Abstract
N-glycanase 1 (NGLY1) Deficiency is a rare monogenic multi-system disorder first described in 2014. NGLY1 is evolutionarily conserved in model organisms. Here we conducted a natural history study and chemical-modifier screen on the Drosophila melanogaster NGLY1 homolog, Pngl. We generated a new fly model of NGLY1 Deficiency, engineered with a nonsense mutation in Pngl at codon 420 that results in a truncation of the C-terminal carbohydrate-binding PAW domain. Homozygous mutant animals exhibit global development delay, pupal lethality and small body size as adults. We developed a 96-well-plate, image-based, quantitative assay of Drosophila larval size for use in a screen of the 2,650-member Microsource Spectrum compound library of FDA approved drugs, bioactive tool compounds, and natural products. We found that the cholesterol-derived ecdysteroid molting hormone 20-hydroxyecdysone (20E) partially rescued the global developmental delay in mutant homozygotes. Targeted expression of a human NGLY1 transgene to tissues involved in ecdysteroidogenesis, e.g., prothoracic gland, also partially rescues global developmental delay in mutant homozygotes. Finally, the proteasome inhibitor bortezomib is a potent enhancer of global developmental delay in our fly model, evidence of a defective proteasome “bounce-back” response that is also observed in nematode and cellular models of NGLY1 Deficiency. Together, these results demonstrate the therapeutic relevance of a new fly model of NGLY1 Deficiency for drug discovery and gene modifier screens.
Keywords: N-glycanse 1, NGLY1, Pngl, Drosophila, disease model
Recessive loss-of-function mutations in the evolutionarily conserved gene NGLY1 result in an ultra-rare genetic disease called NGLY1 Deficiency, which is characterized by global developmental delay, seizures, small head and extremities, chronic constipation, lack of tears, and floppy body (Enns et al. 2014). NGLY1, short for N-glycanase 1, encodes a deglycosylating enzyme that hydrolyzes N-linked glycans from asparagine residues of glycoproteins, liberating oligosaccharides for degradation and recycling (Suzuki et al. 2016). A comprehensive clinical snapshot by National Institutes of Health (NIH) established potential measurable clinical endpoints and a baseline of disease progression in a cohort of 12 patients (Lam et al. 2017).
The pathophysiology of NGLY1 Deficiency has not yet been fully resolved. Before 2014, little was known about NGLY1 function in animal development. Its elucidation is the focus of numerous research groups employing a diversity of disease models, including model organisms and human cells. Two models of the pathogenesis of NGLY1 Deficiency have been proposed.
The first model is rooted in biochemistry and defects in cellular glycoprotein homeostasis (Huang et al. 2015). NGLY1 is an essential component of the conserved protein quality control system known as endoplasmic-reticulum-associated degradation (ERAD), bridging p97/VCP-mediated retrotranslocation of proteins from the ER to the cytoplasm for bulk deglycosylation and subsequent degradation by the ubiquitin-proteasome system (UPS) (Suzuki 2015). The absence of cytoplasmic N-glycanase activity has been proposed to result in inappropriate cleavage of N-glycans from glycoproteins by the downstream cytoplasmic enzyme endo-β-N-acetylglucosaminidase (ENGase), whose normal substrate is soluble oligosaccharide liberated by NGLY1. Such glycoproteins misprocessed by ENGase would retain a single GlcNAc residue that may destabilize proteins and promote their aggregation. Suzuki and colleagues observed evidence of N-GlcNAc misprocessing and accumulation in vitro in NGLY1−/− mouse embryonic fibroblasts (Huang et al. 2015; Fujihira et al. 2017). Based on the collective work of Suzuki and colleagues, inhibition of ENGase has been proposed as a therapeutic thesis for NGLY1 Deficiency (Bi et al. 2017; Fujihira et al. 2017). Indeed, a NGLY1−/− ENGase−/− double mutant mouse is viable while a NGLY1−/− single mutant displays varying degrees of lethality depending on genetic background (Fujihira et al. 2017). However, a NGLY1−/− ENGase−/− double mutant mouse is not healthy. Additional pathogenic mechanisms are required to explain NGLY1 Deficiency.
The second model of pathogenesis is rooted in genetics and defects in the deglycosylation of specific glycoprotein clients, including but not limited to the master regulator of the conserved 26S proteasome “bounce-back” response, NFE2L1 (the transcription factor nuclear factor erythroid 2 like 1 also known as Nrf1) (Radhakrishnan et al. 2010). NRF1 belongs to the ancient basic leucine zipper family of transcription factors that regulate many developmental and stress response pathways in animals (Kim et al. 2016). The fly NRF1 homolog, cap-n-collar (cnc) increases the expression of proteasome subunit genes, as well as oxidative and redox stress response pathways (Grimberg et al. 2011). In an unbiased screen for genetic modifiers of the proteasome bounce-back response in nematodes, Lehrbach and Ruvkun discovered that the nematode homolog of NGLY1, PNG-1, deglycosylates the ER-membrane bound isoform of the nematode homolog of NRF1, SKN-1A. They further demonstrated that deglycosylation of SKN-1A by PNG-1 is required for SKN-1A translocation to the nucleus, and transcriptional activity (Lehrbach and Ruvkun 2016).
In a complementary study, Bertozzi and colleagues revealed that NGLY1 activity is required for NRF1 signaling in mouse embryonic fibroblasts mice in the same manner that PNG-1 activity is required for SKN-1A function in nematodes (Tomlin et al. 2017). In fact, they showed that chemical inhibition of NGLY1 function potentiates cytotoxicity caused by proteasome inhibition in human cancer cell lines (Tomlin et al. 2017), which mirrors the observation in nematodes that png-1 loss-of-function mutants are extremely hypersensitive to proteasome inhibition by bortezomib (Lehrbach and Ruvkun 2016). Jafar-Nejad and colleagues showed that the fly NGLY1/Pngl is required during embryonic and larval development in Drosophila for post-translational processing of Dpp, the fly homolog of the conserved bone morphogen protein (BMP) family (Galeone et al. 2017), opening up the possibility that NGLY1 is required for the function of multiple glycoprotein clients.
Here we carried out phenotyping, high-throughput assay development and a chemical-modifier screen on a new fly model of NGLY1 Deficiency, herein referred to as PnglPL. Of ∼2,650 bioactive compounds, the ecdysteroid insect molting hormone 20-hydroxyecdysone (20E) partially suppressed global developmental delay in mutant homozygotes. Expression of a human NGLY1 transgene in the prothoracic gland (PG) and sites of ecdysteroidogenesis partially rescued global developmental delay in mutant homozygotes. These data indicate that defects in ecdysone-producing tissues contribute to the global developmental delay of the PnglPL flies. PnglPL flies are also hypersensitive to the proteasome inhibitor bortezomib and the organic solvent dimethyl sulfoxide (DMSO). Together, these observations combined with other results in the literature (Owings et al. 2018) can be accommodated by a model wherein NGLY1/pngl is required for NRF1/cnc function in the Drosophila neuroendocrine axis.
Methods
Pngl allele generation
A cassette containing a stop codon and mw+ was cloned into a modified version of pUC57. Homology arms to the ngly1 locus were cloned upstream and downstream of the cassette. The guide RNA (GCTGAGGAATAACTTTCGAT CGG) was cloned into pCDF3 (Port et al. 2014). pCDF3 and pUC57 with Pngl homology arms, stop codon, and mw+ were injected into vas-Cas9 (Bloomington stock #51323). Two independent mw+ F1 strains were established and balanced stocks were created. Sequencing confirmed the integration of the stop codon and mw+ (Figure 1A) immediately 3′ to bp 1906699 (Release 5.57) in chromosome 2R (NT_033778.3).
Figure 1.
Genotyping and phenotyping PnglPL allele. A) We used CRISPR to create an allele of Pngl with a stop codon and white + transgene inserted upstream of the PAW domain. Sequencing confirmed the integration of the stop codon and mw+ immediately 3′ to bp 1906699 (Release 5.57) in chromosome 2R (NT_033778.3). B) The fraction of PnglPL/+ heterozygote, PnglPL/PL homozygote, or PnglPL/PL;Actin > NGLY1 flies that eclose over time normalized to the total surviving flies for each genotype. Flies were grown on standard untreated media. The ratio of flies emerging in this experiment for PnglPL/PL, PnglPL/PL ; Act > hNGLY1, and PnglPL/CyO is 15:40:137 individuals. Ubiquitous expression of a human NGLY1 transgene rescued the 2-day development delay to eclosion observed in PnglPL homozygotes. C) PnglPL homozygous adult flies are smaller than homozygous siblings expressing the human NGLY1 transgene. D) PnglPL homozygotes are 25% smaller than their heterozygous siblings (P < 0.01; Student’s t-test).
Rate of eclosion and rescue by human ngly1 transgene studies
w, PnglPL/CyO; actin-switch-GAL4 males were crossed to PnglPL/CyO,Act-GFP; UAS-human-NGLY1 virgin females. The phenotypes of eclosing flies were recorded on days 9-14 after parents were mated. We found that the actin-switch-gal4 transgenic strain expressed GAL4, despite the absence of RU486.
Fly strains
Actin5C-switch-GAL4 (stock #9431) was obtained from the Bloomington stock center. Pngl excision alleles and UAS-human-NGLY1 were obtained from Dr. Hamed Jafar-Nejad. 2_286-GAL4 driver was obtained from Dr. Kaye Suyama. phm-GAL4 and spok-GAL4 drivers were obtained from Dr. Michael O’Connor.
Larval size measurements
0-4 hr old larvae were placed into petri dishes with standard fly food media for 3 days at 25°. Then, larvae were removed from the food, rinsed in PBS, and placed in PBS to be imaged. The areas of nineteen heterozygous and twenty homozygous larvae were measured in ImageJ.
Plate preparation for chemical modifier screening
100nL compound or DMSO (vehicle) was dispensed from mother plates into wells of 96 well daughter plates using the Echo acoustic dispenser (LabCyte). Then, 100μL of molten standard fly food media (molasses, agar, yeast, propionic acid) lacking cornmeal, but carrying 0.025% bromophenol blue, was dispensed using a MultiFlo (Bio-Tek Instruments). Bromophenol blue dye in the larval gut increases their signal over background later during imaging. Plates were placed on a plate shaker and shaken for 1 min, during which, the molten agar solidified with thoroughly mixed compound/DMSO in each well. Plates were then covered with adhesive aluminum seals and stored at 4° for up to two weeks.
Culturing fly larvae in 96-well plates
PnglPL/CyO, Act-GFP flies were cultured in a large population cage (Genessee) for up to two weeks, where they laid eggs on grape juice agar trays (Genessee) coated with a thin strip of active yeast paste. Egg collections were restricted to ∼6 hr during morning hours and were placed into 25° for ∼24 hr. ∼0-6hr old 1st instar larvae were rinsed off of the trays with room temperature water and collected in funnel-attached 40 micrometer sieves typically used for cell straining. To remove embryo contamination, the larvae/embryo mixture was incubated two times in 10.3% inoculation solution (sodium chloride, sucrose, 10% Triton X-100) for three minutes. Most embryos float to the top of this solution after three minutes, while larvae remain in suspension. Embryos from the mixture’s solution can therefore be removed with a 10ml pipette. Embryos were then added to sorting solution (Polyethylene glycol, COPAS 200x GP sheath reagent, Tween20) and drawn into a BioSorter (Union Biometrica) for sorting and dispensing into 96 well compound/media bearing plates. Three GFP negative homozygotes were gated from heterozygotes and dispensed into the 11 left most columns of the plate, and wells G12 and H12. Three GFP positive heterozygotes were dispensed into wells A12, B12, C12, D12, E12, and F12. Plates were then covered with permeable adhesive seals and incubated at 25° for three days. Approximately 18 plates were dispensed into per day, and larval viability was not affected by their time submerged in dispensing solution (<4 hr).
Preparation of plates for imaging
On the terminal day of the assay (day 3), gas permeable seals were removed and plates were filled with 20% sucrose solution made acidic with hydrochloric acid (pH 2) and carrying defoamer (Five Star Defoamer 105-2 oz). Plates were then vortexed and more solution was added to suspend larvae to a fixed focal plane. Plates were then imaged.
Image and data analysis
The Fly Imager uses a Sony a7r ii camera, controlled over USB by the gphoto software to generate full plate images that are then run through a larval detection algorithm. The algorithm first builds an image of what the well would look like when it is empty. To do this it excludes areas near edges (since those are probably larvae) and interpolates across the gaps. It then looks at the difference between the image and estimated empty background, selecting areas with a high difference to be larvae.
20E feeding and effect on developmental timing
20E (Enzo) was dissolved in 100% ethanol and added to molten standard fly food media at 200µM and added to vials. An equivalent set of vials with an equal amount of ethanol was added for negative controls. 0-6 hr larvae were dispensed with the BioSorter into the vials and incubated at 25° for 12 days. The number of larvae that pupariated were recorded on key days at 8 am, 12 pm, and 4 pm, and the number of adults that eclosed were recorded on days 9 – 12 at a single time.
Rescue of developmental delay with ring and prothoracic gland expression of human NGLY1
Ring gland expression of human NGLY1 was driven using the GAL4/UAS system (Brand and Perrimon 1993) and ring gland drivers 2_286-GAL4, spok-GAL4, and phm-GAL4 in PnglPL/Ex20 compound heterozygotes. Adults were allowed to lay eggs for 8-16 hr, and the genotypes for all eclosing flies were recorded. The number of ring gland rescued flies eclosed was normalized to the number of eclosed sibling homozygotes not carrying the driver.
Bortezomib feeding and effect on developmental timing
Bortezomib (Selleck Chem) was dissolved in DMSO at 100mM stock concentration and added to molten standard fly food media to a set of 35mm petri dishes at testing concentrations while maintaining a final 0.025% DMSO concentration. An equivalent set of 35mm petri dishes with an equal amount of DMSO was added for negative controls. 0-6 hr larvae were dispensed with the biosorter into the 35mm petri dishes and incubated at 25° for 3 days. The number of larvae that were still alive were recorded, and imaged to quantify the sizes with FIJI software.
Data Availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All strains are available upon request. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6227189.
Results
Generation of the PnglPL fly and characterization of its phenotypes
We used CRISPR/Cas9 to create a new allele of Pngl with a premature stop codon and a selectable marker inserted upstream of the PAW domain, herein referred to simply as “PnglPL” (Figure 1A). We rationalized that this truncated allele better reflects patient alleles, as no NGLY1 null alleles have been observed in individuals affected by NGLY1 deficiency. We benchmarked PnglPL against the previously described Pngl genetic null mutant generated by P-element excision (Pnglex20), which causes developmental defects and semi-lethality with few adult escapers (Funakoshi et al. 2010). PnglPL homozygotes are delayed in the larval-to-pupal transition by one day, and delayed to eclosion by 2 days when grown on standard media (Figure 1B). As three-day-old larvae, PnglPL homozygotes are ∼75% the size of their heterozygote siblings (P < 1X10−10, Student’s t-test) (Figure 1D).
Consistent with semi-lethality observed in other Pngl mutants, PnglPL homozygote mutants survive to pupation, but only 32% of flies emerge as adults when reared at 25° (Funakoshi et al. 2010, Galeone et al. 2017). We also found the degree of this lethality in PnglPL homozygote mutants was temperature dependent. Specifically, the pupal lethal phenotype was cold-sensitive, and at temperatures at or below 21° no adults emerged. PnglPL homozygotes that survive to adulthood are sterile, which is also consistent with the effects of previously characterized alleles, Pnglex20 and Pnglex14 (Funakoshi et al. 2010, Galeone et al. 2017).
Time to eclosion in PnglPL homozygotes is completely rescued by ubiquitous expression of a human NGLY1 (hNGLY1) transgene (Figure 1B). The small body size phenotype of PnglPL homozygote adults is also rescued by ubiquitous expression of hNGLY1 (Figure 1C). These results when flies are reared in standard vials suggested phenotypes suitable for high-throughput, whole-organism phenotypic screening at each stage of fly development from 1st instar larvae onwards.
We decided to optimize a high-throughput larval size assay in 96-well plates for several reasons. Assay miniaturization from 30mL vials to 96-well plates involves reducing the number of animals tested by a factor of 10, e.g., 30 animals per vial vs. 3 animals per well. The larval size difference between PnglPL heterozygote larvae vs. PnglPL homozygote larvae was more significant in 96-well plates than the timing to pupation difference or the timing to eclosion difference. A 3-day assay vs. a 7-11 day assay allowed for faster optimization cycle times. Time to pupation and time to eclosion would be secondary assays in vial format to ensure primary screening hits rescue global developmental delay, not just developmental delay in larvae.
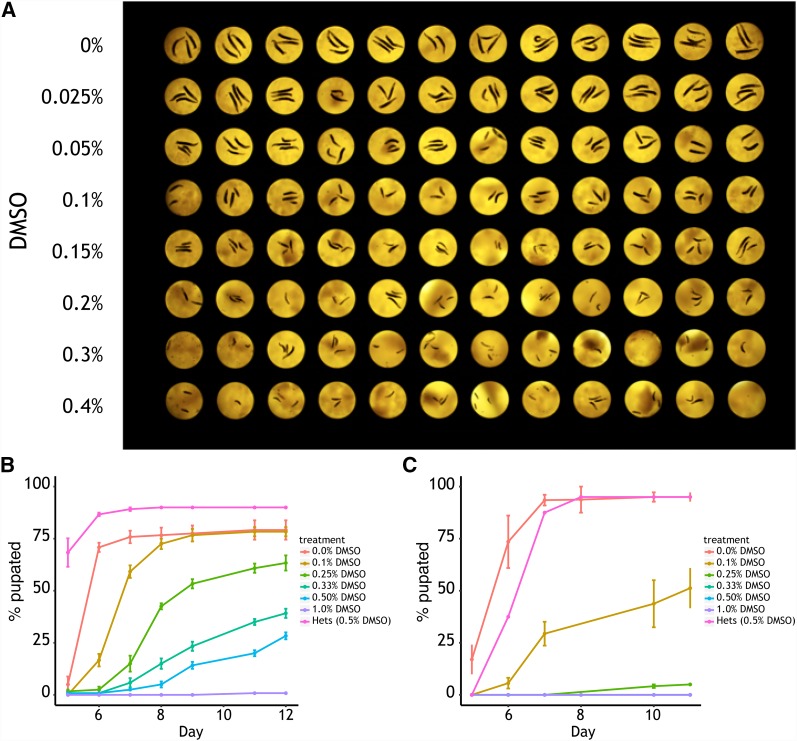
As a prelude to drug screening, we established the tolerability of PnglPL homozygote larvae to dimethyl sulfoxide (DMSO), the organic solvent for almost every compound library, including the Microsource Spectrum collection. The maximum tolerated dose of DMSO, therefore, sets a ceiling on the final assay screening concentration. Unexpectedly, we observed that PnglPL homozygotes are extremely hypersensitive to DMSO compared to the heterozygote control (Figure 2). We estimated a maximum tolerated dose in the PnglPL homozygote of 0.1% v/v DMSO (14mM) (Figure 2A, C). Surprisingly, larvae homozygous for PnglPL are several fold more sensitive than those homozygous for the null allele, Pnglex20 (Figure 2B). The PnglPL truncates the protein before the PAW domain, which binds mannose (Suzuki et al. 2016), but leaves the catalytic domain intact. This suggests to us that at least some of the DMSO sensitivity we observe is not simply due loss of Pngl activity. In comparison, wild-type Drosophila exhibit adult lethality starting at 1% v/v DMSO, larval lethality ranging from 2% v/v to 3% v/v DMSO, and a “no observed adverse effect level” (NOAEL) dose of 0.3% v/v DMSO (Figure S1; Nazir 2003). DMSO could be acting as a general stressor, or possibly inducing oxidoreductive stress, specifically. For the purposes of drug screening, we exploited DMSO as a sensitizer in the larval size assay. Exposure of mutant larvae to 0.1% v/v DMSO, which would entail a final assay screening concentration of 10µM for each library compound, was potent enough to increase the dynamic range of the assay while sparing larval lethality.
Figure 2.
PnglPL homozygotes are hypersensitive to dimethyl sulfoxide (DMSO). A) An image of PnglPL homozygous larvae grown in 96-well plate format on food dosed with different concentrations (0–0.4%) of DMSO. PnglPL homozygous larvae are hypersensitive to DMSO, a decrease in larval size is observed starting at 0.025% DMSO and larval size is decrease as the dose of DMSO is increased. The time to pupation of B) PnglEx20 (n = 3 replicates, 20-30 individuals/replicate) or C) PnglPL (n = 5 replicates, 20-30 individuals/replicate) homozygous larvae reared on food treated with DMSO. Homozygous mutant larvae exhibit hypersensitivity to DMSO showing a delayed time to pupariate and increased lethality.
A high-throughput image-based larval size chemical modifier pilot screen yielded one validated hit, 20-hydroxyecdysone (20E)
We posited that the small larval size phenotype of PnglPL homozygote mutants could be used to discover small-molecule suppressors that provide insight into the pathophysiology of NGLY1/Pngl deficiency in the fly. To that end, we developed a novel image-based assay to culture Drosophila larvae in clear-bottom 96-well plates where each well either contains a unique small molecule or vehicle. Using a 96-well-plate format allowed us to take advantage of existing lab automation for managing multi-well plates in high-throughput screening (HTS) applications, including a whole-organism sorter and dispenser. Our method includes steps to dissolve and dilute pre-existing fly food so that larvae can be floated to a fixed focal plane and then imaged with custom plate imager. Software was written to measure the overall area of floated larvae to enable the identification of small molecules that significantly increase the size of PnglPL homozygote larvae vs. vehicle-fed larvae.
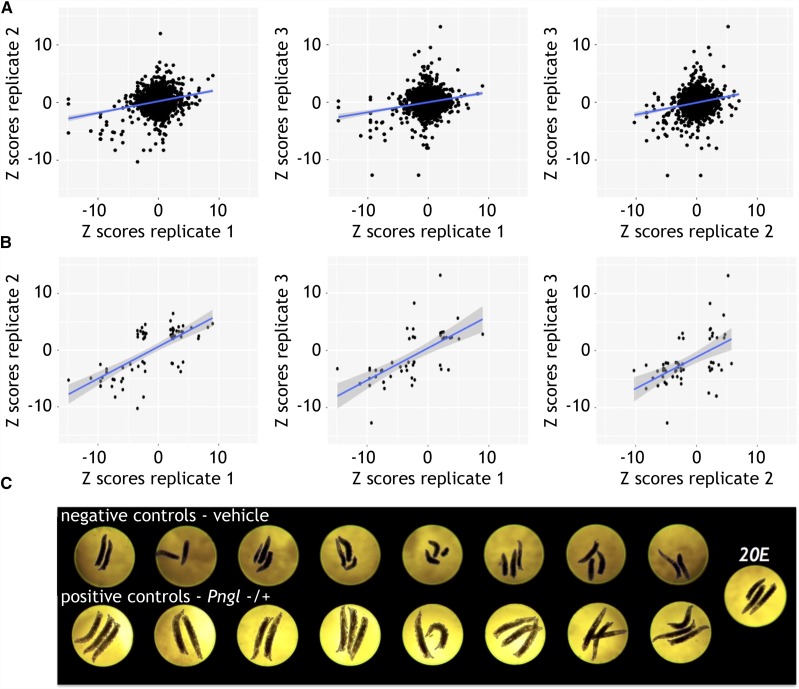
We selected the Microsource Spectrum collection for a pilot screen. The library contains 2,532 unique compounds including ∼600 FDA approved drugs, ∼800 compounds that have reached clinical trial stages in the USA, ∼400 drugs that have been marketed in Europe or Japan but not the USA, ∼600 bioactive tool compounds, and ∼800 natural products. Three larvae per well were cultured in 96-well plates with control wells comprising the two outermost columns. We used PnglPL homozygous larvae fed vehicle (0.1% v/v DMSO) as a negative control. We include two positive control groups: the first group consist of PnglPL/+ heterozygous larvae fed vehicle, and the second group consists of PnglPL homozygous larvae cultured without DMSO. The remaining 80 wells contained PnglPL homozygous larvae fed a unique library compound at 10µM plus 0.1% v/v DMSO. An image of a representative drug screening plate with zoomed-in reference wells is shown in Figure 3.
Figure 3.
Layout of an PnglPL assay plate with examples of controls and pre-hit compound. Larvae were cultured in 96-well plates with negative controls on the left most wells (column 1) which carried PnglPL homozygous with vehicle DMSO at 0.1%. 80 testing wells were in the middle (columns 2-11) which carried PnglPL homozygous larvae with a compound at 1μM, and DMSO at 0.1%. Right most top wells (12A-12F) contain positive control group 1 with PnglPL heterozygous larvae and 0.1% DMSO. Right most bottom wells (12G-12H) contain positive control group 2 with PnglPL homozygous larvae without DMSO. The compound in well G4 was considered a pre-hit compound.
We performed the screen in triplicate, meaning three independent biological replicates. There was statistically significant separation between positive and negative controls (Figure 4A). On average, PnglPL homozygotes were half the size of PnglPL/+ heterozygotes, although some variation in size was observed in each genotype. 75% of all screening plates (73 of 96) had a Z’ factor > 0; most of the screening plates with high variability belonged to the second replicate (Figure 4B). A weak positive correlation existed in pairwise comparisons of each biological replicate when all data points are included (Figure 5A). When we only included wells with Z scores less than -2 or greater than 2, the positive correlation increased significantly on average (Figure 5B). For example, in the pairwise comparison of replicate 1 vs. replicate 2, the correlation improves from R2 = 0.05977 in the full dataset to R2 = 0.48171 in the reduced dataset (with positive and negative controls removed as well).
Figure 4.
The assay had a consistent difference between positive and negative controls. A) A consistent separation between positive and negative controls with Z’Factor >0, indicated an assay that could significantly identify chemical suppressors. B) Three replicates of a 32 plate library were analyzed and 73 of 96 plates had a Z’Factor >0.
Figure 5.
Positive correlation between 3X replicates. A) The three pairwise comparisons of Z-scores show positive correlations indicating that a set of small molecules could modify the small larval size phenotype B) The positive correlations between replicates is more evident when only plotting Z-scores of < -2 or > 2. C) Larval size is partially rescued when 20E is fed to PnglPL homozygous larvae.
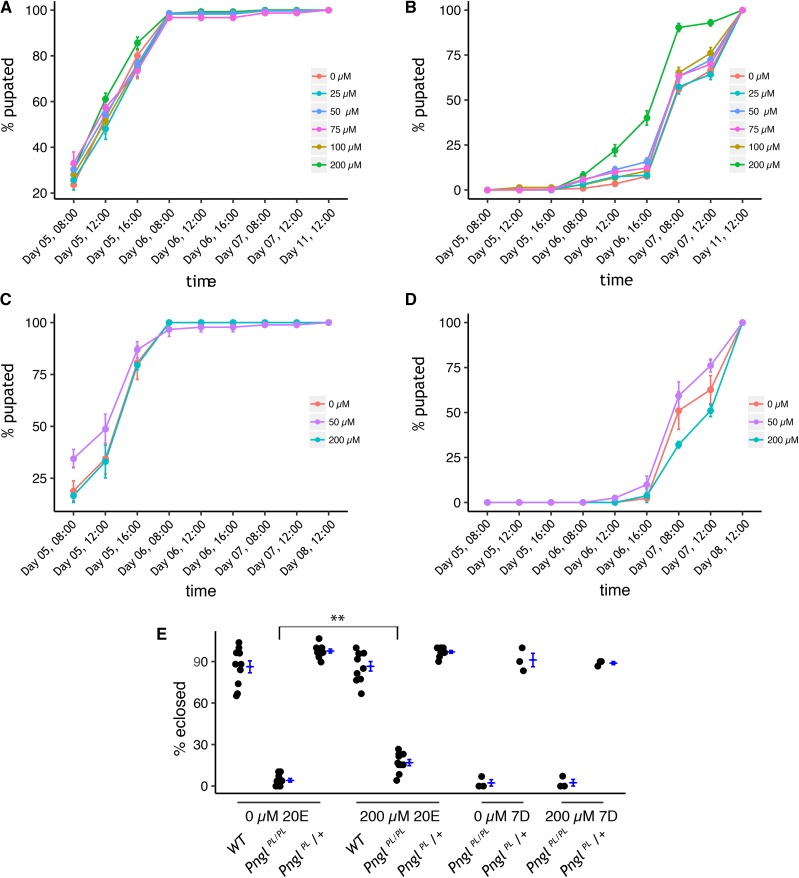
We initially considered 162 pre-hits with a Z score of > 2 in two of three biological replicates as primary screening positives (Table 1). Over two-thirds of those pre-hits proved to be false positives for one of two of the following reasons. First, uneaten fly food in the well occasionally increased the image background artificially inflating the calculated area of the larvae. Second, because Pngl mutants are hypersensitive to DMSO, any failure in compound dispensing or variability in DMSO levels due to hydration resulted in larger larvae. Forty-five compounds with a Z-score of > 2 in two of three replicates were considered further because their wells did not have obvious high background or low/no DMSO. The raw images of the wells containing those 45 compounds were manually inspected, and 18 appeared to have larvae larger than the negative controls in the same plate. Ultimately, these 18 unique compounds were found to have a Z of > 2 in at least two of three replicates. All 18 of the pre-hits from the screen were ordered, dissolved, and retested in attempt to reproduce rescue in vial format with larger numbers of animals (Table 2). One compound, 20-hydroxyecdysone (20E), partially rescued the developmental delay of PnglPL homozygote larvae development to pupae (Figure 6B), but had no effect on developmental timing of PnglPL heterozygote larvae (Figure 6A). Moreover, the suppressive effect of 20E persisted to adulthood, resulting in a statistically significant fourfold increase in eclosion percentage (Figure 6E; P<0.01 Student’s t-test).
Table 1. Whittling Screen pre-hits to a set of promising compounds to test in validation studies.
| Compounds with Z > 2 in 2 of 3 replicates | High background | Low/No DMSO | Without high background or Low/No DMSO | Clear difference from controls found by manual inspection | |
|---|---|---|---|---|---|
| Replicates 1 and 3 | 44 | 30 | 3 | 11 | 5 |
| Replicates 1 and 2 | 42 | 12 | 5 | 25 | 12 |
| Replicates 2 and 3 | 76 | 64 | 3 | 9 | 5 |
| Total | 162 | 106 | 11 | 45 | 22 |
Ultimately 22 compounds were found to have a Z of >2 in two of three replicates and upon manual inspection, the wells with those cpds appeared to have larvae larger than the negative controls. Two of those 22 compounds had a Z of >2 in three of three replicates, were compounded twice in this comparison, and thus the final set of consider further were 18 unique compounds.
Table 2. The 18 compounds that showed promise in the screen and were considered pre-hits. These had a Z-score of >2 in 2 of 3 replicates in the primary screen.
| Common Name | Replicate 1 | Replicate 2 | Replicate 3 | Retest, Phase 1* | Retest, Phase 2 *** | Retest, Phase 3 *** | Retest, Phase 4 **** |
|---|---|---|---|---|---|---|---|
| CRUSTECDYSONE | 3.36 | 2.24 | 3.03 | Small study indicated that 100μM rescued developmental delay to pupation | Trend supported that small size was partially rescued | Trend supported that small size was partially rescued | Positive – conclusive evidence that larval developmental delay and pupal lethality was recued. |
| TETRACYCLINE HUDROCHLORIDE | 2.84 | 1.01 | 2.39 | Z > 2, 3X @50μM | Trend supported that small size was partially rescued | Negative | Negative |
| HARMINE | 3.37 | 1.92 | 2.41 | Z > 2 in >1 replicate | Trend supported that small size was partially rescued | Negative | Negative |
| SULFLURAMID | 4.45 | 6.22 | −0.01 | Z > 2, 3X @ 12.5μM | Larvae appeared larger than controls, but the measured areas were not. | Negative | Not tested |
| URSOCHOLANIC ACID | 8.61 | 7.51 | 1.85 | 25 and 50μM could not be tested b/c of insolubility | Larvae appeared larger than controls, but the measured areas were not. | Negative | Not tested |
| 5a-ANDROSTANE | 6.46 | −1.10 | 2.44 | Z > 2 in >1 replicate | Negative | Not tested | Not tested |
| IRIGENIN TRIMETHYLETHER | 0.73 | 5.62 | 4.90 | Z > 2 in >1 replicate | Negative | Not tested | Not tested |
| PATULIN | −0.43 | 2.22 | 2.63 | Z > 2 in >1 replicate | Negative | Not tested | Not tested |
| ANTIMONY POTASSIUM TARTRATE TRIHYDRATE | 2.30 | 0.57 | 2.66 | Z > 2 in >1 replicate | Negative | Not tested | Not tested |
| ALOGLIPTIN BENZOATE | 2.23 | 8.26 | −2.26 | Z > 2 in >1 replicate | Negative | Not tested | Not tested |
| CEFEPIME HYDROCHLORIDE | 3.25 | −0.12 | 3.85 | Z > 2, 2X, @50μM | Negative | Not tested | Not tested |
| PALMIDROL (5mM) | 3.95 | 1.01 | 5.46 | 10μM did not rescue developmental delay or pupal lethality at 21°C. | Not tested | Not tested | Not tested |
| THIOGUANINE, TIOGUANINE** | 3.51 | −0.91 | 0.80 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| THIOGUANINE, TIOGUANINE | 0.91 | −0.02 | 4.97 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| CAPTAN | 4.11 | −3.41 | 3.36 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| AGARIC ACID | 3.16 | 1.90 | 4.05 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| ZONISAMIDE | 0.42 | 3.19 | 2.55 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| BROXALDINE | 2.05 | −1.05 | 6.62 | Z not >2 in > 1 replicate | Not tested | Not tested | Not tested |
| PICEID | 2.66 | 3.04 | 2.06 | Not tested | Not tested | Not tested | Not tested |
Phase 1 was 3 or 6 reps of a dose response (3.125, 6.25, 12.5, 25, and 50μM) in the primary screen/96 well format and/or tests for rescue of developmental delay (CRUSTECDYSONE and PALMIDROL).
The drug library had thioguanine in 2 different wells. *** Phase 2 and 3 tested whether promising pre-hits could rescue larval size at 10μM or 50μM in petri-dish experiments. ****Phase 4 tested whether promising pre-hits could rescue larval developmental delay and pupal lethality at 22.6° C.
Figure 6.
20-hydroxyecdysone (20E), but not an earlier ecdysone pathway precursor (7-dehydrocholesterol), partially rescues developmental delay and lethality in PnglPL mutants. The time to pupation of A) PnglPL/+ heterozygous or B) PnglPL homozygous larvae reared on food treated with different concentration of 20E (n = 10 replicates, 20-30 individuals/replicate). 20E did not impact developmental rate of heterozygous PnglPL larvae to pupation, but partially rescued development delay of homozygous PnglPL larvae to pupation at 200µM. The time to pupation of C) PnglPL/+ heterozygous or D) PnglPL homozygous larvae reared on food treated with different concentration of 7-d (n = 3 replicates, 25-30 individuals/replicate). 7-d did not impact developmental rate of heterozygous or homozygous PnglPL larvae to pupation. E) The fraction of animals surviving to eclosion. 20E, but not 7-d, rescued larval lethality of PnglPL homozygous at 200µM. (n = 10 replicates, 20-30 individuals/replicate; n = 3 replicates, 25-30 individuals/replicate, respectively).
As a control to rule out a simple ecdysone biosynthesis defect, we showed that the 20E precursor 7-hydroxycholesterol (7D) failed to rescue PnglPL homozygote larvae development to pupae (Figure 6D). If synthesis of 20E is faulty, it is likely at a point downstream of 7D. We could not reproducibly validate any of the other 17 pre-hits, so we focused our efforts on understanding the mechanism-of-action (MoA) of 20E. Therefore, this pilot screen had an extremely low hit rate of 0.04% (1/2532).
20E implicates the fly neuroendocrine axis as particularly sensitive to loss of NGLY1/Pngl function
20E drives metamorphosis in Drosophila and arthropods generally (Faunes and Larraín 2016). Dietary cholesterol forms the basis of 20E, and all 20E precursors are synthesized in the prothoracic gland, an organ that comprises part of the larger ring gland. The immediate 20E precursor, ecdysone or “E”, is packaged into secretory vesicles, secreted, and distributed by the hemolymph throughout the animals. E is converted to 20E in these peripheral tissues, and initiates signaling cascades and gene expression inducing physiological, morphological, and behavioral changes with each molt, or developmental transition.
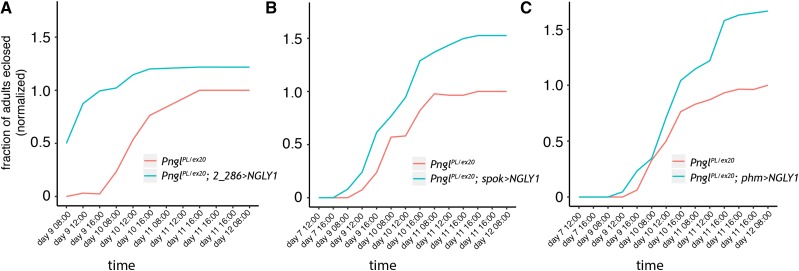
To test whether the 20E insufficiency in Pngl-deficient mutants is autonomous to the ring gland, we expressed a UAS-driven hNGLY1 transgene that can rescue global developmental delay when expressed ubiquitously (Figure 1B, C), in the ring gland with the 2-286-GAL4 driver. To control for off-target mutations due to strain background confounding our results, we tested the trans-heterozygous combination of Pngl alleles, PnglPL/Pnglex20. Most PnglPL/Pnglex20 larvae not expressing the human NGLY1 transgene eclosed on Day 10. In contrast, most PnglPL/Pnglex20 larvae expressing human NGLY1 in the ring gland pupated on Day 9 (Figure 7A). Aside from the ring gland, 2-286-GAL4 also drives expression in the salivary gland, fat body, and cuticle in larvae (Timmons et al. 1997). We observed a similar rescue effect when human NGLY1 transgene expression is driven by two ring-gland-specific drivers, phantom-GAL4 (phm) (Figure 7B) and spookier-GAL4 (spok) (Figure 7C). phm and spok encode cytochrome P450 monooxygenases in the ecdysone biosynthetic pathway (Rewitz et al. 2007). Driving hNGLY expression in PnglPL homozygotes with either phm-GAL4 or spok-GAL4 rescued the pupal lethality phenotype (11.4% and 9.8% eclosed, respectively) compared to sibling controls (6.5% and 6.4% eclipsed, respectively). Together, these data suggest that NGLY1/Pngl is necessary for normal function of the ring gland to enable proper levels of 20E to circulate throughout the developing animal and drive developmental transitions.
Figure 7.
Pngl is necessary for normal function of the ring gland. The fraction of PnglPL / PnglEx20 compound heterozygotes eclosed with human NGLY1 driven by the ring gland driver (blue) A) 2_286-GAL4 B) spookier-GAL4, or C) phantom-GAL4 compared to sibling controls lacking a driver (red). The reported values are normalized to the total number of eclosed PnglPL / PnglEx20 compound heterozygotes for each experiment. A) Compound heterozygous PnglPL / PnglEx20 larvae expressing 2_286 > NGLY1 eclosed earlier and had lower lethality than control flies (64 PnglPL / PnglEx20; 2_286-GAL4 individuals compared to 120 Pngl / CyO siblings; 74 PnglPL / PnglEx20; 2_286 > hNGLY1 individuals compared to 185 Pngl / CyO siblings). Compound heterozygous PnglPL / PnglEx20 larvae expressing A) spookier > NGLY1 or B) phantom > NGLY1 had lower lethality at eclosion than control flies (23 PnglPL / PnglEx20; spok-GAL4 individuals compared to 337 Pngl / CyO siblings; 43 PnglPL / PnglEx20; spok > hNGLY1 individuals compared to 398 Pngl / CyO siblings); and 19 PnglPL / PnglEx20; phm-GAL4 individuals compared to 235 Pngl / CyO siblings; 63 PnglPL / PnglEx20; phm > hNGLY1 individuals compared to 459 Pngl / CyO siblings, respectively).
NGLY1/Pngl deficient flies are hypersensitive to proteasome inhibition
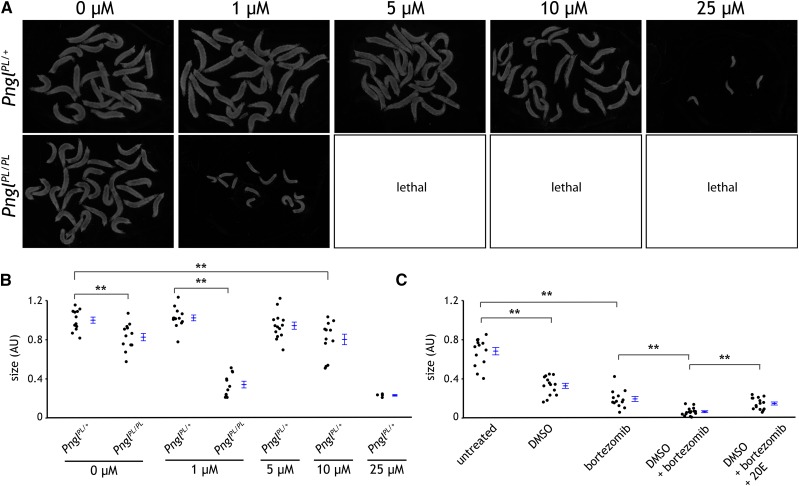
As mentioned above, loss of NGLY1 sensitizes nematodes and human cancer cell lines to proteasome inhibition. We predicted that PnglPL homozygote mutants would exhibit hypersensitivity to bortezomib. Indeed, bortezomib caused 100% lethality of PnglPL homozygous larvae at 5µM, while lethality was not observed in PnglPL/+ heterozygous larvae until a dose of 25µM (Figure 8). In addition, the size of PnglPL homozygous mutants was reduced by <50% when treated with 1µM bortezomib, and to ∼50% PnglPL/+ heterozygous larvae at ∼10µM bortezomib. These data indicate that the half-maximal inhibitory concentration (IC50) of bortezomib to reduce larval growth is ∼10X less in PnglPL homozygote.
Figure 8.
Pngl larvae are ≥10X more sensitive to the proteasome inhibitor bortezomib. Images A) and quantification of larval size B) of 3 day PnglPL/+ heterozygous or age matched PnglPL/PL mutant larvae raised on food treated with 0, 1, 5, 10 or 25µM bortezomib. Black points report the size (in arbitrary units) of individual larvae. Blue lines report the mean and SE of these populations. Bortezomib delays larval developmental progression in PnglPL homozygous larvae more severely than PnglPL/+ heterozygous larvae leading to smaller sized larvae, and is lethal for homozygotes at concentrations equal to or greater than 5 µM. For these experiments, Bortezomib was solubilized in DMSO, resulting in a final concentration of DMSO in treated food of 0.025%, which is below the threshold of effect on PnglPL homozygous larvae. (** significant at P < 0.01; Student’s t-test) C) Quantification of larval size for PnglPL homozygous larvae raised on untreated food, or food treated with 0.1% DMSO, 1 µM bortezomib, 0.1% DMSO and 1 µM bortezomib, or 0.1% DMSO, 1 µM bortezomib, and 133 µM 20E. 20E partially rescued the effect of bortezomib treatment on larval size in PnglPL homozygotes. (** significant at P < 0.01; Student’s t-test).
Treatment of NGLY1/Pngl deficient flies with 20E partially rescues the effect of bortezomib
Given that both 20E and bortezomib can modify the phenotypes associated with mutations in Pngl, we decided to test if treatment with 20E could rescue some of the effect of bortezomib. PnglPL homozygote mutant larvae grown on food treated with both bortezomib and 20E were significantly larger than those grown on food treated with bortezomib alone (Figure 8C).
Discussion
We successfully generated a new Drosophila model of NGLY1 Deficiency, optimized a high-throughput whole-animal phenotypic assay of larval size, and then performed a proof-of-concept drug repurposing screen. While 20E itself should not be considered a drug candidate for NGLY1 Deficiency in humans, the fact that it is a chemical suppressor implicates the neuroendocrine axis in the pathophysiology of Pngl deficiency in flies. In other words, even though 20E is an insect-specific developmental hormone, the neuroendocrine axis and steroid-derived developmental hormones are conserved in mammals and may play a role in NGLY1 Deficiency in humans. Collectively, our findings – most strikingly, hypersensitivity of the PnglPL mutant both to bortezomib and to DMSO – align with results observed in nematodes (Lehrbach and Ruvkun 2016) and human cells (Tomlin et al. 2017) that point to the essential and conserved role of NGLY1 in regulating the function of glycoprotein clients, specifically NRF1 and the proteasome bounce-back response.
In fact, we can already propose a mechanism to link NRF1 function to ecdysteroidogenesis and the neuroendocrine axis in flies. The fly homolog of NRF1 is the longest isoform of cnc, CncC, which contains a conserved N-terminal leucine rich transmembrane region targeting CncC to the ER (Grimberg et al. 2011). Specific loss of CncC in the prothoracic gland reduces the expression of ecdysone biosynthetic genes and results in delayed timing to pupation (Deng and Kerppola 2013). RNA interference of the Colorado potato beetle homolog of CncC also reduced ecdysteroidogenesis pathway gene expression and delayed timing pupation, which could be rescued by 20E supplementation (Sun et al. 2017). We would predict then, that as the functional equivalent of SKN-1A in nematodes and NRF1 in mammals, CncC might also be a substrate for deglycosylation by Pngl in flies. A second testable prediction is that the fly homolog of nematode DDI-1 and human DDI2, rings lost (ringo), acts downstream of Pngl to proteolyze CncC, generating a mature, nuclear-active species.
There are other potential explanations for how 20E might partially rescue global developmental delay in the PnglPL mutant that do not directly involve NRF1/CncC, or that may contribute alongside loss of NRF1/CncC activity. For example, Decapentaplegic (Dpp) signaling is impaired in the ventral mesoderm of Pngl mutant larvae, leading to developmental defects that contribute to the lethality observed in these mutants (Galeone et al. 2017). Aside from local signaling, Dpp also regulates developmental timing. Dpp acts in the prothoracic gland to suppress ecdysone release by repressing the expression of genes required for ecdysteroidogenesis (Setiawan et al. 2017). It is possible defects in Dpp signaling in the prothoracic gland of Pngl mutants could cause the misregulation of ecdysone production.
Alternatively, PnglPL mutants may not package ecdysone into secretory vesicles properly, or may be defective in secreting ecdysone. Second, signal transduction mediated by the interaction between 20E and the ecdysone receptor (EcR) might not be fully operational, and so extra 20E boosts this flawed signaling. Third, ecdysone secretion by the ring gland is induced by two neurons that synapse onto the prothoracic gland and secrete the neuropeptide prothoracicotropic hormone, or PTTH. PTTH contacts the receptor tyrosine kinase Torso to initiate signaling leading to ecdysone secretion. Perhaps there is a flaw in PTTH secretion or Torso signaling. Fourth, damage to developing larval tissues, or starvation, may impinge on 20E to fine tune organism development so that a properly proportioned and nourished animal can develop fully to adulthood. The PnglPL mutant may have damaged tissues, for example through protein-aggregate toxicity or proteasome stress; or it may have some degree of starvation, for example if the gut cannot attain nutrients properly. 20E feeding may bypass delays induced by this hypothetical tissue damage/starvation.
Acknowledgments
We thank the Grace Science Foundation for funding this work. We thank Dr. Kevin Lee for planning and experiment planning. Drs. Hamed Jafar-Nejad, Tadashi Suzuki, and Michael O’Connor kindly provided fly stocks and reagents. Dr. Rebecca Choy provided early guidance on how to scale up fly husbandry/culturing.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.6227189.
Communicating editor: L. Steinmetz
Literature Cited
- Bi Y., Might M., Vankayalapati H., Kuberan B., 2017. Repurposing of Proton Pump Inhibitors as first identified small molecule inhibitors of endo-β-N-acetylglucosaminidase (ENGase) for the treatment of NGLY1 deficiency, a rare genetic disease. Bioorg. Med. Chem. Lett. 27: 2962–2966. 10.1016/j.bmcl.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Deng H., Kerppola T. K., 2013. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 9: e1003263 10.1371/journal.pgen.1003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns G. M., Shashi V., Bainbridge M., Gambello M. J., Zahir F. R., et al. , 2014. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet. Med. 16: 751–758. 10.1038/gim.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunes F., Larraín J., 2016. Conservation in the involvement of heterochronic genes and hormones during developmental transitions. Dev. Biol. 416: 3–17. 10.1016/j.ydbio.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Fujihira H., Masahara-Negishi Y., Tamura M., Huang C., Harada Y., et al. , 2017. Lethality of mice bearing a knockout of the Ngly1-gene is partially rescued by the additional deletion of the Engase gene. PLoS Genet. 13: e1006696 10.1371/journal.pgen.1006696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi Y., Negishi Y., Gergen J. P., Seino J., Ishii K., et al. , 2010. Evidence for an essential deglycosylation-independent activity of PNGase in Drosophila melanogaster. PLoS One 5: e10545 10.1371/journal.pone.0010545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeone A., Han S. Y., Huang C., Hosomi A., Suzuki T., et al. , 2017. Tissue-specific regulation of BMP signaling byDrosophila N-glycanase 1. eLife 6: e27612 10.7554/eLife.27612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg K. B., Beskow A., Lundin D., Davis M. M., Young P., 2011. Basic Leucine Zipper Protein Cnc-C Is a Substrate and Transcriptional Regulator of the Drosophila 26S Proteasome. Mol. Cell. Biol. 31: 897–909. 10.1128/MCB.00799-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Harada Y., Hosomi A., Masahara-Negishi Y., Seino J., et al. , 2015. Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc. Natl. Acad. Sci. USA 112: 1398–1403. 10.1073/pnas.1414593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. M., Han J. W., Chan J. Y., 2016. Nuclear Factor Erythroid-2 Like 1 (NFE2L1): Structure, function and regulation. Gene 584: 17–25. 10.1016/j.gene.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Ferreira C., Krasnewich D., Toro C., Latham L., et al. , 2017. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet. Med. 19: 160–168. 10.1038/gim.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach N. J., Ruvkun G., 2016. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 5: 17721 10.7554/eLife.17721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir A., Mukhopadhyay I., Saxena D. K., Chowdhuri D. K. 2003. Evaluation of the No Observed Adverse Effect Level of Solvent Dimethyl Sulfoxide in Drosophila melanogaster. Toxicol. Mech. Meth 13: 147–152. [DOI] [PubMed] [Google Scholar]
- Owings K. G., Lowry J. B., Bi Y., Might M., Chow C. Y., 2018. Transcriptome and functional analysis in a Drosophila model of NGLY1 deficiency provides insight into therapeutic approaches. Hum. Mol. Genet. 27: 1055–1066. 10.1093/hmg/ddy026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F., Chen H.-M., Lee T., Bullock S. L., 2014. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. PNAS 111: E2967–E2976. 10.1073/pnas.1405500111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S. K., Lee C. S., Young P., Beskow A., Chan J. Y., et al. , 2010. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 38: 17–28. 10.1016/j.molcel.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K. F., O’Connor M. B., Gilbert L. I., 2007. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 37: 741–753. 10.1016/j.ibmb.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Setiawan L., Woods A. L., Hariharan I. K., 2017. The BMP2/4 ortholog Dpp functions as an inter- organ signal that regulates developmental timing in Drosophila. bioRxiv. 10.1101/180562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.-K., Meng Q.-W., Xu Q.-Y., Deng P., Guo W.-C., et al. , 2017. Leptinotarsa cap “n” collar isoform C/Kelch-like ECH associated protein 1 signaling is critical for the regulation of ecdysteroidogenesis in the larvae. Insect Biochem. Mol. Biol. 85: 1–10. 10.1016/j.ibmb.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Suzuki T., 2015. The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder. J. Biochem. 157: 23–34. 10.1093/jb/mvu068 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Huang C., Fujihira H., 2016. The cytoplasmic peptide:N-glycanase (NGLY1) - Structure, expression and cellular functions. Gene 577: 1–7. 10.1016/j.gene.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Becker J., Barthmaier P., Fyrberg C., Shearn A., et al. , 1997. Green fluorescent protein/beta-galactosidase double reporters for visualizing Drosophila gene expression patterns. Dev. Genet. 20: 338–347. [DOI] [PubMed] [Google Scholar]
- Tomlin F. M., Gerling-Driessen U. I. M., Liu Y.-C., Flynn R. A., Vangala J. R., et al. , 2017. Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity. ACS Cent. Sci. 3: 1143–1155. 10.1021/acscentsci.7b00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All strains are available upon request. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6227189.