Abstract
Background and Objective:
Fatigue and physical impairments are a major concern in children with multiple sclerosis (MS) and after acute disseminated encephalomyelitis (post-ADEM). We here aimed to evaluate the interaction between fatigue, exercise capacity, motor performance, neurological status, and quality of life (HRQoL).
Methods:
In this cross-sectional study, data of 38 children (MS n = 22, post-ADEM n = 16), aged 4–17 years attending our national pediatric MS center, were studied. Fatigue was measured with the Pediatric Quality of Life Multidimensional Fatigue Scale, exercise capacity with the Bruce Protocol, motor performance with the Movement Assessment Battery for Children second edition, HRQoL with the Pediatric Quality of Life Questionnaire, and extent of disability with the Expanded Disability Status Scale (EDSS).
Results:
Children with MS and post-ADEM experienced more fatigue (p < 0.001), reduced exercise capacity (p < 0.001), and impaired motor performance (p < 0.001), despite low scores on the EDSS. Fatigue, but not the other parameters, was significantly correlated with HRQoL. Fatigue was not correlated with exercise capacity.
Conclusion:
We confirm the major impact of fatigue on quality of life in children with MS and post-ADEM. Fatigue was not explained by reduced exercise capacity or impaired motor performance. An important finding for clinical practice is that the low EDSS score did not reflect the poor physical functioning.
Keywords: Multiple sclerosis, acute disseminated encephalomyelitis, fatigue, exercise capacity, motor development, EDSS, physical functioning, quality of life
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. It is generally considered as a disease of young adults in their twenties and thirties, although 2%–10% of all MS patients experience their first attack before the age of 18 years.1–3 Pediatric-onset multiple sclerosis (POMS) shows parallels with adult MS but runs a more severe course because the relapse rate is higher, next to a higher lesion load on magnetic resonance imaging (MRI).4,5 Despite the time to the secondary progressive phase in POMS is longer, patients are disabled at a younger age than patients with adult-onset MS.1,6 In addition to mental and cognitive problems, many patients struggle with physical impairments and fatigue.7,8 These problems do not limit themselves to chronic forms of demyelinating disease like MS but are also reported in children after acute disseminated encephalomyelitis (post-ADEM), although rapid motor function recovery after the acute phase in most of the patients seems to be the case.9–11
The Expanded Disease Status Scale (EDSS) is commonly used to evaluate disability of adult and pediatric patients with demyelinating disorders.12 However, it is originally intended for adult MS patients.
As mentioned above, fatigue is a frequently occurring problem in patients with POMS and post-ADEM.13 The cause of fatigue still remains unclear. In a Canadian study of children with MS and monophasic acquired demyelinating syndromes (mono-ADS), a correlation was found between fatigue and physical activity.14 POMS patients were less physically active and scored higher on fatigue scales than patients with mono-ADS. Possibly, due to complaints of fatigue, patients become less physically active and this may lead to decreased exercise capacity.
We assume that physical disturbances as motor problems, fatigue and decreased exercise capacity affect a child’s physical and psychosocial development and hence their quality of life. To the best of our knowledge, there is no data available of motor development in children with POMS and post-ADEM and maximal exercise capacity in children with post-ADEM.
Therefore, we primarily aimed to evaluate whether children with MS and post-ADEM have a reduced exercise capacity and whether this correlates with fatigue. Second, we analyzed the possible relations between quality of life and fatigue, exercise capacity, or motor performance. Finally, we aimed to investigate whether the EDSS is an optimal measurement to determine disabilities in daily life in these children.
Materials and methods
Patients
Children under the age of 18 years were eligible for this cross-sectional study when diagnosed with POMS or ADEM in consensus with the International Pediatric Multiple Sclerosis Study Group 2012 diagnostic criteria.15 Patients with other demyelinating syndromes (e.g. clinically isolated syndromes, neuromyelitis optica) were excluded. All children were evaluated in our national multidisciplinary pediatric MS center as part of routine medical care between June 2013 and December 2015. Assembled clinical parameters consist of patient history and neurological examination performed by a pediatric neurologist. Disability was expressed by the EDSS score.12 Measurements of exercise capacity, motor development, fatigue, and quality of life were administered by a pediatric physical therapist. After the evaluation, tailor-made advice concerning physical therapy and rehabilitation was given.
Written informed consent and permission to use the data for research purposes was obtained from all parents and/or children between 12 and 18 years of age.
Fatigue—Pediatric Quality of Life Multidimensional Fatigue Scale
The Pediatric Quality of Life Multidimensional Fatigue Scale (PedsQL-MFS) was designed as a generic symptom-specific and standardized instrument to measure fatigue in healthy children and in children with acute and chronic health conditions aged 2–18 years. It is validated in Dutch children.16 The PedsQL-MFS comprises three subscales: general fatigue, sleep/rest fatigue, and cognitive fatigue. A total fatigue score is calculated from the subscales. A scale score and total fatigue score of 1 standard deviation (SD) below the mean of healthy age-related reference norm was considered abnormal.
Exercise capacity—Bruce Protocol
The Bruce Protocol was used to test maximal exercise capacity.17 Children were encouraged to perform to exhaustion. The maximal endurance time on the treadmill was used as criterion of exercise capacity. Before and during the test, heart rate (HR) and transcutaneous oxygen saturation were monitored (motion artifact system, type 2001; Respironics Novametrix, Murrysville, PA, USA). HR of ⩾185 beats per minute or loss of coordination, because of excessive fatigue, was taken as maximal performance.18 The standard deviation score (SDS) of the maximal endurance time is calculated using age-related reference values for healthy Dutch children.19,20
Motor performance—Movement Assessment Battery for Children second edition
Motor performance was examined with the Movement Assessment Battery for Children second edition (MABCII). The MABCII is a standardized and age-related norm-referenced test, validated for Dutch children, and developed to classify children according to degree of motor performance.21 The MABCII has three domains: manual dexterity, ball, and balance skills. For each child, the raw item scores were transformed into a domain percentile score and a total percentile score. Scores ⩽fifth percentile denote a definite motor problem, scores between the 6th–16th percentiles denote borderline performance, and scores >16th percentile indicate normal performance.
Quality of life—Pediatric Quality of Life Inventory 4.0
Health-related quality of life (HRQoL) data were collected using the Pediatric Quality of Life Inventory (PedsQL) 4.0.22 Patients as well as one of their parents were asked to fill in the questionnaire. It encompasses 23 items on four Generic Core Scales: physical, emotional, social, and school functioning. A psychosocial functioning scale can be derived from the emotional, social, and school functioning items. All 23 items together provide the total functioning score. The children indicate on a five-point scale the frequency in which they experience a problem, and these scores are linearly transformed to a 0–100 scale. Higher scores indicate better functioning. The Dutch version of the PedsQL has adequate psychometric properties.22 A scale score and total functioning score of 1 SD below the mean of healthy age-related reference norm was taken to indicate impaired HRQoL.23
Data analysis
Analyses were performed using SPSS 23.0 (IBM, Chicago, IL, USA). The Kolmogorov–Smirnov test was used to test whether the data was normally distributed. One-sample t-test was used when comparing continuous data of each measurement to expected average of the reference group. A chi-square test was applied to test whether the distribution of motor performance scores in our population differed significantly from that in the normative population. For correlation analyses, Pearson’s correlation and Spearman’s correlation were used when appropriate. Mann–Whitney U test was used for group comparison. A p value was considered significant <0.05.
Body mass index (BMI) was calculated, and the Dutch Growth Analyzer version 3.5 served to calculate SDS for BMI on the basis of Dutch references.24
Results
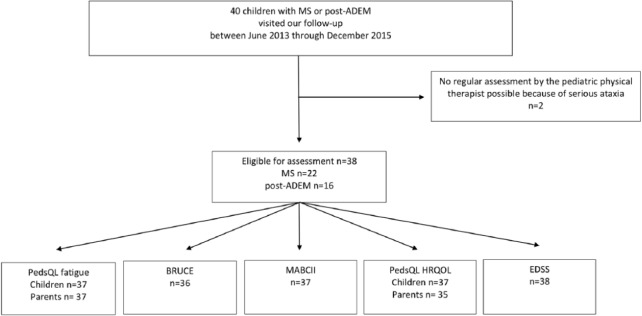
Between June 2013 and December 2015, 40 children were assessed with MS (n = 24) or post-ADEM (n = 16). Two children with MS had no standard assessment by the pediatric physical therapist due to disability caused by serious ataxia (Figure 1). Thus, data of 38 children were eligible for analysis.
Figure 1.
Flowchart: MS: multiple sclerosis; post-ADEM: after acute disseminated encephalomyelitis; PedsQL: Pediatric Quality of Life Inventory; MABCII: Movement Assessment Battery for Children second edition; HRQOL: health-related quality of life; EDSS: Expanded Disability Status Scale.
Relevant baseline characteristics are listed in Table 1. None of the children had concomitant diagnosis influencing cardiopulmonary function.
Table 1.
Baseline characteristics of participants.
| Total group (n = 38) | MS (n = 22) | Post-ADEM (n = 16) | |
|---|---|---|---|
| Boys, n (%) | 13 (34) | 4 (18) | 9 (56) |
| Age in years | 13.4 (9.2 to 15.7) | 14.0 (13.0 to 15.0) | 4.5 (2.3 to 5.9) |
| SDS BMI | 0.8 (0.3 to 1.4) | 1.2 (0.3 to 1.6) | −0.3 (−0.9 to 1.0) |
| Age first symptoms, years | 11.8 (5.0 to 14.1) | 14 (9.0 to 17.0) | 4.5 (1.5 to 11.5) |
| Time from onset to assessment, months | 16.3 (5.1 to 40.5) | 10.2 (4.6 to 21.5) | 40.1 (11.4 to 63.5) |
| DMT in MS at time of assessment, yes, n (%) | 18 (47) | 18 (82) | – |
| Number of episodes within 12 months to assessment, n (%) | |||
| 0 | 15 (40) | 3 (14) | 12 (75) |
| 1 | 18 (47) | 14 (64) | 4 (25) |
| 2 | 3 (8) | 3 (13) | – |
| 3 | 2 (5) | 2 (9) | – |
| EDSS | |||
| 0 | 20 (53) | 10 (46) | 9 (56) |
| 1.0 | 8 (21) | 4 (18) | 5 (31) |
| 1.5 | 7 (18) | 6 (27) | 1 (6) |
| 2.0 | 2 (5) | 2 (9) | – |
| 3.0 | 1 (3) | – | 1 (6) |
| Sports participation at time of assessment, yes, n (%) | 22 (58) | 11 (50) | 11 (69) |
MS: multiple sclerosis; post-ADEM: after acute disseminated encephalitis; SDS: standard deviation score; BMI: body mass index; DMT: disease-modifying therapy; IQR: interquartile range; EDSS: Expanded Disability Status Scale.
Data are presented as median (IQR) unless otherwise stated.
Fatigue—PedsQL-MFS
The PedsQL-MFS was filled in by 37/38 of the children. One child could not fill in the questionnaire due to his young age (<5 years). All parents returned the questionnaire. A total of 15 children and 19 parents indicated fatigue (<−1 SD). Scores on all subscales were significantly lower than the reference group in both patients and parents (Table 2). Parents indicated more often and more severe fatigue for their children than the children themselves. Both children with MS and children post-ADEM experienced greater fatigue than healthy related peers: 8 of 22 children with MS and 7 of 15 children post-ADEM.
Table 2.
Test results: fatigue, exercise capacity, motor performance, quality of life, and EDSS.
| Measurement | Total group children | Total group parents | MS child | MS parents | Post-ADEM child | Post-ADEM parents |
|---|---|---|---|---|---|---|
| PedsQL fatigue | Mean SDS (SD) | Mean SDS (SD) | n (%) < −1 SD | n (%) < −1 SD | n (%) < −1 SD | n (%) < −1 SD |
| n = 37 | n = 38 | n = 22 | n = 22 | n = 15 | n = 16 | |
| Total fatigue score | −0.76 (1.25)*** | −1.22 (1.63)*** | 8 (36) | 10 (46) | 7 (47) | 9 (56) |
| General fatigue | −0.74 (1.17)*** | −1.06 (1.54)*** | 8 (36) | 7 (32) | 6 (40) | 9 (56) |
| Sleep–rest fatigue | −0.56 (1.13)** | −1.04 (1.46)*** | 6 (27) | 9 (41) | 8 (53) | 7 (44) |
| Cognitive fatigue | −0.60 (1.37)** | −0.84 (1.25)*** | 7 (32) | 7 (32) | 6 (40) | 9 (56) |
| Exercise capacity, Bruce | Mean SDS (SD) | n (%) < −1 SD | n (%) < −1 SD | |||
| n = 36 | n = 20 | n = 16 | ||||
| −1.37 (1.09)*** | 17 (85) | 9 (56) | ||||
| MABCII | n (%) | n (%) | n (%) | |||
| n = 37 | n = 21 | n = 16 | ||||
| Total impairment score | ||||||
| Normal | 19 (51.4)*** | 10 (48) | 9 (56) | |||
| Borderline | 5 (13.5)*** | 1 (5) | 4 (25) | |||
| Motor problem | 13 (35.1)*** | 10 (48) | 3 (19) | |||
| Manual dexterity, n (%) | ||||||
| Normal | 24 (64.9)* | 13 (62) | 11 (69) | |||
| Borderline | 8 (21.6)* | 6 (29) | 2 (13) | |||
| Motor problem | 5 (13.5)* | 2 (10) | 3 (19) | |||
| Bal skills, n (%) | ||||||
| Normal | 21 (56.8)*** | 11 (52) | 10 (63) | |||
| Borderline | 7 (18.9)*** | 5 (24) | 2 (12) | |||
| Motor problem | 9 (24.3)*** | 5 (24) | 4 (25) | |||
| Balance skills, n (%) | ||||||
| Normal | 17 (45.9)*** | 5 (24) | 12 (75) | |||
| Borderline | 8 (21.6)*** | 7 (33) | 1 (6) | |||
| Motor problem | 12 (32.4)*** | 9 (43) | 3 (19) | |||
| PedsQL-HRQoL | Mean SDS (SD) | Mean SDS (SD) | n (%) < −1 SD | n (%) < −1 SD | n (%) < −1 SD | n (%) < −1 SD |
| n = 37 | n = 35 | n = 22 | n = 22 | n = 15 | n = 13 | |
| Total functioning score | −0.83 (1.54)*** | −0.73 (1.18)*** | 9 (41) | 8 (36) | 5 (33) | 4 (31) |
| Physical functioning | −1.07 (1.83)*** | −0.53 (1.30)* | 10 (45) | 8 (36) | 6 (40) | 5 (38) |
| Emotional functioning | −0.43 (1.34) | −0.79 (1.07)*** | 4 (18) | 9 (41) | 6 (40) | 6 (46) |
| Social functioning | −0.55 (1.48)* | −0.38 (1.08)* | 7 (32) | 8 (36) | 6 (40) | 3 (23) |
| School functioning | −1.13 (1.70)*** | −0.89 (1.14)*** | 10 (46) | 10 (46) | 10 (67) | 6 (46) |
| Psychosocial functioning | −0.85 (1.52)*** | −0.85 (1.04)*** | 10 (46) | 10 (46) | 6 (40) | 6 (46) |
| EDSS | n (%) | n (%) | n (%) | |||
| n = 38 | n = 22 | n = 16 | ||||
| 0 | 20 (53) | 10 (46) | 10 (63) | |||
| 1 | 8 (21) | 4 (18) | 4 (25) | |||
| 1.5 | 7 (18) | 6 (27) | 1 (6) | |||
| 2 | 2 (5) | 2 (9) | 0 (0) | |||
| 3 | 1 (3) | 0 (0) | 1 (6) | |||
MS: multiple sclerosis; post-ADEM: after acute disseminated encephalomyelitis; MABCII: Movement Assessment Battery for Children second edition; PedsQL: Pediatric Quality of Life Inventory; HRQoL: health-related quality of life; EDSS: Expanded Disability Status Scale; SDS: standard deviation score; SD: standard deviation.
Data are presented as number (%) of patients or mean (SD).
One-sample t-test (compared with zero) or chi-square test (observed vs expected distribution): ***p ⩽ 0.001; **p ⩽ 0.01; *p ⩽ 0.05.
Exercise capacity—Bruce Protocol
Exercise capacity data were analyzed for 36/38 children. Data of two children were not analyzed because they could not reach maximal performance (HR < 185/min). The 36 children performed significantly below reference values (p < 0.001) as shown in Table 2.
Especially children with MS had a limited exercise capacity. Scores below average (<-1 SD) were found in 17 out of 20 (85%) children with MS and in 9 out of 16 (56%) children post-ADEM.
Motor performance—MABCII
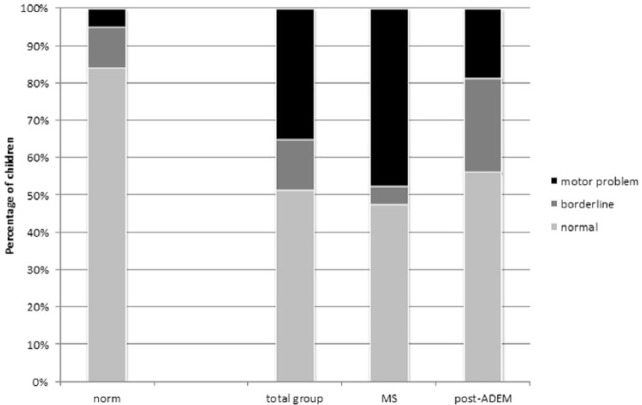
A total of 37 children were tested with the MABCII. One child was not tested because of logistic reasons. In total, 19 of these 37 children (51% vs 84% expected based on reference values) had a total impairment score (TIS) within the normal range, 5 children (14% vs 11% expected) were classified as borderline, and another 13 (35% vs 5% expected) as having a definite motor impairment. This distribution is significantly different from reference values (p ⩽ 0.001). Problems were encountered in all three subscales: manual dexterity, ball, and balance skills as well (p < 0.001) as displayed in Table 2 and Figure 2.
Figure 2.
MABCII—total impairment score (TIS): Left bar “norm”: percentage of children from Dutch reference group. Total group: data of MS and post-ADEM children.
When we added the two children who were excluded from analysis due to serious ataxia, 15 of the 40 children (38%) had a definite motor problem.
Motor performance between the diagnosis groups differed: children with MS had more severe and more often motor impairments than children post-ADEM. Children with MS encountered most problems in balance skills, followed by ball skills and manual dexterity. Children post-ADEM encountered problems in all three subscales, but these problems were more equally distributed over the three subscales.
HRQoL—PedsQL-HRQoL
The PedsQL-HRQoL was completed by 37/38 of the children and 35/38 parents. One child could not fill in the questionnaire because of his age (<5 years) and three parents did not return the questionnaire. An impaired HRQoL (<–1 SD) was indicated by 14 children and 12 parents. Compared with the reference values, scores on all PedsQL scales, except emotional functioning, were significantly lower in children (Table 2). In contrast, the parental scores were significantly lower on all subscales (see Table 2 for descriptive results of children with MS and post-ADEM patients separately).
Expanded disability status—EDSS
All children had an EDSS ⩽ 3.0 (Table 2). A total of 35 patients (92%) did not have disabilities or were not aware of it in daily life (EDSS < 2.0) (see Table 2 for results of children with MS and post-ADEM separately).
Correlations between parameters
The mean SDS of the child-reported PedsQL total fatigue score was significantly correlated with the mean SDS of the PedsQL-HRQoL total score reported by the child (r = 0.704, p < 0.001) and the parent (r = 0.554, p = 0.001). There was no significant correlation between the mean SDS of the PedsQL total fatigue score of the child and mean SDS exercise capacity, MABCII, BMI, or sports participation. Furthermore, there were no correlations found between the subscales of the PedsQL fatigue and above-mentioned parameters (data not shown).
No significant correlation was found between the EDSS and the mean SDS of the PedsQL fatigue total score of the child and parent, exercise capacity, MABCII, PedsQL-HRQoL total score of the child and the parent, BMI, or sports participation (data not shown).
A significant correlation was found between exercise capacity and sports participation (r = 0.365, p = 0.034).
Differences between children with and without fatigue
A significant difference in mean SDS of the PedsQL-HRQoL total score was found between children with and without fatigue (p < 0.001). For the other parameters (exercise capacity, MABCII, BMI, and sports participation), no significant difference was found (data not shown).
Impact of disease duration on outcome parameters
Disease duration differed for children with MS and post-ADEM (Table 1). Therefore, we analyzed the groups separately. Within both groups, there was no significant correlation between any of the outcome parameters and disease duration (data not shown). Disease duration was calculated per group as beneath or above the median.
Impact of disease activity on outcome parameters
The number of demyelinating events within the year to assessment was assessed to express disease activity. This did not correlate with any of the outcome parameters (data not shown).
Discussion
In this cohort of 38 children with MS and post-ADEM, we found that a large proportion of the children experienced fatigue and had reduced exercise capacity. In addition, many children had motor impairments and impaired HRQoL. However, the hypothesis that fatigue and exercise capacity are related in children with MS and post-ADEM was not confirmed. HRQoL was related with fatigue but not with motor performance, the EDSS score, or exercise capacity. Despite the physical impairments measured by the MABCII, in no more than three children (8%), the disability interfered with activities of daily living according to the EDSS results.
Exercise capacity was reduced in both MS and post-ADEM patients. Durstine et al.25 describe that most individuals with a chronic disease or disability become less physically active. This is in line with Grover et al.14 who describe less physical activity in POMS and mono-ADS patients. Physical inactivity can lead to a reduced exercise capacity, which in turn can lead to further inactivity and a decrease in participation in daily life activities. A negative spiral of reduced exercise and physical inactivity may arise.25
In our study, sports participation served as a measure of physical activity and was found related to exercise capacity. This may suggest that exercise capacity and the level of physical activity of children with MS and post-ADEM can be improved with exercise interventions. In other studies of children with chronic systemic inflammatory conditions, exercise interventions indeed improved children’s exercise capacity and physical functioning.25 High-intensity exercise programs appeared to be safe in adult MS patients but need to be investigated in children with MS and post-ADEM.26,27
We did not found a difference in exercise capacity between fatigued and non-fatigued patients, as initially hypothesized. This suggests that fatigue itself is not explained by reduced exercise capacity in our patients and vice versa. The process of inactivity, decreased exercise capacity, and fatigue probably involves a complex interaction including other factors as well. As Grover et al. not only found a correlation between physical activity and fatigue but also with depression, we argue here that psychosocial factors and reduced psychosocial participation may play an important additional role in diminished physical activity and in turn decreased exercise capacity.
This is supported by our findings in HRQoL; children who experienced fatigue reported more problems in not only physical functioning but also in emotional, social, scholastic, and psychosocial functioning. From clinical experience, we observe that children with MS and post-ADEM experience psychological distress and difficulties with coping with the diagnosis or residual deficits. It is described that chronic ill young adolescents feel their chronic condition as “disrupting normal life” and they perceive “discomfort in their own body.”28 Literature of adult patients with MS suggested that exercise could improve physical activity, depression, fatigue, and HRQoL.26 In children without chronic illness, literature shows a positive correlation between physical activity and HRQoL.29 Interventions for these problems in pediatric demyelinating disorders have not been evaluated to date. Disease perception, disease acceptance, and coping might be potential areas in understanding the mechanism of fatigue, diminished physical activity, and reduced exercise capacity.
Physical ability in children with MS and post-ADEM is frequently evaluated with the EDSS. The EDSS contains items on different functional systems that reflect disability of adult patients with MS and includes, for example, sexual functioning.12 In our experience, administration of this scale requires the subject to have adequate language perception and expression, which may not always be the case in a pediatric ADS cohort. In this study, we used the MABCII to measure motor functioning, including manual dexterity, ball, and balance skills. Children with MS showed motor impairments, particularly of balance skills. Children post-ADEM showed motor impairments as well, but these were more equally distributed over the three subscales. A total of 49% of all the children showed severe or borderline deficits on the TIS, which is strikingly high. However, if compared to EDSS scores, only 8% of our cohort has a score ⩾2, which reflects disability which the patient is aware of in daily life.12 A total of 9% of the MS patients scored EDSS ⩾2 versus 6% of the patients post-ADEM, which is low in contrast to the high total MABCII impairment scores (52% and 44%, respectively). This discrepancy confirms that the EDSS is not an optimal measurement for motor deficits in pediatric MS patients and especially not in young patients post-ADEM. The MABCII seems to be a more sensitive measurement for expressing motor deficits and its severity.
In children, it is important to acquire motor skills being able to participate in physical activities and therewith being able to participate with peers. Lack of participation in physical activity has contributed to a decrease in fitness and an increased risk for disease.30 Monitoring of motor performance is important to enable timely intervention.
Several limitations of this study need to be addressed. First, the sample size was relatively small. Second, only sports participation was taken as a measurement of physical activity. Physical activity includes not only sports but also other activities which involve bodily movement, such as playing, home–school transfers, and recreational activities.31 Monitoring physical activity in children is difficult. As far as we know, reference values of activity trackers in Dutch children are not available yet. The use of questionnaires on physical activity is debated because children and adolescents tend to overrate physical activities.32 Moreover, all children were evaluated in our national multidisciplinary pediatric MS center as part of routine medical care. Therefore, we did not have a healthy control group and used published age-related reference values of the healthy population instead. Another limitation of this study is the fact that due to the small sample size, it was not possible to correct for disease-modifying therapy (DMT) in MS patients in the statistical analyses. However, 82% of the MS patients were on DMT, which indicates a relative high group homogeneity.
In conclusion, children with MS and post-ADEM in our study had significant problems on different domains of physical functioning. To further explore the associations between physical functioning (physical activity, exercise capacity, motor development) and psychosocial parameters such as fatigue, depression, anxiety, and coping, larger (multinational) cohorts with longitudinal data are necessary. A possible next step would be to investigate whether an intervention with an exercise program can improve the physical and psychosocial functioning in children with MS and post-ADEM. Finally, our data confirm that the EDSS lacks the sensitivity in children to reflect motor problems. This indicates that other measurements for the assessment of physical impairments, such as the MABCII, are needed.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Leontien CC Toussaint-Duyster, Department of Orthopedics, Section of Physical Therapy, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Yu Yi M Wong, Department of Neurology, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Monique HM Van der Cammen-van Zijp, Department of Orthopedics, Section of Physical Therapy, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Daniëlle Van Pelt-Gravesteijn, Department of Neurology, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Coriene E Catsman-Berrevoets, Department of Pediatric Neurology, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Rogier Q Hintzen, Department of Neurology, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
Rinze F Neuteboom, Department of Pediatric Neurology, Erasmus MC–Sophia Children’s Hospital, Rotterdam, The Netherlands.
References
- 1. Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: Comparison with adult-onset forms. Neurology 2002; 59(12): 1922–1928. [DOI] [PubMed] [Google Scholar]
- 2. Chitnis T, Glanz B, Jaffin S, et al. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler 2009; 15(5): 627–631. [DOI] [PubMed] [Google Scholar]
- 3. Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: A multinational observational study. Lancet Neurol 2007; 6(9): 773–781. [DOI] [PubMed] [Google Scholar]
- 4. Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain 2009; 132(Pt 12): 3392–3400. [DOI] [PubMed] [Google Scholar]
- 5. Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009; 66(1): 54–59. [DOI] [PubMed] [Google Scholar]
- 6. Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007; 356(25): 2603–2613. [DOI] [PubMed] [Google Scholar]
- 7. Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology 2008; 70(20): 1891–1897. [DOI] [PubMed] [Google Scholar]
- 8. Goretti B, Portaccio E, Ghezzi A, et al. Fatigue and its relationships with cognitive functioning and depression in paediatric multiple sclerosis. Mult Scler 2012; 18(3): 329–334. [DOI] [PubMed] [Google Scholar]
- 9. Parrish JB, Weinstock-Guttman B, Smerbeck A, et al. Fatigue and depression in children with demyelinating disorders. J Child Neurol 2013; 28(6): 713–718. [DOI] [PubMed] [Google Scholar]
- 10. Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: A long-term follow-up study of 84 pediatric patients. Neurology 2002; 59(8): 1224–1231. [DOI] [PubMed] [Google Scholar]
- 11. Beatty C, Bowler RA, Farooq O, et al. Long-term neurocognitive, psychosocial, and magnetic resonance imaging outcomes in pediatric-onset acute disseminated encephalomyelitis. Pediatr Neurol 2016; 57: 64–73. [DOI] [PubMed] [Google Scholar]
- 12. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 13. Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology 2001; 56(10): 1308–1312. [DOI] [PubMed] [Google Scholar]
- 14. Grover SA, Aubert-Broche B, Fetco D, et al. Lower physical activity is associated with higher disease burden in pediatric multiple sclerosis. Neurology 2015; 85(19): 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult Scler 2013; 19(10): 1261–1267. [DOI] [PubMed] [Google Scholar]
- 16. Gordijn M, Cremers EM, Kaspers GJ, et al. Fatigue in children: Reliability and validity of the Dutch PedsQL™ Multidimensional Fatigue Scale. Qual Life Res 2011; 20(7): 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973; 85(4): 546–562. [DOI] [PubMed] [Google Scholar]
- 18. Karila C, de Blic J, Waernessyckle S, et al. Cardiopulmonary exercise testing in children: An individualized protocol for workload increase. Chest 2001; 120(1): 81–87. [DOI] [PubMed] [Google Scholar]
- 19. Van der Cammen-van Zijp MH, Ijsselstijn H, Takken T, et al. Exercise testing of pre-school children using the Bruce treadmill protocol: New reference values. Eur J Appl Physiol 2010; 108(2): 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van der Cammen-van Zijp MH, Van den Berg-Emons RJ, Willemsen SP, et al. Exercise capacity in Dutch children: New reference values for the Bruce treadmill protocol. Scand J Med Sci Sports 2010; 20(1): e130–e136. [DOI] [PubMed] [Google Scholar]
- 21. Smits-Engelsman BE. Movement ABC-2-NL: Dutch manual. Amsterdam: Pearson, 2010. [Google Scholar]
- 22. Engelen V, Haentjens MM, Detmar SB, et al. Health related quality of life of Dutch children: Psychometric properties of the PedsQL in the Netherlands. BMC Pediatr 2009; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varni JW, Burwinkle TM, Seid M. The PedsQL as a pediatric patient-reported outcome: Reliability and validity of the PedsQL Measurement Model in 25,000 children. Expert Rev Pharmacoecon Outcomes Res 2005; 5(6): 705–719. [DOI] [PubMed] [Google Scholar]
- 24. Fredriks AM, van Buuren S, Burgmeijer RJ, et al. Continuing positive secular growth change in The Netherlands 1955–1997. Pediatr Res 2000; 47(3): 316–323. [DOI] [PubMed] [Google Scholar]
- 25. Durstine JL, Painter P, Franklin BA, et al. Physical activity for the chronically ill and disabled. Sports Med 2000; 3: 207–219. [DOI] [PubMed] [Google Scholar]
- 26. Yeh EA, Kinnett-Hopkins D, Grover SA, et al. Physical activity and pediatric multiple sclerosis: Developing a research agenda. Mult Scler 2015; 21(13): 1618–1625. [DOI] [PubMed] [Google Scholar]
- 27. Wens I, Dalgas U, Vandenabeele F, et al. High intensity exercise in multiple sclerosis: Effects on muscle contractile characteristics and exercise capacity, a randomised controlled trial. PLoS ONE 2015; 10(9): e0133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venning A, Eliott J, Wilson A, et al. Understanding young peoples’ experience of chronic illness: A systematic review. Int J Evid Based Healthc 2008; 6(3): 321–336. [DOI] [PubMed] [Google Scholar]
- 29. Wafa SW, Shahril MR, Ahmad AB, et al. Association between physical activity and health-related quality of life in children: A cross-sectional study. Health Qual Life Outcomes 2016; 14: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boreham C, Riddoch C. The physical activity, fitness and health of children. J Sports Sci 2001; 19(12): 915–929. [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization. Physical activity 2016. http://www.who.int/dietphysicalactivity/pa/en/
- 32. Chinapaw MJ, Mokkink LB, van Poppel MN, et al. Physical activity questionnaires for youth: A systematic review of measurement properties. Sports Med 2010; 40(7): 539–563. [DOI] [PubMed] [Google Scholar]