microtubules that guide the transport of vesicles from the Golgi apparatus to the cell membrane are enriched in SEPT2. SEPT2 RNA interference decreased the abundance of polyglutaminated microtubules and impaired vesicle delivery [104]. In mouse neurons, SEPT7 acts as a platform for α-tubulin deacetylase, HDAC6, diminishing the stability of microtubules to the level optimal for normal neurogenesis. SEPT7 depletion is associated with significant accumulation of acetylated α-tubulin and delayed growth of microtubules, which results in abnormal neurogenesis, such as impaired elongation and branching in both dendrites and the axon [62].
SEPT9 associates with microtubules and mediates their crosslinking into bundles due to electrostatic interactions between its N-terminus and the C-terminus of β-tubulin [124]. The N-terminal part of SEPT9 contains several repeats of two motifs, K/R-x-x-E/D and R/K-R-x-E, which were found to be critically important for the SEPT9 effect on microtubules in vitro. This result was also confirmed in vivo in MDCK cells. It was shown that SEPT9-dependent formation of microtubule bundles is extremely important for correct axon growth [124]. For other septins, the mechanisms of their binding to microtubules are currently unknown.
The fact that septins participate in the regulation of actin and microtubule cytoskeletons provokes the question concerning their exact role in the coordination of these two systems. For instance, it was shown that SEPT6 and SEPT7 subunits of the septin complex comprise a regulatory module serving for coordinated reorganization of the actin and the microtubular cytoskeletons during axon branching [125]. It is not clear how rearrangements in one of the cytoskeletal systems influence another one. Presumably, the key role belongs to the different rates of the septin, actin, and microtubular cytoskeleton dynamics, which allows them to take turns to provide a scaffolding for assembly, disassembly, and/or modification of the other. It is also possible that septins integrate different signals in order to contribute to the feedback loop including actin and microtubules. For example, all three components of the cytoskeleton are regulated via different branches of the Cdc42 signaling pathway [63, 114, 126, 127].
OTHER FUNCTIONS OF SEPTINS
Mammalian genomes contain numerous septin-encoding genes. Most mRNAs transcribed from these genes undergo splicing and produce various isoforms, the functions of which may differ considerably. For instance, ARTS is an isoform of septin SEPT4, which features a typical GTP-binding motif but lacks the coiled-coil C-terminal domain. Loss of ARTS expression is observed in more than 70% of patients with acute lymphoblastic leukemia, while other SEPT4 isoforms are still expressed [128]. In contrast to other septins, which are usually associated with the cell membrane, ARTS is located in mitochondria. In normal cells, in response to an apoptotic signal, ARTS is released from mitochondria into the cytoplasm, where it binds XIAP1, an apoptosis inhibitor and thus activates apoptosis [129]. The binding of ARTS and XIAP1 is mediated by the С-terminal region of ARTS, called ARTS-IBM (AIBM). A short AIBM-mimicking synthetic peptide has been developed that can trigger apoptosis in tumor cells [130].
Septin M is another mitochondrial septin also produced by alternative splicing of SEPT4 mRNA; in contrast to ARTS, it has a C-terminal coiled coil domain [131]. In the developing mouse and rat brain, septin M is associated with mitochondria until the stage of neuron differentiation, but not after the induction of neurite growth. Since the transportation of mitochondria in dendrites and axons is essential for neuron polarization and differentiation, it was concluded that septin M plays an important role in these processes [131].
SEPT9 modulates the level of epidermal growth factor receptor (EGFR). Cortically located SEPT9-containing septin filaments (most likely composed of SEPT9/SEPT7/SEPT6/SEPT2 octamers) stabilize EGFR and protect it from ubiquitination and subsequent degradation [132]. In a human gastric cancer cell line, SEPT2 stabilizes ErbB2, another receptor of the EGFR family. RNA interference of SEPT2, as well as impaired septin dynamics, lead to ErbB2 ubiquitination, internalization, and lyzosomal degradation [133].
Septins have been shown to perform antimicrobial functions; they can immobilize intracellular pathogenic microorganisms (Fig. 3e). For example, in human cells infected by Shigella flexneri, which causes dysentery, SEPT2/6/7 complexes form filaments that surround the bacteria [134]. This septin cage prevents Shigella division and the formation of actin tails required for its transportation into neighboring cells [134]. Bacteria immobilized within a septin cage are eliminated by autophagy.
CONCLUSIONS
Septins are multifunctional GTP-binding proteins that can form filaments, which are the main form of their functioning. In the recent years, septins have come to be considered the fourth component of the cytoskeleton. The septin cytoskeleton is characterized by several specific features. First, in contrast to actin filaments and microtubules, it is nonpolar. Second, septin cytoskeleton exhibits slow dynamic and relative stability. Third, septins frequently colocalize with actin and microtubular structures, which suggests that the septin cytoskeleton participates in the coordinated regulation of the other two cytoskeleton systems. Finally, due to the great diversity of septins, it is possible for the cytoskeleton to comprise different septin elements, each of which performs a specific function.
Numerous studies have shown that septins are involved in cell compartmentalization by forming local scaffolds for protein anchoring and diffusion barriers that limit the motility of membrane proteins. It should be noted that the functions of scaffolding and diffusion barrier are not mutually exclusive but may be performed simultaneously.
Recent research has implicated septins in different cell processes, as well as in a number of pathological conditions, including tumors, neurological disorders, and infectious diseases. Further studies of septins, especially focused on their structure and the regulation of their intracellular dynamics should help elucidate the mechanisms of their involvement in particular processes and provide new approaches to the diagnostics and therapy of different human diseases.
Acknowledgments
This work was financially supported by State Project no. 0324-2016-0003.
References
- 1.Hartwell LH. Genetic control of the cell division cycle in yeast: 4. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- 2.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haarer BK, Pringle JR. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol Cell Biol. 1987;7:3678–3687. doi: 10.1128/mcb.7.10.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HB, Haarer BK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: Localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford SK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: Localization of the CDC11 gene product and the timing of events at the budding site. Dev Genet. 1991;12:281–292. doi: 10.1002/dvg.1020120405. [DOI] [PubMed] [Google Scholar]
- 6.Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Ding X, Yu W, et al. Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett. 2007;581:5526–5532. doi: 10.1016/j.febslet.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Leipe DD, Wolf YI, Koonin EV, et al. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- 9.Mostowy S, Cossart P. Septins: The fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 10.Saarikangas J, Barral Y. The emerging functions of septins in metazoans. EMBO Rep. 2011;12:1118–1126. doi: 10.1038/embor.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly D, Abdesselam I, Verdier-Pinard P, et al. Septin roles in tumorigenesis. Biol Chem. 2011;392:725–738. doi: 10.1515/BC.2011.073. [DOI] [PubMed] [Google Scholar]
- 12.Mostowy S, Cossart P. Autophagy and the cytoskeleton: New links revealed by intracellular pathogens. Autophagy. 2011;7:780–782. doi: 10.4161/auto.7.7.15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson EA, Petty EM. Conquering the complex world of human septins: implications for health and disease. Clin Genet. 2010;77:511–524. doi: 10.1111/j.1399-0004.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 15.Nishihama R, Onishi M, Pringle JR. New insights into the phylogenetic distribution and evolutionary origins of the septins. Biol Chem. 2011;392:681–687. doi: 10.1515/BC.2011.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;4:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- 17.Casamayor A, Snyder M. Molecular dissection of a yeast septin: Distinct domains are required for septin interaction, localization, and function. Mol Cell Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Kong C, Xie H, et al. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 19.Versele M, Thorner J. Some assembly required: Yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirajuddin M, Farkasovsky M, Hauer F, et al. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- 21.John CM, Hite RK, Weirich CS, et al. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–3307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field CM, Al-Awar O, Rosenblatt J, et al. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertin A, McMurray MA, Grob P, et al. Saccharomyces cerevisiae septins: Supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci U S A. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandrock K, Bartsch I, Bläser S, et al. Characterization of human septin interactions. Biol Chem. 2011;392:751–761. doi: 10.1515/BC.2011.081. [DOI] [PubMed] [Google Scholar]
- 25.Sellin ME, Sandblad L, Stenmark S, et al. Deciphering the rules governing assembly order of mammalian septin complexes. Mol Biol Cell. 2011;22:3152–3164. doi: 10.1091/mbc.E11-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sellin ME, Stenmark S, Gullberg M. Mammalian SEPT9 isoforms direct microtubule-dependent arrangements of septin core heteromers. Mol Biol Cell. 2012;23:4242–4255. doi: 10.1091/mbc.E12-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurray MA, Bertin A, Garcia G, et al. Septin filament formation is essential in budding yeast. Dev Cell. 2011;20:540–549. doi: 10.1016/j.devcel.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MS, Froese CD, Estey MP, et al. SEPT9 occupies the terminal positions in septin octamers and mediates polymerization-dependent functions in abscission. J Cell Biol. 2011;195:815–826. doi: 10.1083/jcb.201106131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata K, Asano T, Nozawa Y, et al. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem. 2004;279:55895–55904. doi: 10.1074/jbc.M406153200. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita M, Field CM, Coughlin ML, et al. Self- and actin-templated assembly of mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 31.Fujishima K, Kiyonari H, Kurisu J, et al. Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. J Neurochem. 2007;102:77–92. doi: 10.1111/j.1471-4159.2007.04478.x. [DOI] [PubMed] [Google Scholar]
- 32.Hsu SC, Hazuka CD, Roth R, et al. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 33.Bläser S, Jersch K, Hainmann I, et al. Human septin-septin interaction: CDCrel-1 partners with KIAA0202. FEBS Lett. 2002;519:169–172. doi: 10.1016/s0014-5793(02)02749-7. [DOI] [PubMed] [Google Scholar]
- 34.Martínez C, Sanjuan MA, Dent JA, et al. Human septin-septin interactions as a prerequisite for targeting septin complexes in the cytosol. Biochem J. 2004;382:783–791. doi: 10.1042/BJ20040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinoda T, Ito H, Sudo K, et al. Septin 14 is involved in cortical neuronal migration via interaction with Septin 4. Mol Biol Cell. 2010;21:1324–1334. doi: 10.1091/mbc.E09-10-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beites CL, Xie H, Bowser R, et al. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- 37.Mizutani Y, Ito H, Iwamoto I, et al. Possible role of a septin, SEPT1, in spreading in squamous cell carcinoma DJM-1 cells. Biol Chem. 2013;394:281–290. doi: 10.1515/hsz-2012-0258. [DOI] [PubMed] [Google Scholar]
- 38.Macedo JNA, Valadares NF, Marques IA, et al. The structure and properties of septin 3: A possible missing link in septin filament formation. Biochem J. 2013;450:95–105. doi: 10.1042/BJ20120851. [DOI] [PubMed] [Google Scholar]
- 39.Zent E, Vetter I, Wittinghofer A. Structural and biochemical properties of Sept7, a unique septin required for filament formation. Biol Chem. 2011;392:791–797. doi: 10.1515/BC.2011.082. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida Marques I, Valadares NF, Garcia W, et al. Septin C-terminal domain interactions: Implications for filament stability and assembly. Cell Biochem Biophys. 2012;62:317–328. doi: 10.1007/s12013-011-9307-0. [DOI] [PubMed] [Google Scholar]
- 41.Hu H, Yu W, Li S, et al. Crystallization and preliminary crystallographic studies of human septin 1 with site-directed mutations. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:128–132. doi: 10.1107/S1744309105043228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia W, Araújo APU, de Lara F, et al. An intermediate structure in the thermal unfolding of the GTPase domain of human septin 4 (SEPT4/Bradeion-beta. forms amyloid-like filaments in vitro. Biochemistry. 2007;46:11101–11109. doi: 10.1021/bi700702w. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita M, Kumar S, Mizoguchi A, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza M, Hyman AA, Glotzer M. GTP binding induces filament assembly of a recombinant septin. Curr Biol. 2002;12:1858–1863. doi: 10.1016/s0960-9822(02)01258-7. [DOI] [PubMed] [Google Scholar]
- 45.Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: Architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478–489. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- 46.Sirajuddin M, Farkasovsky M, Zent E, et al. GTP-induced conformational changes in septins and implications for function. Proc Natl Acad Sci U S A. 2009;106:16592–16597. doi: 10.1073/pnas.0902858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudryashov DS, Reisler E. ATP and ADP actin states. Biopolymers. 2013;99:245–256. doi: 10.1002/bip.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowne-Anderson H, Zanic M, Kauer M, et al. Microtubule dynamic instability: A new model with coupled GTP hydrolysis and multistep catastrophe. Bioessays. 2013;35:452–461. doi: 10.1002/bies.201200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zent E, Wittinghofer A. Human septin isoforms and the GDP-GTP cycle. Biol Chem. 2014;395:169–180. doi: 10.1515/hsz-2013-0268. [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara A, Tanaka Y, Hikawa R, et al. Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton. 2011;68:512–525. doi: 10.1002/cm.20528. [DOI] [PubMed] [Google Scholar]
- 51.Hu Q, Nelson WJ, Spiliotis ET. Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem. 2008;283:29563–29571. doi: 10.1074/jbc.M804962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernández-Rodríguez Y, Momany M. Post-translational modifications and assembly of septin heteropolymers and higher-order structures. Curr Opin Microbiol. 2012;15:660–668. doi: 10.1016/j.mib.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;5682:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 54.Garcia G, Bertin A, Li Z, et al. Subunit-dependent modulation of septin assembly: Budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol. 2011;195:993–1004. doi: 10.1083/jcb.201107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha I, Wang YM, Philp R, et al. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell. 2007;3:421–432. doi: 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Meseroll RA, Occhipinti P, Gladfelter AS. Septin phosphorylation and coiled-coil domains function in cell and septin ring morphology in the filamentous fungus Ashbya gossypii. Eukaryot Cell. 2013;2:182–193. doi: 10.1128/EC.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Gao J, Chung KK, et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muñoz-Soriano V, Nieto-Arellano R, Paricio N. Septin 4, the drosophila ortholog of human CDCrel-1, accumulates in parkin mutant brains and is functionally related to the Nedd4 E3 ubiquitin ligase. J Mol Neurosci. 2012;48:136–143. doi: 10.1007/s12031-012-9788-3. [DOI] [PubMed] [Google Scholar]
- 59.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 60.Dong Z, Ferger B, Paterna JC, et al. Dopamine-dependent neurodegeneration in rats induced by viral vector-mediated overexpression of the parkin target protein, CDCrel-1. Proc Natl Acad Sci U S A. 2003;21:12438–12443. doi: 10.1073/pnas.2132992100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Son JH, Kawamata H, Yoo MS, et al. Neurotoxicity and behavioral deficits associated with Septin5 accumulation in dopaminergic neurons. J Neurochem. 2005;94:1040–1053. doi: 10.1111/j.1471-4159.2005.03257.x. [DOI] [PubMed] [Google Scholar]
- 62.Ageta-Ishihara N, Yamakado H, Morita T, et al. Chronic overload of SEPT4, a parkin substrate that aggregates in Parkinson’s disease, causes behavioral alterations but not neurodegeneration in mice. Mol Brain. 2013;6:35. doi: 10.1186/1756-6606-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joberty G, Perlungher RR, Sheffield PJ, et al. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- 64.Sadian Y, Gatsogiannis C, Patasi C, et al. The role of Cdc42 and Gic1 in the regulation of septin filament formation and dissociation. Elife. 2013;2:e01085. doi: 10.7554/eLife.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dekker C, Stirling PC, McCormack EA, et al. The interaction network of the chaperonin CCT. EMBO J. 2008;27:1827–1839. doi: 10.1038/emboj.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huijbregts RPH, Svitin A, Stinnett MW, et al. Drosophila Orc6 facilitates GTPase activity and filament formation of the septin complex. Mol Biol Cell. 2009;20:270–281. doi: 10.1091/mbc.E08-07-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akhmetova K, Balasov M, Huijbregts RPH, et al. Functional insight into the role of Orc6 in septin complex filament formation in Drosophila. Mol Biol Cell. 2015;26:15–28. doi: 10.1091/mbc.E14-02-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 69.Douglas LM, Alvarez FJ, McCreary C, et al. Septin function in yeast model systems and pathogenic fungi. Eukaryot Cell. 2005;4:1503–1512. doi: 10.1128/EC.4.9.1503-1512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joo E, Tsang CW, Trimble WS. Septins: Traffic control at the cytokinesis intersection. Traffic. 2005;6:626–634. doi: 10.1111/j.1600-0854.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 71.Kinoshita M, Noda M. Roles of septins in the mammalian cytokinesis machinery. Cell Struct Funct. 2001;26:667–670. doi: 10.1247/csf.26.667. [DOI] [PubMed] [Google Scholar]
- 72.Estey M, Di Ciano-Oliveira C, Froese CD, et al. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol. 2010;191:741–749. doi: 10.1083/jcb.201006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tooley AJ, Gilden J, Jacobelli J, et al. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2009;11:17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon MB, Sawada A, Chaturvedi A, et al. Genetic deletion of SEPT7 reveals a cell type-specific role of septins in microtubule destabilization for the completion of cytokinesis. PLoS Genet. 2014;10:e1004558. doi: 10.1371/journal.pgen.1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menon MB, Gaestel M. Sep(t)arate or not: How some cells take septin-independent routes through cytokinesis. J Cell Sci. 2015;128:1877–1886. doi: 10.1242/jcs.164830. [DOI] [PubMed] [Google Scholar]
- 76.Spiliotis ET, Gladfelter AS. Spatial guidance of cell asymmetry: septin GTPases show the way. Traffic. 2012;13:195–203. doi: 10.1111/j.1600-0854.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindsey R, Cowden S, Hernandez-Rodriguez Y, et al. Septins AspA and AspC are important for normal development and limit the emergence of new growth foci in the multicellular fungus Aspergillus nidulans. Eukaryot Cell. 2010;9:155–163. doi: 10.1128/EC.00269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinoshita N, Kimura K, Matsumoto N, et al. Mammalian septin Sept2 modulates the activity of GLAST, a glutamate transporter in astrocytes. Genes Cell. 2004;1:1–14. doi: 10.1111/j.1356-9597.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 79.Dolat L, Hu Q, Spiliotis ET. Septin functions in organ system physiology and pathology. Biol Chem. 2014;395:123–141. doi: 10.1515/hsz-2013-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SK, Shindo A, Park TJ, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilden JK, Peck S, Chen YC, et al. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J Cell Biol. 2012;196:103–114. doi: 10.1083/jcb.201105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mostowy S, Janel S, Forestier C, et al. A role for septins in the interaction between the Listeria monocytogenes invasion protein InlB and the Met receptor. Biophys J. 2011;100:1949–1959. doi: 10.1016/j.bpj.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dolat L, Hunyara JL, Bowen JR, et al. Septins promote stress fiber-mediated maturation of focal adhesions and renal epithelial motility. J Cell Biol. 2014;207:225–235. doi: 10.1083/jcb.201405050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finger FP, Kopish KR, White JG. A role for septins in cellular and axonal migration in C. elegans. Dev Biol. 2003;261:220–234. doi: 10.1016/s0012-1606(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 86.Beites CL, Campbell KA, Trimble WS. The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem J. 2005;385:347–353. doi: 10.1042/BJ20041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amin ND, Zheng YL, Kesavapany S, et al. Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci. 2008;14:3631–3643. doi: 10.1523/JNEUROSCI.0453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito H, Atsuzawa K, Morishita R, et al. Sept8 controls the binding of vesicle-associated membrane protein 2 to synaptophysin. J Neurochem. 2009;108:867–880. doi: 10.1111/j.1471-4159.2008.05849.x. [DOI] [PubMed] [Google Scholar]
- 89.Dent J, Kato K, Peng XR, et al. A prototypic platelet septin and its participation in secretion. Proc Natl Acad Sci U S A. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tokhtaeva E, Capri J, Marcus EA, et al. Septin dynamics are essential for exocytosis. J Biol Chem. 2015;290:5280–5297. doi: 10.1074/jbc.M114.616201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ono R, Ihara M, Nakajima H, et al. Disruption of Sept6, a fusion partner gene of MLL, does not affect ontogeny, leukemogenesis induced by MLL-SEPT6, or phenotype induced by the loss of Sept4. Mol Cell Biol. 2005;24:10965–10978. doi: 10.1128/MCB.25.24.10965-10978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsang CW, Fedchyshyn M, Harrison J, et al. Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol Cell Biol. 2008;23:7012–7029. doi: 10.1128/MCB.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takizawa PA, DeRisi JL, Wilhelm JE, et al. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- 94.Kissel H, Georgescu MM, Larisch S, et al. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–364. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 95.Lhuillier P, Rode B, Escalier D, et al. Absence of annulus in human asthenozoospermia: Case report. Hum Reprod. 2009;24:1296–1303. doi: 10.1093/humrep/dep020. [DOI] [PubMed] [Google Scholar]
- 96.Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berbari NF, O’Connor AK, Haycraft CJ, et al. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu Q, Nelson WJ. The ciliary diffusion barrier: The gatekeeper for the primary cilium compartment. Cytoskeleton (Hoboken) 2011;68:313–324. doi: 10.1002/cm.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghossoub R, Hu Q, Failler M, et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci. 2013;126:2583–2594. doi: 10.1242/jcs.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Q, Milenkovic L, Jin H, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dash SN, Lehtonen E, Wasik AA, et al. Sept7b is essential for pronephric function and devel opment of left-right asymmetry in zebrafish embryogenesis. J Cell Sci. 2014;127:1476–1486. doi: 10.1242/jcs.138495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Higuchi-Sanabria R, Pernice WM, Vevea JD, et al. Role of asymmetric cell division in lifespan control in Saccharomyces cerevisiae. FEMS Yeast Res. 2014;14:1133–1146. doi: 10.1111/1567-1364.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chao JT, Wong AK, Tavassoli S, et al. Polarization of the endoplasmic reticulum by ER-septin tethering. Cell. 2014;158:620–632. doi: 10.1016/j.cell.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 104.Spiliotis ET, Hunt SJ, Hu Q, et al. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cho SJ, Lee H, Dutta S, et al. Septin 6 regulates the cytoarchitecture of neurons through localization at dendritic branch points and bases of protrusions. Mol Cells. 2011;32:89–98. doi: 10.1007/s10059-011-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X, Serwanski DR, Miralles CP, et al. Septin 11 is present in GABAergic synapses and plays a functional role in the cytoarchitecture of neurons and GABAergic synaptic connectivity. J Biol Chem. 2009;284:17253–17265. doi: 10.1074/jbc.M109.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tada T, Simonetta A, Batterton M, et al. Role of septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie Y, Vessey JP, Konecna A, et al. The GTP-binding protein septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 109.Ewers H, Tada T, Petersen JD, et al. A septin-dependent diffusion barrier at dendritic spine necks. PLoS One. 2014;9:1–19. doi: 10.1371/journal.pone.0113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashby MC, Maier SR, Nishimune A, et al. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryu J, Liu L, Wong TP, et al. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 112.Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 113.Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Curr Opin Neurobiol. 2011;21:175–181. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calvo F, Ranftl R, Hooper S, et al. Cdc42EP3/BORG2 and septin network enables mechano-transduction and the emergence of cancer-associated fibroblasts. Cell Rep. 2015;13:2699–2714. doi: 10.1016/j.celrep.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Field CM, Coughlin M, Doberstein S, et al. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 116.Oegema K, Savoian MS, Mitchison TJ, et al. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–552. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mavrakis M, Azou-Gros Y, Tsai FC, et al. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol. 2014;16:322–334. doi: 10.1038/ncb2921. [DOI] [PubMed] [Google Scholar]
- 118.Smith C, Dolat L, Angelis D, et al. Septin 9 exhibits polymorphic binding to F-actin and inhibits myosin and cofilin activity. J Mol Biol. 2015;427:3273–3284. doi: 10.1016/j.jmb.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmidt K, Nichols BJ. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 2004;5:43. doi: 10.1186/1471-2121-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kusch J, Meyer A, Snyder MP, et al. Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 2002;16:1627–1639. doi: 10.1101/gad.222602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pablo-Hernando ME, Arnaiz-Pita Y, Tachikawa H, et al. Septins localize to microtubules during nutritional limitation in Saccharomyces cerevisiae. BMC Cell Biol. 2008;9:55. doi: 10.1186/1471-2121-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spiliotis ET. Regulation of microtubule organization and functions by septin GTPases. Cytoskeleton. 2010;67:339–345. doi: 10.1002/cm.20448. [DOI] [PubMed] [Google Scholar]
- 123.Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bai X, Bowen JR, Knox TK, et al. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol. 2013;203:895–905. doi: 10.1083/jcb.201308068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu J, Bai X, Bowen JR, et al. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol. 2012;22:1109–1115. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 127.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 128.Larisch S. The ARTS connection: Role of ARTS in apoptosis and cancer. Cell Cycle. 2004;3:1021–1023. [PubMed] [Google Scholar]
- 129.Gottfried Y, Rotem A, Lotan R, et al. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Edison N, Reingewertz TH, Gottfried Y, et al. Peptides mimicking the unique ARTS-XIAP binding site promote apoptotic cell death in cultured cancer cells. Clin Cancer Res. 2012;18:2569–2578. doi: 10.1158/1078-0432.CCR-11-1430. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi S, Inatome R, Yamamura H, et al. Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones. Genes Cells. 2003;8:81–93. doi: 10.1046/j.1365-2443.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 132.Diesenberg K, Beerbaum M, Fink U, et al. SEPT9 negatively regulates ubiquitin-dependent downregulation of EGFR. J Cell Sci. 2015;128:397–407. doi: 10.1242/jcs.162206. [DOI] [PubMed] [Google Scholar]
- 133.Marcus EA, Tokhtaeva E, Turdikulova S, et al. Septin oligomerization regulates persistent expression of ErbB2/HER2 in gastric cancer cells. Biochem J. 2016;473:1703–1718. doi: 10.1042/BCJ20160203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sirianni A, Krokowski S, Lobato-Márquez D, et al. Mitochondria mediate septin cage assembly to promote autophagy of Shigella. EMBO Rep. 2016;17:1029–1043. doi: 10.15252/embr.201541832. [DOI] [PMC free article] [PubMed] [Google Scholar]


