Abstract
Purpose
The development of acquired resistance to the first-line epidermal growth factor-tyrosine kinase inhibitor (EGFR-TKI) treatment in non-small-cell lung cancer (NSCLC) is inevitable, and most of these patients needed second-line chemotherapy. Furthermore, the optimum chemotherapeutic regimen is unclear. The aim of this meta-analysis was to evaluate the chemotherapeutic regimens “with-pemetrexed” versus “non-pemetrexed” in advanced NSCLC patients who had progressed after first-line EGFR-TKIs.
Materials and methods
We searched PubMed, Embase, Cochrane Library, and the Web of science for relevant clinical trials. Outcomes analyzed were response rate (RR), disease control rate (DCR), 1-year survival rate (1-year SR), progression-free survival (PFS), and overall survival (OS).
Results
One randomized controlled trial (RCT) and three retrospective studies were included in this meta-analysis, covering a total of 354 patients. The results showed that there was no significant difference between with-pemetrexed arm and non-pemetrexed arm in RR (OR 1.43, 95% CI 0.85–2.41, P=0.18), DCR (OR 1.5, 95% CI 0.94–2.39, P=0.09), and 1-year SR (OR 1.47, 95% CI 0.79–2.74, P=0.22). But the with-pemetrexed chemotherapeutic regimens significantly improved the PFS (HR 0.61, 95% CI 0.46–0.81, P=0.0005) and OS (HR 0.62, 95% CI 0.42–0.90, P=0.01).
Conclusion
The second-line with-pemetrexed chemotherapeutic regimens provided significantly longer PFS and OS than non-pemetrexed chemotherapeutic regimens. These findings indicate that the with-pemetrexed chemotherapeutic regimen may be an optimal second-line chemotherapeutic regimen for patients with advanced NSCLC following EGFR-TKI failure.
Keywords: lung cancer, chemotherapy, pemetrexed, EGFR TKIs, meta-analysis
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide, and non-small-cell lung cancer (NSCLC) represents about 80%–85% of all lung cancers.1 Unfortunately, since the majority of patients are diagnosed at an advanced stage, the opportunity for surgical resection is lost, and the drug therapy is the main treatment option.
During the past few years, the discovery of activating mutations in the kinase domain of the epidermal growth factor receptor (EGFR) gene has changed the treatment strategy for NSCLC, especially adenocarcinoma.2 Recent studies have confirmed that EGFR tyrosine kinase inhibitors (TKIs) when used as first-line treatment for advanced NSCLC patients with activating EGFR mutations provided a significantly superior response rate (RR) and progression-free survival (PFS), as well as better quality-of-life scores.3–7 Therefore, EGFR TKIs have become the preferred first-line treatment for NSCLC patients with EGFR mutations.
However, disease progression occurs after a median of 10–14 months from the beginning of TKI therapy,8 and the development of acquired resistance to the first-line EGFR-TKI treatment is inevitable, and most of these patients needed subsequent salvage therapy. Some new drugs were designed to conquer the mechanism of acquired resistance such as T790M mutation or MET amplification, and the associated clinical trials are still ongoing.9–11 However, these new drugs were not widely used in clinical practice. In addition, not all acquired resistance is related to T790M mutation and the exact mechanism is still unclear.9,12 In these patients, second-line cytotoxic chemotherapy is still the main treatment option. But the optimum chemotherapeutic regimen in these patients is unclear. Pemetrexed is currently used in clinical practice as second-line chemotherapy in patients with NSCLC.13
Some recent clinical trials have been conducted to evaluate the second-line chemotherapeutic regimens with or without pemetrexed for advanced NSCLC patients who had progressed after treatment with first-line EGFR TKIs.14–17 Therefore, we conducted this meta-analysis to compare the chemotherapeutic regimens “with-pemetrexed” versus “non-pemetrexed” in advanced NSCLC patients who had progressed after first-line EGFR-TKIs.
Materials and methods
Search strategy
We searched PubMed, Embase, Cochrane Library, and the Web of science for relevant clinical trials up to March 2017. We used the following keywords: “non-small cell lung cancer OR NSCLC”, “EGFR-TKIs OR gefitinib OR erlotinib”, “progressed OR failure OR acquired resistance”, “chemotherapy OR pemetrexed”. We did not set any language restrictions, and references listed from relevant primary studies and review articles were also examined to find additional publications.
Inclusion criteria
The relevant clinical trials were manually selected carefully based on the following criteria: 1) patients were pathologically confirmed of advanced NSCLC; 2) patients using EGFR-TKIs as first-line therapy and developed acquired resistance or progression of disease; 3) trials comparing pemetrexed singlet or pemetrexed-based combination chemotherapy with non-pemetrexed chemotherapy as second-line chemotherapy (with-pemetrexed vs non-pemetrexed); and 4) the included study has sufficient data for extraction. If multiple publications of the same trial were retrieved or if there was a case mix between publications, only the most recent publication (and the most informative) was included.
Data extraction and quality assessment
Data from the included studies were extracted and summarized independently by two of the authors (Li and Lu). Any disagreement was resolved by the adjudicating senior authors (Luo and Gu). The following information was extracted from each article: 1) basic information such as year of publication, whether the study included randomized controlled trials (RCTs) or was a retrospective study, author name, etc; and 2) information regarding study such as sample size per group, treatment regimen, RR, disease control rate (DCR), 1-year survival rate (1-year SR), PFS, and overall survival (OS). Available information was extracted and recorded to a data collection form and entered into electronic database.
The assessment quality of RCTs was evaluated using the Jadad score.18 When the article attains the score of 3–5 points, its quality is graded as “good”. The methodological quality of retrospective studies was assessed by the modified Newcastle-Ottawa scale.19 A score of 0–9 was allocated to each study, and a score of 6 or more was considered to be of high quality.
Statistical analysis
The event occurrence rate was used for comparing the results of RR, DCR, and 1-year SR in both arms. Hazard ratio (HR) and associated 95% CI were used for comparing PFS and OS in both the arms.
Statistical analyses of the RR, DCR, 1-year SR, PFS, and OS were performed by using the software Review Manager 5.3. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at P<0.10, and heterogeneity was quantified using the I2 statistic. A random-effects model was used if there was heterogeneity between studies; otherwise, a fixed-effects model was used. When PFS and OS could not be extracted from the original papers, we deciphered them from the survival curve as reported by Parmar et al.20 All reported P-values were two-sided and P-values less than 0.05 were considered as statistically significant. Funnel plots were used to screen for potential publication bias.
Results
Characteristics of the included trials
After the selection procedure (Figure 1), one RCT15 and three retrospective studies14,16,17 were considered eligible and included in this meta-analysis. The characteristics and data extracted from these studies are listed in Table 1. Finally, a total of 354 patients from four clinical studies were available for meta-analysis, with 202 in the chemotherapy with-pemetrexed arm and 152 in the chemotherapy non-pemetrexed arm. In these 354 patients, mostly metastatic and stage IV adenocarcinoma, except for 11 patients with stage IIIb in the RCT.15 All of these 354 patients were treated using EGFR-TKIs as first-line therapy, and none of them were treated with any radiation therapy before. After the first-line EGFR-TKIs treatment, the patients presented local progress and distant metastasis, and hence changed to second-line chemotherapy regimens. The regimens included in the with-pemetrexed arm are pemetrexed singlet or pemetrexed-based combination chemotherapy (Table 1). The regimens of the non-pemetrexed arm comprised conventional cytotoxic chemotherapy singlet (eg, docetaxel singlet) or doublet (eg, platinum doublet, navelbine/platinum doublet and platinum+gemcitabine/navelbine/taxotere doublet) (Table 1).
Figure 1.
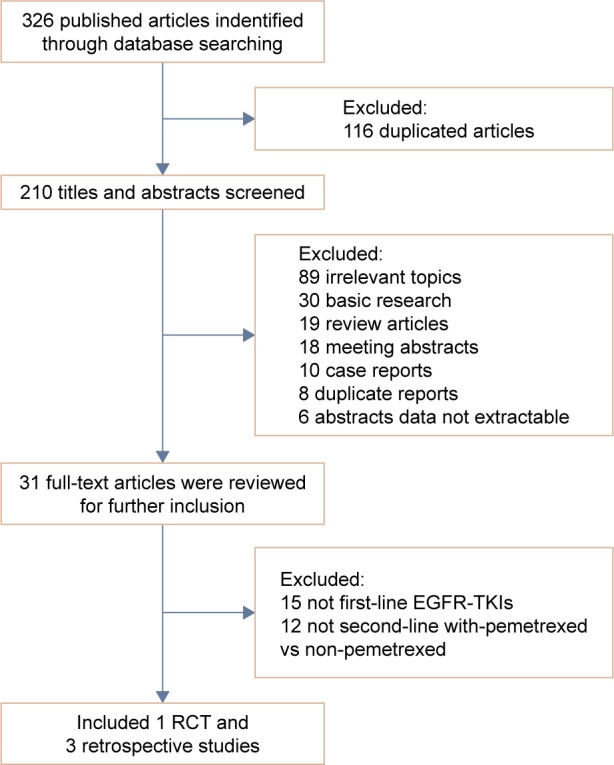
Flowchart of trial selection process.
Abbreviations: EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors; RCT, randomized controlled trial.
Table 1.
Characteristics and data extracted from the studies included in this meta-analysis
| Authors/year | Type | EGFR mutation | Second-line regimens (per arm) | Patients enrolled | RR (%) | DCR (%) | 1-year SR (%) | PFS | OS | Jadad/Ottawa score |
|---|---|---|---|---|---|---|---|---|---|---|
| Dong et al 201415 | RCT | Yes | Pem, docetaxel | 54 55 |
22.2 25.5 |
51.9 52.7 |
25.9 25.5 |
NA | NA | 3 |
| Park et al 201516 | Retrospective | Yes | Pem, platinum doublet | 34 26 |
24 12 |
91 88 |
NA | HR: 0.47 95% CI: 0.26–0.84 |
HR: 0.50 95% CI: 0.22–1.13 |
6 |
| Tseng et al 201617 | Retrospective | Yes | Pem ± platinum ± beva, NVB/platinum doublet | 37 46 |
32.4 17.4 |
78.4 50.0 |
NA | HR: 0.54 95% CI: 0.34–0.86 |
HR: 0.92 95% CI: 0.50–1.68 |
6 |
| Yang et al 201614 | Retrospective | Yes | Pem + platinum, platinum + GEM/NVB/TXT | 77 25 |
26 20 |
54.6 48 |
60.3 40.9 |
HR: 0.78 95% CI: 0.51–1.2 |
HR: 0.47 95% CI: 0.26–0.83 |
6 |
Abbreviations: EGFR, epidermal growth factor receptor; RR, response rate; PFS, progression-free survival; OS, overall survival; DCR, disease control rate; 1-year SR, 1-year survival rate; RCT, randomized controlled trial; HR, hazard ratio; Pem, pemetrexed; Beva, bevacizumab; GEM, gemcitabine; NVB, navelbine; TXT, taxotere; NA, no assessment.
Jadad score was used to assess the study quality of RCT and the score obtained was 3. Three retrospective studies were used to assess Newcastle-Ottawa scale and the score obtained was 6. All these articles were considered to be of high quality.
Response rate
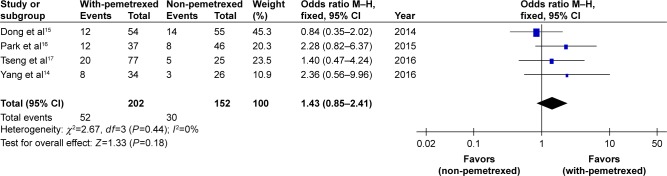
The pooled RR, through which the response rate of the four trials14–17 was analyzed, did not show significant difference between with-pemetrexed arm and non-pemetrexed arm (OR 1.43, 95% CI 0.85–2.41, P=0.18, Figure 2). There was no significant heterogeneity between the trials (P=0.44), and the pooled RR was calculated using fixed-effort model.
Figure 2.
With-pemetrexed vs non-pemetrexed as second-line chemotherapy in RR.
Abbreviations: RR, response rate; M–H, Mantel–Haenszel.
Disease control rate
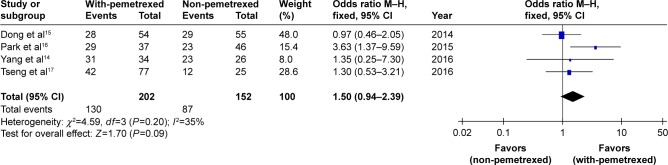
The pooled DCR, through which the disease control rate of the four trials14–17 was analyzed, did not show significant difference between with-pemetrexed arm and non-pemetrexed arm (OR 1.5, 95% CI 0.94–2.39, P=0.09, Figure 3). There was no significant heterogeneity between the trials (P=0.20), and the pooled DCR was calculated using fixed-effort model.
Figure 3.
With-pemetrexed vs non-pemetrexed as second-line chemotherapy in DCR.
Abbreviations: DCR, disease control rate; M–H, Mantel–Haenszel.
One-year survival rate
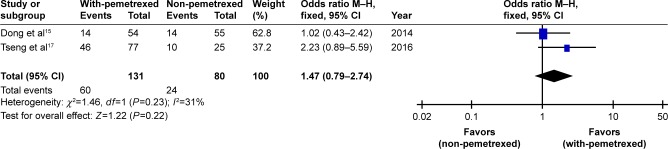
Only two trials reported 1-year SR data.15,17 There was no significant difference in the pooled 1-year SR between with-pemetrexed arm and non-pemetrexed arm (OR 1.47, 95% CI 0.79–2.74, P=0.22, Figure 4). There was no significant heterogeneity between the trials (P=0.23), and the pooled 1-year SR was calculated using fixed-effort model.
Figure 4.
With-pemetrexed vs non-pemetrexed as second-line chemotherapy in 1-year SR.
Abbreviations: 1-year SR, 1-year survival rate; M–H, Mantel–Haenszel.
Progression-free survival
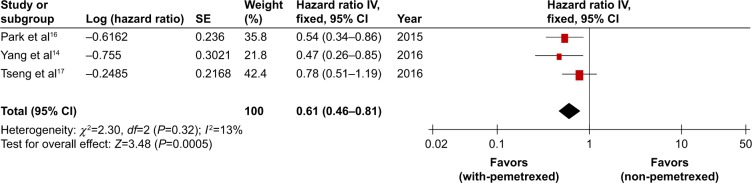
The three retrospective studies14,16,17 included in the analysis provided PFS data and/or survival curves. The PFS was pooled by the hazard ratio (HR) and 95% CI. Compared to non-pemetrexed chemotherapy arm, the with-pemetrexed chemotherapy arm resulted in statistically significant improvement in PFS (HR 0.61, 95% CI 0.46–0.81, P=0.0005, Figure 5) without apparent heterogeneity among the studies (P=0.32), so we performed PFS using fixed-effort model.
Figure 5.
With-pemetrexed vs non-pemetrexed as second-line chemotherapy in PFS.
Abbreviations: PFS, progression-free survival; SE, standard error.
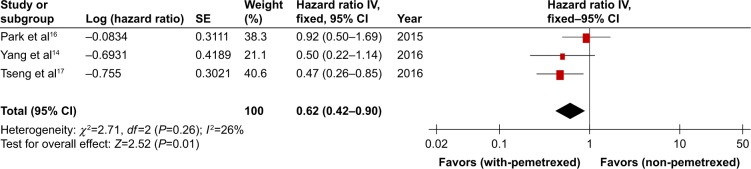
Overall survival
The RCT of Dong et al did not provide the data regarding OS, while the three retrospective studies of Park et al, Yang et al, and Tseng et al14,16,17 provided PFS data and/or survival curves. Compared to non-pemetrexed chemotherapy arm, the with-pemetrexed chemotherapy arm showed statistically significant improvement in OS (HR 0.62, 95% CI 0.42–0.90, P=0.01, Figure 6). The OS was analyzed using fixed-effort model because there was no significant heterogeneity between the trials (P=0.26).
Figure 6.
With-pemetrexed vs non-pemetrexed as second-line chemotherapy in OS.
Abbreviations: OS, overall survival; SE, standard error.
Publication bias
Figure 7 shows a funnel plot of the studies included in this meta-analysis that reported RR. All studies lie inside the 95% CIs, with an approximate even distribution around the vertical, indicating no obvious publication bias. The DCR, 1-year SR, PFS, and OS still did not suggest apparent publication bias in the funnel plot.
Figure 7.
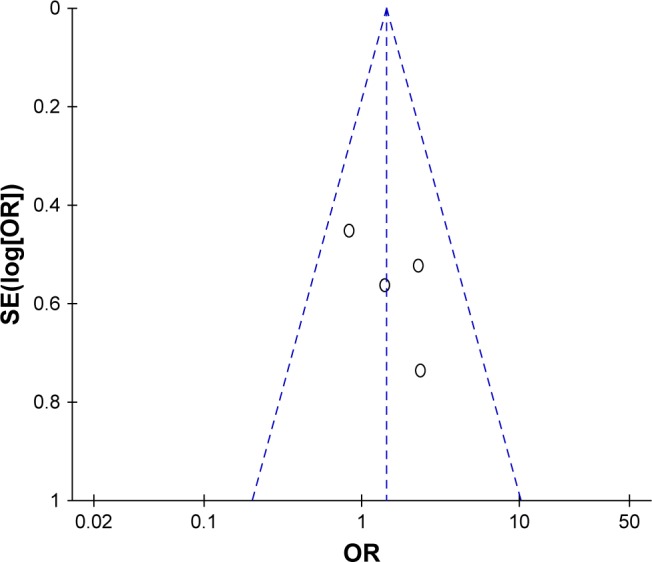
Funnel plots illustrating meta-analysis of RR.
Abbreviations: RR, response rate; SE, standard error; OR, odds ratio.
Discussion
EGFR mutations are most common among NSCLC patients with adenocarcinoma and little or no history of smoking. Molecular testing for mutations in EGFR and other driver oncogenes is now a standard protocol for the initial workup of newly diagnosed adenocarconima. Those with activating EGFR mutations, especially exon 19 deletions and L858R, have broader therapeutic options and improved survival compared with patients without an oncogenic driver.21,22 Three EGFR-TKIs such as erlotinib, gefitinib, and afatinib are approved for first-line treatment of EGFR mutant lung cancer based on multiple Phase III studies demonstrating superiority of EGFR TKIs over chemotherapy in this setting.4–8 Despite EGFR-TKIs showing good efficacy and longer PFS than cytotoxic chemotherapy, acquired resistance to EGFR-TKI treatment was always a concern. For patients with advanced adenocarcinoma harboring susceptible EGFR mutation and who developed acquired resistance to the first-line EGFR-TKIs treatment, cytotoxic chemotherapy was considered to be an effective second-line salvage therapy.
However, the optimum chemotherapeutic regimen in the patients who failed first-line treatment with EGFR-TKIs is inconsistent. A majority of patients in the advanced stage have a lower tolerance for the adverse effects associated with treatment as their organ reserve capacity is low. Selection of an efficacious and well-tolerated chemotherapy regimen is important in advanced patients with NSCLC. Sun et al reported that pemetrexed is the optimal drug with good efficacy and tolerable toxicity if it was used as the post-progression therapy.23 Pemetrexed is a multi-target antifolic acid preparation containing pyrrole pyrimidine that can inhibit key enzymes in the folate-dependent metabolic pathways, thereby affecting tumor growth.24,25 It inhibits the activities of dihydrofolate synthetase, thymidylate synthetase, and glycinamide ribonucleotide formyltransferase. In addition, it transports folate carrier and the cell membrane-folate binding protein transport system into cells to synthesize glutamic acid, which inhibits enzyme activity to prevent tumor growth.26 Clinical trials showed that pemetrexed-based chemotherapy provided longer PFS and OS than gemcitabine-based chemotherapy, while the regimen was used as the first-line therapy for patients with advanced NSCLC.27
In this meta-analysis, we aim to compare the second-line regimens of chemotherapy with pemetrexed versus non-pemetrexed in the RR, DCR, 1-year SR, PFS, and OS after failure of first-line EGFR-TKIs. In the with-pemetrexed arm, the regimens included pemetrexed singlet or pemetrexed-based combination chemotherapy, and the non-pemetrexed arm included conventional cytotoxic chemotherapy singlet or doublet. The results of our meta-analysis showed that there was no significant difference between with-pemetrexed arm and non-pemetrexed arm in RR (OR 1.43, 95% CI 0.85–2.41, P=0.18), DCR (OR 1.5, 95% CI 0.94–2.39, P=0.09), and 1-year SR (OR 1.47, 95% CI 0.79–2.74, P=0.22). But our data and the forest plot indicated that the with-pemetrexed arm tended to show a better RR, DCR, and 1-year SR compares to non-pemetrexed arm. However, this meta-analysis demonstrated that compared with the non-pemetrexed regimens, the second-line regimens of chemotherapy with pemetrexed after failure of first-line EGFR-TKI significantly improved the PFS (HR 0.61, 95% CI 0.46–0.81, P=0.0005) and OS (HR 0.62, 95% CI 0.42–0.90, P=0.01). This may be related to the lower toxicity associated with pemetrexed. However, among the four trials included in this present meta-analysis, only the RCT of Dong et al15 reported the adverse reactions. It was indicated that compared with the pemetrexed group, patients receiving docetaxel experienced significantly higher rates of nausea, myelosuppression, renal damage, and hair loss (all P<0.05).15 So, we assume that good efficacy is an important factor that contributed to prolonged PFS and OS, but it is also reasonable to consider that the lower toxicity of pemetrexed resulted in better PFS and OS. However, although compared with non-pemetrexed regimens, the second-line chemotherapeutic regimens with pemetrexed did not show apparent advantage in RR, DCR, and 1-year SR, they provided significantly longer PFS and OS.
Limitations
First, the number of included trials and the group size was small. After the selection procedure, only one RCT and three retrospective studies were included in this meta-analysis. The with-pemetrexed arm included a total of 202 patients, and the non-pemetrexed arm included a total of 152 patients. In addition, the retrospective nature of the three studies14,16,17 meant that there was an inevitable selection bias. For example, the patient’s individual characteristics, such as age, comorbidities, and performance status, might have influenced the chemotherapy regimen choice made by the clinical physicians, and this could have confounded the outcome of the study. Thus, the results should be interpreted cautiously. If more number of RCTs and patients can be included, the results will be more convincing. Second, the second-line chemotherapeutic regimens after the progression of the first-line TKIs were not uniform in the four trials that were included in this meta-analysis. Indeed, the regimens of the with-pemetrexed arm included pemetrexed singlet or pemetrexed-based combination chemotherapy, and the regimens of the non-pemetrexed arm included conventional cytotoxic chemotherapy singlet or doublet, such as docetaxel singlet, navelbine/platinum doublet, or platinum + gemcitabine/navelbine/taxotere. Current studies show that platinum-based chemotherapeutic regimens are superior for NSCLC.28 Table 1 showed that the chemotherapeutic regimens of with-pemetrexed arm contains 114 patients with platinum, and the non-pemetrexed arm contains 97 patients wih platinum. At present we cannot conduct further stratified analysis according to the usage of platinum in each trial. Therefore, multiple factors contributed to the result in this meta-analysis.
Conclusion
Our meta-analysis showed that compared with non-pemetrexed regimens, the second-line with-pemetrexed chemotherapeutic regimens provided significantly longer PFS and OS in the advanced NSCLC patients who had progressed after first-line treatment with EGFR TKIs. This indicates that the with-pemetrexed chemotherapeutic regimen may be an optimal second-line chemotherapeutic regimen for patients with advanced NSCLC after EGFR-TKI failure.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chen YM. Update of epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer. J Chin Med Assoc. 2013;76(5):249–257. doi: 10.1016/j.jcma.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17(17):5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JY, Shih JY, Yang CH, et al. Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. Int J Cancer. 2010;126(1):247–255. doi: 10.1002/ijc.24657. [DOI] [PubMed] [Google Scholar]
- 11.Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Ther Adv Respir Dis. 2015;9(5):242–250. doi: 10.1177/1753465815587820. [DOI] [PubMed] [Google Scholar]
- 12.Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16(9):e447–e459. doi: 10.1016/S1470-2045(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G. Progress of clinical research in “tailor” chemotherapy for non-small-cell lung cancer. Zhongguo Fei Ai Za Zhi. 2008;11(1):10–13. doi: 10.3779/j.issn.1009-3419.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang CJ, Tsai MJ, Hung JY, et al. Pemetrexed had significantly better clinical efficacy in patients with stage IV lung adenocarcinoma with susceptible EGFR mutations receiving platinum-based chemotherapy after developing resistance to the first-line gefitinib treatment. Onco Targets Ther. 2016;9:1579–1587. doi: 10.2147/OTT.S100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong L, Han ZF, Feng ZH, Jia ZY. Comparison of pemetrexed and docetaxel as salvage chemotherapy for the treatment for nonsmall-cell lung cancer after the failure of epidermal growth factor receptor-tyrosine kinase inhibitors. J Int Med Res. 2014;42(1):191–197. doi: 10.1177/0300060513505808. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Keam B, Kim SH, et al. Pemetrexed singlet versus nonpemetrexed-based platinum doublet as second-line chemotherapy after first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat. 2015;47(4):630–637. doi: 10.4143/crt.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng YH, Hung HY, Sung YC, et al. Efficacy of chemotherapy in epidermal growth factor receptor (EGFR) mutated metastatic pulmonary adenocarcinoma patients who had acquired resistance to first-line EGFR tyrosine kinase inhibitor (TKI) J Chemother. 2016;28(1):50–58. doi: 10.1179/1973947815Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358(9285):870–875. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 22.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JM, Lee KW, Kim JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol. 2009;39(1):27–32. doi: 10.1093/jjco/hyn118. [DOI] [PubMed] [Google Scholar]
- 24.Rollins KD, Lindley C. Pemetrexed: a multitargeted antifolate. Clin Ther. 2005;27(9):1343–1382. doi: 10.1016/j.clinthera.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Socinski MA, Stinchcombe TE, Hayes DN. The evolving role of pemetrexed (Alimta) in lung cancer. Semin Oncol. 2005;32(2 Suppl 2):S16–S22. doi: 10.1053/j.seminoncol.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Goldman ID, Zhao R. Molecular, biochemical, and cellular pharmacology of pemetrexed. Semin Oncol. 2002;29(6 Suppl 18):3–17. doi: 10.1053/sonc.2002.37461. [DOI] [PubMed] [Google Scholar]
- 27.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 28.Goffin J, Lacchetti C, Ellis PM, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5(2):260–274. doi: 10.1097/JTO.0b013e3181c6f035. [DOI] [PubMed] [Google Scholar]