Abstract
G protein-coupled receptors are 7-pass transmembrane receptors that couple to heterotrimeric G proteins to mediate cellular responses to a diverse array of stimuli. Understanding the mechanisms that regulate G protein-coupled receptors is crucial to manipulating their signaling for therapeutic benefit. One key regulatory mechanism that contributes to the functional diversity of many signaling proteins is post-translational modification. Whereas phosphorylation remains the best studied of such modifications, arginine methylation by protein arginine methyltransferases is emerging as a key regulator of protein function. We previously published the first functional evidence that arginine methylation of G protein-coupled receptors modulates their signaling. We report here a third receptor that is regulated by arginine methylation, the Caenorhabditis elegans SER-2 tyramine receptor. We show that arginines within a putative methylation motif in the third intracellular loop of SER-2 are methylated by PRMT5 in vitro. Our data also suggest that this modification enhances SER-2 signaling in vivo to modulate animal behavior. The identification of a third G protein-coupled receptor to be functionally regulated by arginine methylation suggests that this post-translational modification may be utilized to regulate signaling through a broad array of G protein-coupled receptors.
Keywords: tyramine, SER-2, GPCR (G protein-coupled receptor), protein arginine methylation, PRMT
One way in which G protein-coupled receptor (GPCR) signaling can be regulated is through post-translational modification of receptors. Although a large-scale proteomics analysis performed in 2003 identified several GPCRs as substrates of an anti-methylarginine antibody (Boisvert et al. 2003), there was no direct evidence at that time that this receptor class is regulated by methylation. In 2015, we reported the first functional evidence that protein arginine methylation regulates GPCR signaling (Likhite et al. 2015). Methylation of D2-like dopamine receptors (human D2 and C. elegans DOP-3) by protein arginine methyltransferase 5 (human PRMT5 and C. elegans PRMT-5, respectively) promoted signaling in both cell culture and in vivo (Likhite et al. 2015). The D2-like dopamine receptor family was identified as a possible substrate for PRMT5 in a bioinformatics analysis that examined GPCRs for predicted methylation motifs (RGG or RXR) in their intracellular domains. The human D2 receptor was found to have a putative methylation motif in its third intracellular loop that is conserved in the corresponding receptor sequences from other mammalian, vertebrate and invertebrate species, including the corresponding C. elegans D2-like dopamine receptor, DOP-3. The third intracellular loop of both the human D2 and C. elegans DOP-3 receptors was methylated by human PRMT5 in vitro, and changing the conserved arginines of the putative methylation motif to alanines diminished receptor methylation. Correspondingly, changing these arginines to disrupt the methylation motif also diminished signaling through both of these receptors (Likhite et al. 2015). Combined, the results of that study revealed that arginine methylation promotes signaling through the D2 receptor to dampen cAMP signaling in cultured human cells, and also promotes DOP-3 signaling to regulate C. elegans behavior.
PRMT5 is a type 2 protein arginine methyltransferase (PRMT) that transfers two methyl groups from S-adenosyl-L-methionine (SAM) to form symmetric dimethylarginines (SDMAs) (Branscombe et al. 2001). This modification can be added to arginines in glycine- and arginine-rich motifs, in proline-, glycine-, and methionine-rich motifs, and even in the absence of any recognizable motif (Bedford and Clarke 2009; Wang et al. 2013; Wang et al. 2014). PRMT5 has been shown to influence gene expression, snRNP biogenesis, the DNA damage response, and germ cell development (Meister et al. 2001; Fabbrizio et al. 2002; Ancelin et al. 2006; Tee et al. 2010; He et al. 2011; Huang et al. 2011). In their study of D2-like dopamine receptors, Likhite et al. (2015) added to the growing list of PRMT5 substrates and described the founding members of a new class of proteins – GPCRs – that are functionally regulated by arginine methylation.
The bioinformatics analysis performed by Likhite et al. (2015) identified 300 human GPCRs and 64 C. elegans GPCRs that contain an intracellular RGG or RXR putative methylation motif. Many of the identified C. elegans receptors are predicted or known to bind biogenic amines including dopamine, serotonin, octopamine and tyramine (Chase and Koelle 2007). In humans, tyramine has been considered a trace amine because it is found at low levels. However, a new family of GPCRs, the trace amine-associated receptors (TAARs), was discovered in 2001, suggesting that tyramine may act as a classical neurotransmitter in vertebrates (Borowsky et al. 2001). In addition, there is evidence that tyramine plays an important physiological role in humans and has been linked to human disorders such as hypertensive crisis and attention deficit hyperactivity disorder (ADHD) (Blackwell and Mabbitt 1965; Burchett and Hicks 2006; Berry 2007; D’Andrea et al. 2013). In C. elegans, tyramine also acts as a neurotransmitter and is considered the invertebrate counterpart of adrenaline (Roeder et al. 2003; Alkema et al. 2005; Roeder 2005). Once thought to act only as the precursor to octopamine, it is now clear that tyramine signaling modulates numerous C. elegans behaviors, ranging from the inhibition of egg laying to the formation and retrieval of imprinted memories (Rex et al. 2004; Alkema et al. 2005; Rex et al. 2005; Chase and Koelle 2007; Wragg et al. 2007; Pirri et al. 2009; Ringstad et al. 2009; Donnelly et al. 2013; Jin et al. 2016).
The C. elegans genome encodes three tyraminergic GPCRs, SER-2, TYRA-2 and TYRA-3, and one ligand-gated ion channel, LGC-55, that bind tyramine (Rex and Komuniecki 2002; Tsalik et al. 2003; Rex et al. 2004; Wragg et al. 2007; Pirri et al. 2009; Donnelly et al. 2013). The most extensively characterized of the GPCRs is the SER-2 receptor, which is expressed in a subset of sensory neurons, interneurons and motor neurons, as well as head muscles and pharyngeal cells (Altun-Gultekin et al. 2001; Rex and Komuniecki 2002; Tsalik et al. 2003; Rex et al. 2004; Alkema et al. 2005; Donnelly et al. 2013; Wilson et al. 2017). Among tyramine-regulated behaviors, a role for SER-2 has been shown in mediating tyramine (TA) -induced immobilization (Donnelly et al. 2013) and in antagonizing serotonin (5-HT) -stimulated pharyngeal pumping (Rex et al. 2004). In these studies, ser-2 loss-of-function (lof) animals were resistant to the paralytic effects of exogenous TA (Donnelly et al. 2013) and the addition of TA did not antagonize 5-HT-stimulated pumping in ser-2(lof) animals (Rex et al. 2004), respectively. In both cases, expression of a wild-type ser-2 transgene rescued the behavioral phenotypes, demonstrating that they were specific to the loss of SER-2 receptor function.
While exploring their environments during forward locomotion, C. elegans display a foraging behavior in which they move their nose from side-to-side (Croll and Smith 1978). This foraging behavior is inhibited while animals reverse in response to light anterior mechanosensory stimulation, termed anterior touch (Chalfie et al. 1985; Alkema et al. 2005). Suppression of head movements while reversing in response to touch could help an animal escape from nematophagous fungi that can trap worms with constricting hyphal rings (Barron 1977). Extensive circuit-level analyses have revealed a critical role for TA and LGC-55 in suppressing foraging behavior in response to anterior touch; animals unable to synthesize TA and animals lacking LGC-55 do not suppress head oscillations during this backing response (Alkema et al. 2005; Pirri et al. 2009). Although not seen by Alkema et al. (2005), it has been reported that animals also suppress foraging while reversing in response to nose touch (Rex et al. 2004). The explanation for the difference is not clear, but could be the result of nuanced differences in the execution of the nose touch assay. Rex et al. (2004) found that ser-2(lof) animals continued to display foraging behavior while reversing following nose touch, unlike the wild-type animals in their study, and expression of a wild-type ser-2 transgene rescued the behavioral phenotype (Rex et al. 2004). We also have observed that, in contrast to wild-type animals, ser-2(lof) animals do not cease foraging while backing in response to nose touch.
Following the bioinformatics analysis of Likhite et al. (2015), it was unknown if any of the other GPCRs identified to contain an intracellular RGG or RXR putative methylation motif are functionally regulated by arginine methylation, similar to the D2-like dopamine receptors. Herein, we report that the C. elegans SER-2 tyramine receptor is also regulated by methylation. We show that human PRMT5 methylates a portion of the third intracellular loop of SER-2 in vitro, and that the conserved arginines within the predicted methylation motif are required for methylation by PRMT5. Using C. elegans behavior as a readout for nervous system function, we show that PRMT-5 also promotes tyraminergic signaling through the C. elegans SER-2 receptor in vivo, and that changing the predicted arginine methylation target sites in SER-2 diminished its ability to regulate C. elegans behavior. Together, our data reveal a third receptor that appears to be functionally regulated by protein arginine methylation. This work suggests that arginine methylation may be a widespread post-translational modification used to regulate the activity of GPCRs.
Materials And Methods
C. elegans Culture
Strains were maintained at 20° under standard conditions on nematode growth media (NGM) agar plates seeded with OP50 E. coli bacteria (Brenner 1974).
Strains
Strains used in this study include: N2 Bristol wild-type, OH313 ser-2(pk1357), FG129 prmt-5(gk357), FG807 ser-2(pk1357);prmt-5(gk357), FG808 ser-2(pk1357);udEx460[ser-2p::ser-2,elt-2::GFP], FG809 ser-2(pk1357);udEx461[ser-2p::ser-2,elt-2::GFP], FG810 ser-2(pk1357);udEx462[ser-2p::ser-2,elt-2::GFP], FG811 ser-2(pk1357);udEx463[ser-2p::ser-2(R245A/R247A),elt-2::GFP], FG812 ser-2(pk1357);udEx464[ser-2p::ser-2(R245A/R247A),elt-2::GFP], FG813 ser-2(pk1357);udEx465[ser-2p::ser-2(R245A/R247A),elt-2::GFP], FG814 prmt-5(gk357);udEx466[ser-2p::prmt-5,elt-2::GFP], FG815 prmt-5(gk357);udEx467[ser-2p::prmt-5,elt-2::GFP] and FG816 prmt-5(gk357);udEx468[ser-2p::prmt-5,elt-2::GFP].
Transgenic Strains
Germline transformations were performed as previously described (Mello et al. 1991). For prmt-5 and ser-2 rescue experiments, pJM67 elt-2::gfp plasmid (25 ng/μl) (Fukushige et al. 1998) was used as the co-injection marker, along with either the prmt-5 or ser-2 rescuing plasmid (50 ng/μl).
Plasmid Construction
pFG2:
The ∼5.3 kb glr-1 promoter was cut out of C06E1xP, first by digesting with SalI and blunting with Klenow, followed by digestion with PstI. This fragment was gel purified and ligated into the PstI/SmaI sites of pPD49.26 (Fire Lab C. elegans Vector Kit, Addgene).
pFG102:
The ser-2 cDNA (isoform e) was PCR amplified from ser-2 in pFLAG (gift from Rick Komunicki) (Rex et al. 2004) with primers designed to incorporate a 5′ KpnI site and a 3′ SacI site, and subcloned into these sites of pFG2.
pFG288:
The ∼2.2 kb ser-2 promoter (Donnelly et al. 2013) was PCR amplified from N2 genomic DNA, incorporating a 5′ BamHI and a 3′ HindIII site, and subcloned into these sites of pPD49.26 (Fire Lab C. elegans Vector Kit, Addgene).
pFG289:
The cDNA encoding ser-2 (isoform e) was isolated from pFG102 with KpnI/SacI, and subcloned into these sites of pPD49.26 (Fire Lab C. elegans Vector Kit, Addgene).
pFG290 ser-2p::ser-2:
The ∼2.2 kb ser-2 promoter (Donnelly et al. 2013) was PCR amplified from N2 genomic DNA, incorporating a 5′ BamHI and a 3′ HindIII site, and subcloned into these sites of pFG289.
pFG291 ser-2p::ser-2(R245A/R247A):
Site-directed mutagenesis (QuikChange, Stratagene) was used to incorporate the R245A and R247A substitutions into the ser-2p::ser-2 plasmid pFG290.
pFG292 ser-2p::prmt-5:
The cDNA encoding prmt-5 was isolated from pFG66 (Likhite et al. 2015) with NheI/KpnI, and inserted into these sites of pFG288.
pFG293 GST-S-SER-2 3ICL-S 30 aa:
A portion of the cDNA encoding the third intracellular loop (ICL) of SER-2 (amino acid residues 240 to 269 of isoform e) was amplified by PCR from ser-2p::ser-2 (pFG290), incorporating sequence encoding an N- and C-terminal S-tag (amino acids KETAAAKFERQHMDS) as well as a 5′ BamHI and a 3′ XmaI site. This DNA was then inserted into the corresponding sites of pGEX-5X-3.
pFG294 GST-S-SER-2(R245A/R247A) 3ICL-S 30 aa:
A portion of the cDNA encoding the third intracellular loop (ICL) of SER-2(R245A/R247A) (amino acid residues 240 to 269 of isoform e) was amplified by PCR from ser-2p::ser-2(R245A/R247A) (pFG291), incorporating sequence encoding an N- and C-terminal S-tag (amino acids KETAAAKFERQHMDS) as well as a 5′ BamHI and a 3′ XmaI site. This DNA was then inserted into the corresponding sites of pGEX-5X-3.
All constructs were verified by sequencing where appropriate.
Behavioral Assays
All behavioral assays were performed on at least three separate days, in parallel with controls. Assays were performed at room temperature using young adult animals aged 24 hr post the L4 larval stage. For all behavioral experiments the combined data of ≥ 3 transgenic lines is shown, and the number of transgenic animals assayed in each experiment is indicated within the figure legends. In all cases n ≥ 24 for non-transgenic animals. The Student’s two-tailed t-Test and one-way Anova with Tukey’s Honestly Significant Difference (HSD) were used for statistical analyses.
To quantify resistance to tyramine-induced immobilization, young adult animals were transferred to agar plates supplemented with 12 mM tyramine. Approximately 8 animals were transferred to assay plates and scored for locomotion every minute for a 10 min period. Animals were scored as immobilized if there was no sustained forward or backward locomotion in a 5 sec interval (Donnelly et al. 2013). Tyramine plates were prepared by autoclaving 1.7% agar in water, cooling to ∼55° and adding glacial acetic acid to a concentration of 2 mM and tyramine-hydrochloride (Sigma-Aldrich) to a concentration of 12 mM.
Pharyngeal pumping was measured by washing young adult animals aged 24 hr post the L4 larval stage off of a seeded plate with M9 buffer. Animals were washed twice in M9 buffer (Wood 1988) and then incubated in ligand for 20 min. Animals were then spun down and transferred onto agar pads and the number of pumps per 20 sec was counted. A pump was defined as the movement of the pharyngeal grinder. The ligands tyramine-hydrochloride (Sigma-Aldrich) and serotonin creatinine sulfate monohydrate (Sigma-Aldrich) were prepared at the indicated concentrations in M9 buffer.
The nose touch assay was performed essentially as described (Kaplan and Horvitz 1993; Hart et al. 1995). Briefly, young adult animals were transferred to agar plates spread with 100 μl OP50 and allowed to recover for 5 min. An arm hair was placed in the path of a forward-moving animal to allow a “nose-on” collision. The presence or absence of foraging (classified as the continuous movement of the head in an exploratory fashion) while reversing was recorded. Five trials per animal and ≥30 animals per genotype were scored as follows: no foraging (no head movement while reversing) and foraging (head movement while reversing). Occasionally, animals did not reverse upon nose touch and were scored as no response.
Protein Purification
Overnight cultures of E. coli expressing either GST, GST-SER-2240-269 [GST-S-SER-2 3ICL-S (30 amino acids)] or GST-SER-2240-269(R245A/R247A) [GST-S-SER-2(R245A/R247A) 3ICL-S (30 amino acids)] were pelleted, resuspended in lysis buffer (1x PBS/1 M NaCl, 1 mM PMSF, 1 mM DTT, 1 mg/ml lysozyme) and subjected to two rounds of French Press lysis. Lysates was centrifuged at 30,000 × g for 30 min at 4°. Supernatants were incubated with Glutathione Sepharose High Performance beads (Amersham Biosciences) that had been equilibrated with wash buffer (1x PBS/1 M NaCl, 0.02% v/v Triton X-100, 1 mM DTT). Unbound sample was allowed to flow through and the resin was washed 3x with 10 mL of wash buffer. GST-tagged proteins were eluted with elution buffer (50 mM Tris pH 8, 200 mM NaCl, 0.01% v/v Triton X-100, 1 mM DTT, 15 mM glutathione). Fractions containing the protein eluate were dialyzed in dialysis buffer (1x PBS, 15% glycerol) for storage at -80°.
In vitro Methylation
The in vitro methylation assay was performed essentially as described (Likhite et al. 2015) in a total volume of 20 μl with 6 μg of substrate (50 ng of SmB’ (Goulet et al. 2007)), 210 ng of recombinant human PRMT5 complex (Active Motif), and 5.5 μCi of S-[methyl-3H]adenosyl-L-methionine (55 to 85 Ci/mmol; PerkinElmer) in 1x methylation buffer [150 mM NaCl, 50 mM Tris-HCl (pH 8), 1 mM EDTA]. Reactions were incubated at 37° for 4 hr, resolved by SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were sprayed with 6% PPO enhance reagent (2,5-Diphenyloxazole, in isopropanol) three times at 10 min intervals before being exposed to Kodak BioMax MS film with a BioMax Transcreen LE Intensifying Screen at -80° for two weeks and subsequently developed. Band intensities were quantified with Bio-Rad ImageLab software and were normalized according to gel loading.
Western Blotting
Following film exposure for quantification of methylation signal, the PVDF membrane was washed two times with 100% methanol and two times in PBS-T to remove the PPO. The washed membrane was then blocked with blocking solution (5% milk in PBS-T) for one hour at room temperature. Polyclonal anti-GST primary antibody (Abcam, ab19256) was used at a 1:20,000 dilution. The secondary antibody (horseradish peroxidase-conjugated light-chain specific mouse anti-rabbit IgG, Bio-Rad) was used at a 1:10,000 dilution. The chemiluminescent reaction was performed using Amersham ECL Prime Western Blotting Detecting Reagent (GE Healthcare).
Bioinformatics Analysis
TMpred (Prediction of Transmembrane Regions and Orientation) (Hofmann and Stoffel 1993) and TMHMM (Predication of Transmembrane Helices in Proteins, v2.0) (Krogh et al. 2001) were used (with the default model parameters) to predict the locations of transmembrane domains (TMDs).
Data and Reagent Availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of this article are represented fully within the article and its figures. Figure S1 shows the sequence alignments of the predicted arginine methylation motifs from related receptors, as included in the Discussion. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6193037.
Results
PRMT5 methylates the C. elegans SER-2 receptor
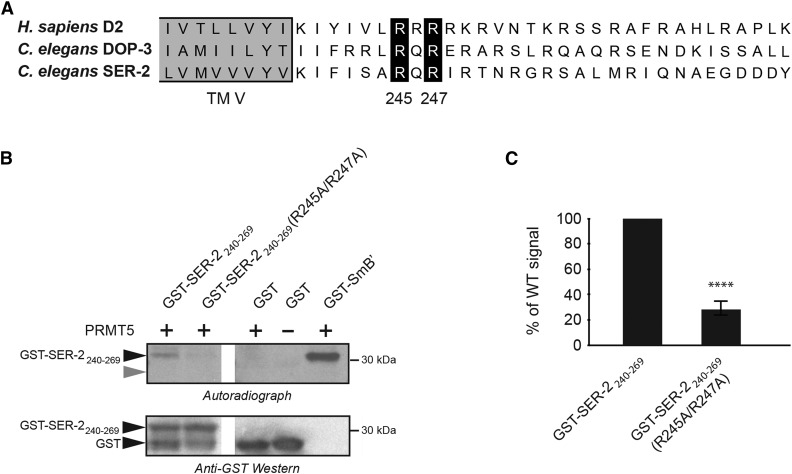
We previously reported the PRMT5-dependent methylation of two D2-like dopamine GPCRs, the human D2 and C. elegans DOP-3 receptors (Likhite et al. 2015). These receptors contain an arginine methylation motif in the third intracellular loop that is highly conserved across species (Likhite et al. 2015). We wished to investigate the possible methylation of another GPCR, the C. elegans SER-2 tyramine receptor. SER-2 has a predicted methylation motif in the third intracellular loop with identical placement to those seen in the human D2 and C. elegans DOP-3 receptors (Figure 1A). To test the ability of the methyltransferase PRMT5 to methylate the SER-2 receptor, we performed an in vitro methylation assay. A recombinant fragment of the third intracellular loop of the SER-2 receptor [amino acid residues 240-269, flanked both amino- and carboxy- terminally with an S-tag to increase solubility, and fused to glutathione S-transferase (GST)] was methylated by PRMT5 (Figure 1B). To determine whether the arginines of the predicted methylation motif (Arg245 and Arg247) were necessary for SER-2 methylation, we generated a recombinant fragment in which these arginines were changed to alanines (R245A/R247A). SER-2 receptor methylation was markedly diminished when the two conserved arginine residues were replaced with alanines (Figure 1B). Quantification of the bands from three independent experiments showed that less than 30% of the wild-type SER-2240-269 signal was present when SER-2240-269(R245A/R247A) was used as substrate (Figure 1C). These data establish the third intracellular loop of the SER-2 receptor as a substrate for PRMT5 in vitro and suggest that Arg245 and Arg247 are key sites of methylation within this region.
Figure 1.
Human PRMT5 methylates the C. elegans SER-2 receptor in vitro. (A) Alignment showing conservation of the predicted arginine methylation motifs of the human (D2) and C. elegans (DOP-3) D2-like dopamine receptors, along with the C. elegans SER-2 tyramine receptor. Resides Arg245 and Arg247 of SER-2 are indicated and lie within a conserved RXR motif, in which X can be any amino acid residue. The entire third intracellular loop of SER-2 is comprised of 133 amino acids (representing residues 239-371); only residues 239-269 of the third intracellular loop are shown. The gray shading indicates the end of transmembrane domain five (TM V) of the receptors. (B) Representative blot for the in vitro methylation assay. A wild-type and mutant recombinant fragment of the third intracellular loop of the C. elegans SER-2 receptor [amino acid residues 240-269, flanked both amino- and carboxy- terminally with an S-tag to increase solubility, and fused to glutathione S-transferase (GST)] were used in an in vitro methylation assay with active recombinant human PRMT5. There are no arginines within the S-tag. A GST-tagged portion of SmB’ protein, a robust PRMT5 substrate (Goulet et al. 2007), served as the positive control and GST was used as the negative control. The autoradiograph shows that the wild-type GST-SER-2240-269 fragment was methylated by PRMT5, while methylation of GST-SER-2240-269(R245A/R247A) was significantly diminished (P ≤ 0.0001). Gray arrowhead indicates the molecular weight position of GST in the autoradiograph, which was not methylated. Anti-GST Western blotting of the polyvinylidene difluoride (PVDF) membrane was used to normalize values for equivalent substrate loading. Figure panels were made from a single exposure of the membrane; lanes unrelated to this study were cut from the image. GST-SmB’ does not appear on the Western because much less was used as substrate and loaded relative to the other lanes. Molecular mass markers (kDa) are indicated on the right. (C) Quantification of the degree of methylation of the receptor fragments was based on densitometric analysis of the autoradiographs. The degree of GST-SER-2240-269(R245A/R247A) methylation was 30% of that of the wild-type (WT) fragment. Error bar represents the standard error of the mean (SEM) from three independent experiments. **** P ≤ 0.0001.
C. elegans PRMT-5 contributes to the regulation of locomotion by exogenous tyramine
Having identified the SER-2 receptor as a substrate for PRMT5-mediated methylation in vitro, we wished to determine the extent to which arginine methylation affects SER-2 signaling in vivo. To do this, we examined C. elegans behaviors that are modulated by tyramine signaling through the SER-2 receptor, in animals lacking the protein arginine methyltransferase PRMT-5.
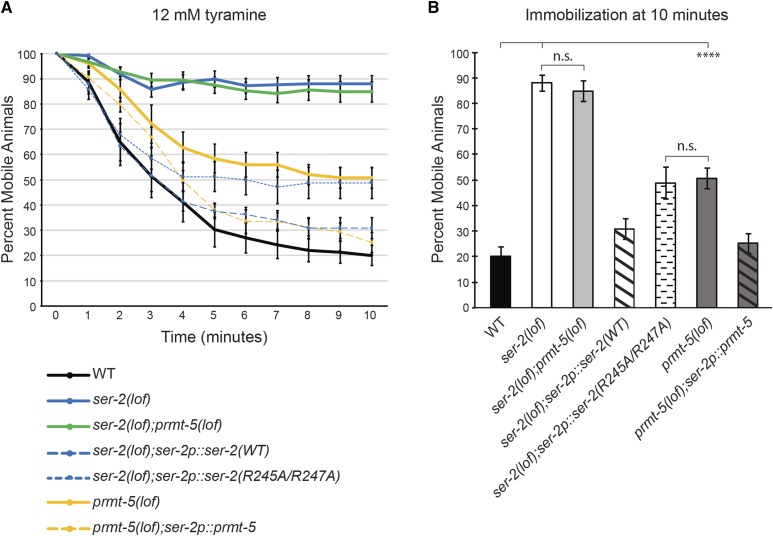
Tyramine (TA) modulates C. elegans locomotor behavior by activating SER-2 in the GABAergic motor neurons (Donnelly et al. 2013). Wild-type animals become immobilized on plates containing exogenous tyramine, while ser-2(lof) animals are resistant to this paralysis (Donnelly et al. 2013). To determine if protein arginine methylation contributes to TA-induced immobilization through SER-2 signaling in vivo, we tested the effect of exogenous TA on animals lacking PRMT-5. prmt-5(lof) animals displayed an intermediate level of immobilization when compared to wild-type and ser-2(lof) animals (Figure 2). Animals lacking both the SER-2 receptor and PRMT-5 [ser-2(lof);prmt-5(lof) double mutants] displayed immobilization levels similar to those of the ser-2(lof) single mutants, suggesting that these two proteins function in the same pathway. The partial tyramine resistance observed in prmt-5(lof) animals is consistent with PRMT-5 playing a role in promoting SER-2-mediated tyramine signaling.
Figure 2.
C. elegans lacking PRMT-5 are less susceptible to tyramine-induced immobilization. (A) prmt-5 loss-of-function animals display an intermediate immobilization phenotype when compared to wild-type and ser-2(lof) animals (P ≤ 0.001 when comparing prmt-5(lof) animals to either ser-2(lof) or wild-type animals across time). Restoring WT SER-2 function (ser-2p::ser-2) fully rescued tyramine-induced immobilization (P > 0.2 when compared to wild-type animals across time). ser-2(lof) animals expressing SER-2(R245A/R247A) displayed a partial resistance to tyramine-induced immobilization, similar to prmt-5(lof) animals (P > 0.8 across time). The percentage of mobile animals on 12 mM tyramine plates at each time-point is shown. (B) The bar graph shows the percentage of animals that became immobilized on 12 mM tyramine plates at the 10 min endpoint displayed in panel A. Alleles used: prmt-5(gk357) and ser-2(pk1357). WT = the N2 wild-type strain. For rescue experiments, the combined data of three independent transgenic lines and n ≥ 78 transgenic animals are shown. Error bars represent the standard error of the mean (SEM). **** P ≤ 0.0001. n.s. = not significant.
To determine whether PRMT-5 regulates tyramine-modulated paralysis by acting in the same cells as SER-2, we used the ser-2 promoter (ser-2p, 2.2 kb upstream of the first translational start site (Donnelly et al. 2013)) to drive prmt-5 cDNA expression and restore PRMT-5 function in ser-2-expressing cells. prmt-5(lof) animals expressing the ser-2p::prmt-5 transgene displayed an immobilization phenotype similar to that of wild-type animals (Figure 2). Using the ser-2 promoter to restore SER-2 (isoform e) receptor expression [ser-2p::ser-2(WT)] in ser-2(lof) animals also fully rescued TA-induced immobilization (Figure 2). To determine if the arginines of the predicted PRMT-5 methylation motif (Arg245 and Arg247) contributed to SER-2 function in vivo, we generated a SER-2(R245A/R247A) mutant receptor using site-directed mutagenesis. ser-2(lof) animals expressing the ser-2p::ser-2(R245A/R247A) transgene phenocopied prmt-5(lof) animals (Figure 2). Taken together, these results suggest that Arg245 and Arg247 in the predicted PRMT-5 methylation motif of the third intracellular loop of the SER-2 receptor contribute to its signaling potential in vivo. Our data are consistent with methylation of these arginines by PRMT-5 playing a role in promoting SER-2 signaling.
C. elegans PRMT-5 promotes tyramine-mediated inhibition of serotonin-stimulated pharyngeal pumping
Pharyngeal pumping is a cycle of contraction and relaxation of the pharyngeal muscle that transports bacteria from the pharynx of the worm into its intestine. Exogenous serotonin (5-HT) can stimulate pharyngeal pumping by mimicking the presence of food, while the addition of exogenous TA antagonizes 5-HT-stimulated pumping (Horvitz et al. 1982). However, consistent with SER-2 expression in pharyngeal muscles (Tsalik et al. 2003), TA does not inhibit 5-HT-stimulated pumping in ser-2(lof) animals (Rex et al. 2004).
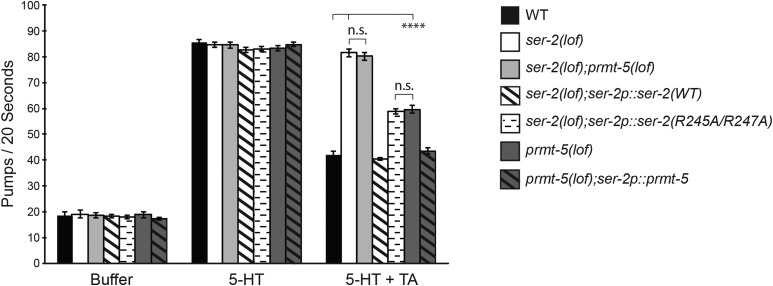
To determine if protein arginine methylation promotes TA-mediated inhibition of 5-HT-stimulated pharyngeal pumping, we first tested the effect of exogenous TA on animals lacking PRMT-5 function. Similar to the TA-induced immobilization experiments (Figure 2), prmt-5(lof) animals displayed an intermediate pharyngeal pumping phenotype when compared to wild-type and ser-2(lof) animals (Figure 3). prmt-5(lof) animals expressing the ser-2p::prmt-5 transgene to restore PRMT-5 function in ser-2-expressing cells showed a pharyngeal pumping rate similar to wild-type animals in the presence of 5-HT and TA, consistent with PRMT-5 regulating pharyngeal pumping by acting in the same cells as SER-2. To assess the contribution of Arg245 and Arg247 to SER-2 function, we compared TA inhibition of 5-HT-stimulated pharyngeal pumping in ser-2(lof) animals expressing the ser-2p::ser-2(WT) vs. ser-2p::ser-2(R245A/R247A) transgene. While ser-2(lof) animals expressing wild-type SER-2 displayed a pumping rate similar to wild-type animals, ser-2(lof) animals expressing SER-2(R245A/R247A) were again similar to animals lacking PRMT-5 (Figure 3). Combined, these results suggest that PRMT-5 promotes SER-2 signaling in pharyngeal cells, and that the two arginines in the predicted methylation motif are important for SER-2 receptor signaling in vivo.
Figure 3.
C. elegans PRMT-5 promotes TA inhibition of 5-HT-stimulated pharyngeal pumping. Animals were incubated in M9 buffer, 5-HT (10 mM), or TA (2 mM) + 5-HT (10 mM). The number of pumps per 20 sec was counted. In the presence of 5-HT + TA, prmt-5(lof) animals displayed an intermediate pharyngeal pumping phenotype when compared to wild-type and ser-2(lof) animals (P ≤ 0.0001 when comparing prmt-5(lof) animals to either ser-2(lof) or wild-type animals). prmt-5(lof) animals expressing ser-2p::prmt-5 showed a pharyngeal pumping rate similar to wild-type animals (P > 0.5) in the presence of 5-HT and TA. Restoring WT SER-2 function [ser-2p::ser-2(WT)] fully rescued TA-mediated inhibition of 5-HT stimulation (P > 0.07). ser-2(lof) animals expressing SER-2(R245A/R247A) displayed a partial TA-mediated inhibition, similar to prmt-5(lof) animals (P > 0.6). The pumps per 20 sec is shown. Alleles used: prmt-5(gk357) and ser-2(pk1357). WT = the N2 wild-type strain. For rescue experiments, the combined data of three independent transgenic lines and n ≥ 42 transgenic animals are shown. Error bars represent the standard error of the mean (SEM). **** P ≤ 0.0001. n.s. = not significant.
Loss of PRMT-5 function leads to continued foraging behavior in response to nose touch
Consistent with SER-2 expression in both the neurons and muscles of the head that affect head movement (Tsalik et al. 2003; Rex et al. 2004; Donnelly et al. 2013; Wilson et al. 2017), ser-2(lof) animals fail to suppress foraging behavior while reversing in response to nose touch (Rex et al. 2004). To determine if protein arginine methylation regulates C. elegans foraging behavior, we first assessed the extent to which prmt-5(lof) animals suppress foraging in response to nose touch. While only 16% of wild-type animals continued foraging while reversing, 51% of prmt-5(lof) animals continued foraging as they reversed following nose touch (Figure 4). By comparison, 60% of ser-2(lof) animals did not suppress foraging while reversing. Restoration of PRMT-5 function in ser-2-expressing cells of prmt-5(lof) animals (by expressing the ser-2p::prmt-5 transgene) suppressed foraging to the extent seen in wild-type animals. Furthermore, while expression of wild-type SER-2 in ser-2(lof) animals suppressed foraging to wild-type levels, ser-2(lof) animals expressing SER-2(R245A/R247A) did not fully suppress foraging, similar to prmt-5(lof) animals. Taken together, these data are consistent with a role for arginine methylation promoting SER-2 signaling to dampen foraging behavior in response to nose touch.
Figure 4.
C. elegans PRMT-5 suppresses foraging in response to nose touch. The presence or absence of foraging behavior following nose touch was scored during the reversal response. prmt-5(lof) animals displayed an intermediate foraging phenotype when compared to wild-type and ser-2(lof) animals (P ≤ 0.01 when comparing prmt-5(lof) animals to either ser-2(lof) or wild-type animals). prmt-5(lof) animals expressing ser-2p::prmt-5 showed a foraging rate similar to wild-type animals (P > 0.8). Restoring wild-type SER-2 function [ser-2p::ser-2(WT)] in ser-2(lof) animals fully rescued the suppression of foraging (P > 0.6 when compared to wild-type animals). ser-2(lof) animals expressing SER-2(R245A/R247A) displayed an intermediate degree of foraging, similar to prmt-5(lof) animals (P > 0.2). No Foraging = inhibition of head movement while reversing; Foraging = continuous head movement while reversing; No Response = no reversal upon nose touch. Alleles used: prmt-5(gk357) and ser-2(pk1357). WT = the N2 wild-type strain. For rescue experiments, the combined data of three independent transgenic lines and n ≥ 45 transgenic animals (5 trials per animal) are shown. Error bars represent the standard error of the mean (SEM). ** P ≤ 0.01. n.s. = not significant.
Discussion
Our lab previously reported the first functional evidence that signaling through GPCRs, specifically D2-like dopamine receptors (human D2 and C. elegans DOP-3), is regulated by protein arginine methylation (Likhite et al. 2015). Herein, we provide evidence that a third GPCR, the C. elegans SER-2 tyramine receptor, is also functionally regulated by protein arginine methylation. We show that human PRMT5 methylates the third intracellular loop of the SER-2 receptor in vitro, and that mutating the conserved arginines (Arg245/Arg247) of the putative methylation motif reduced this methylation (Figure 1). C. elegans PRMT-5 also promotes SER-2 regulated behaviors in vivo, and changing the conserved arginines of SER-2 reduced its ability to regulate these behaviors (Figures 2-4). This work, combined with our previous study (Likhite et al. 2015), suggests that protein arginine methylation may serve as an important regulatory means to modulate the activity of a diversity of GPCRs.
Natural predators of wild C. elegans include nematophagous fungi (Barron 1977) armed with constricting hyphal rings that can capture worms (Thorn andBarron 1984). Mechanosensory stimulation of the hyphal rings, such as by nematodes, triggers their constriction to facilitate prey capture (Schmidt et al. 2007). Therefore, it has been proposed that C. elegans may elude capture by suppressing their head movements while reversing after they encounter these fungi, allowing them to escape without triggering ring constriction. One of the main neuron pairs involved in the suppression of head movements is the set of tyraminergic RIM interneurons. Specifically, RIM-ablated animals fail to suppress foraging behavior while reversing in response to light anterior touch, suggesting that TA release from the RIMs inhibits these head oscillations (Alkema et al. 2005).
Nose touch is primarily sensed by the ASH polymodal nociceptive sensory neurons (with a small contribution from the FLP and OLQ sensory neurons) (Kaplan and Horvitz 1993). The RIM interneurons appear to be part of a “disinhibitory” circuit that serves to tonically dampen locomotor reversals (Piggott et al. 2011). In this model, when a relatively weak stimulus (e.g., nose touch) is encountered, the ASH nociceptors signal to the AIBs which, in turn, inhibit the RIMs (Piggott et al. 2011). With the RIMs silenced, reversals are enabled through a parallel stimulatory circuit (ASH to the AVA/AVD/AVE command interneurons) (Piggott et al. 2011). It was proposed that, in this scenario, the inhibition of RIM also allows for head oscillations (foraging) while animals reverse in response to nose touch (Piggott et al. 2011), which would be consistent with the behavioral observations of Alkema et al. (2005).
However, it was reported that the RIMs can in fact be stimulated or inhibited by AIB, depending on the strength of the sensory stimulus delivered to the ASH sensory neurons (Piggott et al. 2011). For example, the ASHs also detect high osmolarity (Bargmann et al. 1990; Kaplan and Horvitz 1993; Hart et al. 1999; Hilliard et al. 2005), which is a more noxious stimulus than nose touch (Mellem et al. 2002). In response to the stronger stimulus of osmotic shock delivered to ASH, AIB stimulates RIM (Piggott et al. 2011). The presumed associated release of TA from RIM then inhibits foraging while animals reverse in response to high osmolarity (Piggott et al. 2011). We propose that nose touch signaling through ASH can indeed inhibit foraging behavior, as first reported by Rex et al. (2004) and repeated here (Figure 4), but that its ability to do so is dependent upon whether the strength of the mechanosensory stimulation is sufficient to elicit tyramine release from RIM. Nuanced differences in the execution of the nose touch assay, including the source and thickness of the hair presented as the obstacle for the “nose-on” collision, influence the efficacy of the nose touch response (D. M. Ferkey, unpublished observations) and likely lead to a difference in signal strength through ASH. We suggest a model in which nose touch is detected by ASH and, if it generates a strong enough signal that AIB promotes tyramine release from RIM, it activates the SER-2 receptors found on C. elegans head muscles (Tsalik et al. 2003; Rex et al. 2004; Donnelly et al. 2013; Wilson et al. 2017) to inhibit foraging behavior. This model would be consistent with a general need to inhibit foraging while reversing as a survival mechanism, regardless of whether that reversal was triggered by activation of ALM/AVM (anterior touch) or ASH (nose touch or osmotic shock). In each case, activation of the sensory neurons would trigger a reversal response meant to elude danger. Further supporting this model, we note the discrepancy between labs for whether foraging is inhibited while animals reverse in response to high osmolarity; Alkema et al. (2005) did not see suppression of foraging in wild-type animals under their assay conditions, while Piggott et al. (2011) did. Again, small differences in the set-up or execution of the assay could affect the strength of the signal delivered to the ASHs.
Tyramine receptors have been identified in numerous invertebrate species, including fruit flies (Saudou et al. 1990), honeybees (Blenau et al. 2000) and cockroaches (Blenau et al. 2017), suggesting that tyramine’s role as a neurotransmitter extends beyond C. elegans. Like SER-2, each of these receptors also has a putative methylation motif within its third intracellular loop (Figure S1), suggesting that methylation may regulate tyraminergic signaling across multiple phyla. Interestingly, these tyramine receptors, along with the human D2 and C. elegans DOP-3 receptors, all signal through Gαi/o, which traditionally functions to inhibit adenylyl cyclase and decrease cAMP production (Saudou et al. 1990; Malek et al. 1993; Voss et al. 1993; Vanden Broeck et al. 1995; Blenau et al. 2000; Bofill-Cardona et al. 2000; Rex and Komuniecki 2002; Neve et al. 2004; Likhite et al. 2015). One possibility is that arginine methylation may preferentially modulate signaling through Gαi/o-coupled receptors. However, as only a limited number of receptors have been examined to date, it is also possible that methylation regulates G protein-coupled signaling broadly. Consistent with the latter, many of the GPCRs identified by Likhite et al. (2015) to contain a putative methylation motif do not couple with Gαi/o.
BLAST (Basic Local Alignment Search Tool) analysis identified the serotonin 1A (5-HT1A) receptor as the closest human homolog of the tyraminergic SER-2 receptor (Likhite et al. 2015). Similar to the invertebrate TA receptors, the 5-HT1A receptor couples to Gαi/o to mediate inhibitory neurotransmission (Barnes and Sharp 1999). The 5-HT1A receptor also has a putative methylation motif within its third intracellular loop (Figure S1), suggesting that even though the receptor binds a different biogenic amine, its regulation by methylation has likely been conserved through evolution.
Although not examined here, the most recently reported SER-2-regulated behavior is the formation and retrieval of imprinted olfactory memories (Jin et al. 2016). Exposing juvenile C. elegans to pathogenic bacteria early in their life leads to a long-lasting aversion of the bacteria, with sensory neurons signaling to both AIB and the tyraminergic RIM interneurons to form the imprinted olfactory memory (Jin et al. 2016). The RIMs, which are necessary for memory formation, release tyramine that signals through the SER-2 receptor expressed on the AIY interneurons (Jin et al. 2016). The SER-2 receptor (and AIY interneurons) is necessary for retrieval of the olfactory memory. However, an additional tyraminergic GPCR, TYRA-2, is also required for imprinted olfactory aversion (Jin et al. 2016). We have found that, like SER-2, TYRA-2 contains a putative methylation motif in its third intracellular loop (Figure S1), suggesting that methylation of these receptors may contribute to the formation and/or retrieval of imprinted memories. For example, the introduction of pathogenic bacteria may alter the methylation status of these receptors, perhaps in a cell-specific manner.
The addition of a methyl group to an arginine residue removes a hydrogen bond donor and decreases the electrostatic surface potential at the residue, resulting in a change in size and hydrophobicity that can affect its interaction with binding partners (Bedford and Clarke 2009). Thus, protein arginine methylation plays a key role in regulating protein-protein interactions, and could regulate the activity of GPCRs by modulating the binding (or activation) of G proteins or accessory regulator proteins that interact with the third intracellular loop. Previous studies have also shown that arginine methylation can regulate the local phosphorylation state of target proteins. For example, PRMT1 (a type 1 PRMT) -mediated methylation of FOXO transcription factors (Yamagata et al. 2008; Takahashi et al. 2011) or BAD (BCL-2 antagonist of cell death) (Sakamaki et al. 2011) blocks their phosphorylation by Akt (also known as protein kinase B). In these cases, the methylated arginines lie within the phosphorylation motif. PRMT1-mediated arginine methylation of hnRNPK (heterogeneous nuclear ribonucleoprotein K) also blocks phosphorylation (by PKCδ) of a nearby serine (Yang et al. 2014). The predicted arginine methylation motif of the SER-2 receptor (Arg245 and Arg247) lies between two predicted sites of phosphorylation in the third intracellular loop (Ser243 and Thr250) (Gattiker et al. 2002; Rex et al. 2004). Thus, another possibility is that SER-2 methylation by PRMT-5 could regulate the local phosphorylation state of these residues to regulate receptor signaling. Finally, since GPCR phosphorylation can lead to receptor desensitization and subsequent downregulation (Moro et al. 1993; Ferguson 2001), methylation of GPCRs could antagonize phosphorylation to regulate cell-surface expression of receptors.
The work described here provides evidence of a third GPCR that is functionally regulated by arginine methylation. In humans, GPCRs are the largest family of tractable drug targets (Overington et al. 2006; Lagerström and Schiöth 2008) and are the target of over 30% of all marketed pharmaceuticals (White 2005). Given the therapeutic success associated with targeting enzymes that catalyze the post-translational modification of proteins, such as histone deacetylases (Bose et al. 2014), to treat disease, our findings may influence the development of innovative approaches to modulate G protein-coupled signaling for therapeutic benefit. Notably, methylation appears to have a modulatory effect on GPCR signaling, rather than being an absolute requirement for signaling (Likhite et al. 2015) (Figures 2-4). Therefore, a new generation of treatments based on manipulating PRMT activity and/or GPCR methylation status (mimicking, promoting or blocking) could allow for finer control over the level of signaling than receptor agonists or antagonists can provide.
Acknowledgments
We thank Aditi Chaubey for helpful feedback on this manuscript and Paul Cullen, Paul Gollnick, Doug Portman, Keith Nehrke, Andrew Samuelson and Andrew Wojtovich for valuable discussions. We thank Rick Komunicki and the Caenorhabditis Genetics Center for reagents. We are grateful to Paul Gollnick for technical assistance with protein purification, and Maggie Postolache and Mauricio Suarez for help with statistical analyses. This work was supported by the National Institutes of Health (R21MH101386-01A1 to D.M.F.).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.6193037.
Communicating editor: D. Fay
Literature Cited
- Alkema M. J., Hunter-Ensor M., Ringstad N., Horvitz H. R., 2005. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260. 10.1016/j.neuron.2005.02.024 [DOI] [PubMed] [Google Scholar]
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., et al. , 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969. [DOI] [PubMed] [Google Scholar]
- Ancelin K., Lange U. C., Hajkova P., Schneider R., Bannister A. J., et al. , 2006. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8: 623–630. 10.1038/ncb1413 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Thomas J. H., Horvitz H. R., 1990. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 55: 529–538. 10.1101/SQB.1990.055.01.051 [DOI] [PubMed] [Google Scholar]
- Barnes N. M., Sharp T., 1999. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. 10.1016/S0028-3908(99)00010-6 [DOI] [PubMed] [Google Scholar]
- Barron G. L., 1977. Nematophagous fungi: Endoparasites of Rhabditis terricola. Microb. Ecol. 4: 157–163. 10.1007/BF02014285 [DOI] [PubMed] [Google Scholar]
- Bedford M. T., Clarke S. G., 2009. Protein arginine methylation in mammals: who, what, and why. Mol. Cell 33: 1–13. 10.1016/j.molcel.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. D., 2007. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev. Recent Clin. Trials 2: 3–19. 10.2174/157488707779318107 [DOI] [PubMed] [Google Scholar]
- Blackwell B., Mabbitt L. A., 1965. Tyramine in Cheese Related to Hypertensive Crises after Monoamine-Oxidase Inhibition. Lancet 1: 938–940. 10.1016/S0140-6736(65)91257-2 [DOI] [PubMed] [Google Scholar]
- Blenau W., Balfanz S., Baumann A., 2000. Amtyr1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J. Neurochem. 74: 900–908. 10.1046/j.1471-4159.2000.0740900.x [DOI] [PubMed] [Google Scholar]
- Blenau W., Balfanz S., Baumann A., 2017. PeaTAR1B: Characterization of a Second Type 1 Tyramine Receptor of the American Cockroach, Periplaneta americana. Int. J. Mol. Sci. 18: 2279 10.3390/ijms18112279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Cardona E., Kudlacek O., Yang Q., Ahorn H., Freissmuth M., et al. , 2000. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J. Biol. Chem. 275: 32672–32680. 10.1074/jbc.M002780200 [DOI] [PubMed] [Google Scholar]
- Boisvert F. M., Cote J., Boulanger M. C., Richard S., 2003. A proteomic analysis of arginine-methylated protein complexes. Mol. Cell. Proteomics 2: 1319–1330. 10.1074/mcp.M300088-MCP200 [DOI] [PubMed] [Google Scholar]
- Borowsky B., Adham N., Jones K. A., Raddatz R., Artymyshyn R., et al. , 2001. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 98: 8966–8971. 10.1073/pnas.151105198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P., Dai Y., Grant S., 2014. Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol. Ther. 143: 323–336. 10.1016/j.pharmthera.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branscombe T. L., Frankel A., Lee J. H., Cook J. R., Yang Z., et al. , 2001. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J. Biol. Chem. 276: 32971–32976. 10.1074/jbc.M105412200 [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett S. A., Hicks T. P., 2006. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 79: 223–246. 10.1016/j.pneurobio.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., et al. , 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5: 956–964. 10.1523/JNEUROSCI.05-04-00956.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D. L., Koelle M. R., 2007. Biogenic amine neurotransmitters in C. elegans (February 20, 2007), WormBook, ed. The C. elegans Research Community WormBook 10.1895/wormbook.1.132.1, http://www.wormbook.org. [DOI]
- Croll N. A., Smith J. M., 1978. Integrated behaviour in the feeding phase of Caenorhabditis elegans (Nematoda). J. Zool. 184: 507–517. 10.1111/j.1469-7998.1978.tb03304.x [DOI] [Google Scholar]
- D’Andrea G., D’Amico D., Bussone G., Bolner A., Aguggia M., et al. , 2013. The role of tyrosine metabolism in the pathogenesis of chronic migraine. Cephalalgia 33: 932–937. 10.1177/0333102413480755 [DOI] [PubMed] [Google Scholar]
- Donnelly J. L., Clark C. M., Leifer A. M., Pirri J. K., Haburcak M., et al. , 2013. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 11: e1001529 10.1371/journal.pbio.1001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrizio E., El Messaoudi S., Polanowska J., Paul C., Cook J. R., et al. , 2002. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 3: 641–645. 10.1093/embo-reports/kvf136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. S., 2001. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53: 1–24. [PubMed] [Google Scholar]
- Fukushige T., Hawkins M. G., McGhee J. D., 1998. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev. Biol. 198: 286–302. [PubMed] [Google Scholar]
- Gattiker A., Gasteiger E., Bairoch A., 2002. ScanProsite: a reference implementation of a PROSITE scanning tool. Appl. Bioinformatics 1: 107–108. [PubMed] [Google Scholar]
- Goulet I., Gauvin G., Boisvenue S., Cote J., 2007. Alternative splicing yields protein arginine methyltransferase 1 isoforms with distinct activity, substrate specificity, and subcellular localization. J. Biol. Chem. 282: 33009–33021. 10.1074/jbc.M704349200 [DOI] [PubMed] [Google Scholar]
- Hart A. C., Kass J., Shapiro J. E., Kaplan J. M., 1999. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J. Neurosci. 19: 1952–1958. 10.1523/JNEUROSCI.19-06-01952.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A. C., Sims S., Kaplan J. M., 1995. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85. 10.1038/378082a0 [DOI] [PubMed] [Google Scholar]
- He W., Ma X., Yang X., Zhao Y., Qiu J., et al. , 2011. A role for the arginine methylation of Rad9 in checkpoint control and cellular sensitivity to DNA damage. Nucleic Acids Res. 39: 4719–4727. 10.1093/nar/gkq1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. A., Apicella A. J., Kerr R., Suzuki H., Bazzicalupo P., et al. , 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24: 63–72. 10.1038/sj.emboj.7600493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Stoffel W., 1993. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe Seyler 374: 166. [Google Scholar]
- Horvitz H. R., Chalfie M., Trent C., Sulston J. E., Evans P. D., 1982. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014. 10.1126/science.6805073 [DOI] [PubMed] [Google Scholar]
- Huang J., Vogel G., Yu Z., Almazan G., Richard S., 2011. Type II arginine methyltransferase PRMT5 regulates gene expression of inhibitors of differentiation/DNA binding Id2 and Id4 during glial cell differentiation. J. Biol. Chem. 286: 44424–44432. 10.1074/jbc.M111.277046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Pokala N., Bargmann C. I., 2016. Distinct Circuits for the Formation and Retrieval of an Imprinted Olfactory Memory. Cell 164: 632–643. 10.1016/j.cell.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. M., Horvitz H. R., 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90: 2227–2231. 10.1073/pnas.90.6.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E. L., 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305: 567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- Lagerström M. C., Schiöth H. B., 2008. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7: 339–357 (erratum: Nat. Rev. Drug Discov. 7: 542) 10.1038/nrd2518 [DOI] [PubMed] [Google Scholar]
- Likhite N., Jackson C. A., Liang M. S., Krzyzanowski M. C., Lei P., et al. , 2015. The protein arginine methyltransferase PRMT5 promotes D2-like dopamine receptor signaling. Sci. Signal. 8: ra115 10.1126/scisignal.aad0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek D., Munch G., Palm D., 1993. Two sites in the third inner loop of the dopamine D2 receptor are involved in functional G protein-mediated coupling to adenylate cyclase. FEBS Lett. 325: 215–219. 10.1016/0014-5793(93)81076-C [DOI] [PubMed] [Google Scholar]
- Meister G., Eggert C., Buhler D., Brahms H., Kambach C., et al. , 2001. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11: 1990–1994. 10.1016/S0960-9822(01)00592-9 [DOI] [PubMed] [Google Scholar]
- Mellem J. E., Brockie P. J., Zheng Y., Madsen D. M., Maricq A. V., 2002. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron 36: 933–944. 10.1016/S0896-6273(02)01088-7 [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro O., Lameh J., Sadee W., 1993. Serine- and threonine-rich domain regulates internalization of muscarinic cholinergic receptors. J. Biol. Chem. 268: 6862–6865. [PubMed] [Google Scholar]
- Neve K. A., Seamans J. K., Trantham-Davidson H., 2004. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 24: 165–205. 10.1081/RRS-200029981 [DOI] [PubMed] [Google Scholar]
- Overington J. P., Al-Lazikani B., Hopkins A. L., 2006. How many drug targets are there? Nat. Rev. Drug Discov. 5: 993–996. 10.1038/nrd2199 [DOI] [PubMed] [Google Scholar]
- Piggott B. J., Liu J., Feng Z., Wescott S. A., Xu X. Z., 2011. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 147: 922–933. 10.1016/j.cell.2011.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirri J. K., McPherson A. D., Donnelly J. L., Francis M. M., Alkema M. J., 2009. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron 62: 526–538. 10.1016/j.neuron.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex E., Hapiak V., Hobson R., Smith K., Xiao H., et al. , 2005. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J. Neurochem. 94: 181–191. 10.1111/j.1471-4159.2005.03180.x [DOI] [PubMed] [Google Scholar]
- Rex E., Komuniecki R. W., 2002. Characterization of a tyramine receptor from Caenorhabditis elegans. J. Neurochem. 82: 1352–1359. 10.1046/j.1471-4159.2002.01065.x [DOI] [PubMed] [Google Scholar]
- Rex E., Molitor S. C., Hapiak V., Xiao H., Henderson M., et al. , 2004. Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J. Neurochem. 91: 1104–1115. 10.1111/j.1471-4159.2004.02787.x [DOI] [PubMed] [Google Scholar]
- Ringstad N., Abe N., Horvitz H. R., 2009. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325: 96–100. 10.1126/science.1169243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T., 2005. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50: 447–477. 10.1146/annurev.ento.50.071803.130404 [DOI] [PubMed] [Google Scholar]
- Roeder T., Seifert M., Kahler C., Gewecke M., 2003. Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 54: 1–13. 10.1002/arch.10102 [DOI] [PubMed] [Google Scholar]
- Sakamaki J., Daitoku H., Ueno K., Hagiwara A., Yamagata K., et al. , 2011. Arginine methylation of BCL-2 antagonist of cell death (BAD) counteracts its phosphorylation and inactivation by Akt. Proc. Natl. Acad. Sci. USA 108: 6085–6090. 10.1073/pnas.1015328108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F., Amlaiky N., Plassat J. L., Borrelli E., Hen R., 1990. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 9: 3611–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. R., Dorfelt H., Perrichot V., 2007. Carnivorous fungi from Cretaceous amber. Science 318: 1743 10.1126/science.1149947 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Daitoku H., Hirota K., Tamiya H., Yokoyama A., et al. , 2011. Asymmetric arginine dimethylation determines life span in C. elegans by regulating forkhead transcription factor DAF-16. Cell Metab. 13: 505–516. 10.1016/j.cmet.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Tee W. W., Pardo M., Theunissen T. W., Yu L., Choudhary J. S., et al. , 2010. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 24: 2772–2777. 10.1101/gad.606110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn R. G., Barron G. L., 1984. Carnivorous mushrooms. Science 224: 76–78. 10.1126/science.224.4644.76 [DOI] [PubMed] [Google Scholar]
- Tsalik E. L., Niacaris T., Wenick A. S., Pau K., Avery L., et al. , 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263: 81–102. 10.1016/S0012-1606(03)00447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Broeck J., Vulsteke V., Huybrechts R., De Loof A., 1995. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J. Neurochem. 64: 2387–2395. 10.1046/j.1471-4159.1995.64062387.x [DOI] [PubMed] [Google Scholar]
- Voss T., Wallner E., Czernilofsky A. P., Freissmuth M., 1993. Amphipathic alpha-helical structure does not predict the ability of receptor-derived synthetic peptides to interact with guanine nucleotide-binding regulatory proteins. J. Biol. Chem. 268: 4637–4642. [PubMed] [Google Scholar]
- Wang M., Fuhrmann J., Thompson P. R., 2014. Protein arginine methyltransferase 5 catalyzes substrate dimethylation in a distributive fashion. Biochemistry 53: 7884–7892. 10.1021/bi501279g [DOI] [PubMed] [Google Scholar]
- Wang M., Xu R. M., Thompson P. R., 2013. Substrate specificity, processivity, and kinetic mechanism of protein arginine methyltransferase 5. Biochemistry 52: 5430–5440. 10.1021/bi4005123 [DOI] [PubMed] [Google Scholar]
- White A., 2005. Biotechnology and pharmaceutical companies take aim at promising drug targets, IBC Life Sciences, Westborough, MA. [Google Scholar]
- Wilson M. A., Iser W. B., Son T. G., Logie A., Cabral-Costa J. V., et al. , 2017. skn-1 is required for interneuron sensory integration and foraging behavior in Caenorhabditis elegans. PLoS One 12: e0176798 10.1371/journal.pone.0176798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, W. B., 1988 The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
- Wragg R. T., Hapiak V., Miller S. B., Harris G. P., Gray J., et al. , 2007. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J. Neurosci. 27: 13402–13412. 10.1523/JNEUROSCI.3495-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Daitoku H., Takahashi Y., Namiki K., Hisatake K., et al. , 2008. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol. Cell 32: 221–231. 10.1016/j.molcel.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Yang J. H., Chiou Y. Y., Fu S. L., Shih I. Y., Weng T. H., et al. , 2014. Arginine methylation of hnRNPK negatively modulates apoptosis upon DNA damage through local regulation of phosphorylation. Nucleic Acids Res. 42: 9908–9924. 10.1093/nar/gku705 [DOI] [PMC free article] [PubMed] [Google Scholar]