The authors discuss the diagnosis, the role of chemotherapy, and the role of emerging therapies in the treatment of metastatic soft tissue sarcoma.
Abstract
Soft tissue sarcomas represent a group of heterogeneous mesenchymal tumors that occur rarely in adults. While a variety of histological subtypes exist, some of the most common are leiomyosarcoma and liposarcoma. For eligible patients, standard first-line treatment of metastatic disease has typically comprised anthracycline-containing regimens. While traditional cytotoxic chemotherapy has been the mainstay of treatment in metastatic soft tissue sarcoma, emerging targeted and novel therapies are creating new frontiers of treatment for a variety of histological subtypes. Olaratumab (Lartruvo, Eli Lilly) in combination with doxorubicin represents a new potential first-line treatment option. Second-line therapy is often histology-driven, and novel treatment options include trabectedin (Yondelis, Janssen) and eribulin (Halaven, Eisai). This review discusses the diagnosis, role of chemotherapy in unresectable and metastatic disease, and role of emerging therapies in the treatment of metastatic soft tissue sarcoma.
INTRODUCTION
Adult sarcomas are a rare class of malignant tumors that account for approximately 1% of adult malignancies, and about 80% of these originate from soft tissue.1 In the United States in 2017, there were an estimated 12,390 new cases and 4,990 deaths due to sarcoma in adults.2
Most of these tumors arise in the limbs; however, they can occur in any part of the body, including the abdominal cavity, retroperitoneum, thoracic, and head and neck regions. The incidence of de novo metastatic disease in this group of tumors is around 10%, with 83% of these metastases located in the lung. An additional 25% of cases will develop metastatic disease after the initial treatment for curative intent of the primary tumor.3,4
Few patients with resectable oligometastatic lung disease will have the potential for cure, or they will alternatively enter a prolonged remission period with reported five-year survival rates ranging from 25% to 40%.5,6 The vast majority of patients who present with metastatic soft tissue sarcoma (STS) will unfortunately succumb to complications of the disease. For this group of patients, systemic therapy offers palliation to diminish symptoms and improve quality of life.
In this review, we will summarize the palliative systemic treatment options for disseminated metastatic STS with information pertaining to regimens newly approved by the Food and Drug Administration (FDA).
GENERAL PRINCIPLES
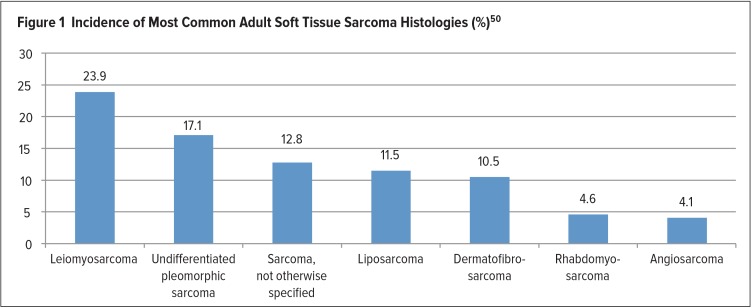
STS comprises a group of heterogeneous tumors with more than 50 different histological subtypes that can be classified according to the soft tissue of origin. The most common subtypes found in adults are described in Figure 1.
Figure 1.
Incidence of Most Common Adult Soft Tissue Sarcoma Histologies (%)50
In the case of unresectable disseminated disease, a biopsy should be performed before initiating treatment. A core biopsy is preferred; a fine-needle aspiration (FNA) is not recommended for initial diagnostic purposes because multiple studies have shown inferior diagnostic accuracy for FNA compared with the core biopsy.7,8 Of note, definitive diagnosis may require flow cytometry, cytogenetics, or molecular analyses for chromosomal translocations, all of which may require an incisional biopsy to be performed.
Some patients with metastatic STS may remain asymptomatic for a prolonged period even without active treatment. This group of patients may be watched closely, particularly if they have a low burden of disease.
With multiple histological types, STS has varied responses to therapy. Selection of a treatment regimen must be based on several factors, including histology, disease biology, and patient preferences. Moreover, prognostic factors for longer survival are different than the factors predicting response to systemic therapy.9 This may indicate that survival is associated more with disease biology than with type or response to systemic therapy.
TREATMENT PRINCIPLES
A majority of existing studies do not address the variety of histological subtypes of STS during the analysis of the data, including reported response rates and outcomes. Only recently have various studies started to recognize the concept of histology-directed treatment. One should recognize that different histological subtypes exhibit different patterns of response to chemotherapy; therefore, the choice of treatment in these types of tumors should be histologically driven.
For many years, cytotoxic therapy consisting of doxorubicin and ifosfamide was among the few options to treat this type of solid tumor. However, recent advances in molecular pathogenesis and the subsequent development of novel and targeted therapies have added to the armamentarium of treatment options in the management of advanced STS in adults.
CYTOTOXIC SYSTEMIC CHEMOTHERAPY
Single-Agent Regimens
Few single-agent chemotherapies have shown a reasonable response rate with acceptable toxicity to justify their use in the treatment of metastatic STS. Two of the most commonly used single agents are doxorubicin and ifosfamide.
Doxorubicin is considered to be the oldest drug therapy used for metastatic STS, with the first data published in the 1970s.10 Doxorubicin is an anthracycline antibiotic that exerts its antineoplastic activity by inhibition of topoisomerase, which prevents DNA religation during DNA replication, resulting in DNA strand breaks.11 Most of the trials evaluating single-agent doxorubicin in adult STS showed response rates in the 10% to 25% range, and the dose shown to be effective with acceptable toxicity is 60 mg/m2.12–16 Adverse events associated with doxorubicin include myelosuppression, mucositis, nausea/vomiting, and cardiotoxicity.11 Pegylated liposomal doxorubicin has a better toxicity profile compared with unencapsulated doxorubicin because it is less cardiotoxic. When the two regimens were compared in the treatment of adults with advanced or metastatic STS of varying subtypes, 50 mg/m2 of pegylated liposomal doxorubicin demonstrated similar efficacy to unencapsulated doxorubicin, with response rates of 14% and 12%, respectively, when gastrointestinal stromal tumors (GISTs) were excluded.12
Ifosfamide has also been used in the treatment of metastatic STS. Ifosfamide is an agent derived from nitrogen mustard that exerts its antineoplastic activity by alkylating DNA.11 Common adverse events associated with ifosfamide include myelosuppression, neurotoxicity, and hemorrhagic cystitis. The agent is typically administered with mesna to decrease the potential for hemorrhagic cystitis. Ifosfamide has a response rate of 18% at 5 g/m2 as a first-line therapy.17 A trial compared ifosfamide in two doses (3 g/m2 infusion over four hours daily for three days, or 9 mg/m2 continuous infusion over 72 hours) to doxorubicin 75 mg/m2 every three weeks in patients with various STS subtypes.18 There was no difference in the primary endpoint of progression-free survival (PFS) among the three groups, but the rate of adverse events was higher with both ifosfamide doses, including grade 4 neutropenia, grade 4 febrile neutropenia, and grade 3/4 encephalopathy. As a second-line treatment after doxorubicin failure, ifosfamide has demonstrated response rates ranging from 7% to 41%, with different doses used in each trial.19–21 A retrospective study evaluating high-dose ifosfamide, defined as administering a total dose of 14 g/m2 over six days every 21 days, in the second- and third-line treatment of patients with refractory STS revealed that this dosing scheme can have a particular advantage in synovial sarcoma.22 Of the patients with synovial sarcoma evaluated, 40% had a partial response and 40% had stable disease. Adverse events with high-dose ifosfamide were similar to those reported with other ifosfamide doses in STS, with neutropenia and neurological toxicity being among the most commonly reported serious adverse events.
Gemcitabine, a deoxycytidine analogue that inhibits DNA synthesis,23 has limited activity as monotherapy. A phase 2 trial evaluated intravenous (IV) gemcitabine 1,000 mg/m2 over 30 minutes on days 1 and 8 every 21 days in patients with metastatic leiomyosarcoma. The median PFS and overall survival (OS) were three months and 13.9 months, respectively.24 The most common grade 3/4 adverse events included neutropenia, thrombocytopenia, and alanine aminotransferase (ALT) elevation. One potential modality that has been theorized to improve the efficacy of gemcitabine is administration at a rate of 10 mg/m2 per minute to maximize the formation rate of the active triphosphate metabolite of gemcitabine.25 A phase 2 trial evaluating fixed-dose rate gemcitabine demonstrated a positive tumor growth control rate in patients with leiomyosarcoma, with 7% and 21% of patients demonstrating a partial response and stable disease, respectively.
As a single agent, paclitaxel can be utilized in the treatment of angiosarcoma. Paclitaxel exerts its anticancer activity by stabilizing microtubules via the inhibition of the depolymerization process during the mitotic phase of the cell cycle.26 In a phase 2 study evaluating paclitaxel 80 mg/m2 IV given over one hour weekly in patients with advanced or metastatic angiosarcoma, paclitaxel demonstrated two-month and four-month PFS rates of 74% and 45%, respectively.27 Anemia, neutropenia, and asthenia were among the most common adverse events reported in the trial.
Other single-agent drugs that have been investigated and have shown activity in metastatic STS include dacarbazine, temozolomide, vinorelbine, cisplatin, and carboplatin.28–34 All are associated with response rates ranging between 5% and 20%.
Combination Chemotherapy
Many phase 2 and 3 trials have evaluated a variety of chemotherapy combinations in an effort to improve response rates and OS. The typical backbone of combination regimens is doxorubicin with an alkylating agent. Controversy has surrounded the clinical benefits of this approach. Some combination chemotherapy trials have produced superior response rates in the combination arm compared with the single-therapy arm. Until recently, none of the previous trials had shown a significant survival advantage over single-agent regimens. Furthermore, combination chemotherapy regimens have the potential for greater risk of serious treatment-related toxicities. The single exception is the combination of olaratumab (Lartruvo, Eli Lilly) and doxorubicin, which was reported recently in a phase 2 randomized trial to have an OS advantage over a single agent, doxorubicin, with acceptable toxicity.35
A meta-analysis conducted by the Cochrane Collaboration reviewed eight randomized trials reported between 1976 and 1995 comparing single-agent doxorubicin with various doxorubicin-containing combination chemotherapy regimens.36 In these trials, single-agent doxorubicin was compared with a range of doxorubicin-containing combinations that included vincristine, vindesine, cyclophosphamide, streptozotocin, mitomycin-C, cisplatin, and ifosfamide. Combination regimens were consistently associated with higher rates of gastroenterological and hematologic toxicities, while the better response rates associated with combination therapy were minimal and depended on the statistical model used (fixed effects model ORresp, 1.29; 95% confidence interval [CI], 1.03–1.60; P = 0.03; random effects model ORresp, 1.26; 95% CI, 0.96–1.67; P = 0.10). There was no statistically significant difference in the one-year (ORmortality, 0.87; 95% CI, 0.73–1.05; P = 0.14) or two-year mortality rates (ORmortality, 0.84; 95% CI, 0.67–1.06; P = 0.13).
Gemcitabine plus docetaxel can be considered in patients who cannot tolerate doxorubicin because of their cardiac history. The GeDD trial compared monotherapy with doxorubicin to combination therapy with gemcitabine and docetaxel.37 In this multicenter, randomized, phase 3 trial, patients with various metastatic STS histologies received either doxorubicin 75 mg/m2 IV every 21 days or gemcitabine 675 mg/m2 IV over 90 minutes on days 1 and 8 plus docetaxel 75 mg/m2 IV over 60 minutes on day 8 every 21 days. No significant difference in PFS at 24 weeks was observed between the doxorubicin arm (46.3% [95% CI, 37.5–54.6]) and the combination therapy arm (46.4% [95% CI, 37.5–54.8]). The authors concluded that doxorubicin should remain the standard first-line treatment of metastatic STS, but gemcitabine/docetaxel can be considered an acceptable alternative in patients with cardiac dysfunction in whom doxorubicin is contraindicated.
NEW PHARMACOTHERAPIES
Olaratumab
Olaratumab is an immunoglobulin G monoclonal antibody that was approved by the FDA in 2016.38 Olaratumab targets a specific subtype of the platelet-derived growth factor receptor (PDGFR) alpha receptor.39 The recommended dose is 15 mg/kg infused IV over 60 minutes on days 1 and 8 of a 21-day cycle. A randomized, open-label phase 2 study compared single-agent doxorubicin therapy with the combination of doxorubicin and olaratumab in patients with locally advanced or unresectable STS not amenable to curative surgical resection or radiation therapy.35 The most common histological subtypes in this study included leiomyosarcoma (38%), liposarcoma (18%), and undifferentiated pleomorphic sarcoma (18%). Olaratumab was administered at a dose of 15 mg/kg IV on days 1 and 8 of a 21-day cycle continuously until disease progression and doxorubicin was administered at a dose of 75 mg/m2 on day 1 of a 21-day cycle for up to eight cycles. The primary endpoint was PFS; OS was a secondary endpoint. Combination therapy demonstrated an increase in median PFS (6.6 months versus 4.1 months; hazard ratio [HR], 0.67) compared with doxorubicin monotherapy. Initial use of combination therapy showed a near doubling of median OS (26.5 months versus 14.7 months; HR, 0.46; 95% CI, 0.30–0.71; P = 0.0003), despite accounting for crossover to olaratumab in the single-agent doxorubicin arm. Combined therapy was associated with increased side effects, including infusion-related reactions (13% versus 0%), neutropenia (58% versus 35%), and mucositis (53% versus 35%). The authors concluded that the addition of olaratumab to doxorubicin in the treatment of adult STS not amenable to curative resection or radiation therapy improved OS and had an acceptable toxicity profile.
Trabectedin
Trabectedin (Yondelis, Janssen) is a newer antineoplastic agent recently approved in the United States to treat advanced liposarcoma and leiomyosarcoma in patients who have already been treated with an anthracycline-based regimen.40 Trabectedin is a marine-derived tetrahydroisoquinolone alkaloid that binds to the minor groove of DNA, which disrupts the function of DNA binding proteins and leads to cell-cycle perturbation and apoptosis.41 It is currently approved for administration at a dose of 1.5 mg/m2 IV over 24 hours on day 1 of a 21-day cycle. Administration is recommended via a central venous line because trabectedin is a vesicant.
A multicenter, randomized, open-label, phase 2 study evaluated the safety and efficacy of trabectedin compared with best supportive care in adults with advanced translocation-related sarcoma who did not respond to available standard chemotherapy.42 A variety of STS histological subtypes were eligible, including myxoid/round-cell liposarcoma, synovial sarcoma, alveolar rhabdomyosarcoma, and Ewing sarcoma. The primary endpoint was PFS. Trabectedin was administered at a dose of 1.2 mg/m2 IV over 24 hours. Seventy-six patients were randomized: 39 received trabectedin, and 37 received best supportive care. Patients with primarily myxoid/round-cell liposarcoma and synovial sarcoma who received trabectedin had a significantly longer median PFS compared with best supportive care (5.6 months versus 0.9 months; HR, 0.07; 95% CI, 0.30–0.16; P < 0.0001). OS had not yet been reached in the trabectedin group (95% CI, 12.8–not reached [NR]) compared with eight months in patients receiving best supportive care (7.0–NR; HR, 0.42; 95% CI, 0.18–0.98; P = 0.04). Grade 3/4 treatment-related adverse events in patients receiving trabectedin included neutropenia (67%), febrile neutropenia (14%), thrombocytopenia (17%), ALT elevations (61%), aspartate aminotransferase (AST) elevations (41%), and creatinine phosphokinase (CPK) elevations (6%). The authors of this study concluded that trabectedin significantly improved PFS in adults with metastatic translocation-related sarcoma that had progressed with standard first-line chemotherapy compared with best supportive care.
A multicenter, randomized, open-label, phase 3 trial compared trabectedin with dacarbazine in patients with advanced leimoyosarcoma or liposarcoma who had been treated previously with standard chemotherapy, including previous anthracycline-based chemotherapy.43 Patients were randomized 2:1 to receive either trabectedin 1.5 mg/m2 IV over 24 hours or dacarbazine 1,000 mg/m2; both drugs were administered on day 1 of a 21-day cycle. The primary endpoint was OS, with PFS as a secondary endpoint. Although this trial showed significantly higher median PFS (4.2 months versus 1.5 months) with trabectedin compared with dacarbazine, median OS was not significantly different (12.4 months versus 12.9 months). The most common treatment-emergent adverse events of grade 3/4 in the trabectedin group included neutropenia (37%), thrombocytopenia (17%), anemia (14%), ALT elevations (26%), AST elevations (13%), and CPK elevations (5.3%). Elevations in AST and ALT were transient, and despite the elevations in CPK only 1.2% of patients receiving trabectedin experienced rhabdomyolysis. The authors concluded that trabectedin demonstrated superior disease control compared with dacarbazine in adults with previously treated metastatic liposarcoma and leiomyosarcoma.
Eribulin
Eribulin (Halaven, Eisai) is a nontaxane microtubule inhibitor that represents another potential treatment option in metastatic STS. It was approved in the United States in 2016 for the treatment of inoperable liposarcoma in patients who had been treated previously with an anthracycline-based regimen.44 A randomized, open-label, phase 3 trial compared the safety and efficacy of eribulin with dacarbazine in patients with advanced leiomyosarcoma or liposarcoma who had received at least two prior systemic chemotherapy regimens, including an anthracycline, unless contraindicated.45 Eribulin was administered at a dose of 1.4 mg/m2 IV on days 1 and 8 of a 21-day cycle, and dacarbazine was administered at a dose of 850 mg/m2, 1,000 mg/m2, or 1,250 mg/m2 on day 1 of a 21-day cycle. The primary endpoint was OS; PFS was a secondary endpoint. A total of 452 patients were randomized: 228 received eribulin, and 224 received dacarbazine. Treatment benefits for eribulin in this study were limited to the subgroup of patients with liposarcoma and not those with leiomyosarcoma. The OS benefit was greater in patients receiving eribulin than in those receiving dacarbazine (15.6 months versus 8.4 months; HR, 0.51; 95% CI, 0.35–0.75) in patients with liposarcoma. Median PFS was 2.6 months in both treatment groups (HR, 0.88; 95% CI, 0.71–1.09; P = 0.23). Discontinuation of eribulin secondary to adverse events and dose reduction occurred in 8% and 26% of patients, respectively. Common grade 3/4 adverse events experienced by patients receiving eribulin included neutropenia (35.4%), anemia (7%), fatigue (3%), and peripheral neuropathy (1.8%). The authors concluded that eribulin improved OS compared with dacarbazine in adults with advanced STS.
Pazopanib
Pazopanib (Votrient, Novartis) is a multitargeted tyrosine kinase inhibitor (TKI) that targets and inhibits vascular endothelial growth factor receptors (VEGFR), PDGFR, fibroblast growth factor receptors, and c-Kit. It is indicated for the treatment of patients with advanced STS who have received prior chemotherapy, except for patients with liposarcoma.46 A randomized, double-blind, placebo-controlled, phase 3 study by the European Organization for Research and Treatment of Cancer compared pazopanib 800 mg orally daily until disease progression or intolerable toxicity with placebo in patients with various histological STS subtypes; patients with liposarcoma were excluded.47 The primary endpoint was PFS, with OS as a secondary endpoint. A total of 372 patients were randomized 2:1—246 to pazopanib and 123 to placebo. Patients receiving pazopanib exhibited significantly better median PFS compared with placebo (4.6 months versus 1.6 months; HR, 0.31; 95% CI, 0.24–0.40; P < 0.001). However, there was no significant difference in OS (12.5 months versus 10.7 months; HR, 0.86; 95% CI, 0.67–1.11; P = 0.25). Common grade 3/4 adverse events experienced in patients receiving pazopanib included fatigue (13%), hypertension (7%), diarrhea (5%), and anorexia (6%). The authors concluded that pazopanib is an effective option in the management of previously treated adult nonadipocytic STS.
Regorafenib
Regorafenib (Stivarga, Bayer Healthcare) is another multitargeted TKI that exerts its antineoplastic activity by targeting and inhibiting VEGFR, c-Kit, BRAF, RET, and PDGFR.48 While regorafenib is not yet approved by the FDA for the treatment of non-GIST STS, its safety and efficacy have been evaluated in a randomized, placebo-controlled, phase 2 trial in patients with liposarcoma, leimyosarcoma, synovial sarcoma, or other types of STS that had been treated previously with a variety of agents.49 Patients were randomized to receive either regorafenib 160 mg orally daily for 21 days followed by seven days off or matching placebo. A total of 181 patients were randomized to treatment; 89 received regorafenib and 92 received placebo. The primary endpoint was PFS; OS was a secondary endpoint. Compared with placebo, regorafenib demonstrated a significant benefit in PFS among patients with leiomyosarcoma (3.7 months versus 1.8 months; HR, 0.46; 95% CI, 0.46–0.80; P = 0.0045) and synovial sarcoma (5.6 months versus 1.0 months; HR, 0.10; 95% CI, 0.03–0.35; P < 0.0001). Patients with liposarcoma did not experience a benefit in PFS compared with placebo (1.1 months versus 1.7 months; HR, 0.89; 95% CI, 0.48–1.64; P = 0.70). Common grade 3/4 adverse events experienced by patients in the regorafenib arm included arterial hypertension (19%), hand–foot syndrome (15%), asthenia (13%), and myalgia (96%).
Table 1 summarizes key clinical characteristics of the novel and targeted agents for the treatment of STS, while Table 2 presents major findings of the studies of these agents.
Table 1.
Novel and Targeted Agents for the Treatment of Soft-Tissue Sarcomas
| Drug Name | Indicated Sarcoma Histology | Dosing | Mechanism of Action | Adverse Events* | Additional Comments |
|---|---|---|---|---|---|
| Olaratumab (Lartruvo, Eli Lilly)35,38 | Any histological subtype for which an anthracycline-containing regimen is appropriate | 15 mg/kg IV days 1 and 8 every 21 days | Inhibits PDGFRα | Nausea, fatigue, neutropenia, mucositis, alopecia, vomiting, anemia, constipation, diarrhea, decreased appetite, pyrexia, abdominal pain, febrile neutropenia, cardiac dysfunction† |
|
| Trabectedin (Yondelis, Janssen)40,42 | Leiomyosarcoma, liposarcoma | 1.5 mg/m2 CIVI over 24 hours every 21 days | Alkylating agent, binds to minor groove of DNA | Nausea, decreased appetite, constipation, malaise, vomiting, anemia, myalgia, pyrexia, diarrhea, neutropenia, ALT increase, AST increase, thrombocytopenia, lymphopenia |
|
| Eribulin (Halaven, Eisai)44,45 | Liposarcoma | 1.4 mg/m2 days 1 and 8 every 21 days | Nontaxane microtubule inhibitor | Neutropenia, fatigue, nausea, alopecia, constipation, pyrexia, anemia, asthenia, decreased appetite, peripheral neuropathy |
|
| Pazopanib (Votrient, Novartis)46,47 | Leiomyosarcoma | 800 mg orally daily | Inhibits VEGFR, PDGFR, FGFR, c-Kit | Fatigue, diarrhea, nausea, weight loss, hypertension, anorexia, hair hypopigmentation, vomiting, dysgeusia |
|
| Regorafenib (Stivarga, Bayer Healthcare)48,49 | Leiomyosarcoma, synovial sarcoma | 160 mg orally daily for 3 weeks, then 1 week off | Inhibits c-Kit, VEGFR, PDGFR, RET, BRAF | Asthenia, pain, myalgia, anorexia, abdominal pain, diarrhea, mucositis, hand–foot syndrome, hypertension, dyspnea, hypophosphatemia |
|
Common all-grade adverse events observed in > 20% of patients receiving drug in clinical trial.
All-grade adverse events observed in > 20% of patients receiving olaratumab/doxorubicin.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; CIVI = continuous intravenous infusion; CYP3A4 = cytochrome P450 3A4; FGFR = fibroblast growth factor receptor; IV = intravenous; PDGFR = platelet-derived growth factor receptor; RET = rearranged during transfection; VEGFR = vascular endothelial growth factor receptor.
Table 2.
Studies of Novel Agents in the Treatment of Metastatic Soft Tissue Sarcoma in Adults
| Study | Design | Regimen | Median PFS | Median OS | Response Rates |
|---|---|---|---|---|---|
| Tap et al.35 | Phase 1b/2, randomized, open-label | Olaratumab 15 mg/kg IV days 1 and 8 and doxorubicin 75 mg/m2 day 1 every 21 days | 6.6 months | 26.5 months | ORR, 18.2% |
| Kawai et al.42 | Phase 2, randomized, open-label | Trabectedin 1.2 mg/m2 CIVI over 24 hours every 21 days | 5.6 months | Not reached | PR, 8% SD, 57% |
| Demetri et al.43 | Phase 3, randomized, open-label, active-controlled | Trabectedin 1.5 mg/m2 CIVI over 24 hours every 21 days | 4.2 months | 12.4 months | ORR, 9.9% SD, 51% |
| Schöffski et al.45 | Phase 3, randomized, open-label, active-controlled | Eribulin 1.4 mg/m2 IV days 1 and 8 every 21 days | 2.6 months | 13.5 months | PR, 4% SD, 52% |
| PALETTE47 | Phase 3, randomized, double-blind, placebo-controlled | Pazopanib 800 mg orally daily | 4.6 months | 12.5 months | PR, 6% SD, 67% |
| REGOSARC49 | Phase 2, randomized, double-blind, placebo-controlled | Regorafenib 160 mg orally daily for 21 days on and 7 days off | LMS: 3.7 months SS: 5.6 months |
LMS: 21 months SS: 13.4 months |
LMS: PR, 0%; SD, 86% SS: PR, 8%; SD, 77% |
CIVI = continuous IV infusion; IV = intravenous; LMS = leiomyosarcoma; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; PR = partial response; SD = stable disease; SS = synovial sarcoma.
CONCLUSION
The treatment of metastatic STS has seen promising advances with the approval of several novel pharmacological agents in recent years. Prior to these new drug approvals, the standard of care, first-line treatment for patients with metastatic STS was doxorubicin with ifosfamide, single-agent doxorubicin, or gemcitabine plus docetaxel. In patients with a contra indication to anthracycline-based therapy (e.g., patients with heart failure), a gemcitabine-based chemotherapy regimen could be considered for first-line therapy. Patients diagnosed with angiosarcoma may also be treated with paclitaxel as a first-line therapy. Finally, patients with a poor performance status or multiple comorbidities for whom chemotherapy is being considered can also be treated with pegylated liposomal doxorubicin or single-agent gemcitabine.
As a general initial approach to therapy, all patients with STS should be evaluated for eligibility to receive doxorubicin as first-line therapy. Patients with disease that is not amenable to curative resection or radiation therapy who are eligible to receive doxorubicin can also be considered for combination therapy with olaratumab. It is still important to keep in mind that synovial sarcoma and myxoid/round-cell liposarcoma may be more sensitive to combination therapy with doxorubicin and ifosfamide than to doxorubicin with olaratumab, as these tumor types may be more sensitive to ifosfamide. However, there has yet to be a study comparing these two combinations.
Histology-driven therapy is now generally considered to be a second-line consideration; however, it may still be recommended as an initial approach to therapy for some commonly anthracycline-resistant histologies, such as alveolar soft part sarcomas, solitary fibrous tumor/hemangiopericytomas, and clear cell sarcomas. Such patients may benefit from newer treatments, such as pazopanib.
Following progression of disease on first-line therapy, a variety of treatment options may be appropriate. For example, eribulin or trabectedin can be considered for second-line treatment of liposarcomas. In addition, trabectedin can also be considered for second-line treatment of leiomyosarcomas. Pazopanib can be considered for second-line therapy for a variety of STS subtypes, except liposarcoma. Regorafenib, while it has been studied and demonstrated a benefit in patients with leiomyosarcoma and synovial sarcoma, is not yet FDA-approved for this indication.
Overall, the recent approval of several new agents has expanded the number of options available for the treatment of metastatic STS in adults.
Footnotes
Disclosures: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC Press, International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Siegel RL, Miller KD, Jermal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Vezeridis MP, Moore R, Karakousis CP. Metastatic patterns in soft-tissue sarcomas. Arch Surg. 1983;118:915–918. doi: 10.1001/archsurg.1983.01390080023007. [DOI] [PubMed] [Google Scholar]
- 4.Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3:353–366. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Vogt-Moykopf I, Bülzebruck H, Merkle NM, et al. Results of surgical treatment of pulmonary metastases. Eur J Cardiothorac Surg. 1988;2:224–232. doi: 10.1016/1010-7940(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Choong PF, Pritchard DJ, Rock MG, et al. Survival after pulmonary metastasectomy in STS. Prognostic factors in 214 patients. Acta Orthop Scand. 1995;66:561–568. doi: 10.3109/17453679509002316. [DOI] [PubMed] [Google Scholar]
- 7.Rougraff BT, Aboulafia A, Biermann JS, et al. Biopsy of soft tissue masses: evidence-based medicine for the Musculoskeletal Tumor Society. Clin Orthop Relat Res. 2009;467:2783–2791. doi: 10.1007/s11999-009-0965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakely PE, Jr, Kneisl JS. Soft tissue aspiration cytopathology. Cancer. 2000;90:292–298. [PubMed] [Google Scholar]
- 9.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced STS: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens—a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin RS, Wiernik PH, Bachur NR. Adriamycin: a new effective agent in the therapy of disseminated sarcomas. Med Pediatr Oncol. 1975;1:63–76. doi: 10.1002/mpo.2950010109. [DOI] [PubMed] [Google Scholar]
- 11.Chabner BA, Bertino J, Cleary J, et al. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. Brunton L, Chabner B, Knollmann B, editors. New York, New York: McGraw-Hill; 2011. [Google Scholar]
- 12.Judson I, Radford JA, Harris M, et al. Randomised phase 2 trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic STS: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37:870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 13.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced STSs. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 14.Borden EC, Amato DA, Edmonson JH, et al. Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer. 1990;66:862–867. doi: 10.1002/1097-0142(19900901)66:5<862::aid-cncr2820660509>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Mouridsen HT, Bastholt L, Somers R, et al. Adriamycin versus epirubicin in advanced STSs. A randomized phase 2/phase 3 study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol. 1987;23:1477–1483. doi: 10.1016/0277-5379(87)90089-7. [DOI] [PubMed] [Google Scholar]
- 16.Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic STSs. J Clin Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 17.Bramwell VH, Mouridsen HT, Santoro A, et al. Cyclophosphamide versus ifosfamide: a randomized phase 2 trial in adult soft-tissue sarcomas. The European Organization for Research and Treatment of Cancer [EORTC], Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 1993;31(suppl 2):S180–S184. [PubMed] [Google Scholar]
- 18.Lorigan P, Verweij J, Papai Z, et al. Phase 3 trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic STS: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Vadhan-Raj S, Papadopolous N, et al. High-dose ifosfamide in bone and STSs: results of phase 2 and pilot studies— dose-response and schedule dependence. J Clin Oncol. 1997;15:2378–2384. doi: 10.1200/JCO.1997.15.6.2378. [DOI] [PubMed] [Google Scholar]
- 20.Keohan ML, Taub RN. Chemotherapy for advanced sarcoma: therapeutic decisions and modalities. Semin Oncol. 1997;24:572–579. [PubMed] [Google Scholar]
- 21.van Oosterom AT, Mouridsen HT, Nielsen OS, et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first-and second-line chemotherapy in advanced STS patients. Eur J Cancer. 2002;38:2397–2406. doi: 10.1016/s0959-8049(02)00491-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Chang MH, Baek KK, et al. High-dose ifosfamide as second- or third-line chemotherapy in refractory bone and STS patients. Oncology. 2011;80:257–261. doi: 10.1159/000328795. [DOI] [PubMed] [Google Scholar]
- 23.Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanism of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 24.Patel SR, Gandhi V, Jenkins J, et al. Phase 2 clinical investigation of gemcitabine in advanced STSs and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483–3489. doi: 10.1200/JCO.2001.19.15.3483. [DOI] [PubMed] [Google Scholar]
- 25.Ferraresi V, Ciccarese M, Cercato MC, et al. Gemcitabine at fixed dose-rate in patients with advanced STSs: a mono-institutional phase 2 study. Cancer Chemother Pharmacol. 2008;63:149–155. doi: 10.1007/s00280-008-0723-9. [DOI] [PubMed] [Google Scholar]
- 26.Jordan MA, Toso RJ, Thrower D, et al. Mechanism of mitotic block and inhibition cell proliferation by Taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penel N, Bui BN, Bay J, et al. Phase 2 trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;25:5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb JA, Benjamin RS, Baker LH, et al. Role of DTIC (NSC-45388) in the chemotherapy of sarcomas. Cancer Treat Rep. 1976;60:199–203. [PubMed] [Google Scholar]
- 29.Buesa JM, Mouridsen HT, van Oosterom AT, et al. High-dose DTIC in advanced soft-tissue sarcomas in the adult. A phase 2 study of the E.O.R.T.C. Soft Tissue and Bone Sarcoma Group. Ann Oncol. 1991;2:307–309. doi: 10.1093/oxfordjournals.annonc.a057942. [DOI] [PubMed] [Google Scholar]
- 30.Garcia del Muro X, Lopez-Pousa A, Martin J, et al. A phase 2 trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced STS: a study by the Spanish Group for Research on Sarcomas. Cancer. 2005;104:1706–1712. doi: 10.1002/cncr.21384. [DOI] [PubMed] [Google Scholar]
- 31.Casanova M, Ferrari A, Spreafico F, et al. Vinorelbine in previously treated advanced childhood sarcomas: evidence of activity in rhabdomyosarcoma. Cancer. 2002;94:3263–3268. doi: 10.1002/cncr.10600. [DOI] [PubMed] [Google Scholar]
- 32.Anderson SE, Keohan ML, D’Adamo DR, et al. A retrospective analysis of vinorelbine chemotherapy for patients with previously treated soft-tissue sarcomas. Sarcoma. 2006;2006:15947. doi: 10.1155/SRCM/2006/15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thigpen JT, Blessing JA, Beecham J, et al. Phase 2 trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol. 1991;9:1962–1966. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein D, Cheuvart B, Trump DL, et al. Phase 2 trial of carboplatin in soft-tissue sarcoma. Am J Clin Oncol. 1990;13:420–423. doi: 10.1097/00000421-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramwell VH, Anderson D, Charette ML, et al. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic STS. Cochrane Database Syst Rev. 2003;3:CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddon BM, Whelan J, Strauss SJ, et al. GeDDiS: A prospective randomised controlled phase 3 trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic STSs (EudraCT 2009-014907-29) J Clin Oncol. 2015;33(suppl) doi: 10.1016/S1470-2045(17)30622-8. abstract 10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lartruvo (olaratumab) prescribing information. Indianapolis, Indiana: Eli Lilly and Company; Oct, 2016. [Google Scholar]
- 39.Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yondelis (trabectedin) prescribing information. Horsham, Pennsylvania: Janssen Products; May, 2017. [Google Scholar]
- 41.D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–2163. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 42.Kawai A, Araki N, Sugiura H, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study. Lancet Oncol. 2015;16:406–416. doi: 10.1016/S1470-2045(15)70098-7. [DOI] [PubMed] [Google Scholar]
- 43.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase 3 randomized multicenter clinical trial. J Clin Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halaven (eribulin) prescribing information. Woodcliff Lake, New Jersey: Eisai Ltd; Aug, 2017. [Google Scholar]
- 45.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 46.Votrient (pazopanib) prescribing information. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation; May, 2017. [Google Scholar]
- 47.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo controlled, phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 48.Stivarga (regorafenib) prescribing information. Whippany, New Jersey: Bayer HealthCare Pharmaceuticals, Inc; Apr, 2017. [Google Scholar]
- 49.Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced STS (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1732–1742. doi: 10.1016/S1470-2045(16)30507-1. [DOI] [PubMed] [Google Scholar]
- 50.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of STSs, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]