Abstract
Background/Aims
This study aimed to describe the health-related quality of life (HRQoL) outcomes for Korean chronic hepatitis C patients and to investigate the impact of patient and virus-related factors on HRQoL.
Methods
HRQoL was assessed in 235 hepatitis C virus (HCV)-infected patients from seven nationwide tertiary hospital, including those with liver cirrhosis and hepatocellular carcinoma (HCC), using the Shor-Form 36 (SF-36) version 2 and the European quality of life questionnaire-5 dimensions (EQ-5D-3L).
Results
The SF-36 physical (48.8±8.3) and mental (46.2±11.7) component summary scores of the HCV-infected patients were below normal limits. Of the eight domains, general health, vitality, and mental health tended to show low scores. Patients with decompensated cirrhosis had the lowest HRQoL, while HCC and chronic hepatitis patients had similar HRQoL results. The EQ-5D index was low (0.848±0.145) in the HCV infected patients. Multivariable analysis showed age ≤65 years, high monthly family income (>$2,641), low comorbidity score, and sustained virologic response (SVR) were independently associated with favorable HRQoL.
Conclusions
HRQoL in Korean patients with chronic HCV infection was low and was affected by cirrhosis severity, SVR, and comorbidity as well as income, which had the strongest effect. Therefore, HRQoL may be improved by antiviral therapy with reasonable costs to prevent cirrhosis progression.
Keywords: Quality of life, Hepatitis C, Republic of Korea
INTRODUCTION
About 185 million people worldwide are estimated to have chronic hepatitis C virus (HCV) infection.1 Of these patients, 20% to 30% progress to liver cirrhosis (LC), which may subsequently lead to hepatic decompensation and hepatocellular carcinoma (HCC).2 With a growing emphasis on patient-centered medicine, patient illness and treatment experiences, expressed as patients-reported outcome measures (PROM), are gaining importance more than ever before3 and should be investigated in addition to liver-related mortality and morbidity.
Health-related quality of life (HRQoL) is a quantitative PROM, usually measured by standardized psychometric questionnaires. The Health Survey Short Form-36 (SF-36) and the European quality of life questionnaire-5 dimensions (EQ-5D) are two of the most commonly employed HRQoL measurement tools. However, as HRQoL measurements reflect not only an individual’s perception but also a population’s resources and policies,4 results may vary for specific diseases across different regions and race. Thus, though Western HRQoL studies have reported impaired HRQoL in chronic hepatitis C (CHC) patients by disease severity when compared to the general population,5 these results may not be reflective of the HRQoL in South Korean CHC patients, which has not been investigated.
Additionally, antiviral therapy impacts the HRQoL of CHC patients. Despite approval of potent direct acting antiviral agents (DAAs), interferon (IFN) based therapy was the only available option in South Korea until 2015. Notably, IFN treatment has frequent adverse events, negatively impacting HRQoL in CHC patients. In addition, DAAs are costly. Therefore, HRQoL measurements in CHC patients are needed to estimate the cost-effectiveness of DAA treatments in distinct global regions with different socioeconomical conditions.
Thus, this study aimed to describe the HRQoL outcomes for Korean CHC patients via SF-36 and EQ-5D, compare the HRQoL in CHC patients with that of the general Korean population, and investigate the impact of patient-related and virus-related factors on the HRQoL.
MATERIALS AND METHODS
1. Patients
In this multicenter cross-sectional study, 235 patients over 18 years of age with chronic HCV infection (positive HCV RNA), including those with LC and HCC, were prospectively enrolled at ambulatory outpatient clinics from November 2014 to March 2015. This nationwide study included seven tertiary institutions located in the capital area (Seoul National University Bundang Hospital, Soonchunhyang University Bucheon Hospital and Inje University Ilsan Paik Hospital), three metropolitan cities (Inje University Busan Paik Hospital in Busan, Chungnam National University Hospital in Daejeon, Keimyung University Hospital in Daegu), and one province area (Chonbuk National University Hospital in Jeonju) of South Korea. This study was approved by the Institutional Review Board (IRB) of the seven hospitals, and written informed consent was obtained from all participants prior to this study.
2. Collection of clinical data
Demographic and socioeconomic characteristics including age, gender, education, monthly family income, health insurance status, employment status, comorbidities for calculating Charlson comorbidity Index,6 smoking and drinking status, and regular exercise habits of the subjects were obtained from the questionnaire.
Each subject’s HCV status, HCV genotype, viral load, and antiviral treatment experience were collected from his/her medical record.
Liver disease status was classified into five categories: CHC, compensated LC, decompensated LC, HCC, and liver-transplanted. LC was defined by histological examination or by one or more clinical findings of portal hypertension:7 (1) cirrhotic appearance of the liver with splenomegaly on imaging study (ultrasonography, computed tomography or magnetic resonance image); (2) thrombocytopenia (platelet <120,000/mm3); (3) presence of esophagogastric varices on endoscopy; (4) presence of ascites; and (5) presence of portosystemic encephalopathy. Among them, decompensated LC was defined as those with complications of LC such as presence of significant jaundice (total bilirubin, >2 mg/dL), ascites, variceal bleeding, or portosystemic encephalopathy. HCC was diagnosed based on histological findings or typical imaging characteristics as defined by the Korean Liver Cancer Study Group and the National Cancer Center guidelines,8 which is similar to the American Association for the Study of Liver Diseases guideline.
3. Assessment of HRQoL in CHC patients
The SF-36 version 2 questionnaire9,10 is a self-administered questionnaire comprised of a total of 36 items with two (physical and mental) components. The physical component summary (PCS) includes the four domains of physical functioning (PF), physical role functioning (RP), bodily pain (BP), and general health (GH). The mental component summary (MCS) includes four domains of vitality (VT), social functioning (SF), emotional role (RE), and mental health (MH). Each raw domain score can be converted into a 0–100 scale, with a higher score indicating a higher health status. The usage of the questionnaire survey and software for analysis were licensed by Optuminsight Life Sciences, Inc (Lincoln, RI, USA).
The EQ-5D is a measure of health status consisting of two parts: EQ-5D descriptive system and EQ-5D visual analogue scale (VAS). The descriptive system includes11,12 mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. In this study, a 3-level scoring scale was used for each dimension, in which 1, 2, or 3 represented “no problems,” “some or moderate problems,” or “extreme problems,” respectively. An EQ-5D score of 1 denoted “full health” (11111 state) and 0 denoted “death.” Once the data was collected, a scoring function was used to assign a value (EQ-5D index score) to self-reported health states from a set of population-based preference weights.12 The Korean version of EQ-5D has been validated and included in the Korea National Health and Nutrition Examination Survey (KNHANES) since 2005.12,13
4. Statistical analysis
Descriptive analysis for continuous variables was performed with mean±standard deviation for normally distributed data and interquartile range and median for non-normally distributed data. Categorical variables were described by counts and related percentages. Comparisons of HRQoL score among groups defined by liver disease status or by IFN-based antiviral treatment experience were performed by one-way analysis of variance (ANOVA) and by Scheffe post hoc analysis. Multivariable binary regression analysis was performed to predict favorable PCS or MCS, respectively. Additionally, multivariable linear regression was performed to estimate independent association of each variable with EQ-5D index. A p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata version 14.0 (College Station, TX, USA).
RESULTS
1. Patient characteristics
The baseline characteristics of 235 patients with chronic HCV infection were summarized in Table 1. The mean age was 60.5±11.1 years and 45.1% of the subjects were males (Table 1). Median Charlson comorbidity index score was 1.0 (range, 0 to 9; data not shown), and 62 patients (26.4%) showed high comorbidity index (>2). Private health insurance was held by 146 patients (62.1%), and 109 patients (46.4%) had an occupation with steady income. A total of 181 patients (77%) responded that their monthly family income was less than 3,000,000 Korean Won (KRW, about $2,641).
Table 1.
Demographic and Clinical Characteristics of 235 Patients with Chronic Hepatitis C Virus Infection in South Korea
| Characteristics | Value |
|---|---|
| Age, yr | 60.5±11.1 |
| Sex | |
| Male | 106 (45.1) |
| Female | 129 (54.9) |
| Body mass index, kg/m2 | 23.6±3.4 |
| Smoking (never) | 128 (54.5) |
| Alcohol drinking (never) | 114 (48.5) |
| Regular exercise (≥1 times/wk) | 134 (57.0) |
| Charlson comorbidity index (>2) | 62 (26.4) |
| Employed | 109 (46.4) |
| Education (high school graduate) | 44 (18.7) |
| Having spouse | 173 (73.6) |
| Monthly family income, KRW* | |
| <1,000,000 | 71 (30.2) |
| 1,000,000–1,999,999 | 58 (24.7) |
| 2,000,000–2,999,999 | 52 (22.1) |
| 3,000,000–3,999,999 | 19 (8.1) |
| 4,000,000–4,999,999 | 8 (3.4) |
| 5,000,000–5,999,999 | 12 (5.1) |
| >6,000,000 | 15 (6.4) |
| Underlying liver disease | |
| Chronic hepatitis | 139 (59.1) |
| Liver cirrhosis, compensated | 44 (18.7) |
| Liver cirrhosis, decompensated | 9 (3.8) |
| Liver cancer | 41 (17.4) |
| Liver transplantation | 2 (0.9) |
| HCV genotype | |
| 1 | 119 (50.6) |
| 2 | 95 (40.4) |
| 3 | 1 (0.4) |
| 6 | 1 (0.4) |
| Not tested | 19 (8.1) |
| HCV RNA titer, IU/mL | |
| <100,000 | 37 (15.7) |
| 100,000–650,000 | 49 (20.9) |
| >650,000 | 130 (55.3) |
| Not tested | 19 (8.1) |
| Interferon-based antiviral treatment | |
| Naïve | 98 (41.7) |
| Treatment-experienced (non-responder, intolerance) | 52 (22.1) |
| On treatment | 49 (20.9) |
| Sustained virologic response | 36 (15.3) |
Data are presented as mean±SD or number (%).
KRW, Korean Won; HCV, hepatitis C virus.
The currency exchange rate of 1 KRW to U.S. Dollar (USD) was approximately 0.0008 (1,000,000 KRW=880.3 USD).
Liver disease status was chronic hepatitis in 139 patients (59.1%), compensated LC in 44 (18.7%), decompensated LC in nine (3.8%), and HCC in 41 patients (17.4%) at time of survey. Two subjects (0.9%) had undergone liver transplantation and had no recurrence of cirrhosis or cancer. Distribution of liver disease status was typical of that of CHC patients in Korea as well as in other regions. Major HCV genotypes were genotype 1 (119, 50.6%), and 2 (95, 40.4%), reflecting a typical distribution found in Korean CHC patients. IFN-based antiviral treatment-naïve and -experienced patients were 98 (41.7%), and 137 (58.3%), respectively. Of the 137 treated patients, 36 (15.3%) achieved sustained virologic response (SVR), 52 (22.1%) showed no response or intolerance, and 49 (20.9%) were on-treatment at the time of survey.
2. HRQoL in Korean patients with chronic HCV infection
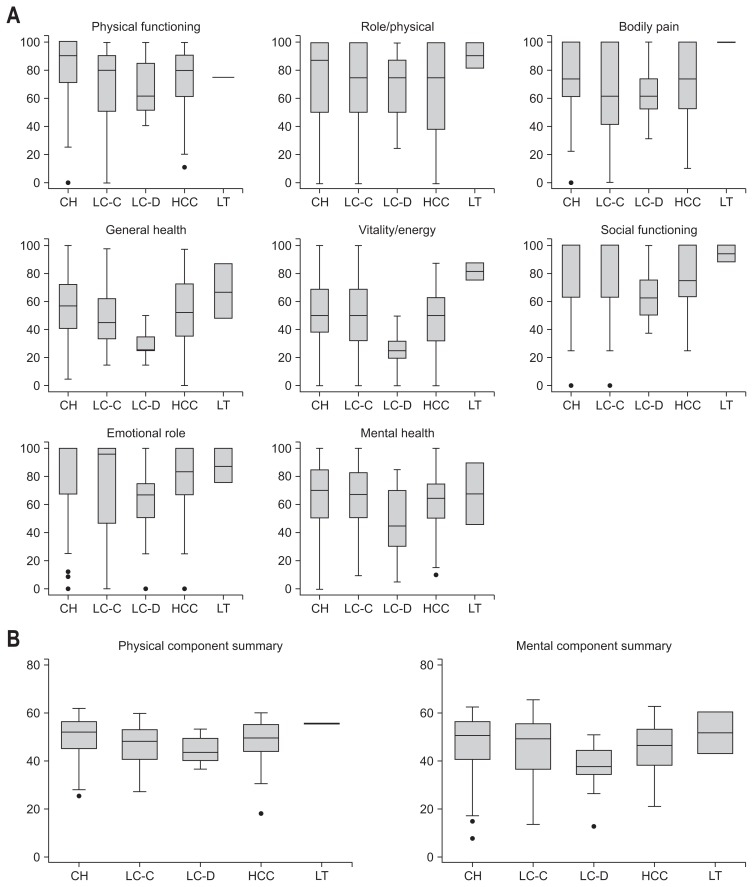
HRQoL scores in CHC patients were below normal ranges regardless of measurement tool used (Fig. 1A). By SF-36 measurements, patients with chronic HCV infection scored 76.6±22.8 in PF, 72.0±30.8 in RP, 72.3±26.9 in BP, 53.5±21.7 in GH, 50.2±24.2 in VT, 80.2±24.5 in SF, 77.0±30.5 in RE and 65.0±23.5 in MH domain. Of these, GH, VT, and MH domains showed especially low scores. Summary scores showed a PCS of 48.8±8.3 and a MCS of 46.2±11.7 in HCV infected Korean patients (Table 2, Fig 1B).
Fig. 1.
Health-related quality of life measured by the Short Form 36 version 2 according to liver disease status in Korean patients with chronic hepatitis C infection. (A) Individual domain scores. (B) Summary scores.
CH, chronic hepatitis (n=139); LC-C, compensated liver cirrhosis (n=44); LC-D, decompensated liver cirrhosis (n=9); HCC, hepatocellular carcinoma (n=41); LT, liver transplantation recipient (n=2). Detailed information is provided in Table 2.
Table 2.
Health-Related Quality of Life Assessed by the SF-36 According to Liver Disease Severity in Korean Patients with Chronic HCV Infection
| Total HCV-infected patients (n=235) | Chronic hepatitis (n=139) | Compensated LC (n=44) | Decompensated LC (n=9) | Hepatocellular carcinoma (n=41) | Liver transplantation (n=2) | p-value | |
|---|---|---|---|---|---|---|---|
| SF-36 domain score | |||||||
| PF | 76.6±22.8 | 80.6±21.5 | 70.9±25.5 | 65.5±21.0 | 71.7±22.7 | 75.0±0.0 | 0.024 |
| RP | 72.0±30.8 | 74.7±30.9 | 69.6±29.7 | 65.3±29.2 | 66.2±32.4 | 90.7±13.3 | 0.418 |
| BP | 72.3±26.9 | 74.7±25.9 | 65.5±28.8 | 63.2±20.2 | 72.0±28.7 | 100.0±0.0 | 0.131 |
| GH | 53.5±21.7 | 56.7±20.8 | 48.5±20.5 | 29.7±10.2* | 52.4±23.8 | 67.0±29.3 | 0.001 |
| VT | 50.2±24.2 | 52.2±23.4 | 49.3±27.7 | 25.7±14.5* | 48.0±21.8 | 81.3±8.8 | 0.007 |
| SF | 80.2±24.5 | 81.5±24.4 | 81.8±25.1 | 66.7±21.7 | 76.2±24.8 | 93.8±8.8 | 0.289 |
| RE | 77.0±30.5 | 79.8±30.0 | 73.1±32.7 | 60.2±30.8 | 75.2±29.4 | 87.5±17.7 | 0.290 |
| MH | 65.0±23.5 | 67.5±22.3 | 63.2±26.9 | 49.4±26.0 | 62.0±22.2 | 67.5±31.8 | 0.163 |
| SF-36 summary score | |||||||
| PCS | 48.8±8.3 | 50.0±8.1 | 46.6±8.1 | 45.0±6.1 | 47.6±9.0 | 55.3±0.03 | 0.041 |
| MCS | 46.2±11.7 | 47.1±11.5 | 46.0±12.7 | 36.8±11.5 | 45.1±10.7 | 51.8±12.4 | 0.113 |
| EQ-5D profile (11111) | 82 (34.9) | 59 (42.5) | 11 (25.0) | 1 (11.1) | 11 (26.8) | 0 | 0.043 |
| EQ-5D VAS | 67.9±17.8 | 69.5±15.5 | 65.7±20.0 | 58.3±13.7 | 66.3±22.2 | 85±7.1 | 0.148 |
| EQ-5D index | 0.848±0.145 | 0.880±0.105 | 0.775±0.225* | 0.786±0.134 | 0.829±0.123 | 0.910±0.004 | <0.001 |
Data are presented as mean±SD or number (%).
SF-36, Short Form 36; HCV, chronic hepatitis C virus; LC, liver cirrhosis; PF, physical functioning; RP, physical role functioning; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, emotional role; MH, mental health; PCS, physical component summary; MCS, mental component summary; EQ-5D, European quality of life questionnaire-5 dimensions; VAS, visual analogue scale.
p-value <0.05 compared with the chronic hepatitis C group using Scheffe post hoc analysis.
Detailed explanation about tests and a survey license request are available at each website (SF-36, https://campaign.optum.com/content/optum/en/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html; EQ-5D, https://euroqol.org).
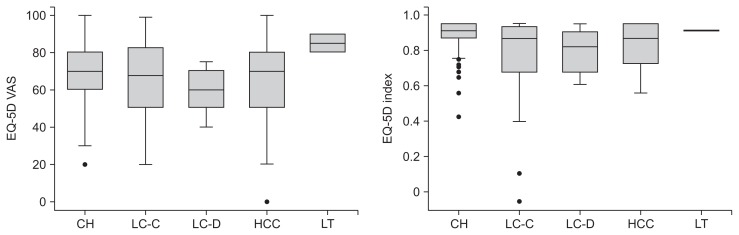
By EQ-5D, HCV infected subjects showed impaired HRQoL (Table 2, Fig 2). Only 34.9% of HCV infected subjects expressed no problem in all EQ-5D domains (11111), and mean VAS was 67.9±17.8. The mean EQ-5D index score, which ranged from 0 to 1 similar with utility score, was 0.848±0.145.
Fig. 2.
Health-related quality of life measured by the European quality of life questionnaire-5 dimensions (EQ-5D) according to liver disease status in Korean patients with chronic hepatitis C infection.
VAS, visual analogue scale; CH, chronic hepatitis (n=139); LC-C, compensated liver cirrhosis (n=44); LC-D, decompensated liver cirrhosis (n=9); HCC, hepatocellular carcinoma (n=41); LT, liver transplantation recipient (n=2). Detailed information is provided in Table 2.
3. Impact of liver disease status and IFN-based antiviral treatment outcome on HRQoL scores in Korean patients with chronic HCV infection
There were significant differences by ANOVA in SF-36 domains PF, GH, and VT, as well as PCS among different liver disease statuses (Table 2, Fig. 1). LC patients tended to have lower scores in all domains when compared to chronic hepatitis or HCC patients. GH and VT scores in the decompensated LC group were significantly lower (GH, 29.7±10.2; VT, 25.7±14.5) than those of all other liver disease groups by post hoc analysis (Table 2, Fig. 1A). MH and MCS scores of the decompensated LC group tended to be low, but this was not statistically significant, probably due to the small sample size of the decompensated cirrhosis group (Table 2, Fig. 1).
Similarly, EQ-5D measurements showed that the proportion of subjects with best scores (score 1) in all domains was significantly different among the five liver disease status groups (p=0.043) (Table 2). EQ-5D index was significantly different among the groups, and the lowest score was recorded in the compensated LC group (0.775±0.225, p<0.001) (Table 2, Fig. 2). EQ-VAS did not indicate HRQoL difference based on disease severity (p=0.148) (Table 2, Fig. 2).
The effect of antiviral treatment in Korean patients with HCV infection was described in Table 3. SF-36 domains RP, GH, VT, and SF showed significant differences according to antiviral treatment results. Moreover, RP and VT in subjects who had SVR were significantly higher than that of treatment-naïve subjects (Table 3). Nonetheless, SF-36 summary score and EQ-5D results (profile, VAS, and index) did not significantly differ according to antiviral treatment.
Table 3.
Health-Related Quality of Life According to the Results of Interferon-Based Antiviral Therapy in Korean Patients with Chronic HCV Infection
| Antiviral treatment naïve (n=98) | On treatment (n=49) | Sustained virologic responders (n=36) | Treatment failure or intolerance (n=52) | p-value | |
|---|---|---|---|---|---|
| SF-36 domain score | |||||
| PF | 73.8±24.4 | 81.4±19.7 | 81.5±23.7 | 74.0±20.9 | 0.109 |
| RP | 67.2±32.8 | 67.2±32.0 | 87.2±22.9* | 75.1±27.3 | 0.005 |
| BP | 71.7±27.0 | 69.0±27.1† | 77.9±26.6 | 72.6±26.7 | 0.503 |
| GH | 50.2±21.9 | 58.5±18.9 | 59.8±17.1 | 50.5±25.0 | 0.030 |
| VT | 47.2±23.5 | 48.5±24.5 | 60.4±23.3† | 50.4±24.7 | 0.041 |
| SF | 77.4±26.1 | 79.3±24.8 | 90.6±18.3 | 78.8±23.7 | 0.045 |
| RE | 74.8±31.6 | 73.0±32.3 | 86.6±27.1 | 78.5±27.9 | 0.167 |
| MH | 62.6±24.6 | 64.5±22.7 | 70.7±23.7 | 66.3±21.9 | 0.341 |
| SF-36 summary score | |||||
| PCS | 47.7±8.9 | 49.5±7.5 | 51.6±7.1 | 48.2±8.3 | 0.084 |
| MCS | 45.0±11.8 | 44.9±12.7 | 50.2±10.5 | 46.7±10.9 | 0.110 |
| EQ-5D profile (11111) | 34 (34.7) | 18 (36.7) | 15 (41.7) | 15 (28.9) | 0.651 |
| EQ-5D VAS | 65.8±18.4 | 68.5±16.9 | 68.1±16.1 | 72.8±19.0 | 0.242 |
| EQ-5D index | 0.834±0.138 | 0.870±0.121 | 0.875±0.156 | 0.835±0.166 | 0.277 |
Data are presented as mean±SD or number (%).
HCV, chronic hepatitis C virus; SF-36, Short Form 36; PF, physical functioning; RP, physical role functioning; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, emotional role; MH, mental health; PCS, physical component summary; MCS, mental component summary; EQ-5D, European quality of life questionnaire-5 dimensions; VAS, visual analogue scale.
p-value <0.05 compared with the treatment naïve group and the on-treatment group using Scheffe post hoc analysis;
p-value <0.05 compared with the treatment naïve group using Scheffe post hoc analysis.
4. Independent factors affecting HRQoL scores in Korean patients with chronic HCV infection
According to multivariable regression analysis, favorable physical status measured by SF-36 (above normal PCS) was independently affected by age, monthly family income, comorbidity, and antiviral treatment outcome (Table 4). A high monthly family income and SVR led to favorable mental status, but age and comorbidity did not.
Table 4.
Independent Factors Affecting the Physical and Mental Scores of the SF-36 in Korean Patients with Chronic HCV Infection
| Favorable physical status (above normal PCS) | Favorable mental status (above normal MCS) | |
|---|---|---|
| Age >65 yr | 0.38 (0.20–0.73)† | 1.05 (0.59–1.92) |
| Female sex | 0.92 (0.48–1.76) | 0.66 (0.37–1.16) |
| Education (>high school graduate) | 1.31 (0.50–3.48) | 0.95 (0.43–2.09) |
| Monthly family income >3,000,000 KRW* | 4.13 (1.48–11.55)† | 3.08 (1.42–6.68)† |
| Comorbidity score (per 1 increase) | 0.68 (0.55–0.86)† | 0.90 (0.73–1.10) |
| Advanced liver disease (decompensated LC or HCC) | 2.20 (0.89–5.45) | 0.96 (0.43–2.14) |
| Sustained virologic response | 4.22 (1.36–1.00)† | 2.26 (1.01–5.06) |
SF-36, Short Form 36; HCV, chronic hepatitis C virus; PCS, physical component summary; MCS, mental component summary; KRW, Korean Won; LC, liver cirrhosis; HCC, hepatocellular carcinoma.
The currency exchange rate of 1 KRW to U.S. Dollar (USD) was approximately 0.0008 (3,000,000 KRW=2,641 USD);
p-value <0.05.
Notably, independent factors for EQ-5D index were not identical to those for SF-36. As shown in Table 5, old age and an increase in comorbidity negatively affected both EQ-5D index and SF-36, but monthly family income and SVR, which significantly affected SF-36, did not affect EQ-5D index. Instead, an education level above high school increased EQ-5D index significantly (Table 5).
Table 5.
Independent Factors Affecting the EQ-5D Index in Korean Patients with Chronic HCV Infection
| β | p-value | |
|---|---|---|
| Age >65 yr | −0.06 (−0.10 to −0.20)† | 0.004† |
| Female sex | −0.01 (−0.04 − 0.04) | 0.998 |
| Education (>high school) | 0.05 (0.01 to 0.10)† | 0.042† |
| Monthly family income >3,000,000 KRW* | 0.02 (−0.03 to 0.06) | 0.519 |
| Comorbidity score (per 1 increase) | −0.16 (−0.03 to −0.02)† | 0.022† |
| Advanced liver disease (decompensated LC or HCC) | 0.02 (−0.03 to 0.07) | 0.470 |
| Sustained virologic response | 0.02 (−0.03 to 0.07) | 0.384 |
EQ-5D, European quality of life questionnaire-5 dimensions; HCV, chronic hepatitis C virus; KRW, Korean Won; LC, liver cirrhosis; HCC, hepatocellular carcinoma.
The currency exchange rate of 1 KRW to U.S. Dollar (USD) was approximately 0.0008 (3,000,000 KRW=2,641 USD);
p-value <0.05.
DISCUSSION
In the present cross-sectional study, HRQoL in Korean ambulatory patients with chronic HCV infection was impaired in all domains. This was the first study to determine HRQoL in CHC patients with dual measurement tools (SF-36v2 and EQ-5D), allowing for comparison between HCV infected subjects and the general population reference in South Korea. Results showed that age and comorbidity negatively affected HRQoL in HCV infected subjects regardless of the measurement tool used. However, monthly family income, education, and SVR differed in their impact on HRQoL between the two measurement tools. Although LC patients showed lower HRQoL than chronic hepatitis or HCC patients, advanced liver disease per se was not an independent factor of HRQoL measured by SF-36v2 and EQ-5D in Korean HCV infected patients.
To define HRQoL in chronic liver disease patients, several disease specific tools have been previously developed, such as the liver disease QoL (LDQoL),14 sickness impact profile,15 multidimensional fatigue index-20, and liver disease symptom index.16 Nevertheless, there is no consensus as to which measurement tool is the most superior as results from such tools, given their specificity, are difficult to generalize.17 In the present study, we used two generalized tools to measure HRQoL because those tests were mostly validated in Korean general population.12,18,19 Although SF-36 and EQ-5D do not define disease statuses, our study showed that these two methods could successfully represent the impaired HRQoL in Korean patients infected with HCV.
Results from the most recent and largest pooled analysis using SF-36 and EQ-5D and involving 448 Korean healthy controls18 showed SF domain scores of 52.2±8.0 on PCS, 49.9±8.3 on MCS, and an EQ-5D index of 0.945±0.091. Thus, in every SF domain, Korean healthy controls scored higher than the CHC patients included in this study. Nonetheless, a head-to-head comparison was not acceptable due to the much younger age of these healthy controls (44.5±15.5 vs CHC patients 60.5±11.1) (Table 1). According to data from the KNHANES, HRQoL measured by EQ-5D19 in elderly people (>65 years) of the general population resulted in an EQ-5D index of 0.84 (standard error [SE], 0.007)12 and EQ-VAS of 66.3 (SE, 0.8). Our results from CHC patients (EQ-5D index, 0.858±0.145; VAS, 67.9±17.8) were similar to these values.
In Western countries, patients with chronic HCV infection have been shown to have severe impairments in every SF-36 domain compared to patients with other chronic diseases such as diabetes or rheumatic arthritis as well as healthy controls.3 Mental score was especially worse in HCV infected patients regardless of liver disease.20 In contrast, according to our study, HCV infection reduced both PCS and MCS in Korean patients. Moreover, the difference between mental domain scores found in chronic liver disease patients and the general population18 (−3.7) was not significantly higher than the difference found in between the physical domain scores of the two groups (−3.4). Mean differences in detailed eight domains between the two groups ranged from −3.9 to −17.0. However, the VT domain, which had the largest difference between patients and general population in Western studies, showed the smallest difference (−3.9) between South Korean CHC patients and healthy controls. This finding might be caused by the different mode of HCV transmission. Intravenous drug users, a significant proportion (30% to 40%) of the HCV infected population in Western countries, tend to be vulnerable to mental stress and show a high prevalence of depression, which may contribute to significant mental health impairment. However, intravenous drug users account for only 3.9% of patients with chronic HCV infection in Korea.21
In our study, EQ-5D was unable to parse out differences in HRQoL among different liver disease status groups. Nonetheless, according to SF-36 measurements, among the liver status groups, the lowest GH and VT scores (reflecting physical and mental status respectively) were found in decompensated LC patients, even when compared to HCC patients, and this difference was significant. This was consistent with previous Korean reports.22 Furthermore, though not statistically significant, the decompensated LC group had the lowest MCS as well as the lowest score in every mental domain. In other words, the major characteristic of HRQoL in decompensated LC patients measured by SF-36v2 was an impaired mental status. This contrasts with a previous study that showed greatest impairment in SF, PF, and pain domains, which are more physical impairments.23 As our study population was surveyed at outpatient clinics for ambulatory patients, the physical dysfunction in admitted decompensated LC patients of the previous study may have been masked.
As shown in previous studies, successful antiviral therapy improved HRQoL in HCV infected patients (Table 3). Of note, MCS in patients, including those who were receiving IFN-based antiviral treatment at time of survey, was similar to that of treatment naïve patients. The only impaired component in patients during antiviral therapy when compared to treatment-naïve patients was pain (BP, Table 3). In contrast to Western countries where every SF-36 domain was impaired during antiviral therapy24,25 with a high rate of depression (20%),26 Asian patients have been reported to experience rare psychiatric symptoms (4%) during antiviral therapy.27 Instead, HRQoL in Korean HCV patients was mainly affected by economic issues as in other countries. Subjects with a high monthly family income (>3,000,000 KRW) were significantly younger (56.1±10.5 years vs 61.8±11.0 years, p<0.001, data not shown), less frequent in advance liver disease (9.3% vs 24.9%, p=0.014, data not shown), and with higher educational level (48.2% vs 9.9%, p<0.001, data not shown) rather than those with low income (≤3,000,000 KRW/month). Nevertheless, the independent effect of the economic factor was consistently observed in other multivariable models considering the interaction between monthly family income and the other related factors (data not shown). Old age and comorbidities did lower physical QOL, but it did not affect mental QOL (Table 4).
Although this is the first study to compare HRQoL in HCV infected patients to that of the general Korean population, the present study had several limitations. First, the number of patients with decompensated cirrhosis, HCC, and liver transplantation was small because these patients compose a small fraction of HCV patients and are less likely to be managed in outpatient clinics. Moreover, the proportion of patients who had achieved SVR was low (15.3%) because they do not visit clinics as frequently as those who have not reached SVR. Nevertheless, this study showed that advanced liver disease and successful antiviral treatment had crucial impacts on HRQoL in HCV infected patients. The second limitation of this study was its cross-sectional design. A longitudinal study design would be the most accurate way to detect HRQoL change in each person, but this is difficult to perform due to the long course of chronic HCV infection. Therefore, further studies on the serial change of HRQoL after DAA therapy are needed to confirm the impact of viral eradiation on physical and mental health status in Korean patients like in other Asian.28
In conclusion, HRQoL in Korean patients with chronic HCV infection was lower than in the general population, both physically and mentally. HRQoL was affected by the severity of cirrhosis, monthly income, comorbidities, and SVR. Therefore, by providing appropriate antiviral therapy before the development of advanced liver disease, QoL may be expected to improve in Korean CHC patients. Nonetheless, a systematic evaluation for the cost-effectiveness of DAA therapeutics is warranted as economic issue was the most affecting factor on QoL in Koreans.
ACKNOWLEDGEMENTS
This study was supported by a grant from Bristol-Myers-Squibb and a grant for the Chronic Infectious Disease Cohort Study (Korea HCV Cohort Study, 2017E5100100) from the Korea Centers for Disease Control and Prevention. The authors are grateful to the devoted research coordinators (Da-Seul Lee, Dawoon Jeong, Jiyoung Paik, Ye Young Lee, Ae Ran An, Hyoyoung Kang, Hyeyoung Gye, and Sujin Lee).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gane E, Kershenobich D, Seguin-Devaux C, et al. Strategies to manage hepatitis C virus (HCV) infection disease burden-volume 2. J Viral Hepat. 2015;22(Suppl 1):46–73. doi: 10.1111/jvh.12352. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley D, Elliott L, Cunningham-Burley S, Whittaker A. Health-related quality of life for individuals with hepatitis C: a narrative review. Int J Drug Policy. 2015;26:936–949. doi: 10.1016/j.drugpo.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Measuring healthy days: population assessment of health-related quality of life. Atlanta: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 5.Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 7.Suk KT, Baik SK, Yoon JH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18:1–21. doi: 10.3350/kjhep.2012.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis. 2014;32:764–777. doi: 10.1159/000368020. [DOI] [PubMed] [Google Scholar]
- 9.Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M. Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med. 2004;203:189–194. doi: 10.1620/tjem.203.189. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Jo MW, Lee SI. Psychometric properties of the Korean Short Form-36 health survey version 2 for assessing the general population. Asian Nurs Res (Korean Soc Nurs Sci) 2013;7:61–66. doi: 10.1016/j.anr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Norman R, Cronin P, Viney R, King M, Street D, Ratcliffe J. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12:1194–1200. doi: 10.1111/j.1524-4733.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12:1187–1193. doi: 10.1111/j.1524-4733.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 13.Ock M, Jo MW, Lee S. Measuring Health Related Quality of Life Using EQ-5D in South Korea. J Health Tech Assess. 2013;1:103. [Google Scholar]
- 14.Gralnek IM, Hays RD, Kilbourne A, et al. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease: the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 15.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness impact profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 16.van der Plas SM, Hansen BE, de Boer JB, et al. Generic and disease-specific health related quality of life in non-cirrhotic, cirrhotic and transplanted liver patients: a cross-sectional study. BMC Gastroenterol. 2003;3:33. doi: 10.1186/1471-230X-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orr JG, Homer T, Ternent L, et al. Health related quality of life in people with advanced chronic liver disease. J Hepatol. 2014;61:1158–1165. doi: 10.1016/j.jhep.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Kim SO, Lee SI, Jo MW. Deriving a mapping algorithm for converting SF-36 scores to EQ-5D utility score in a Korean population. Health Qual Life Outcomes. 2014;12:145. doi: 10.1186/s12955-014-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014. [PubMed] [Google Scholar]
- 20.Tillmann HL, Wiese M, Braun Y, et al. Quality of life in patients with various liver diseases: patients with HCV show greater mental impairment, while patients with PBC have greater physical impairment. J Viral Hepat. 2011;18:252–261. doi: 10.1111/j.1365-2893.2010.01292.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SS, Jeong SH, Jang ES, et al. Treatment rate and factors related to interferon-based treatment initiation for chronic hepatitis C in South Korea. J Med Virol. 2016;88:275–281. doi: 10.1002/jmv.24335. [DOI] [PubMed] [Google Scholar]
- 22.Park CK, Park SY, Kim ES, et al. Assessment of quality of life and associated factors in patients with chronic viral liver disease. Korean J Hepatol. 2003;9:212–221. [PubMed] [Google Scholar]
- 23.Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34:1123–1132. doi: 10.1111/j.1365-2036.2011.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinakos E, Gigi E, Lalla T, et al. Health-related quality of life in Greek chronic hepatitis C patients during pegylated interferon and ribavirin treatment. Hippokratia. 2010;14:122–125. [PMC free article] [PubMed] [Google Scholar]
- 25.John-Baptiste AA, Tomlinson G, Hsu PC, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439–2448. doi: 10.1038/ajg.2009.346. [DOI] [PubMed] [Google Scholar]
- 26.Evon DM, Ramcharran D, Belle SH, et al. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the Virahep-C study. Am J Gastroenterol. 2009;104:2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen NH, VuTien P, Garcia RT, et al. Response to pegylated interferon and ribavirin in Asian American patients with chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat. 2010;17:691–697. doi: 10.1111/j.1365-2893.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, Stepanova M, Chan HL, et al. Patient-reported outcomes in Asian patients with chronic hepatitis C treated with ledipasvir and sofosbuvir. Medicine (Baltimore) 2016;95:e2702. doi: 10.1097/MD.0000000000002702. [DOI] [PMC free article] [PubMed] [Google Scholar]