Abstract
Background
In dialysis patients, the obesity-survival paradox still requires an explanation. Anemia and high doses of erythropoiesis-stimulating agents (ESAs) are associated with worse outcomes in the hemodialysis (HD) population. In the present study, we explored the relation between obesity and anemia control in a sample of maintenance HD patients in Egypt.
Methods
This multicenter observational study included 733 patients on maintenance HD from 9 hemodialysis centers in Egypt. Clinical and laboratory data as well as average doses of ESAs and parenteral iron were recorded. The erythropoietin resistance index (ERI) was calculated.
Results
Obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, was present in 22.6% of the studied population. The target hemoglobin level (10.0–11.5 g/dL) was achieved in 27.3% of non-obese and 25.3% of obese patients, with no significant difference. The median serum ferritin and the values of transferrin saturation index did not differ significantly between these two groups. The weekly ESA dose was significantly lower in obese than in non-obese patients (P = 0.0001). A trend toward higher ESA doses and ERI values was observed in patients with lower BMIs (P < 0.0001). Multiple linear regression revealed that the BMI and urea reduction ratio were the strongest predictors of the ERI.
Conclusion
Our study adds more evidence to obesity-associated advantages in HD patients. BMI may determine ESA response, with better responses observed in patients with higher BMIs.
Keywords: Body mass index, Erythropoiesis-stimulating agents, Obesity, Renal Dialysis
Introduction
Obesity is the leading cause of increased mortality worldwide because of its association with inflammatory-metabolic disorders such as hypertension, cardiovascular disease, dyslipidemia, glucose intolerance, and certain cancers. In chronic kidney disease (CKD), there is a strong correlation between the body mass index (BMI) and the relative risk of disease progression [1]. With the increasing number of obese dialysis patients, mortality was expected to be much higher in these patients than in non-obese patients. However, contrary to this expectation, epidemiologic studies have demonstrated the protective benefit of obesity in the hemodialysis (HD) population [2]. The exact reasons for this obesity-survival paradox remain unclear. Studies exploring the mechanisms of the reverse epidemiology of obesity among maintenance HD patients are required.
Anemia and poor nutritional status are strong predictors of worse outcomes in the CKD population [3]. The health effects of anemia in HD patients have been widely studied, and anemia is recognized to have a significant impact on the disease burden, considerably increasing morbidity and mortality and reducing the quality of life [4]. The association between anemia and mortality risk may be mediated through cardiovascular disease, malnutrition, and inflammation [5]. Better management of malnutrition and anemia has been reported to improve the survival of dialysis patients [6].
Erythropoiesis-stimulating agents (ESAs) are the main treatment used to manage anemia in HD patients [7]. However, an association between higher ESA doses and mortality has been observed in many interventional studies [7–10]. In recent years, there has been growing interest in defining the effective factors and optimal ESA doses that will help patients reach the hemoglobin target without increasing their mortality [11].
We hypothesized that the obese HD population would have better control of anemia, and that this could be the link between obesity and better survival. In the present study, we aimed to evaluate anemia status in a sample of maintenance HD patients in Egypt, in order to explore any possible relationship between obesity and anemia control.
Methods
This observational multicenter study was conducted under routine clinical practice conditions at 9 hemodialysis centers in Egypt. Data were collected during March 2017. The study was approved by the institutional ethical committee. Patients’ demographic data (age, sex, weight, height, and duration of HD), clinical and laboratory results, and data corresponding to anemia (hemoglobin [Hb] levels, ferritin levels, and the transferrin saturation index [TSAT]) were obtained from the patients’ medical records. Based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines in 2012, the target Hb level was considered to be 10.0–11.5 g/dL [12].
Treatment of anemia was monitored in terms of the doses of ESAs and parenteral iron in the past 12 months, and the average dose was calculated. The ESA dose was expressed as IU/kg/week (darbepoetin alfa doses were converted as μg/kg/week × 200) [13]. The erythropoietin resistance index (ERI) was calculated as the average weekly ESA dose per kg body weight (wt) divided by the average Hb level (ERI = [ESA/wt]/Hb) [14]. Adequacy of iron therapy was defined as a serum ferritin level greater than 200 μg/L and a TSAT level greater than 20% [15]. The BMI was calculated as the weight in kilograms divided by the square of the height in meters. Patients were classified as obese and non-obese based on the BMI cutoff of 30 kg/m2. Patients were further allocated to 1 of 6 subgroups according to their BMIs: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and first-, second-, and third-degree obese (BMI 30.0–34.9 kg/m2, 35.0–39.9 kg/m2, and > 40 kg/m2, respectively).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The distributions of tested variables were examined by Shapiro– Wilk or Kolmogorov–Smirnov tests for normality as appropriate. Quantitative data are presented as the mean and standard deviation or median and interquartile range for variables with Gaussian and non-Gaussian distributions, respectively. On the other hand, qualitative data are presented as numbers and percentages. The significance of differences between continuous variables was assessed with an independent samples t test for normally distributed variables. The Mann–Whitney U test was used for data that did not follow a normal distribution. The chi-square test was used for the comparison of qualitative variables. The Kruskal–Wallis test was used to compare the distributions of two or more groups that were not normally distributed. Pearson’s and Spearman correlations were used to examine the correlations between variables according to their distribution. Multiple stepwise linear regression analysis was performed to discover the most significant predictor of the ERI, and ERI was entered as the dependent variable.
Results
Seven hundred and thirty-three patients from 9 hemodialysis centers in Egypt were recruited. Obesity, defined as a BMI ≥ 30 kg/m2, was present in 166 patients (about 22.6% of the studied population).
Table 1 describes the baseline demographic, clinical, and laboratory characteristics of the included participants. The obese and non-obese groups did not differ significantly in age. Our study included 288 women and 445 men. Obesity was significantly more prevalent in the women: 29.1% of them were obese, compared to only 18.4% of the men (P = 0.001). The prevalence rates of diabetes and hypertension were 23.5% and 52.4%, respectively, in the obese group, and were 22.4% and 47.6% in the non-obese group, with no significant difference. There were no differences between the obese and non-obese groups in the urea reduction ratio (URR), serum creatinine, albumin, Ca, or parathormone levels. The serum phosphorus level was significantly higher in the obese group (P = 0.005).
Table 1.
Baseline characteristics of hemodialysis (HD) patients with and without obesity
| Variable | Non-obese | Obese | P value |
|---|---|---|---|
| Subject | 567 (77.4) | 166 (22.6) | |
| Age (yr) | 52 ± 13.4 | 56 ± 10.7 | 0.155* |
| Duration of HD (mo) | 60 (16–228) | 56 (18–204) | 0.568† |
| Sex | |||
| Female | 204 (36.0) | 84 (50.6) | 0.001‡ |
| Male | 363 (64.0) | 82 (49.4 ) | |
| Diabetes mellitus | |||
| No | 440 (77.6) | 127 (76.5) | 0.935‡ |
| Yes | 127 (22.4) | 39 (23.5) | |
| Hypertension | |||
| No | 298 (52.6) | 79 (47.6) | 0.357‡ |
| Yes | 269 (47.4) | 87 (52.4) | |
| Serum creatinine (mg/dL) | 7.2 (2–17) | 7.6 (3–14.8) | 0.275† |
| URR (%) | 58 (25–87) | 60 (27–82) | 0.251† |
| Serum albumin (g/dL) | 4 ± 0.64 | 4.2 ± 0.47 | 0.279* |
| Serum Ca (mg/dL) | 9.3 ± 1.5 | 9.0 ± 1.3 | 0.492* |
| Serum phosphorus (mg/dL) | 4.8 (2.0–9.3) | 5.4 (2.8–10.5) | 0.005† |
| iPTH (pg/mL) | 299.0 (4.7–3,576.0) | 281.0 (16.0–1,654.0) | 0.888† |
Data are presented as number (%), mean ± standard deviation, or median (range).
iPTH, intact parathyroid hormone; URR, urea reduction ratio.
Analyzed by
Student t test,
Mann–Whitney U test,
chi-square test.
Anemia parameters are displayed in Table 2. The mean Hb concentrations were 10.2 ± 2 g/dL and 10.3 ± 2.4 g/dL in the non-obese and obese groups, respectively, with no significant difference. The median serum ferritin and TSAT values did not differ significantly between the groups. The target Hb level (10.0–11.5 g/dL) was achieved in 27.3% of the non-obese patients (155 patients) and 25.3% of the obese patients (42 patients), with no significant difference. The dose of parenteral iron did not differ significantly between the obese and non-obese groups. Adequate iron status during ESA treatment was achieved in 66.7% of non-obese and 52.4% of obese patients, with no significant difference. Most of the patients treated with ESAs were receiving epoetin alfa (98% of the patients included in this survey). The weekly ESA dose was significantly lower in the obese group than in the non-obese group (P = 0.0001).
Table 2.
Parameters of anemia in obese and non-obese hemodialysis patients
| Variable | Non-obese | Obese | P value |
|---|---|---|---|
| Subject | 567 (77.4) | 166 (22.6) | |
| Hb (g/dL) | 10.2 ± 2.3 | 10.3 ± 2.4 | 0.999* |
| Patient with target Hb | 155 (27.3) | 42 (25.3) | 0.785‡ |
| Serum ferritin (μg/L) | 429 (29–2,100) | 368 (32–1,813) | 0.268† |
| TSAT (%) | 29 (7–63) | 26 (11–51) | 0.603† |
| Patients with adequate iron status | 378 (66.7) | 87 (52.4) | 0.402‡ |
| Dose of iron (mg/wk) | 46 (0–125) | 30 (0–125) | 0.474† |
| ESA dose (unit/kg body weight/wk) | 96 (12–354) | 62 (21–164) | < 0.001† |
| ESA dose (unit/wk) | 6,000 (1,000–12,000) | 4,000 (2,000–1,400) | 0.049† |
| Erythropoietin resistance index | 8.0 (1–42.0) | 4.3 (1.7–24.8) | < 0.001† |
Data are presented as number (%), mean ± standard deviation, or median (range).
ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; TSAT, transferrin saturation.
Analyzed by
Student t test,
Mann–Whitney U test,
chi-square test.
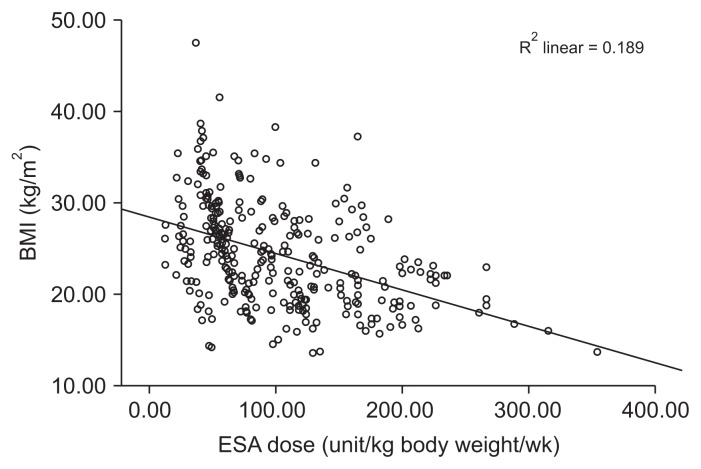
Table 3 displays the correlations of the BMI with different variables. Obesity increased with aging. Higher serum creatinine and phosphorus levels were associated with increasing BMIs. Dialysis adequacy (URR) exhibited a significant positive correlation with the BMI. Regarding anemia parameters, the Hb, serum ferritin, TSAT, and iron dose values did not correlate with the BMI. A significant negative correlation was observed between the ESA dose and BMI (Fig. 1).
Table 3.
Correlation between body mass index (BMI) and other variables
| Variable | BMI | |
|---|---|---|
|
| ||
| Rho | P value | |
| Age | 0.125 | 0.001 |
| Duration of hemodialysis | 0.071 | 0.057 |
| Serum albumin | 0.151 | 0.001 |
| Urea reduction ratio | 0.095 | 0.015 |
| Serum creatinine | 0.098 | 0.013 |
| Serum Ca | 0.081 | 0.771 |
| Serum phosphorus | 0.133 | 0.005 |
| Hemoglobin | 0.024 | 0.520 |
| Serum ferritin | 0.016 | 0.767 |
| TSAT | −0.014 | 0.872 |
| Iron dose | 0.037 | 0.729 |
| ESA dose | −0.467 | < 0.001 |
| Erythropoietin resistance index | −0.325 | < 0.001 |
ESA, erythropoiesis-stimulating agent; TSAT, transferrin saturation.
Probability of Spearman correlation.
Figure 1.
The relationship between body mass index (BMI) and erythropoiesis-stimulating agent (ESA) dose.
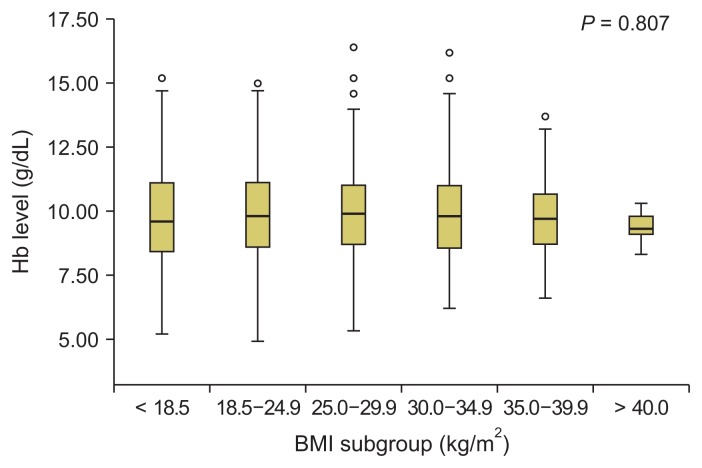
Fig. 2 demonstrates the hemoglobin levels in the BMI subgroups of HD patients. The median Hb level was 9.6 g/dL (5.2–15.2 g/dL) in underweight patients, 9.6 g/dL (4.9–14.7 g/dL) in normal-weight patients, 10.2 g/dL (5.3–15.2 g/dL) in overweight patients, 10.3 g/dL (6.2–16.2 g/dL) in first-degree obese patients, 10.6 g/dL (6.6–12.1 g/dL) in second-degree obese patients, and 9.3 g/dL (8.9–9.8 g/dL) in third-degree obese patients (those with BMIs > 40 kg/m2). The median Hb level did not differ significantly among the BMI subgroups.
Figure 2.
Hemoglobin (Hb) level in different body mass index (BMI) subgroups.
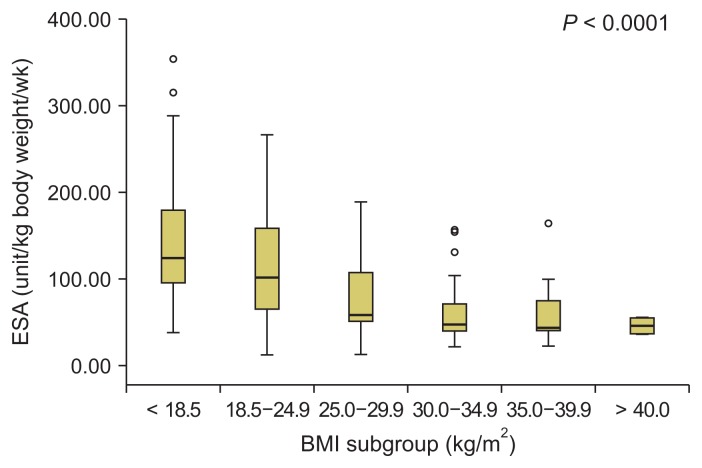
Fig. 3 demonstrates the ESA doses in the different BMI subgroups. The weekly ESA dose/kg decreased significantly as the BMI increased across subgroups. This significant difference continued until a BMI of 29.9 kg/m2. Above this cut-off point (i.e., among the three obese subgroups with BMIs ≥ 30 kg/m2), no significant difference in the ESA dose was observed. The median weekly ESA dose/kg was 123.7 IU (38.1–354.43 IU) in underweight patients, 101.4 IU (12.6–266.6 IU) in normal-weight patients, and 58.8 IU (12.5–188.9 IU) in overweight patients. First-, second- and third-degree obese patients had lower median levels of ESA/kg/week: 47.1 IU (21.62–156.4 IU), 34.3 IU (22.6–164.3 IU), and 43.3 IU (36.4–55.1 IU), respectively.
Figure 3.
Erythropoietin (ESA) dose in different body mass index (BMI) subgroups.
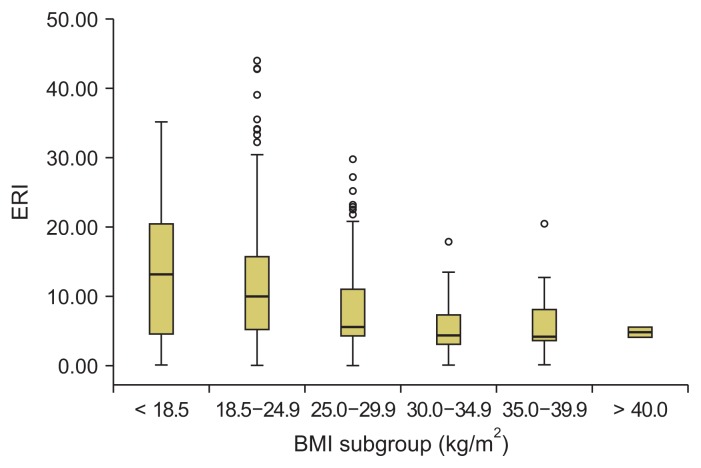
Fig. 4 demonstrates the ERI values in the different BMI subgroups. The ERI decreased significantly as the BMI increased. The median ERI (minimum–maximum) was 13.2 (0–69) in the BMI subgroup ≤ 18 kg/m2, 9.9 (0–54.4) in the BMI subgroup 18–24.9 kg/m2, 5.5 (0–31.9) in the BMI subgroup 25–29.9 kg/m2, and 4.3 (0–20.6) in the BMI subgroup 30–34.9 kg/m2. However, in the three subgroups with BMIs ≥ 30 kg/m2, no significant difference in the ESA dose was observed.
Figure 4.
Erythropoietin resistance index (ERI) in different body mass index (BMI) subgroups.
Discussion
This observational multicenter study included 733 HD patients from 9 hemodialysis centers in Egypt. All available clinical and laboratory data were obtained, as well as the 12-month average doses of parenteral iron and ESA therapy. Obesity, defined as the BMI ≥ 30 kg/m2, was present in about 22.6% of the studied population. Data from the United States indicate that the incidence and prevalence rates of obesity in maintenance dialysis patients largely exceed the corresponding figures in the general population [16]. In a retrospective, observational, cross-sectional study of 190 patients on maintenance HD recruited from 5 Spanish dialysis centers, 38% of the patients were overweight (BMI ≥ 25 kg/m2) [17]. Postorino et al [18] reported in 2009 that the prevalence of overweight in patients on HD had reached levels of up to 30%.
In general, the age of patients on HD has increased in last decade. In France, the mean age of patients on dialysis was reported to be 70.2 years [19], and in the United Kingdom it was around 65 years [20]. The obese patients in our study seemed to be older, but the difference was not significant. Of note, age and duration of dialysis had a significant positive correlation with the BMI, which may have contributed to the better survival of obese dialysis patients. The patients enrolled in this study were mostly males (60.7%). In 2000, Arnold [21] reported that fewer women than men were undergoing HD treatment, despite the higher proportion of women in the general population across all age groups. The survival advantage that women have over men in the general population was found to be markedly diminished in HD patients [22]. Of all 206,374 HD patients included in the Dialysis Outcomes and Practice Patterns Study (DOPPS), 121,566 were men and 84,808 were women, equivalent to a proportion of 41% women. Compared to women, men on dialysis were younger and less frequently obese [23]. In our studied population, obesity was also more prominent in women.
Since the introduction of ESAs in the late 1980s, it has been possible for HD patients to achieve desirable Hb levels. As a result, the mean Hb level in HD patients has improved significantly over the past two decades [24]. The KDIGO guidelines for the management of anemia in patients with ESKD on HD recommend a Hb target of 10.0–11.5 g/dL [12]. This requirement was achieved in 26.9% of our studied population. About 27.3% of non-obese patients (155 patients) and 25.3% of obese patients (42 patients) reached the target, with no significant difference. In 2017, Nafar et al [25] enrolled a total of 7,009 prevalent HD patients and found that the Hb levels of 55% of the patients were within the target values. In developing countries, anemia in HD patients may be exacerbated by environmental factors related to nutrition and a higher prevalence of infectious diseases.
ESA maintenance dosing can be challenging in HD patients due to variations in patient response. In our patients, the median ESA dose and ERI value were significantly higher in non-obese than obese patients, with a significant negative correlation between the ESA dose and BMI (Fig. 1). This increment in the erythropoietin dose in non-obese patients seemed not to result in better anemia control, as there was no improvement in the Hb level in this group. Our results were further supported by investigating the influence of the BMI on the Hb level, ESA dose, and ERI in different BMI subgroups (Fig. 2–4). There were no significant differences in Hb levels among patients in the different BMI subgroups. Patients’ ESA requirements and ERI values continued to decline significantly with increasing BMIs until a BMI of 29.9 kg/m2. Although the ESA dose continued to decline, it did not differ significantly among patients with first-, second-, and third-degree obesity. Moreover, Hb levels failed to correlate with BMI values in our studied population (Supplementary Table 1), although both BMI values and Hb levels correlated negatively with the weekly ESA dose (Supplementary Table 2). According to these results, it seems that the BMI is related to the ESA dose determination, rather than the achieved Hb level in HD patients.
A wide variety of factors have been reported to influence the response to ESAs in HD patients, such as iron deficiency, secondary hyperparathyroidism, dialysis adequacy, systemic inflammation, malnutrition, and drugs [26]. We found no significant differences between obese and non-obese patients in factors affecting the response to ESAs, such as iron status, hyperparathyroidism, serum albumin levels, creatinine levels, and dialysis adequacy.
The most common cause of ESA hyporesponsiveness in HD patients is iron deficiency. Konjin et al [27] and Pannen and Robotham [28] reported that iron indices (TSAT%, serum transferrin, serum ferritin) can predict ESA resistance. In our study, obese and non-obese patients achieved similarly adequate iron stores, with no significant difference. Obese and non-obese patients did not differ significantly in their serum ferritin levels or TSAT percentages. These results suggest that iron status did not contribute to the difference in ESA response between the obese and non-obese groups.
The median percentage of the URR in our study was slightly higher in obese than in non-obese patients, with no statistically significant difference. On the other hand, we did observe a significant positive correlation between the BMI and the URR. In 2007, Lowrie et al [29] suggested that smaller patients may require proportionately greater total doses than larger patients to achieve comparable survival. Obesity may also be associated with more efficient disposal of lipophilic uremic toxins [30]. On the other hand, the dialysis dose and nutrition are considered to be interrelated, as nutrition is inextricably linked to the adequacy of HD treatment [31], and increased fat stores in HD patients usually reflect a well-preserved appetite [32]. Ifudu et al [33] demonstrated that the dialysis adequacy in ESRD patients receiving ESA treatment correlated directly with the patients’ Hb and hematocrit levels. The weekly ESA dose in our HD patients had a significant negative correlation with the URR. Our results confirm that adequate dialysis reduces the need for erythropoietin.
Hyperparathyroidism inhibits erythropoiesis secondary to bone marrow fibrosis [34,35]. We found that intact parathyroid hormone (iPTH) levels did not differ significantly between obese and non-obese patients. In addition, the weekly ESA dose did not correlate with PTH levels. Thus, PTH levels did not seem to determine ESA responsiveness in our patients.
Serum albumin not only is an indicator of nutritional status in HD patients, but also has been reported to be a good predictive parameter of epoetin responsiveness [36]. In 2012, Mallick et al [37] found that patients with higher albumin levels and transferrin saturation levels had better responses to ESAs. In our study, serum albumin levels did not differ significantly between obese and non-obese patients (Table 1), but correlated positively with the BMI (Table 3) and Hb levels (Supplementary Table 1). The ESA dose was not found to correlate with serum albumin levels (Supplementary Table 2). According to our results, albumin levels were related to BMI values and Hb levels, but not to ESA responsiveness.
Multiple linear regression analysis revealed that the BMI and URR were the strongest predictors of the ERI (Table 4). In 2013, do Sameiro-Faria et al [38] evaluated 191 HD patients and 25 healthy individuals, and found that Hb, BMI, and albumin levels were independent variables associated with ESA doses.
Table 4.
Multiple linear regression analysis with erythropoietin resistance index as dependent variable
| Model | Unstandardized coefficients | Standardized coefficients | t | P value | |
|---|---|---|---|---|---|
|
|
|
||||
| B | SE | Beta | |||
| (Constant) | 39.506 | 7.317 | 5.399 | 0.000 | |
| Age | −0.115 | 0.054 | −0.146 | −2.116 | 0.036 |
| BMI | −0.395 | 0.132 | −0.207 | −2.999 | 0.003 |
| URR | −20.017 | 8.227 | −0.168 | −2.433 | 0.016 |
| Albumin | −0.047 | 1.127 | −0.003 | −0.041 | 0.967 |
| Serum ferritin | −0.002 | 0.002 | −0.076 | −1.129 | 0.260 |
BMI, body mass index; URR, urea reduction ratio; SE, standard error.
In the past few years, two initial studies with small sample sizes elucidated a potential relation between the BMI and resistance to ESAs in HD patients [38,39]. Our data indicated that the BMI determines the ESA response, with better responses observed in patients with higher BMIs. This favorable ESA response may reflect a modulated response in obesity mediated by autocrine regulation. Leptin, which is higher in overweight patients, has been shown to stimulate erythropoiesis [39]. Obesity also provides protection against malnutrition. Hyporesponsiveness to ESAs in non-obese HD patients may simply be a marker of poor underlying overall health. Malnutrition-inflammation complex syndrome is more relevant in non-obese patients, and can reduce ESA responsiveness.
In conclusion, our study adds to the evidence of obesity-associated advantages. Anemia control was achieved in obese dialysis patients with lower ESA doses. The strength of this study is that a large number of HD patients from nine HD centers were recruited. However, this observational study only identified a possible association between the ESA dose and BMI. Prospective studies are needed to provide evidence in this context and to investigate the mechanisms linking BMI values and different measures of body composition (especially fat mass) with ESA requirements in HD patients.
Supplementary Data
Acknowledgments
The author would like to thank Dr. Magdy Hegazy (Alexandria medical director in Minstry of Health and Population), Brigadier General Dr. Mohamed Shawky Ali Elmasry (director of Mansoura Military Hospital), Affaf Fahmy (nursing director of nephrology unit), Hanan Nagah (veterinary student), Soha Ali (pharmacy student), Heba Magdy El Bahnasawy (dentistry student), Menna Magdy El Bahnasawy (medical student), and Mr. Rabia Ramadan.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 2.Park J, Ahmadi SF, Streja E, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–425. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanni A, Mannucci I, Verzola D, et al. Protein-energy wasting and mortality in chronic kidney disease. Int J Environ Res Public Health. 2011;8:1631–1654. doi: 10.3390/ijerph8051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–960. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Collins AJ. Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodi-alysis patients. Kidney Int. 2004;65:626–633. doi: 10.1111/j.1523-1755.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 9.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 11.Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide) line(s) Kidney Int. 2012;82:952–960. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline anaemia of chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 13.Aljama P, Bommer J, Canaud B, et al. Practical guidelines for the use of NESP in treating renal anaemia. Nephrol Dial Transplant. 2001;16(Suppl 3):22–28. doi: 10.1093/ndt/16.suppl_3.22. [DOI] [PubMed] [Google Scholar]
- 14.Chait Y, Kalim S, Horowitz J, et al. The greatly misunderstood erythropoietin resistance index and the case for a new responsiveness measure. Hemodial Int. 2016;20:392–398. doi: 10.1111/hdi.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo V, Martin M, Rufino M, et al. High prevalence of overweight in a stable Spanish hemodialysis population: a cross sectional study. J Ren Nutr. 2003;13:52–59. doi: 10.1053/jren.2003.50004. [DOI] [PubMed] [Google Scholar]
- 18.Postorino M, Marino C, Tripepi G, Zoccali C CREDIT (Calabria Registry of Dialysis and Transplantation) Working Group. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 19. [Network and epidemiologic information in nephrology: Kidney report 2009]. French In. Nephrol Ther. 2011;7(Suppl 2):S43–S214. doi: 10.1016/S1769-7255(11)70009-8. [DOI] [PubMed] [Google Scholar]
- 20.The Renal Association. [Date accessed: 20 October 2017];UK Renal Registry Report. 2008 Available at: http://www.renalreg.com/Reports/2008.html.
- 21.Arnold K. Journal to encourage analysis by sex/ethnicity. J Natl Cancer Inst. 2000;92:1561. doi: 10.1093/jnci/92.19.1561. [DOI] [PubMed] [Google Scholar]
- 22.Taking sex into account in medicine. Lancet. 2011;378:1826. doi: 10.1016/S0140-6736(11)61795-9. [DOI] [PubMed] [Google Scholar]
- 23.Hecking M, Bieber BA, Ethier J, et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11:e1001750. doi: 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA. Changing patterns of anemia management in US hemodialysis patients. Am J Med. 2012;125:906–914.e9. doi: 10.1016/j.amjmed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Nafar M, Samavat S, Khoshdel A, Alipour-Abedi B. Anemia evaluation and erythropoietin dose requirement among hemodialysis patients: a multicenter study. Iran J Kidney Dis. 2017;11:56–65. [PubMed] [Google Scholar]
- 26.Szczech LA, Barnhart HX, Sapp S, et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 2010;77:239–246. doi: 10.1038/ki.2009.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konijn AM, Carmel N, Levy R, Hershko C. Ferritin synthesis in inflammation. II. Mechanism of increased ferritin synthesis. Br J Haematol. 1981;49:361–370. doi: 10.1111/j.1365-2141.1981.tb07238.x. [DOI] [PubMed] [Google Scholar]
- 28.Pannen BH, Robotham JL. The acute-phase response. New Horiz. 1995;3:183–197. [PubMed] [Google Scholar]
- 29.Lowrie EG, Li Z, Ofsthun N, Lazarus JM. Measurement of dialyzer clearance, dialysis time, and body size: death risk relationships among patients. Kidney Int. 2004;66:2077–2084. doi: 10.1111/j.1523-1755.2004.00987.x. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P, Lindholm B. Resolved: being fat is good for dialysis patients: the Godzilla effect: con. J Am Soc Nephrol. 2008;19:1062–1064. [PubMed] [Google Scholar]
- 31.Carrero JJ, Qureshi AR, Axelsson J, et al. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr. 2007;85:695–701. doi: 10.1093/ajcn/85.3.695. [DOI] [PubMed] [Google Scholar]
- 32.Azar AT, Wahba K, Mohamed AS, Massoud WA. Association between dialysis dose improvement and nutritional status among hemodialysis patients. Am J Nephrol. 2007;27:113–119. doi: 10.1159/000099836. [DOI] [PubMed] [Google Scholar]
- 33.Ifudu O, Feldman J, Friedman EA. The intensity of hemodialysis and the response to erythropoietin in patients with end-stage renal disease. N Engl J Med. 1996;334:420–425. doi: 10.1056/NEJM199602153340702. [DOI] [PubMed] [Google Scholar]
- 34.Muirhead N, Hodsman AB, Hollomby DJ, Cordy PE. The role of aluminium and parathyroid hormone in erythropoietin resistance in haemodialysis patients. Nephrol Dial Transplant. 1991;6:342–345. doi: 10.1093/ndt/6.5.342. [DOI] [PubMed] [Google Scholar]
- 35.Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med. 1993;328:171–175. doi: 10.1056/NEJM199301213280304. [DOI] [PubMed] [Google Scholar]
- 36.Macdougall LG, Moodley G, Eyberg C, Quirk M. Mechanisms of anemia in protein-energy malnutrition in Johannesburg. Am J Clin Nutr. 1982;35:229–235. doi: 10.1093/ajcn/35.2.229. [DOI] [PubMed] [Google Scholar]
- 37.Mallick S, Rafiroiu A, Kanthety R, Iqbal S, Malik R, Rahman M. Factors predicting erythropoietin resistance among maintenance hemodialysis patients. Blood Purif. 2012;33:238–244. doi: 10.1159/000335256. [DOI] [PubMed] [Google Scholar]
- 38.do Sameiro-Faria M, Ribeiro S, Rocha-Pereira P, et al. Body mass index and resistance to recombinant human erythropoietin therapy in maintenance hemodialysis patients. Ren Fail. 2013;35:1392–1398. doi: 10.3109/0886022X.2013.828267. [DOI] [PubMed] [Google Scholar]
- 39.Vega A, Ruiz C, Abad S, et al. Body composition affects the response to erythropoiesis-stimulating agents in patients with chronic kidney disease in dialysis. Ren Fail. 2014;36:1073–1077. doi: 10.3109/0886022X.2014.917937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.