Abstract
Unlike the classical nuclear envelope with two membranes found in other eukaryotic cells, most nematode sperm nuclei are not encapsulated by membranes. Instead, they are surrounded by a nuclear halo of unknown composition. How the halo is formed and regulated is unknown. We used forward genetics to identify molecular lesions behind three classical fer (fertilization defective) mutations that disrupt the ultrastructure of the Caenorhabditis elegans sperm nuclear halo. We found fer-2 and fer-4 alleles to be nonsense mutations in mib-1. fer-3 was caused by a nonsense mutation in eri-3. GFP::MIB-1 was expressed in the germline during early spermatogenesis, but not in mature sperm. mib-1 encodes a conserved E3 ubiquitin ligase homologous to vertebrate Mib1 and Mib2, which function in Notch signaling. Here, we show that mib-1 is important for male sterility and is involved in the regulation or formation of the nuclear halo during nematode spermatogenesis.
Keywords: spermatogenesis, E3 ubiquitin ligase, nuclear envelope, C. elegans
One hallmark of eukaryotic cells is the nuclear envelope, a specialized extension of the endoplasmic reticulum consisting of two lipid bilayers and a perinuclear space (Hetzer 2010). The essential role of the nuclear envelope is to compartmentalize the nucleus from the cytoplasm. Therefore, the nuclear envelope evolved in the last eukaryotic common ancestor and was critical to the evolution of the wide variety of eukaryotic organisms alive today (Baum and Baum 2014). However, there are exceptions to this paradigm. All nematode classes except Enoplida have sperm devoid of a nuclear envelope at maturity (Justine 2002; Yushin and Malakhov 2014). Instead of two lipid bilayers, nematode sperm nuclei are surrounded by a halo of electron-dense material that also encapsulates the sperm centrioles (Wolf et al. 1978). The halo is thought to contain RNA (Ward et al. 1981).
Nearly forty years after its initial discovery, little is known about the molecular makeup and developmental regulation of the perinuclear halo. Two components have been shown to localize to the nuclear halo, the centrosome component SPD-2 and the novel protein SPE-11, which is one of the few paternally provided proteins in the embryo (Browning and Strome 1996; Sadler and Shakes 2000; McNally et al. 2012), but any role of these two proteins in the organization of the nuclear halo is unknown. One intriguing hypothesis is that the sperm nuclear halo serves as a sink for paternally provided mRNA, siRNA, and piRNA molecules that are delivered to the zygote through sperm (Stoeckius et al. 2014). Here, we employed a forward genetic approach in C. elegans to identify additional players in the formation of the perinuclear halo of nematode sperm.
C. elegans has long been appreciated as an excellent model to study gametogenesis (Hirsh et al. 1976). A large number of mutations have been isolated in C. elegans genes required for spermatogenesis, called fer or spe for fertilization or spermatogenesis defective, respectively (Argon and Ward 1980; L’Hernault et al. 1988). These mutations are specific to spermatogenesis and result in hermaphrodites that lay unfertilized oocytes. The temperature sensitive period for fer mutants corresponds to the timing of spermatogenesis in hermaphrodites and the fertilization defect can be rescued by mating mutant hermaphrodites to wild-type males (Argon and Ward 1980).
We were particularly interested in three genes, fer-2, fer-3, and fer-4, because of their striking ultrastructural mutant phenotype (Ward et al. 1981). When sperm from fer-2, fer-3, or fer-4 mutant males grown at 25° are examined by electron microscopy, the RNA halo that normally surrounds centrioles and condensed chromatin in mature spermatids is absent (Ward et al. 1981). In place of the halo, large tubules of straight hollow cylinders accumulate around the condensed chromatin of spermatids and sperm (Ward et al. 1981). The nature of the perinuclear tubules is unknown; with diameters of about 50 nm (Ward et al. 1981), they are unlike other described tubular cellular components. The working model, as proposed by Ward et al. (1981), is that in fer-2, fer-3, or fer-4 mutant sperm, tubules form from aberrant polymerized components of the ribonucleoprotein complexes that normally make the perinuclear halo. We hypothesized that determining the molecular identity of fer-2, fer-3, and fer-4 gene products would elucidate molecular mechanisms of the formation of the normal perinuclear halo and/or the abnormal tubules that form in the mutant sperm. Here we report that fer-2 and/or fer-4 is a mutation in the predicted E3 ubiquitin ligase mib-1 and that fer-3 is a mutation in eri-3, a member of a Dicer-associated complex.
Materials And Methods
Strains
C. elegans strains were grown on nematode growth medium plates spotted with OP50 bacteria and maintained at 15° unless otherwise noted (Stiernagle 2006). N2 was used as the wild-type control strain (Brenner 1974). Strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). Strains BA2 fer-2(hc2), BA3 fer-3(hc3), BA4 fer-4(hc4), BA547 fer-2(hc2); him-5(e1490), and BA562 fer-4(hc4); him-5(e1490) were previously described (Argon and Ward 1980). WM172 eri-3(tm1361) II was originally made by Shohei Mitani (National Bioresource Project at the Tokyo Women’s Medical University).
Whole genome sequencing
For genomic DNA preps, nearly starved animals from five plates (5 cm each) were washed in M9 (Stiernagle 2006), pelleted, resuspended in 200 μL buffer ATL (QIAGEN), and subjected to three freeze-thaw cycles. Genomic DNA was purified following the “animal tissue” protocol of the QIAGEN DNeasy Blood & Tissue Kit with an added RNAse A (10 mg/ml) incubation at room temperature for 15 min after the Proteinase K incubation. Genomic DNA was sent to the Functional Genomics Laboratory at QB3-Berkeley, fragmented into 300-600 bp pieces, and cloned into multiplexed libraries. The libraries were sequenced using paired end reads of 150 base pairs on an Illumina HiSeq 2500. Sequences were aligned, filtered and tabulated following the CloudMap pipeline (Minevich et al. 2012) on the public Galaxy server (Afgan et al. 2016).
CRISPR/Cas9 mutagenesis and genome editing
CRISPR/Cas9 was used to generate a null mutation in mib-1 and to fuse gfp to the 5′ or 3′ end of the mib-1 open reading frame (Paix et al. 2015; 2016). Custom guide crRNA and universal tracrRNA were synthesized by Integrated DNA Technologies or Dharmacon. For the mib-1(yc44) mutation, the crRNA guide sequence was CGUAAUACCACCUCGAAAAC. For the mib-1::gfp(yc46) fusion, the sequence of the crRNA was AUUGAUAUUCACGAGUAGAU. For gfp::mib-1(yc51) we used ACAAAAAUGAACGGAGUAGC as the crRNA. Purified Cas9-NLS protein was obtained from QB3-Berkeley. To make the repair templates for the homology-directed insertion, gfp sequences were amplified from pDD282 (a gift from Bob Goldstein; Addgene plasmid # 66823) (Dickinson et al. 2015) using Phusion DNA Polymerase (Thermo Fisher Scientific) and primers with overhangs consisting of 58-60 base pairs of C. elegans homology flanking the predicted CRIPSR/Cas9 cut-site (Paix et al., 2015). The QIAquick PCR Purification Kit (Qiagen) was used to clean the PCR product. In brief, we injected young adult hermaphrodite gonads with 9 μM or 17.5 μM crRNA:tracrRNA:Cas9 complexes along with 0.67 μM of the repair template for the gfp insertions (Paix et al. 2015; 2016). To follow CRISPR efficiency we used the dpy-10 co-CRISPR approach (Arribere et al. 2014). Both a dpy-10 crRNA sequence (1:12 ratio for dpy-10 crRNA:mib-1 crRNA) and 0.5 μM ssDNA oligo as a dpy-10 repair template were added to the injection mix (Arribere et al. 2014). The dpy-10 mutation was removed from the strains by crossing to N2 or him-8(e1489) males. The following three strains were generated: UD549 mib-1(yc44), UD563 mib-1::gfp(yc46); him-8(e1489), and UD577 gfp::mib-1(yc51), him-8(e1489).
Fertilization and brood size phenotypes
To quantify the temperature-sensitive fertilization defects, assayed strains were initially cultured at 15° on NGM plates seeded with OP50. Twenty L2 hermaphrodites from each strain were singled onto their own plates. For the hc2/yc44 complementation test, we singled 40 F1 L2s expecting half would be males. Half of singled L2s were then raised at 25° and the other half at 15°. After 48 hr, hermaphrodites raised at 25° were transferred to a fresh plate. The oocytes remaining on the original plate were scored as “fertilized” or “not fertilized” based on the globular and opaque appearance of unfertilized oocytes and the development of fertilized oocytes into L1 progeny within 24 hr. Oocytes laid within a 2-day window were added together. Plates with less than a total of 25 oocytes were excluded from the data. Also excluded were counts from F1s that were not cross progeny, which was verified by Sanger sequencing. For L2s raised at 15°, counts started 72 hr after individuals were singled to account for the delay in development.
Microscopy
We performed immunofluorescence staining on dissected male germlines from L4 males grown at 20°. Dissection, fixation and immunofluorescence were performed as described (Jaramillo-Lambert et al. 2007) with the following alterations. Samples were fixed for 5 min at room temperature in 2% paraformaldehyde in egg buffer [118 mM NaCl, 48 mM KCl2, 2 mM CaCl2, 2 mM MgCl2, 5 mM HEPES at pH 7.4]. We used a 0.7% BSA/1x PBS + 0.1% Tween20 solution for blocking. Rabbit anti-GFP antibody (NovusBiologicals NB600-308) was used at a 1:500 dilution and donkey anti-rabbit antibody Alexa Fluor 488 (Invitrogen A21206) was used at 1:500 for the secondary. DNA was stained with DAPI (final concentration of 0.2 ng/uL). Images were collected with a 63× Plan Apo 1.40 NA objective on an DM6000 epifluorescence compound microscope (Leica) with AF6000 software (Leica). Images were uniformly enhanced using the levels command in Adobe Photoshop.
Data Availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article and figures.
Results And Discussion
fer-2 is a mutation in mib-1
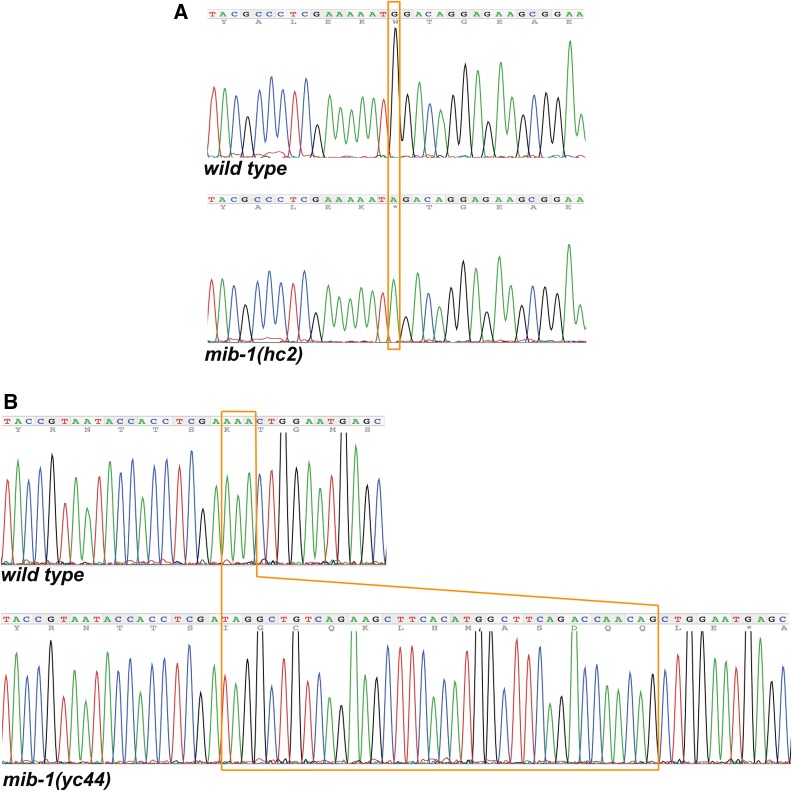
We set out to identify the molecular lesions of fer-2, fer-3, and fer-4 using a whole-genome sequencing approach. Libraries were prepared from fer-2(hc2) or fer-3(hc3) genomic DNA for Illumina paired-end sequencing. The sequences were aligned and analyzed using the CloudMap pipeline (Minevich et al. 2012) which identified over 5,000 single nucleotide polymorphisms (SNP) in the sequenced fer-2(hc2) strain when compared to the reference N2, wild-type genome. Only a single SNP in the fer-2(hc2) sequence data set was predicted to cause a nonsense mutation in an open reading frame. This SNP was a C to T transition at position 11,297,830 of chromosome III. It is predicted to change the tryptophan of codon number 460 in the mib-1 gene to a stop codon. Thus, we hypothesized that fer-2(hc2) is an allele of mib-1.
We have high confidence in the mib-1 mutation for the following three reasons. 1. The mutation was covered 105 times in the whole-genome dataset and confirmed by Sanger sequencing (Figure 1A). 2. Although fer-2 was originally mapped to chromosome IV (Argon and Ward 1980), three-point mapping later placed fer-2 between tra-1 and dpy-18, close to position 7.2 cM on chromosome III (Hodgkin 1993). This position is within a cM of mib-1. 3. Based on the fer-2(hc2) phenotype, we predicted that fer-2 transcripts would be enriched in the male germline. We therefore examined a list of 864 spermatogenesis-enriched genes previously identified in a microarray study (Reinke et al. 2004). mib-1 is the only one of the 864 transcripts that maps between tra-1 and dpy-18 on chromosome III. Thus, fer-2 maps near mib-1 and mib-1 is the only gene in the region enriched in spermatogenesis expression lists.
Figure 1.
Mutations in mib-1. Sanger sequence outputs for wild type and mib-1 mutant strains. (A) hc2 is a C to T transition at position 11,297,830 of chromosome III. The opposite coding strand sequence is shown that leads to a Tryptophan to stop codon nonsense mutation. (B) yc44 is a 38 bp insertion (boxed) in the sixth exon of mib-1 that is quickly followed by a stop codon.
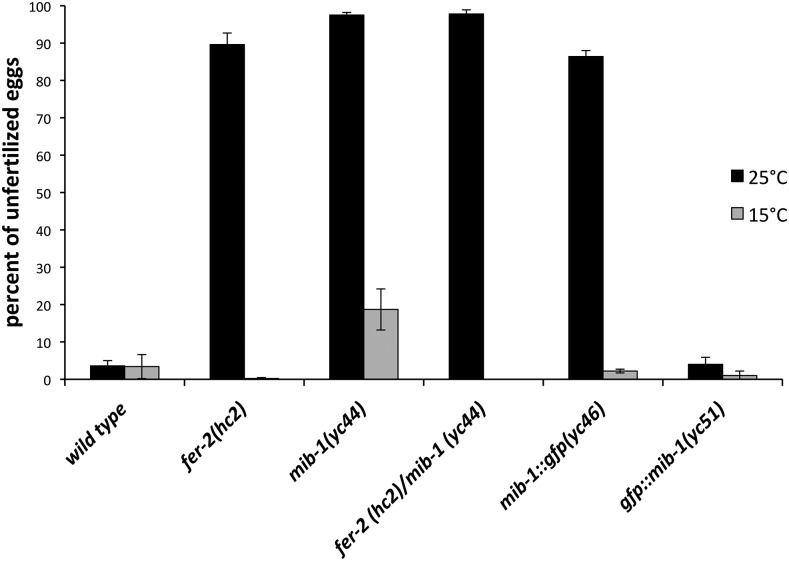
To further test whether fer-2 is mib-1, one would traditionally attempt to rescue fer-2(hc2) with wild-type genomic DNA of the mib-1 locus or to phenocopy the fer-2(hc2) phenotype with mib-1(RNAi). However, extrachromosomal arrays are suppressed in germ lines and RNAi is very inefficient in C. elegans sperm. In addition, no known alleles of mib-1 had been previously identified or characterized. We therefore generated a new insertion/deletion allele of mib-1 through imprecise non-homologous end joining repair after inducing a double strand break using CRISPR/Cas9. We isolated a mutant allele, mib-1(yc44) with a 38 base pair insertion of the dpy-10 repair template that was used in our co-CRISPR approach (Arribere et al. 2014). The yc44 insertion into the sixth exon of mib-1 is predicted to cause a frame shift and is therefore likely a null allele (Figure 1B). mib-1(yc44) animals laid about 18% unfertilized oocytes when raised at 15°, but laid an average of 97% unfertilized oocytes at 25° (Figure 2). Thus, mib-1(yc44) phenocopied fer-2(hc2) as a temperature-sensitive spermatogenesis defective mutant.
Figure 2.
mib-1 is required for male fertility. The mean percent of unfertilized oocytes from the total number of oocytes plus embryos laid by hermaphrodites is shown. For each bar, progeny from at least 10 hermaphrodites were scored at 15° and 25°C. Error bars are the standard error of the mean.
As final confirmation that fer-2(hc2) is an allele of mib-1, we crossed males homozygous for the C to T SNP on chromosome III to mib-1(yc44) hermaphrodites, and raised the F1 progeny at 25°. The fer-2(hc2)/mib-1(yc44) heterozygotes laid 97.8% unfertilized oocytes at the restrictive temperature (Figure 2). Thus, fer-2(hc2) failed to complement mib-1(yc44) and we concluded that hc2 is an allele of mib-1.
Our results suggest that one fer-2(hc2) strain has been mislabeled since its original isolation (Argon and Ward 1980). We ordered all available strains carrying hc2 or hc4 from the Caenorhabditis Genetics Center and sequenced mib-1 for the C to T mutation that causes the premature stop codon (Figure 1A) that we found in hc2. We identified the C to T mutation in BA2 fer-2(hc2), BA4 fer-4(hc4), and BA562 fer-4(hc4); him-5(e1490). However, the mutation was absent in BA547 fer-2(hc2); him-5(e1490). Thus, an unidentified different lesion, perhaps outside of mib-1, likely causes the temperature-sensitive male sterile phenotype in the BA547 strain and it is not possible to determine whether we identified the original fer-2(hc2) or fer-4(hc4) lesion. We henceforth refer to both fer-2 and fer-4 as mib-1.
Localization of MIB-1 during spermatogenesis
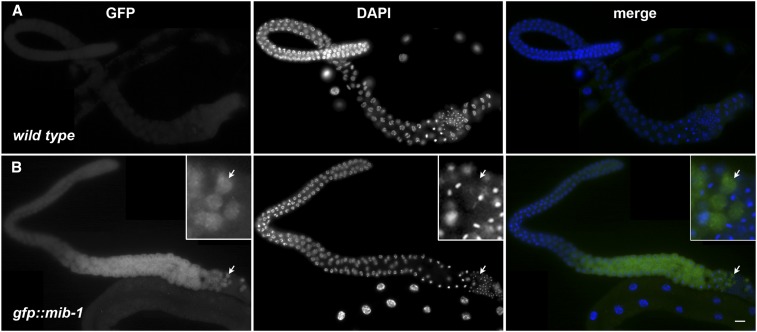
After determining the fer-2 phenotype was due to a mutation in mib-1, CRISPR/Cas9 gene editing was used to tag the C-terminus of endogenous MIB-1 with GFP. Animals homozygous for mib-1::gfp(yc46) as the only source of MIB-1 lay an average of 86% unfertilized oocytes at 25° (Figure 2), suggesting that MIB-1::GFP is nonfunctional. We proceeded to use CRISPR/Cas9 to tag MIB-1 with GFP at the N-terminus. gfp::mib-1(yc51) hermaphrodites laid wild-type percentages of fertilized embryos at both the permissive and restrictive temperatures (Figure 2), suggesting GFP::MIB-1 is functional. We next visualized GFP::MIB-1 by immunofluorescence with anti-GFP antibodies because of a weak GFP signal in live animals. GFP::MIB-1 was highly expressed in the proximal arm of the male gonad, but not in mature sperm (Figure 3). The timing of GFP::MIB-1 expression was consistent with a role in spermatogenesis. We observed cytoplasmic GFP::MIB-1 expression starting at mid-pachytene of male gonads. The signal persisted throughout late pachytene, diplotene, and diakinesis. The last visible signal detected was in the cytoplasmic residual body. We did not detect GFP::MIB-1 in mature spermatids. GFP::MIB-1 is similarly expressed in the L4 hermaphrodite gonad during spermatogenesis. The nuclear halo is formed shortly after spermatids bud off the residual body (Wolf et al. 1978; Ward et al. 1981). Thus, the timing of expression and the depositing of GFP::MIB-1 into the residual body is consistent with a model where MIB-1 needs to be turned off to stabilize its targets and allow its targets to function in the normal formation of the nuclear halo. In mib-1 mutants, the targets would be prematurely active, leading to the gross ultrastructural deformities previously described for fer-2 and fer-4 (Ward et al. 1981).
Figure 3.
GFP::MIB-1 is expressed in the male germline. (A) wild type and (B) gfp::mib-1. Anti-GFP immunofluorescence showing GFP::MIB-1 expression in the proximal arm of male germline. GFP is green and DAPI-stained nuclei are blue in the merge. Scale bar is 10 μm. In the inset, a group of four residual bodies marked with an arrow is enlarged.
Possible mechanisms for MIB-1 and the sperm nuclear halo
C. elegans mib-1 encodes an E3 ubiquitin protein ligase conserved to vertebrate Mib1 and Mib2 (Berndt et al. 2011). Mutations in the human MIB1 gene cause left ventricular noncompaction cardiomyopathy (Luxán et al. 2013). Mib1 ubiquitinates the Notch ligands Delta and Jagged and targets them for endocytosis, turning off Notch signaling in zebrafish and mammals (Itoh et al. 2003; Kang et al. 2013). However, there are no major Notch-related phenotypes reported in MIB2−/− knockout mice, suggesting that Mib2 acts synthetically to regulate Notch signaling and/or targets at least one alternate substrate (Koo et al. 2005; Guo et al. 2016). Our results show that C. elegans mib-1 mutants have a fertilization defective phenotype that is distinct from any previously described Notch pathway mutant in C. elegans (Greenwald 2005). Thus, it appears that mib-1 has an additional role in nematodes to function in perinuclear halo formation during spermatogenesis. A recent report showed that lesions in mib-1 are also the cause of the spe-16(hc54) spermatogenesis-defective phenotype (Ratliff et al. 2018). A phenotypic analysis of spe-16 showed that it had the same temperature-dependent, male-sterile phenotype as previously reported for fer-2 and -4 (Ratliff et al. 2018), but whether spe-16 disrupts the formation of the sperm nuclear halo was not tested. Similar to our findings, Ratliff et al. (2018) showed that GFP::MIB-1 localized diffusely in the male germline, but not in sperm. In addition, they found that mib-1 has a function in the Delta/Notch pathway, as loss-of-function alleles of mib-1 partially suppressed gain-of-function lin-12 vulva developmental defects (Ratliff et al. 2018). Finally, Ratliff et al. (2018) showed that MIB-1 can ubiquitinate itself in vitro. However, the exact in vivo target(s) of MIB-1 during spermatogenesis and nuclear halo formation remains to be determined.
We also cloned fer-3(hc3) by whole-genome sequencing. We had very little mapping data to guide us for fer-3. However, scanning the CloudMap output of SNPs between fer-3(hc3) and wild type led to the identification of a nonsense mutation in eri-3. We found a T to C transition at position 1,123,966 of chromosome II, which changes codon 69 of the eri-3 gene from a serine to a proline. eri-3 was previously reported to have a temperature sensitive spermatogenesis mutant phenotype (Pavelec et al. 2009). We crossed fer-3(hc3) into eri-3(tm1361) and the alleles failed to complement; F1 heterozygote hermaphrodites laid unfertilized embryos at 25°. An additional group also reported that fer-3(hc3) is an allele of eri-3 using the same complementation test (Conine et al. 2013). ERI-3 is a component of the 26G-RNA ALG-3/4 pathway and mutations in alg-3/4 lead to a similar nuclear halo defect in spermatozoa, suggesting that they are in the same pathway (Conine et al. 2013). Perhaps, MIB-1 and ERI-3 function to load specific paternal RNAs into the nuclear halo of sperm that are then contributed to the zygote (Stoeckius et al. 2014). The expression of MIB-1 during spermatogenesis and a common downstream phenotype suggests a potential role in post-transcriptional regulation of proteins required for normal sperm morphology. It could therefore be informative in the future to identify the targets of MIB-1 and any possible relationship between MIB-1 and the 26G-RNA ALG-3/4 pathway.
Acknowledgments
We thank David Fay and members of the Fay lab for hosting DAS on sabbatical where the project was initiated, especially John Yochem who helped analyze the whole-genome sequences. We thank QB3 Berkeley for preparing libraries and Illumina sequencing. We thank Venecia A. Valdez and Michael Paddy for help with the imaging. We thank members of the Starr lab for reading the manuscript and support throughout the project. This work was supported by the National Institutes of Health (grant number R01 GM073874).
Footnotes
Communicating editor: D. Fay
Literature Cited
- Afgan E., Baker D., van den Beek M., Blankenberg D., Bouvier D., et al. , 2016. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 44: W3–W10. 10.1093/nar/gkw343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argon Y., Ward S., 1980. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics 96: 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X. H., Artiles K. L., Hartman P. S., et al. , 2014. Efficient Marker-Free Recovery of Custom Genetic Modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. 10.1534/genetics.114.169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum D. A., Baum B., 2014. An inside-out origin for the eukaryotic cell. BMC Biology 2014 12:1 12: 1 10.1186/s12915-014-0076-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J. D., Aoyagi A., Yang P., Anastas J. N., Tang L., et al. , 2011. Mindbomb 1, an E3 ubiquitin ligase, forms a complex with RYK to activate Wnt/β-catenin signaling. J. Cell Biol. 194: 737–750. 10.1083/jcb.201107021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics. 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H., Strome S., 1996. A sperm-supplied factor required for embryogenesis in C. elegans. Development 122: 391–404. [DOI] [PubMed] [Google Scholar]
- Conine C. C., Moresco J. J., Gu W., Shirayama M., Conte D., Jr, et al. , 2013. Argonautes Promote Male Fertility and Provide a Paternal Memory of Germline Gene Expression in C. elegans. Cell 155: 1532–1544. 10.1016/j.cell.2013.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., Goldstein B., 2015. Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics 200: 1035–1049. 10.1534/genetics.115.178335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I., 2005. LIN-12/Notch signaling in C. elegans, WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.10.1 http://www.wormbook.org. [Google Scholar]
- Guo B., McMillan B. J., Blacklow S. C., 2016. Structure and function of the Mind bomb E3 ligase in the context of Notch signal transduction. Curr. Opin. Struct. Biol. 41: 38–45. 10.1016/j.sbi.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer M. W., 2010. The Nuclear Envelope. Cold Spring Harb. Perspect. Biol. 2: a000539 10.1101/cshperspect.a000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim D., Klass M., 1976. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49: 200–219. 10.1016/0012-1606(76)90267-0 [DOI] [PubMed] [Google Scholar]
- Hodgkin J., 1993. Molecular cloning and duplication of the nematode sex-determining gene tra-1. Genetics 133: 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Kim C.-H., Palardy G., Oda T., Jiang Y.-J., et al. , 2003. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4: 67–82. 10.1016/S1534-5807(02)00409-4 [DOI] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Ellefson M., Villeneuve A. M., Engebrecht J., 2007. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308: 206–221. 10.1016/j.ydbio.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Justine J.-L., 2002. Male and Female Gametes and fertilisation, pp. 73–120 in The Biology of Nematodes, CRC Press, Boca Raton, FL. [Google Scholar]
- Kang K., Lee D., Hong S., Park S.-G., Song M.-R., 2013. The E3 Ligase Mind Bomb-1 (Mib1) Modulates Delta-Notch Signaling to Control Neurogenesis and Gliogenesis in the Developing Spinal Cord. J. Biol. Chem. 288: 2580–2592. 10.1074/jbc.M112.398263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B.-K., Yoon K.-J., Yoo K.-W., Lim H.-S., Song R., et al. , 2005. Mind Bomb-2 Is an E3 Ligase for Notch Ligand. J. Biol. Chem. 280: 22335–22342. 10.1074/jbc.M501631200 [DOI] [PubMed] [Google Scholar]
- L’Hernault S. W., Shakes D. C., Ward S., 1988. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 120: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxán G., Casanova J. C., Martínez-Poveda B., Prados B., D’Amato G., et al. , 2013. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 19: 193–201. 10.1038/nm.3046 [DOI] [PubMed] [Google Scholar]
- McNally K. L. P., Fabritius A. S., Ellefson M. L., Flynn J. R., Milan J. A., et al. , 2012. Kinesin-1 Prevents Capture of the Oocyte Meiotic Spindle by the Sperm Aster. Dev. Cell 22: 788–798. 10.1016/j.devcel.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. 10.1534/genetics.112.144204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Folkmann A., Rasoloson D., Seydoux G., 2015. High Efficiency, Homology-Directed Genome Editing in Caenorhabditis elegans Using CRISPR-Cas9 Ribonucleoprotein Complexes. Genetics 201: 47–54. 10.1534/genetics.115.179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Schmidt H., Seydoux G., 2016. Cas9-assisted recombineering in C. elegans: genome editing using in vivo assembly of linear DNAs. Nucleic Acids Res. 44: e128 10.1093/nar/gkw502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelec D. M., Lachowiec J., Duchaine T. F., Smith H. E., Kennedy S., 2009. Requirement for the ERI/DICER Complex in Endogenous RNA Interference and Sperm Development in Caenorhabditis elegans. Genetics 183: 1283–1295. 10.1534/genetics.109.108134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M., Hill-Harfe K. L., Gleason E. J., Ling H., Kroft T. L., et al. , 2018. MIB-1 Is Required for Spermatogenesis and Facilitates LIN-12 and GLP-1 Activity in Caenorhabditis elegans. Genetics 209: 173–193. 10.1534/genetics.118.300807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V., Gil I. S., Ward S., Kazmer K., 2004. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323. 10.1242/dev.00914 [DOI] [PubMed] [Google Scholar]
- Sadler P. L., Shakes D. C., 2000. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development 127: 355–366. [DOI] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi: 10.1895/wormbook.1.101.1 10.1895/wormbook.1.101.1 [DOI]
- Stoeckius M., Grün D., Rajewsky N., 2014. Paternal RNA contributions in the Caenorhabditis elegans zygote. EMBO J. 33: 1740–1750. 10.15252/embj.201488117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Argon Y., Nelson G. A., 1981. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J. Cell Biol. 91: 26–44. 10.1083/jcb.91.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N., Hirsh D., McIntosh J. R., 1978. Spermatogenesis in males of the free-living nematode, Caenorhabditis elegans. J. Ultrastruct. Res. 63: 155–169. 10.1016/S0022-5320(78)80071-9 [DOI] [PubMed] [Google Scholar]
- Yushin V. V., Malakhov V. V., 2014. The origin of nematode sperm: Progenesis at the cellular level. Russ. J. Mar. Biol. 40: 71–81. 10.1134/S1063074014020114 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article and figures.