Abstract
Genetic linkage maps are essential for comparative genomics, high quality genome sequence assembly and fine scale quantitative trait locus (QTL) mapping. In the present study we identified and genotyped markers via restriction-site associated DNA (RAD) sequencing and constructed a genetic linkage map based on 1,597 SNP markers of an interspecific F2 cross of two closely related Lake Victoria cichlids (Pundamilia pundamilia and P. sp. ‘red head’). The SNP markers were distributed on 22 linkage groups and the total map size was 1,594 cM with an average marker distance of 1.01 cM. This high-resolution genetic linkage map was used to anchor the scaffolds of the Pundamilia genome and estimate recombination rates along the genome. Via QTL mapping we identified a major QTL for sex in a ∼1.9 Mb region on Pun-LG10, which is homologous to Oreochromis niloticus LG 23 (Ore-LG23) and includes a well-known vertebrate sex-determination gene (amh).
Keywords: Genetics of Sex, amh, Cichlidae, RAD, recombination rate, sex chromosome evolution, sex determination, synteny, XY system
The haplochromine cichlid lineage of the East African Great Lakes is famous for forming large adaptive radiations often in exceptionally short time, resulting in several hundred species each in Lakes Malawi and Victoria, and dozens of species each in several smaller East African Lakes (Kocher 2004; Seehausen 2015). The Lake Victoria haplochromine cichlid radiation stands out in being the youngest (∼15,000 years) showing a high degree of diversity in morphology, behavior and ecology (Greenwood 1974; Seehausen 1996). An abundance of studies have been published on the evolution of Lake Victoria cichlids, providing insight to colonization history (Nagl et al. 2000; Seehausen et al. 2003; Verheyen et al. 2003; Meier et al. 2017b), species formation (Seehausen et al. 1997; Seehausen and van Alphen 1999; Seehausen et al. 1999; Selz et al. 2014a), the interaction of sexual and natural selection (Seehausen 2000, Seehausen et al. 2008, Maan and Seehausen 2011), and the role of hybridization between distant relatives (Seehausen et al. 2003, Keller et al. 2013, Selz et al. 2014b, Meier et al. 2017a, Meier et al. 2017b). Recently, several cichlid genomes were published (Brawand et al. 2014), among them one from Lake Victoria. This genome is being used to investigate the genomic landscape of speciation (Meier et al. 2018). Detailed genetic linkage maps offer a powerful tool to improve the quality of genome assemblies (Fierst 2015) and set the framework for quantitative trait loci (QTL) localization. In the past decade, a number of genetic linkage maps have been published for haplochromine cichlids using various molecular genetic markers (Streelman et al. 2003; Sanetra et al. 2009; O’Quin et al. 2013; Henning et al. 2014; 2017). For Lake Victoria cichlids three linkage maps based on two interspecific F2 hybrid crosses were published. The first was based on an F2 cross between Paralabidochromis chilotes and Paralabidochromis sauvagei and contained 184 microsatellites and two SNP markers with a mean marker spacing of 6.09 cM on 25 linkage groups (Kudo et al. 2015). The two others were based on F2 crosses between Paralabidochromis sauvagei and Pundamilia cf. nyererei (Henning et al. 2014) and Paralabidochromis chilotes and Pundamilia cf. nyererei (Henning et al. 2017). Linkage maps were constructed with 867 and 752 single-nucleotide polymorphism (SNP) markers resulting in a mean marker spacing of 1.30 and 1.09 cM, respectively on 22 linkage groups (Henning et al. 2014; 2017). These linkage maps were then used to identify QTL, such as for lateral stripes, lip size, and head morphology (Henning et al. 2014; 2017) as well as sex determination (Kudo et al. 2015). None of the linkage maps has been used to improve the Lake Victoria haplochromine genome assembly.
In haplochromine cichlids, some polymorphic color patterns are genetically linked to sex determination and are associated with segregating polymorphisms in sex determination (Holzberg 1978; Seehausen et al. 1999; Lande et al. 2001; Streelman et al. 2003; Kocher 2004). These observations supported the hypothesis that the rapid evolution of sex determination systems might play a role in the very rapid speciation of haplochromine cichlids (Seehausen et al. 1999; Lande et al. 2001; Kocher 2004; Ser et al. 2010). A high diversity of sex determination systems and high sex chromosome turnover rates are known in fish, including cichlids, with a variety of environmental and genomic factors resulting in male or female phenotypes (reviewed e.g., in Heule et al. 2014a). In cichlids, very closely related species, populations within the same species, and even individuals within a population, can have different sex determination mechanisms or non-homologous sex chromosomes. This is evidenced by the presence of both XX-XY and ZZ-ZW sex determination systems within haplochromines of Lakes Victoria and Malawi and in oreochromine cichlids (Seehausen et al. 1999; Lande et al. 2001; Cnaani et al. 2008; Roberts et al. 2009; Ser et al. 2010). Some candidates for genetic sex determination in cichlids exist and could be associated with respective chromosomes, suggesting both XY and ZW systems. Across different species of Oreochromis, sex determination loci have been repeatedly mapped to three different linkage groups (LGs), namely LG 1 (XY), LG 3 (ZW), and LG 23 (XY) (Cnaani et al. 2008). In haplochromine cichlids, sex determination loci mainly mapped to LG 5 (ZW and XY) and LG 7 (XY) (Ser et al. 2010; Kudo et al. 2015; Roberts et al. 2016; Böhne et al. 2016; Peterson et al. 2017). Some genes that have repeatedly evolved as master sex determination genes in teleost fishes (Kikuchi and Hamaguchi 2013; Heule et al. 2014a) seem to play a role in sex determination in cichlids as well. Recent results published on Astatotilapia calliptera, a haplochromine cichlid from Lake Malawi, and Oreochromis niloticus, a distant relative of the East African adaptive radiations, indicate that two of these candidate genes, the gonadal soma-derived factor (gsdf) and the anti Müllerian hormone (amh) might have been re-used as sex determination loci (Eshel et al. 2014; Peterson et al. 2017). Those genes are often derived by duplication or allelic diversification from genes with a known function in sex differentiation or gonad development (Heule et al. 2014a).
In the present study we construct a linkage map of an interspecific F2 cross between two very closely related Lake Victoria cichlid species (Pundamilia pundamilia and P. sp. ‘red head’). The map was built using 1,597 SNPs identified and genotyped via restriction-site associated DNA (RAD) sequencing with an average marker distance of 1.01 cM. We then used the linkage map to anchor the scaffolds of the P. nyererei reference genome to the 22 linkage groups of the map and to perform a QTL analysis for putative sex determination loci in Pundamilia. We identify the LG determining sex in a Lake Victoria cichlid cross, as well as potential candidate genes for sex determination and put these findings into the context of sex determination evolution within a rapidly radiating clade of fish.
Materials and Methods
Mapping family and RAD sequencing
The genetic cross was started with a lab bred Pundamilia sp. ‘red head’ (Seehausen 1996) male from Zue Island in Lake Victoria (lab strain established from wild caught fishes by OS in 1993, 4th or 5th lab generation) and a wild P. pundamilia female caught by OS at Makobe Island in Lake Victoria in 2003. Eggs were removed from the female’s mouth five days after spawning and reared in isolation from the adults. After reaching maturity, four F1 individuals were crossed, resulting in two F2 families with together more than 300 individuals. When F2 individuals were adult and sexually mature sex was determined based on coloration, then sedated fish were killed with MS222 (50 mg/L for sedation; 300 mg/L for euthanization), and a fin clip was taken and stored in 98% ethanol for genetic analyses (Animal Permit numbers: BE18/15, BE47/11, LU04/07, LU02/13). Genomic DNA of 218 F2 progeny, the four F1 parents, and the two F0 grandparents was extracted using phenol-chloroform (Sambrook and Russell 2001). Restriction-site associated DNA (RAD) sequencing libraries were prepared following Marques et al. (2016) using a protocol slightly modified from Baird et al. (2008). In brief, genomic DNA was digested with SbfI followed by shearing and size selection of 300 to 500 bp. Equimolar proportions of DNA from 11 to 48 individuals carrying different barcode sequences were pooled into one library. Each library was amplified in four reactions of 50 µl aliquots. A total of nine libraries were single-end sequenced (100 bp) each on a single lane of an Illumina HighSequation 2500 platform either at the Next Generation Sequencing Platform of the University of Bern or at the Genomic Technologies Facility of the University of Lausanne. Some individuals and all F0 grandparents were sequenced in up to three libraries to increase coverage. Together with each library, we sequenced about 10% reads of bacteriophage PhiX genomic DNA (Illumina Inc.) to increase complexity at the first 10 sequenced base pairs. During read processing, PhiX reads were further utilized to recalibrate libraries to equalize base quality scores across Illumina lanes utilizing GATK version 3.2 (McKenna et al. 2010).
Sequence processing and genotyping
Before recalibration, read qualities were inspected using fastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and filtered using FASTX Toolkit 0.0.13 (http://hannonlab.cshl.edu/fastx_toolkit/index.html) requiring a minimum quality of 10 at all bases and of 30 in at least 95% of the read. After PhiX removal, reads were demultiplexed, cleaned, and trimmed to 92 bp with process_radtags implemented in Stacks v1.26 (Catchen et al. 2013). Reads were mapped against the P. nyererei reference genome (Brawand et al. 2014) using bowtie2 version 2.2.6 (using default parameters except for setting –N 1 allowing one mismatch in a seed alignment to increase sensitivity; Langmead and Salzberg 2012). Mapped reads of individuals run in multiple libraries were merged using Picard tools version 1.97 and filtered for a mapping quality of at least 30. After the filtering pipeline we were left with a total of 719,720,265 sequences across the nine RAD libraries (on average 79,970,000 reads per library). For the female and male parental samples, 1,364,225 and 6,459,242 reads respectively, were mapped and remained after filtering. For the 222 progeny individuals (including the F1) we obtained on average 2,008,826 reads per individual. All 224 individuals (218 F2, the two grandparents and four F1) were genotyped using freebayes version 1.0.0 (Garrison and Marth 2012). As a first filter, sites were kept if bi-allelic, had less than 50% missing data, a quality of more than 2, a minor allele frequency of more than 5%, and a minimal depth of 3. Utilizing a script established to filter freebayes genotype calls based on RAD sequencing (https://github.com/jpuritz/dDocent/blob/master/scripts/dDocent_filters), genotypes were further excluded (thresholds given in brackets) on criteria related to allelic balance at heterozygote sites (< 0.28 allele balance between reads), quality vs. depth (ratio <0.5), strand presentation (overlapping forward and reverse reads), and site depth (one standard deviation from mean and a quality score lower than twice the depth first, followed by an additional maximum mean depth cutoff of 67). Multi-allelic variants and indels were removed, resulting in 7,401 SNPs. Of the 7,401 filtered SNPs 2,052 were alternative homozygous in the grandparents, and were used to build the genetic linkage map.
Linkage map
A linkage map was constructed with JoinMap 4.0 (Van Ooijen 2006) using 212 F2 progeny derived from two F1 families. Out of the 224 genotyped individuals (including the 2 F0 and 4 F1), 2 F1 and 6 F2 were removed due to missing data (>25%). Out of the 2,052 loci homozygous for alternative alleles in the grandparents, we placed 1,597 in the final linkage map. Loci were excluded if positioned identically with another locus. Markers showing segregation distortion (χ2 test, P < 0.001) were excluded for linkage map reconstruction. Linkage groups were identified based on an independent logarithm of odds (LOD) threshold of 12. Unlinked markers were excluded. The strongest cross-link (SCL) in the final map is 5.4. The linkage map was built using the regression mapping algorithm, a recombination frequency smaller than 0.40, and an LOD larger than 3. Up to three rounds of marker positioning were conducted with a jump threshold of 5. A ripple was performed after the addition of each new marker. Map distances were calculated using the Kosambi mapping function. All markers resolved onto 22 linkage groups were matched to positions in the Oreochromis niloticus genome using a chain file (Brawand et al. 2014) with liftover (UCSC Genome Browser LiftOver tool; Hinrichs et al. 2006), which converts genome coordinates between different assemblies. This matching allows examining the synteny between Pundamilia and Oreochromis chromosomes and comparisons with other published studies.
Anchoring of reference scaffolds
In order to reconstruct a chromosomal reference genome for Pundamilia, we used the linkage map to anchor the scaffolds of the Pundamilia genome from Brawand et al. (2014) onto the 22 Pundamilia linkage groups (Pun-LGs) identified during mapping (see paragraph above). We ordered and oriented the scaffolds with ALLMAPS (Tang et al. 2015). Gaps between the scaffolds were then estimated using interpolated recombination rate estimates based on the conversion between map distances (cM) and physical distances (bp) as implemented in the ALLMAPS function “estimategaps” (Tang et al. 2015). We caution, that gap sizes estimated from interpolated recombination rates are associated with high uncertainty. In addition to an improved reference version, resolving linkage groups, we compiled a chain file for converting positions on the original Pundamilia nyererei reference (Brawand et al. 2014) to our new reference (Pundamilia reference version 2.0). Chain files were produced with ALLMAPS and in the opposite direction using chainSwap from kentUtils (https://github.com/ENCODE-DCC/kentUtils). We could then use the chain file to liftover the position of all 7,401 genotyped loci, using Picard liftoverVcf (http://broadinstitute.github.io/picard/index.html). In addition, we generated a new version of the NCBI Pundamilia nyererei RefSeq annotation file with the positions for reference version 2.0 by lifting over the positions from the NCBI PunNye1.0 annotation release 101 (https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Pundamilia_nyererei/101/#BuildInfo) using the UCSC liftOver tool (Hinrichs et al. 2006) and custom-made chain files (see Table 2). By comparing physical (bp) and recombination distances (cM), we estimated recombination rates along the different linkage groups. First, we pruned the linkage map for markers generating negative recombination rates and markers that were less than 20 kb apart. Then we fitted a cubic smoothing spline to the physical (bp) and recombination (cM) distances using the R function “smooth.spline” setting the smoothing parameter (spar) to 0.7 and inferred the recombination positions in cM for the genomic positions as the first derivative of the “predict.smooth.spline” function.
Table 2. List of genomic resources provided with this manuscript and available at the dyrad repository https://doi.org/10.5061/dryad.59q56g6.
| Type | Name |
|---|---|
| Text file giving the position of 1,597 loci on the Pundamilia linkage map and the respective positions on Pundamilia and Oreochromis references | P_cross.MarkerPositions.txt |
| Fasta file of the improved Pundamilia reference genome (v2.0) | P_nyererei_v2.fasta.gz |
| Chain files to convert position between original (v1.0) and new reference (v2.0) | P_nyererei_v1.To.P_nyererei_v2.chain, P_nyererei_v2.To.P_nyererei_v1.chain |
| Annotation file matching reference v2.0 position with the NCBI annotation release 101 | P_nyererei_v2.gff.gz |
| Text file giving the extrapolated recombination rates along Pundamilia reference genome | P_nyererei_v2.RecRates.txt |
QTL mapping of sex
QTL mapping of the sex-determining region was performed with Rqtl (Broman et al. 2003) based on 209 individuals (3 F2 were discarded prior to analysis as they were juveniles) and 1,597 SNP markers. 137 males and 72 females were included. Sex was mapped by standard interval mapping as a binary trait and significance was determined by permutation (n = 1000). Bayesian confidence intervals were estimated as implemented in Rqtl and the highest LOD score was used to calculate the percent variance explained following 1 – 10-2 LOD / n (Broman and Sen 2009). Plotting phenotypic sex against the genotypes for the marker most strongly associated with sex, revealed two individuals labeled as females, but carrying a male genotype. Those individuals were dissected and the post-hoc inspection revealed undeveloped gonads. The same plot also revealed both males (n = 74) and females (n = 32) that were heterozygous at the locus strongly associated with sex. To investigate if sex in those individuals, was explained by another locus, we extracted the genotypes of these individuals, sub-setting the data set and repeated the interval mapping. Further, we made use of 366 markers positioned on the linkage group containing the sex QTL and investigated segregation patterns at those loci in more detail in the larger of our mapping families (n = 122 F2 offspring). Based on the improved, annotated reference (v.2.0) we determined the number of annotated genes in the QTL interval and screened for candidate genes for sex determination.
Data availability
All genomic resources (see Table 2), the genotype (vcf format), and phenotype file are available at the dyrad repository https://doi.org/10.5061/dryad.59q56g6. Raw read sequencing files (fastq files for all 224 individuals) are deposited on short read archive SRA accession SRP136207. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6221921.
Results and Discussion
Linkage map
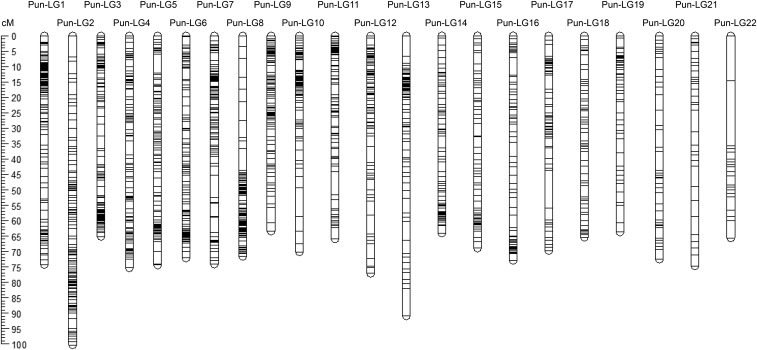
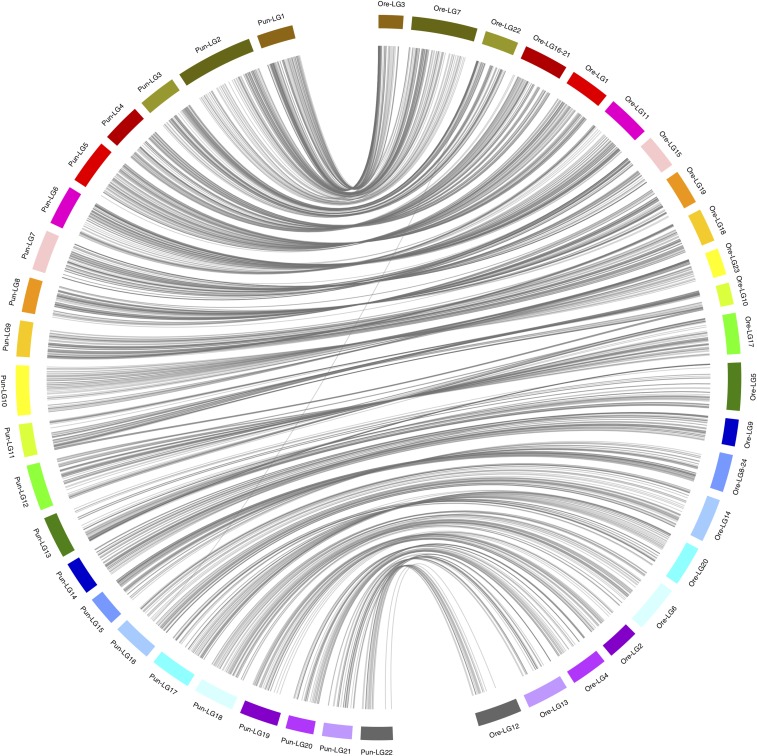
The linkage map comprises 22 linkage groups containing 1,597 markers with an average marker distance of 1.01 cM adding up to a total map length of 1593.72 cM (Figure 1, Table 1). It is slightly longer than other maps published on Lake Victoria cichlids (1130.63 cM in Henning et al. 2014, 1133.2 cM in Kudo et al. 2015 and 1225.68 cM in Henning et al. 2017), but contains more markers with a lower average marker distance (1.30 cM (Henning et al. 2014), 1.09 cM (Henning et al. 2017) and 6.09 cM (Kudo et al. 2015)). The detection of 22 linkage groups is consistent with the expected number of chromosomes in haplo-tilapiine cichlids (Guyon et al. 2012). Out of 1,597 markers used to build the Pundamilia linkage map, 1,182 markers could be positioned onto Oreochromis niloticus linkage groups (Ore-LG). Figure 2 reveals extensive synteny between the chromosomes of these distantly related cichlid species. The linkage map presented here will facilitate comparative genomics and will enable comparisons of previous QTL results with newly established results (for an example see paragraph below on QTL for sex-determination) using Ore-LGs as a reference point.
Figure 1.
Linkage map indicating the positioning of 1,597 markers and Kosambi mapping length (cM) of 22 linkage groups.
Table 1. Summary of length and number of markers for each linkage group. Synteny between this study (Pun-LG) and the Oreochromis niloticus reference (Ore-LG) is indicated.
| Pundamilia LG | Oreochromis LG | length [cM] | # SNPs |
|---|---|---|---|
| Pun-LG1 | Ore-LG3 | 74.182 | 120 |
| Pun-LG2 | Ore-LG7 | 100.271 | 103 |
| Pun-LG3 | Ore-LG22 | 65.086 | 100 |
| Pun-LG4 | Ore-LG16-21 | 75.325 | 94 |
| Pun-LG5 | Ore-LG1 | 74.372 | 90 |
| Pun-LG6 | Ore-LG11 | 72.082 | 90 |
| Pun-LG7 | Ore-LG15 | 74.093 | 88 |
| Pun-LG8 | Ore-LG19 | 71.579 | 88 |
| Pun-LG9 | Ore-LG18 | 63.352 | 82 |
| Pun-LG10 | Ore-LG23 | 70.089 | 77 |
| Pun-LG11 | Ore-LG10 | 65.94 | 76 |
| Pun-LG12 | Ore-LG17 | 77.124 | 75 |
| Pun-LG13 | Ore-LG5 | 90.878 | 74 |
| Pun-LG14 | Ore-LG9 | 63.956 | 71 |
| Pun-LG15 | Ore-LG8-24 | 70.914 | 61 |
| Pun-LG16 | Ore-LG14 | 72.914 | 60 |
| Pun-LG17 | Ore-LG20 | 69.652 | 58 |
| Pun-LG18 | Ore-LG6 | 65.41 | 54 |
| Pun-LG19 | Ore-LG2 | 63.732 | 44 |
| Pun-LG20 | Ore-LG4 | 72.481 | 39 |
| Pun-LG21 | Ore-LG13 | 74.665 | 33 |
| Pun-LG22 | Ore-LG12 | 65.627 | 20 |
| 1593.724 | 1597 |
Figure 2.
Synteny plot showing the correspondence of Pundamilia linkage groups (Pun-LG) with Oreochromis niloticus linkage groups (Ore-LG). Lines indicate markers used in linkage map construction, which could be positioned in the Pundamilia reference (v2.0) and lifted over to the Oreochromis reference.
Improvement of the genomic resources for Lake Victoria cichlids (Pundamilia)
The Pundamilia linkage map provides a new chromosome framework for whole genome sequence assembly and map integration with more anchoring points then previous published maps. The anchored genome encompasses 78.7% of the total bases (653,642,680 bp) of the original P. nyererei reference genome based on 383 anchored scaffolds, of which 233 are now oriented. This is a slightly higher fraction than in the Lake Malawi cichlid Metriaclima zebra, where 564,259,264 bp (66.5%) of the genome sequence could be anchored to linkage groups (O’Quin et al. 2013). The mean marker density is 2.4 per megabase (Mb). The 6,853 remaining scaffolds could not be anchored due to lack of informative markers. This improved resolution of the new reference assembly (v2.0) will greatly facilitate genome scan approaches in Lake Victoria cichlids. Such approaches rely on the information from neighboring genomic positions to identify signatures of selection due to genetic hitchhiking. Any approaches evaluating or making use of linkage information, like linkage disequilibrium (LD) based genome scans, association studies or evaluations of the genomic landscape of divergence will now become more powerful. Together with the improved reference, we provide chain files to liftover positions from the previous version (v1.0) to the new chromosome level resolved reference version (v2.0). We further provide a matching annotation file based on the NCBI annotation (see Table 2 for a complete list of all genomic resources). Finally, we estimated recombination rates and show that those are highly variable across the genome ranging from 0 to 9.4 cM/Mb (Table 2), with a mean recombination rate of 2.3 cM/Mb. Knowledge of fine-scale patterns of recombination rate variation (see Figure 4C) will be useful for future studies of adaption and speciation (Stapley et al. 2017) in the exceptional species radiation of Lake Victoria cichlids (Meier et al. 2018).
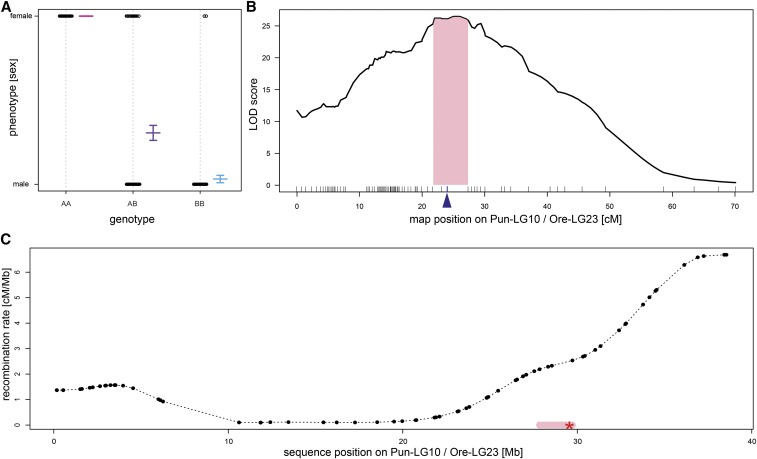
Figure 4.
A) Phenotypic effect of genotypes at the locus most strongly associated with sex. The plot identifies two females as likely phenotyping errors and 106 individuals heterozygote at that locus. B) Plot of LOD scores indicating the region of strongest association with sex on Pun-LG10 (Ore-LG23). The 95% Bayesian confidence interval is highlighted in light pink. Marker loci are indicated along the x-axis. The locus shown in panel A is indicated by a blue arrow. C) Variation in recombination rates (sex-averaged) along Pun-LG10 (Ore-LG23). The Bayesian confidence interval (pink highlight) is situated next to a region of low recombination. The red star indicates the position of amh (candidate gene for sex determination).
Characterization of sex-determination in Pundamilia
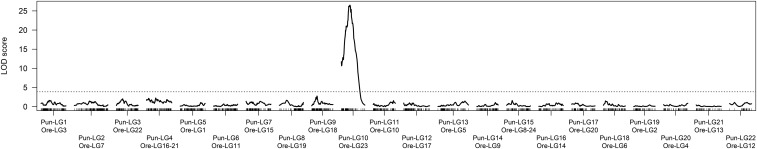
Our knowledge of sex determination in Pundamilia, a prime model system of sympatric speciation in Lake Victoria, had been limited. Here, we mapped sex to Pun-LG10, which is homologous with Ore-LG23 (Figure 3; P << 0.001, LOD = 26.5). We did not find any further associations on any of the other LGs. Ore-LG23 has been previously identified as one potential XY sex-determining LG in Oreochromis (Cnaani et al. 2008; Palaiokostas et al. 2013) and in four cichlid tribes from Lake Tanganyika overexpression of male specific genes accumulates on Ore-LG23 (Böhne et al. 2014). Early work on sex determination in Lake Victoria cichlids had suggested polymorphisms in several unlinked genomic regions to be associated with sex, and invoked a major effect locus and some modifiers (Seehausen et al. 1999). Recent QTL mapping identified genomic regions involved in sex determination in Lake Victoria cichlids on Ore-LG5 and Ore-LG2 (Kudo et al. 2015) or on derived, female specific B chromosomes (Yoshida et al. 2011). Ore-LG5 was repeatedly found to be involved in sex-determination (both ZW or XY) in other cichlids, e.g., in the riverine haplochromine cichlids Astatotilapia burtoni and Astatotilapia calliptera from Lakes Tanganyika and Malawi and associated rivers (Roberts et al. 2016; Böhne et al. 2016; Peterson et al. 2017), in Cyprichromis leptosoma from Lake Tanganyika (Gammerdinger et al. 2018) and in Labeotropheus trewavasae and across some Metriaclima species from Lake Malawi (Ser et al. 2010; Parnell and Streelman 2013). We find no indications of sex determining factors on Ore-LG5 in Pundamilia.
Figure 3.
QTL mapping of sex. LOD scores across the 22 linkage groups are shown. Genome-wide significance levels are indicated by horizontal lines (alpha = 0.05 dotted line). Marker loci are indicated along the x-axis.
The mapping interval (Bayesian confidence interval of 5.7 cM, 21.7 to 27.4 cM; Figure 4B) in total covers four markers and spans a region of ∼1.9 Mb (Figure 4C). The marker showing the strongest association with sex in our study (Figure 4A) explains 44% of the phenotypic variance in sex. We have misidentified two likely sub-adults (gonads appear not to be develeoped on post-hoc inspection. However, sex is not entirely explained by this marker as we also identified 106 individuals, both males and females, which are heterozygous at this position. Repeating the mapping procedure for those heterozygous individuals again identified a region on Pun-LG10 (Ore-LG23) as weakly associated with sex (P = 0.177, LOD = 3.33, position right to previous interval at 28.8 cM). This suggests that none of the markers used to build the linkage map are determining sex directly, but that the causal locus can be found close by and indicates that there are no further major genetic determiners of sex segregating in this cross. Investigating the segregation patterns in the larger of the F2 mapping-families (n = 122) more in detail revealed that the loci selected to build the map (reciprocally homozygous in F0 female (AA) and male (BB) and heterozygous in both F1 (AB)) segregate as expected in a 50:50 ratio of AA:AB in F2 females and AB:BB in F2 males (Figure 5A). However, evaluating segregation patterns of the additional markers genotyped but not used for the construction of the linkage map, indicate that the sex determination system on Pun-LG10 is male heterogametic (XY, Figure 5B). We identified 57 loci between 0 and 35 Mb that were homozygous in the F0 and F1 females and heterozygous in the F0 and F1 males; these markers are similarly homozygous in all F2 females and heterozygous in all F2 males, consistent with females being XX and males being XY (Figure 5B, the plot also shows 13 loci > 35 Mb). Additional evidence comes from markers heterozygous in the F0 female (AB) and homozygous in the F0 male (BB), for which we find all 35 loci for positions < 33 Mb heterozygous (AB) for both, the male and the female F1 individual of the mapping family. The heterozygous loci in both, male and female, F1 are a segregation pattern only consistent with male heterogametic (XY) segregation. If females would be heterogametic (ZW) those loci would need to be homozygous (BB) in one of the F1 sexes and not heterozygous (AB) in both as observed in the first 33 Mb of Pun-LG10 including the mapping interval of our sex QTL. Sex-averaged recombination rates around the QTL are similar to genome wide average rates. However, recombination rates to one side of the QTL are low and even close to zero within 20 Mb proximity to the mapping interval (Figure 4C). Such a pattern, potentially due to suppressed recombination in the heterogametic sex (males), might indicate initial steps toward the evolution of a heteromorphic (degenerated) sex (Y) chromosome (Charlesworth 1991).
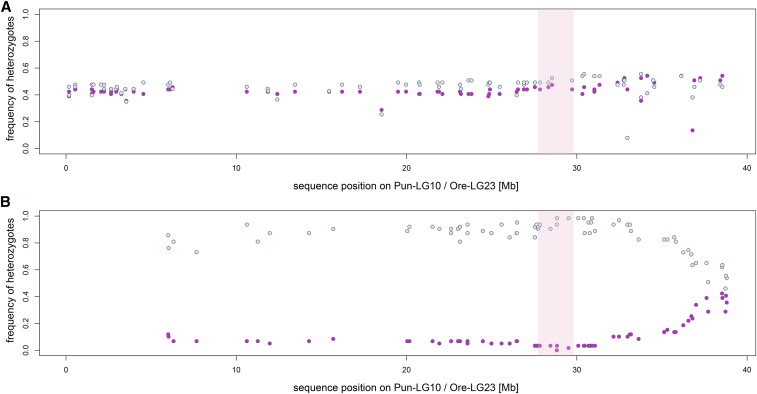
Figure 5.
Frequency of heterozygote individuals (n = 122), separated by sex (63 males: light blue, 59 females: pink) for markers selected by their segregation pattern in the larger mapping family and by their position on Pun-LG10 (Ore-LG23). A) 78 markers, selected as reciprocally homozygous (AA/BB) in the F0 and heterozygote in both, the male and the female F1 (AB/AB), segregate as expected in a 50:50 ratio of AA:AB in F2 females and AB:BB in F2 males, resulting in frequency of heterozygous F2 individuals around 0.5 for both sexes. B) 70 markers, selected as homozygote in the F0 and F1 females and heterozygote in the F0 and F1 males, segregate similarly in the F2; i.e., the frequency of heterozygous individuals is approaching 0 in females and 1 in males for positions < 35 Mb. The QTL region (Bayesian confidence interval) for sex determination in Pundamilia is located between 27.8 and 29.7 Mb (shaded region).
Within our mapping interval of ∼1.9 Mb, 65 genes, based on the NCBI annotation for the new Pundamilia reference assembly, can be found (Table S1). Among them is the anti-müllerian hormone (amh), a master gene for sex determination in other fish. Amh is part of the transforming growth factor beta pathway, responsible for the regression of Müllerian ducts in tetrapods (Josso et al. 2001). Even though teleost fish do not have Müllerian ducts, the amh pathway has a prominent role in sex determination for several distantly related fish species. In the Japanese pufferfish (Takifugu rubripes), a mutation in the receptor of the amh (amhrII) determines sex (Kamiya et al. 2012). The amhy (Y chromosome-specific anti-müllerian hormone) gene has been inserted upstream of amh in the cascade of male development in the neotropical silverside Odonthestes hatcheri (Hattori et al. 2012). Similarly, in Oreochromis niloticus, a Y-linked duplicate of amh acts as a major sex determination locus (Eshel et al. 2012; Li et al. 2015). In Oryzias luzonensis, a mutation of an amh related ligand gsdfy is responsible for sex determination (Myosho et al. 2012). The same ligand is suggested to be involved in sex determination in the haplochromine cichlid Astatotilapia calliptera (Peterson et al. 2017). Beside the two master sex determination genes in Oreochromis niloticus on LG23 (amh) and in Astatotilapia calliptera on LG7 (gsdf) (Peterson et al. 2017), no other candidate for sex determination have been shown to be directly involved in sex determination in any other cichlid species (Heule et al. 2014b, Böhne et al. 2016, Gammerdinger et al. 2018, but see Böhne et al. 2014). They might act as so-called “newcomers” (Herpin and Schartl 2015). Our results indicate that in the Lake Victoria cichlid Pundamilia Pun-LG10 (Ore-LG 23) acts as an (evolving) sex chromosome, even though it might not be the only region controlling sex in Pundamilia. The anti-müllerian hormone amh (or a derived copy) appears to be a strong candidate influencing sexual development in Pundamilia, but further work is warranted to characterize the genomic candidate region and the impact of this candidate gene on sex determination.
A recent meta-analysis showed that transitions between sex determination systems are frequent across fish species, including transitions to and between heteromorphic sex chromosomes (Pennell et al. 2018). In cichlids a high turnover of sex determination systems was described in Lake Malawi (Ser et al. 2010), Lake Tanganyika (Böhne et al. 2014; Gammerdinger et al. 2018), and in oreochromine cichlids (Cnaani et al. 2008). Neither amh, nor Pun-LG10 or a homologous region was invoked in sex determination in other Lake Victoria cichlids that have previously been used for mapping sex (Kudo et al. 2015; Yoshida et al. 2011). This circumstance implies that multiple sex determining systems segregate among Lake Victoria cichlids as well. This is consistent with early work on sex determination in this group (Seehausen et al. 1999). Given the extreme youth of the Lake Victoria species radiation (∼15,000 years; Seehausen 2006), this may be surprising at first. Recent work, however, has shown that much of the genetic variation in the radiation is much older than the species radiation and originated from a hybridization event between two anciently divergent cichlid lineages from which all 500+ species of the radiation evolved (Meier et al. 2017a). It is tempting to speculate that the variation in sex determination systems between and within species of this radiation traces its roots to these ancient lineages too, something that should be tested in the future.
Acknowledgments
We thank Katie Peichel for her critical input during the analysis of the data and manuscript preparation. We thank Andreas Taverna, Alan Smith, and Ola Svensson for fish maintenance and breeding. We thank David Marques for lifting over the annotation file. We thank the team from the Genetic Diversity Center (GDC) at ETH Zürich for bioinformatics support. Genomic analyses were performed using the computing infrastructure of the GDC and the Euler computer cluster at ETH Zurich. JS was supported by the German Research Foundation grant SCHW-1690/1-1 and Leibniz association grant SAW-2017-ZFMK-4. This work was supported by the Swiss Science Foundation grant SNSF 31003A_163338 awarded to OS.
Author’s contributions
PGDF, JS, MPH, and JIM performed the experiment and the analysis. OS conceived the original idea, made the F1 cross and supervised the project. PGDF and JS took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.6221921.
Communicating editor: R. Doerge
Literature Cited
- Baird N. A., Etter P. D., Atwood T. S., Currey M. C., Shiver A. L., et al. , 2008. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3: e3376 10.1371/journal.pone.0003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhne A., Sengstag T., Salzburger W., 2014. Comparative transcriptomics in East African cichlids reveals sex-and species-specific expression and new candidates for sex differentiation in fishes. Genome Biol. Evol. 6: 2567–2585. 10.1093/gbe/evu200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhne A., Wilson C. A., Postlethwait J. H., Salzburger W., 2016. Variations on a theme: Genomics of sex determination in the cichlid fish Astatotilapia burtoni. BMC Genomics 17: 883 10.1186/s12864-016-3178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand D., Wagner C. E., Li Y. I., Malinsky M., Keller I., et al. , 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature 513: 375–381. 10.1038/nature13726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinf. 19: 889–890. 10.1093/bioinformatics/btg112 [DOI] [PubMed] [Google Scholar]
- Broman K. W., Sen S., 2009. A Guide to QTL Mapping with R/qtl, Vol. 46 Springer, New York: 10.1007/978-0-387-92125-9 [DOI] [Google Scholar]
- Catchen J., Hohenlohe P. A., Bassham S., Amores A., Cresko W. A., 2013. Stacks: an analysis tool set for population genomics. Mol. Ecol. 22: 3124–3140. 10.1111/mec.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., 1991. The evolution of sex chromosomes. Science 251: 1030–1033. 10.1126/science.1998119 [DOI] [PubMed] [Google Scholar]
- Cnaani A., Lee B.-Y., Zilberman N., Ozouf-Coastaz C., Hulata G., et al. , 2008. Genetics of sex determination in tilapiine species. Sex Dev. 2: 43–54. 10.1159/000117718 [DOI] [PubMed] [Google Scholar]
- Eshel O., Shirak A., Weller J. I., Hulata G., Ron M., 2012. Linkage and physical mapping of sex region on LG23 of Nile Tilapia (Oreochromis niloticus). G3 (Bethesda) 2: 35–42. 10.1534/g3.111.001545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel O., Shirak A., Dor L., Band M., Zak T., et al. , 2014. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus). BMC Genomics 15: 774 10.1186/1471-2164-15-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L., 2015. Using linkage maps to correct and scaffold de novo genome assemblies: methods, challenges, and computational tools. Front. Genet. 6: 220 10.3389/fgene.2015.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammerdinger W. J., Conte M. A., Sandkam B. A., Ziegelbecker A., Koblmüller S., et al. , 2018. Novel sex chromosomes in three cichlid fishes from Lake Tanganyika. J. Hered. 1: 12. [DOI] [PubMed] [Google Scholar]
- Garrison, E., and G. Marth, 2012 Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207.3907 [q-bio.GN]
- Greenwood P. H., 1974. Cichlid fishes of Lake Victoria, East Africa: the biology and evolution of a species flock. Bull. Brit. Mus. Zool. (Suppl. 6): 1–134. [Google Scholar]
- Guyon R., Rakotomanga M., Azzouzi N., Coutanceau J. P., Bonillo C., et al. , 2012. A high-resolution map of the Nile tilapia genome: a resource for studying cichlids and other percomorphs. BMC Genomics 13: 222 10.1186/1471-2164-13-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R. S., Murai Y., Oura M., Masuda S., Majhi S. K., et al. , 2012. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 109: 2955–2959. 10.1073/pnas.1018392109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning F., Lee H. J., Franchini P., Meyer A., 2014. Genetic mapping of horizontal stripes in Lake Victoria cichlid fishes: benefits and pitfalls of using RAD markers for dense linkage mapping. Mol. Ecol. 23: 5224–5240. 10.1111/mec.12860 [DOI] [PubMed] [Google Scholar]
- Henning F., Machado‐Schiaffino G., Baumgarten L., Meyer A., 2017. Genetic dissection of adaptive form and function in rapidly speciating cichlid fishes. Evolution 71: 1297–1312. 10.1111/evo.13206 [DOI] [PubMed] [Google Scholar]
- Herpin A., Schartl M., 2015. Plasticity of gene‐regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 16: 1260–1274. 10.15252/embr.201540667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heule C., Salzburger W., Böhne A., 2014a Genetics of sexual development – an evolutionary playground for fish. Genetics 196: 579–591. 10.1534/genetics.114.161158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heule C., Göppert C., Salzburger W., Böhne A., 2014b Genetics and timing of sex determination in the East African cichlid fish Astatotilapia burtoni. BMC Genet. 15: 140 10.1186/s12863-014-0140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs A. S., Karolchik D., Baertsch R., Barber G. P., Bejerano G., et al. , 2006. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 1: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg S., 1978. A field and laboratory study of the behaviour and ecology of Pseudotropheus zebra (Boulenger), an endemic cichlid of Lake Malawi (Pisces; Cichlidae). J. Zoological Syst. Evol. Res. 16: 171–187. 10.1111/j.1439-0469.1978.tb00929.x [DOI] [Google Scholar]
- Josso N., di Clemente N., Gouédard L., 2001. Anti-Müllerian hormone and its receptors. Mol. Cell. Endocrinol. 179: 25–32. 10.1016/S0303-7207(01)00467-1 [DOI] [PubMed] [Google Scholar]
- Kamiya T., Kai W., Tasumi S., Oka A., Matsunaga T., et al. , 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8: e1002798 10.1371/journal.pgen.1002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller I., Wagner C. E., Greuter L., Mwaiko S., Selz O. M., et al. , 2013. Population genomic signatures of divergent adaptation, gene flow and hybrid speciation in the rapid radiation of Lake Victoria cichlid fishes. Mol. Ecol. 22: 2848–2863. 10.1111/mec.12083 [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Hamaguchi S., 2013. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 242: 339–353. 10.1002/dvdy.23927 [DOI] [PubMed] [Google Scholar]
- Kocher T. D., 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5: 288–298. 10.1038/nrg1316 [DOI] [PubMed] [Google Scholar]
- Kudo Y., Nikaido M., Kondo A., Suzuki H., Yoshida K., et al. , 2015. A microsatellite-based genetic linkage map and putative sex-determining genomic regions in Lake Victoria cichlids. Gene 560: 156–164. 10.1016/j.gene.2015.01.057 [DOI] [PubMed] [Google Scholar]
- Lande R., Seehausen O., Van Alphen J. J., 2001. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica 112–113: 435–443. 10.1023/A:1013379521338 [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S., 2012. Fast gapped-read alignment with Bowtie 2. Nature Met. 9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Sun Y., Zhao J., Shi H., Zeng S., et al. , 2015. A tandem duplicate of Anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 11: e1005678 10.1371/journal.pgen.1005678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan M. E., Seehausen O., 2011. Ecology, sexual selection and speciation. Ecol. Lett. 14: 591–602. 10.1111/j.1461-0248.2011.01606.x [DOI] [PubMed] [Google Scholar]
- Marques D. A., Lucek K., Meier J. I., Mwaiko S., Wagner C. E., et al. , 2016. Genomics of rapid incipient speciation in sympatric threespine stickleback. PLoS Genet. 12: e1005887 10.1371/journal.pgen.1005887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., et al. , 2010. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. I., Marques D. A., Mwaiko S., Wagner C. E., Excoffier L., et al. , 2017a Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8: 14363 10.1038/ncomms14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. I., Sousa V. C., Marques D. A., Selz O. M., Wagner C. E., et al. , 2017b Demographic modelling with whole‐genome data reveals parallel origin of similar Pundamilia cichlid species after hybridization. Mol. Ecol. 26: 123–141. 10.1111/mec.13838 [DOI] [PubMed] [Google Scholar]
- Meier J. I., Marques D. A., Wagner C. E., Excoffier L., Seehausen O., 2018. Genomics of parallel ecological speciation in Lake Victoria cichlids. Mol. Biol. Evol. 10.1093/molbev/msy051 [DOI] [PubMed] [Google Scholar]
- Myosho T., Otake H., Masuyama H., Matsuda M., Kuroki Y., et al. , 2012. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191: 163–170. 10.1534/genetics.111.137497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl S., Tichy H., Mayer W. E., Takezaki N., Takahata N., et al. , 2000. The origin and age of haplochromine fishes in Lake Victoria, East Africa. Proc. Biol. Sci. 267: 1049–1061. 10.1098/rspb.2000.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Quin C., Drilea A. C., Conte M. A., Kocher T. D., 2013. Mapping of pigmentation QTL on an anchored genome assembly of the cichlid fish, Metriaclima zebra. BMC Genomics 14: 287 10.1186/1471-2164-14-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaiokostas C., Bekaert M., Khan M. G., Taggart J. B., Gharbi K., et al. , 2013. Mapping and validation of the major sex-determining region in Nile tilapia (Oreochromis niloticus L.) using RAD sequencing. PLoS One 8: e68389 (erratum: PLoS One 8) 10.1371/journal.pone.0068389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell N. F., Streelman J. T., 2013. Genetic interactions controlling sex and color establish the potential for sexual conflict in Lake Malawi cichlid fishes. Heredity 110: 239–246. 10.1038/hdy.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell M. W., Mank J. E., Peichel C. L., 2018. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. (in press) 10.1111/mec.14540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. N., Cline M. E., Moore E. C., Roberts N. B., Roberts R. B., 2017. Genetic sex determination in Astatotilapia calliptera, a prototype species for the Lake Malawi cichlid radiation. Sci. Nat. 104: 41 10.1007/s00114-017-1462-8 [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Ser J. R., Kocher T. D., 2009. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science 326: 998–1001. 10.1126/science.1174705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. B., Juntti S. A., Coyle K. P., Dumont B. L., Stanley M. K., et al. , 2016. Polygenic sex determination in the cichlid fish Astatotilapia burtoni. BMC Genomics 17: 835 10.1186/s12864-016-3177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W., 2001. Molecular Cloning: A Laboratory Manual, Ed. 3rd Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sanetra M., Henning F., Fukamachi S., Meyer A., 2009. A microsatellite-based genetic linkage map of the cichlid fish, Astatotilapia burtoni (Teleostei): a comparison of genomic architectures among rapidly speciating cichlids. Genetics 182: 387–397. 10.1534/genetics.108.089367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O., 1996. Lake Victoria Rock Cichlids: Taxonomy, Ecology and Distribution. Verduyn Cichlids, Zevenhuizen, Zuidplas, The Netherlands. [Google Scholar]
- Seehausen O., Van Alphen J. J., Witte F., 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277: 1808–1811. 10.1126/science.277.5333.1808 [DOI] [Google Scholar]
- Seehausen O., van Alphen J. J., 1999. Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity in Lake Victoria? Ecol. Lett. 2: 262–271. 10.1046/j.1461-0248.1999.00082.x [DOI] [Google Scholar]
- Seehausen O., van Alphen J. J. M., Lande R., 1999. Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol. Lett. 2: 367–378. 10.1046/j.1461-0248.1999.00098.x [DOI] [Google Scholar]
- Seehausen O., 2000. Explosive speciation rates and unusual species richness in haplochromine cichlid fishes: effects of sexual selection. Adv. Ecol. Res 31: 237–274. 10.1016/S0065-2504(00)31015-7 [DOI] [Google Scholar]
- Seehausen O., Koetsier E., Schneider M. V., Chapman L. J., Chapman C. A., et al. , 2003. Nuclear markers reveal unexpected genetic variation and a Congolese-Nilotic origin of the Lake Victoria cichlid species flock. Proc. Biol. Sci. 270: 129–137. 10.1098/rspb.2002.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O., 2006. African cichlid fish: a model system in adaptive radiation research. Proc. Biol. Sci. 273: 1987–1998. 10.1098/rspb.2006.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O., Terai Y., Magalhaes I. S., Carleton K. L., Mrosso H. D., et al. , 2008. Speciation through sensory drive in cichlid fish. Nature 455: 620–626. 10.1038/nature07285 [DOI] [PubMed] [Google Scholar]
- Seehausen O., 2015. Process and pattern in cichlid radiations–inferences for understanding unusually high rates of evolutionary diversification. New Phytol. 207: 304–312. 10.1111/nph.13450 [DOI] [PubMed] [Google Scholar]
- Selz O. M., Pierotti M. E., Maan M. E., Schmid C., Seehausen O., 2014a Female preference for male color is necessary and sufficient for assortative mating in two cichlid sister species. Behav. Ecol. 25: 612–626. 10.1093/beheco/aru024 [DOI] [Google Scholar]
- Selz O. M., Thommen R., Maan M. E., Seehausen O., 2014b Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish. J. Evol. Biol. 27: 275–289. 10.1111/jeb.12287 [DOI] [PubMed] [Google Scholar]
- Ser J. R., Roberts R. B., Kocher T. D., 2010. Multiple interacting loci control sex determination in lake Malawi cichlid fish. Evol. 64: 486–501. 10.1111/j.1558-5646.2009.00871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley J., Feulner P. G., Johnston S. E., Santure A. W., Smadja C. M., 2017. Variation in recombination frequency and distribution across eukaryotes: patterns and processes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372: 20160455 (corrigenda: Philos. Trans. R. Soc. Lond. B Biol. Sci. 373: 20170360) 10.1098/rstb.2016.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streelman J. T., Albertson R. C., Kocher T. D., 2003. Genome mapping of the orange blotch colour pattern in cichlid fishes. Mol. Ecol. 12: 2465–2471. 10.1046/j.1365-294X.2003.01920.x [DOI] [PubMed] [Google Scholar]
- Tang H., Zhang X., Miao C., Zhang J., Ming R., et al. , 2015. ALLMAPS: robust scaffold ordering based on multiple maps. Genome Biol. 16: 3 10.1186/s13059-014-0573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen J. W., 2006, p. 33 in JoinMap 4. Software for the calculation of genetic linkage maps in experimental populations, Kyazma BV, Wageningen, Netherlands. [Google Scholar]
- Verheyen E., Salzburger W., Snoeks J., Meyer A., 2003. Origin of the superflock of cichlid fishes from Lake Victoria, East Africa. Science 300: 325–329. 10.1126/science.1080699 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Terai Y., Mizoiri S., Aibara M., Nishihara H., et al. , 2011. B chromosomes have a functional effect on female sex determination in Lake Victoria cichlid fishes. PLoS Genet. 7: e1002203 10.1371/journal.pgen.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All genomic resources (see Table 2), the genotype (vcf format), and phenotype file are available at the dyrad repository https://doi.org/10.5061/dryad.59q56g6. Raw read sequencing files (fastq files for all 224 individuals) are deposited on short read archive SRA accession SRP136207. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6221921.