Abstract
Metabolic flexibility, the capacity to adapt to fuel availability for energy production, is crucial for maintaining whole-body energy homeostasis. An inability to adequately promote FA utilization is associated with lipid accumulation in peripheral tissues and contributes to the development of insulin resistance. In vivo assays to quantify whole-body lipid oxidation in mouse models of insulin resistance are lacking. We describe a method for assessing whole-body FA oxidation in vivo, as well as tissue-specific lipid uptake in conscious mice. The method relies on intravenous administration of [9,10-3H(N)]palmitic acid combined with a non-β-oxidizable palmitate analog, [1-14C]2-bromopalmitic acid. Pretreatment with etomoxir, a CPT1 inhibitor that prevents the shuttling of FAs into mitochondria, markedly reduced the appearance of the β-oxidation product 3H2O in circulation and reduced lipid uptake by oxidative tissues including heart and soleus muscle. Whole-body fatty oxidation was unaltered between chow- or high-fat-fed WT and transgenic mice expressing a mutant form of the AMPK γ3 subunit (AMPKγ3R225Q) in skeletal muscle. High-fat feeding increased lipid oxidation in WT and AMPKγ3R225Q transgenic mice. In conclusion, this technique allows for the assessment of the effect of pharmaceutical agents, as well as gene mutations, on whole-body FA oxidation in mice.
Keywords: diet effects/lipid metabolism, fatty acids/oxidation, metabolic kinetics
The ability to readily switch between glucose and lipids as fuel source to maintain whole-body energy homeostasis is impaired in metabolic diseases such as obesity and T2D where the oversupply and possible underutilization of FAs is linked to insulin resistance (1). Thus, understanding of the contribution of key metabolic tissues such as skeletal muscle, liver, and adipose tissue to whole-body FA oxidation (FAO) is relevant for the development of therapeutic approaches (2–6). Whole-body and tissue-specific glucose metabolism can be assessed with sensitive in vivo methods such as hyperinsulinemic-euglycemic clamp utilizing radioactive tracers ([3-3H]glucose and 2[14C]deoxyglucose) (7), but comparable methods for reliable in vivo assessments of lipid metabolism in mice are lacking.
Indirect calorimetry in metabolic chambers based on measures of O2 consumption and CO2 production allow for an estimate of whole-body fuel preference. However, this methodology does not provide for an assessment of tissue-specific FAO (8). In primary cells, as well as in isolated tissues or tissue homogenates, the uptake and fate of radiolabeled FAs can be readily assessed by quantifying the incorporation into triglycerides or the production of by-products of the β-oxidation enzymatic reactions (9, 10). Lipid tracer dilution studies assess the turnover rate of plasma FFAs in humans and dogs in vivo (11, 12). Furthermore, whole-body oxidation can be estimated using mass balance techniques with 14C-labeled FAs (i.e., the clearance of the β-oxidation by-product 14CO2 from the system) (13–15). An in vivo assay has been developed to assess tissue-specific plasma FFA utilization in anesthetized rats via a short-term intravenous administration of 14C-palmitate as well as the non-β-oxidizable analog 3H-2-bromopalmitate functioning as a proxy for lipid uptake (16). The rate of whole-body FAO was also assessed by measuring the production of 3H2O from the infusion of 3H-palmitate in conscious catheterized rats (17). However, these techniques have not been adapted for mouse models, limiting the ability to assess effects of gene ablation or overexpression on whole-body lipid metabolism. Tools to study lipid metabolism in a tissue-specific manner, as well as at the whole-body level, are useful to investigate the metabolic cause and consequence of conditions such as dyslipidemia, obesity, and T2D.
AMP-activated protein kinase (AMPK) is a major energy sensor in the cell and orchestrates the regulation of glucose and lipid metabolism to match the energy demand (18). Due to the role of AMPK in insulin-independent and exercise-mediated glycolysis and lipid oxidation, strategies to activate this pathway have been of interest over the last decades (19–23). AMPK is a heterotrimeric protein complex formed by a catalytic α and regulatory β and γ subunits, with α and β subunits encoded by two genes (α1 and α2 or β1 and β2) and the γ subunit encoded by three genes (γ1, γ2, and γ3) (24, 25). Naturally occurring mutations in the AMPKγ3 subunit that alter functional and metabolic properties of skeletal muscle, the main expression site of this subunit, have been identified in pigs (26, 27). Transfection of AMPK trimers containing α2, β2, and a mutant (R225Q) form of γ3 in COS cells increases AMPK activity and markedly diminishes AMPK dependence on AMP, as compared with trimers containing a WT γ3 subunit. While the expression of the AMPKγ3R225Q mutation alters metabolic properties of skeletal muscle (28, 29), the effects of this mutation on whole-body lipid oxidation are unknown. This is physiologically relevant given the potential utility of AMPK activators for the treatment of metabolic diseases such as T2D or obesity.
In this study, we present a method to assess whole-body FAO in conscious mice using 3H-palmitate adapted to the high rate of metabolism in mice. We validate the sensitivity of the assay in WT mice pretreated with etomoxir, an inhibitor of CPT1-mediated mitochondrial lipid transport and β-oxidation. In addition, we assess the effect of skeletal muscle overexpression of the AMPKγ3R225Q mutation on whole-body lipid metabolism in chow- or high-fat-fed mice.
MATERIALS AND METHODS
Animals
Animal experiments were approved by the regional animal ethical committee at Stockholm North. Five to 6 month old male C57BL/6J mice (Charles River, Germany) were used to establish the conditions for the in vivo assay and test the effects of etomoxir on whole-body lipid metabolism. AMPKγ3R225Q mice (5–6 months of age) and WT littermates (28) fed chow (R34, Lantmännen, Sweden) or, beginning at 17 weeks of age, high-fat diet containing 55% fat (TD.93075, Harlan Laboratories) for 6 weeks were studied. Mice were genotyped by PCR as described previously (28). Animals were group-housed in a temperature- and light-controlled environment (12 h light/12 h dark) and had ad libitum access to food and water.
In vivo FAO in conscious mice
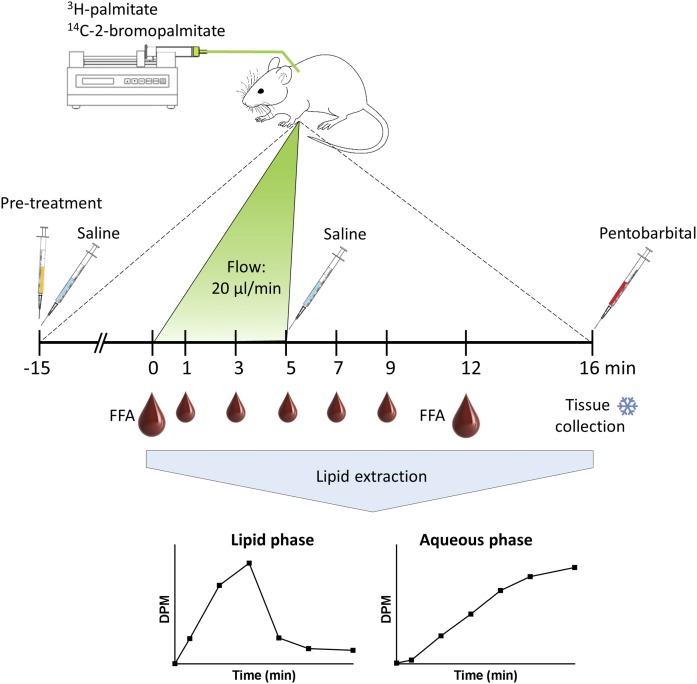
Right jugular vein catheterization was performed on isoflurane-anesthetized mice with 0.3 mm inner diameter Micro-Renathane catheters (AgnTho’s, Sweden). Catheters were secured on the back of the animal through a small incision in the skin. Carprofen analgesia was administered during the surgery, and analgesic treatment was continued for 2 days. Mice were left to recover and monitored for at least 4 days postsurgery in single cages. Mice were fasted for 2 h (8 AM to 10 AM) prior to the experiment. Thereafter, body weight was recorded, glycemia was measured (OneTouch Ultra 2 glucose meter, LifeScan), and 2 h-fasted blood samples were collected for the determination of plasma FFAs. Blood samples were subjected to centrifugation for 6 min at 10,000g (4°C), and plasma was stored at −80°C. Infusate per mouse was prepared with 107 DPM of [9,10-3H(N)]palmitic acid (NET043001MC, PerkinElmer, CA) and 107 DPM of [1-14C]2-bromopalmitic acid (MC 451, Moravek Inc., CA) dried under nitrogen steam and reconstituted in 100 µl of saline containing 1.2% BSA and 0.15 mM palmitate. Before the start of the infusion of the tracer (t = 0), the catheter was flushed with saline, and the infusion rate was set to 20 μl/min. Blood samples (15 µl) were collected from the tail tip using heparinized capillaries at t = 0, 1, 3, 5, 7, 9, and 12 min, and whole blood was directly frozen in liquid N2 (Fig. 1). At t = 5 min, the pump (Univentor, Malta) was disconnected, and the catheter was flushed with saline. An additional blood sample was obtained at t = 12 and subjected to centrifugation for the determination of plasma FFA levels. After the last blood sample collection, mice were euthanized via an injection of pentobarbital sodium into the jugular vein (t = 16 min), and skeletal muscle [tibialis anterior (TA), extensor digitorum longus (EDL), and soleus], gonadal adipose tissue [white adipose tissue (WAT)], liver, and heart were dissected, cleaned from blood, and quickly frozen in liquid N2. In some cases, mice were injected with 5 mg/kg (+)-etomoxir sodium salt hydrate (Sigma-Aldrich, Germany) dissolved in saline into the jugular vein 15 min prior to the tracer infusion.
Fig. 1.
Schematic diagram outlining the in vivo assay to determine FAO in conscious mice. Radioactive lipid tracer (107 DPM of 3H-palmitate and 107 DPM of 14C-2-bromopalmitate) is administered through a jugular vein catheter at a flow rate of 20 µl/min for 5 min. Blood samples are collected throughout the infusion period and up to t = 12 min, after which the mouse is euthanized via pentobarbital injection. Tissues are collected and frozen in liquid nitrogen. Lipid extraction performed on blood and tissue samples allows for the separation of infused radioactive lipids from the β-oxidation by-product 3H2O that accumulates in the aqueous phase.
Blood processing and tracer analysis
Lipid extraction was performed as described previously (30) on whole-blood samples collected during the experiment, and the infused radioactive lipids were separated from the β-oxidation by-product (3H2O in the aqueous phase). Samples were rotated overnight at room temperature with an addition of 300 µl of isopropanol/0.1% acetic acid. Samples were then rotated for an additional 10 min after an addition of 600 µl of hexane and 150 µl of 1 M KCl. The phases were allowed to separate, and the upper organic phase containing the lipids was collected, vacuum-dried for 1 h, and reconstituted in 50 µl of methanol:chloroform (1:1) before being transferred into scintillation vials. The lower phase (aqueous phase) was treated with 300 µl of 1 M NaOH and shaken for 30 min at 50°C to dissolve cell debris. After neutralization with 300 µl of 1 M HCl, the samples were transferred into scintillation vials, and radioactivity was measured (1414 Win Spectral Liquid Scintillation Counter, Wallac). Based on the assumption that trace amounts of [9,10-3H(N)]palmitic acid and [1-14C]2-bromopalmitic acid behave like endogenous FAs as observed in previous studies in rats (16), the rate of FAO was calculated with the individual specific activity of 3H-palmitate, and appearance of 3H2O in the blood were normalized by the baseline FFA level and body weight for each mouse. The following formula was used, where d[3H2O]/dt0,9 is obtained from the linear regression of the appearance of 3H2O in the blood between t = 0 and t = 9, describes the clearance of 3H-palmitate from the blood during the same time frame, FFAt0 represents the plasma FFAs at baseline, and BW refers to the body weight of the mouse.
Tissue processing
Lipid extraction was performed as described above on weighed and disrupted tissue pieces (TissueLyzer; Qiagen, Hilden, Germany) to quantify 3H-palmitate and 3H2O in each tissue. For the quantification of 14C-2-bromopalmitate uptake in each tissue, samples were weighed, digested in 300 µl of 1 M NaOH for 30 min at 50°C, and neutralized with 300 µl of 1 M HCl before being transferred into scintillation vials.
FFA quantification
Circulating FFAs were measured using the colorimetric Free Fatty Acid Kit ab65341 (Abcam, UK) according to the manufacturer’s instructions.
Glycogen assay
Skeletal muscle glycogen was measured in EDL muscle (10–15 mg) using a glycogen assay kit according to the manufacturer’s instructions (Abcam, Cambridge, UK).
Statistical analysis
All data are presented as mean ± SEM. Differences between two groups were determined by Student’s t-test. Differences between more than two groups were determined by two-way ANOVA followed by Bonferroni’s posthoc test. Differences were considered statistically significant at P < 0.05. Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software Inc., CA).
RESULTS
Inhibition of in vivo FAO by etomoxir
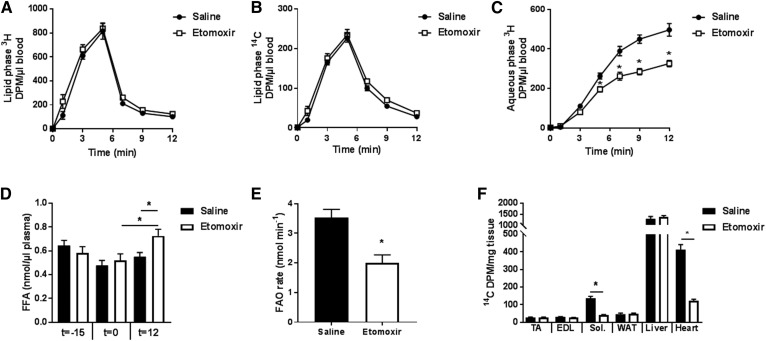
Conscious male WT mice were treated with etomoxir (5 mg/kg) or saline 15 min prior to the experiment. Kinetics of 3H-palmitate and 14C-2-bromopalmitate were determined in blood samples for each mouse (Fig. 2A, B). The continuous jugular vein infusion of 3H-palmitate resulted in a sharp increase in 3H-specific activity in the blood during the first 5 min, followed by a drop after the infusion was stopped (Fig. 2A). Similar curves were obtained with 14C-2-bromopalmitate (Fig. 2B). Etomoxir pretreatment was without effect on the appearance of labeled lipids in the circulation (Fig. 2A, B). We next determined the appearance of 3H2O in blood over time. 3H2O appearance in blood was detected 3 min following the initiation of the infusion and further increased at 12 min following the infusion (Fig. 2C). Abundance of 3H2O in the blood was significantly decreased by 33% in the etomoxir pretreated animals compared with saline-treated mice, indicating effective blockage of the mitochondrial FA transporter CPT1 and β-oxidation of 3H-palmitate (Fig. 2C). To calculate the rate of whole-body FAO, the circulating level of FFAs was determined in both groups. At the time of the pretreatment (t = −15min), as well as at baseline, levels of plasma FFAs were comparable between groups (Fig. 2D). At the end of the procedure, plasma FFA levels were elevated after etomoxir treatment compared with baseline or saline pretreatment (Fig. 2D). Etomoxir treatment reduced the rate of FAO (44%) (Fig. 2E). The infusion of 3H-palmitate was combined with the non-β-oxidizable FFA analog, 14C-2-bromopalmitate functioning as a proxy for tissue-specific FA uptake. Quantification of 14C-2-bromopalmitate in individual tissues revealed that etomoxir treatment reduced FA uptake by soleus muscle and heart. The uptake of 14C-2-bromopalmitate by TA and EDL muscle, as well as gonadal adipose tissue and liver, was unaltered by etomoxir treatment (Fig. 2F).
Fig. 2.
Etomoxir inhibits whole-body FAO. Kinetics of the appearance of 3H-palmitate (A) and 14C-2-bromopalmitate (B) in the blood. C: Determination of appearance of the β-oxidation by-product 3H2O in the blood (aqueous phase) of 2 h fasted male C57Bl/6J mice pretreated with either saline (black circles) or the CPT1-inhibitor etomoxir (white squares). D: Plasma FFA levels were measured at the time of pretreatment (t = −15), the beginning of the tracer infusion (t = 0), and 12 min after the infusion onset (t = 12). E: Rate of whole-body FAO. F: Tissue-specific uptake of non-β-oxidizable 14C-2-bromopalmitate of saline- or etomoxir-treated mice. Filled bars, saline; open bars, etomoxir. Results are shown as mean ± SEM. * P < 0.05. n = 7 mice. DPM, disintegrations per minute; Sol, soleus.
Skeletal muscle overexpression of AMPKγ3R225Q does not affect whole-body FAO
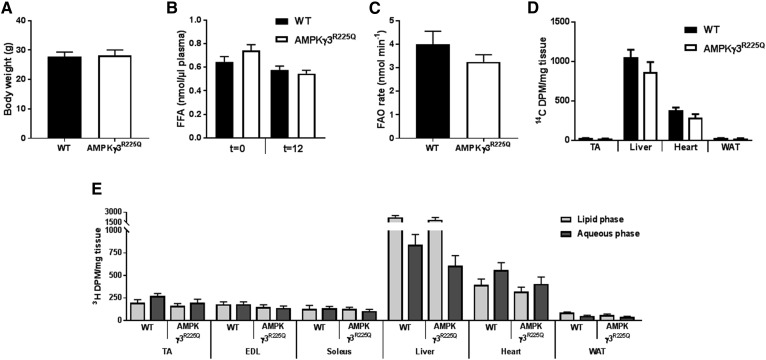
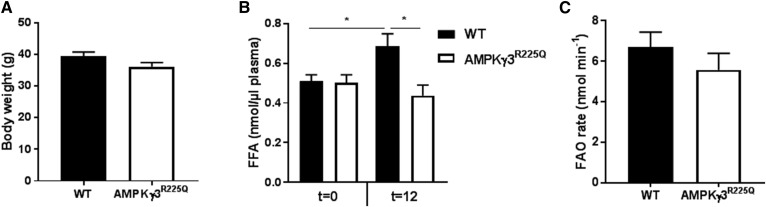
The effects of AMPKγ3R225Q on the rate of whole-body FAO was determined. Body weight and basal plasma FFA levels were similar between chow-fed AMPKγ3R225Q and WT mice (Fig. 3A, B). Glycogen content was increased in EDL muscle from AMPKγ3R225Q as compared with WT mice (0.62 ± 0.07 vs. 0.88 ± 0.05 mg/g wet weight, respectively; P = 0.01). Moreover, the rate of FAO, assessed via the appearance of 3H2O in the blood, was similar between chow-fed AMPKγ3R225Q and WT mice (Fig. 3C). Tissue-specific uptake of 14C-2-bromopalmitate was similar between AMPKγ3R225Q and WT mice (Fig. 3D). To further assess whether the oxidation potential of skeletal muscle from AMPKγ3R225Q mice is increased, the fate of 3H-palmitate in tissues was assessed. Accumulation of 3H-palmitate and 3H2O in tissue homogenates was similar between genotypes. However, TA muscle and heart oxidized 3H-palmitate to a greater relative extent as compared with EDL, soleus, liver, and WAT (Fig. 3E). High-fat feeding increased body weight as compared with chow-fed mice, independent of genotype (Fig. 4A). High-fat feeding also increased blood glucose levels independent of genotype (11.7 ± 0.7 and 11.9 ± 0.4 mM for high-fat-fed versus 9.6 ± 0.3 and 9.3 ± 0.5 mM for chow-fed AMPKγ3R225Q and WT mice, respectively; P < 0.05) Basal (t = 0) plasma FFA levels were similar between AMPKγ3R225Q and WT mice irrespective of diet (Figs. 3B, 4B). The rate of whole-body FAO was similar between high-fat fed AMPKγ3R225Q and WT mice (Fig. 4C). Nevertheless, high-fat diet increased the rate of FAO, given that high-fat-fed mice oxidized significantly more 3H-palmitate compared with chow-fed mice irrespective of genotype (Figs. 3C, 4C).
Fig. 3.
Whole-body FAO is unaltered by skeletal muscle expression of AMPKγ3R225Q in chow-fed mice. Body weight (A), plasma FFAs (B), and whole-body FAO rate (C) of 2 h fasted male chow-fed WT (filled bars) or skeletal muscle-specific AMPKγ3R225Q transgenic (open bars) mice. D: The total accumulation of 14C was quantified in the tissues. E: Lipid extraction was performed on tissues and 3H abundance was quantified in the lipid phase (light gray) and aqueous phase (dark gray). Results are shown as mean ± SEM. n = 5–8 mice. DPM, disintegrations per minute.
Fig. 4.
Whole-body FAO is unaltered by skeletal muscle expression of AMPKγ3R225Q in high-fat-fed mice. Body weight (A), plasma FFAs (B), and whole-body FAO rate (C) of male 2 h fasted WT (filled bars) or skeletal muscle-specific AMPKγ3R225Q transgenic (open bars) mice after 6 weeks of high-fat feeding. Results are shown as mean ± SEM. * P < 0.05. n = 9–10 mice.
DISCUSSION
Methods to study whole-body lipid metabolism are critical to advance the understanding of the pathogenesis of obesity and T2D. However, approaches to determine the effects of pharmacological or genetic strategies on lipid metabolism in vivo in mouse models are lacking. In this study, we present an in vivo method for the assessment of whole-body FAO in conscious mice. Quantification of 3H2O production and determination of the rate of FAO is achieved via a jugular vein infusion of 3H-palmitate and collection of blood and multiple tissue samples. The simultaneous administration of the non-β-oxidizable 14C-2-bromopalmitate allows for the quantification of FA uptake by individual tissues. The acute pretreatment of conscious mice with etomoxir, a CPT1 inhibitor, reduced whole-body FAO, establishing the reliability and sensitivity of the technical approach presented in this study. Additionally, we show that skeletal muscle expression of a mutant form of the AMPK γ3 isoform (AMPKγ3R225Q) does not alter whole-body FAO in either chow-fed or high-fat-fed mice.
Etomoxir reduces palmitate oxidation both in vitro and in vivo by blocking the uptake of long-chain FAs into mitochondria (16, 31, 32). Short- and long-term pharmacological inhibition of CPT1 (33), as well as heterozygous deletion of the skeletal muscle isoform Cpt1b (34), suppresses palmitate oxidation. In diet-induced obese CPT1b+/− mice, the suppression of palmitate oxidation is accompanied by increased glucose oxidation in skeletal muscle (34). Etomoxir reduced FA uptake in heart and soleus muscle, consistent with an earlier study in rats (16) that provided evidence for reduced tissue-specific clearance of FFA in oxidative tissues such as heart, with no change in nonoxidative tissues such as glycolytic EDL muscle (16). This indicates that oxidative tissues are the main consumers of FFA as a fuel source for oxidative metabolism under resting conditions. On the whole-body level, we found the overall clearance of 14C-2-bromopalmitate from the circulation unaltered, suggesting that the contribution of red oxidative skeletal muscle to whole-body FA uptake appears to be minor. Moreover, FAO by liver, a tissue that mainly drives whole-body FA uptake, was unaltered by etomoxir. Among all tissues studied, the accumulation of 3H2O was greatest in liver, confirming earlier work in rat (16). Altering lipid oxidation in additional tissues, including liver and glycolytic skeletal muscle, by higher doses of etomoxir or alternatively tissue-specific inhibitors of CPT1 isoforms may have a greater effect on whole-body metabolism.
AMPK activation increases the abundance of mitochondrial enzymes and lipid metabolism in skeletal muscle (28, 35, 36). Previously, we have reported that in AMPK transgenic mice, overexpression of the R225Q mutant form results in increased glucose uptake and oleate oxidation in isolated EDL muscle of high-fat-fed animals that are furthermore protected from insulin resistance. This is presumably due to increased lipid oxidation and reduced intramuscular lipids (28, 29). The elevated lipid oxidation in glycolytic skeletal muscle of AMPKγ3R225Q mice is associated with increased expression of PGC-1α and several transcription factors that regulate mitochondrial biogenesis (37), consistent with increased citrate synthase activity in longissimus dorsi muscle of Hampshire pigs where the AMPKγ3 mutation was first described (38). Here, we report that the whole-body FAO rate is unaltered between AMPKγ3R225Q and WT littermates under either chow-fed or high-fat-fed conditions. Although this result was unexpected, it underscores the importance of the contribution of heart, liver, and other tissues with a higher oxidative capacity as compared with skeletal muscle to whole-body FAO. Moreover, the AMPKγ3R225Q mutant is overexpressed in glycolytic skeletal muscle and not present in highly oxidative organs (28). Tracer distribution analyses in the individual tissues of WT and AMPKγ3R225Q mice reveal that heart was the organ with the highest accumulation of the oxidation by-product in comparison to the abundance of 3H-palmitate. While AMPK activation in glycolytic skeletal muscle did not increase whole-body FAO, we cannot exclude the possibility that increasing AMPK activity in oxidative skeletal muscle through alternative means may have an effect. Moreover, time-restricted feeding to promote changes in energy expenditure and fuel utilization (39), disruption of the insulin-signaling pathway (40), or deletion of ACC, a target of AMPK, may elevate whole-body FAO (41).
Short-term high-fat feeding is associated with a shift in whole-body metabolism from glucose utilization toward lipids, concomitant with increased adiposity, hepatic steatosis, and impaired glucose tolerance (42, 43). Conversely, longer-term high-fat diets are associated with reduced lipid oxidation and skeletal muscle insulin resistance (44, 45). We report that high-fat diet led to a robust increase in whole-body FAO in WT and AMPKγ3R225Q mice as compared with respective chow-fed control mice. The elevation in whole-body FAO was consistent with previous reports in rodents (42, 43) and may be associated with increased mitochondrial FAO capacity in skeletal muscle (42).
Over recent years, the assessment of FA metabolism using stable isotopes coupled to mass spectrometry analysis has been developed (46, 47). This methodology allows for noninvasive and prolonged measurements, but comes at high costs for specialized equipment. The method established herein presents the advantage of being accessible to laboratories that routinely use radioactive tracers. In conclusion, we introduce an in vivo technique that allows for an assessment of whole-body lipid oxidation in conscious mice based on the oxidation of 3H-palmitate. Application of this methodology for metabolic studies of genetically modified mouse strains may reveal novel insights into the regulation of lipid metabolism. In addition, this technique is a potent tool for the validation of novel metabolically active compounds to improve lipid metabolism for the treatment of obesity and T2D.
Footnotes
Abbreviations:
- AMPK
- AMP-activated protein kinase
- EDL
- extensor digitorum longus
- FAO
- FA oxidation
- TA
- tibialis anterior
This work was funded by the Novo Nordisk Foundation NNF14OC0009941; Torsten Söderbergs Foundation M71/15; Karolinska Institutet Strategic Research Program in Diabetes 2009-1068; Swedish Research Council Grant 2015 00165; and Swedish Diabetes Foundation Grant DIA2015-032. The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Goodpaster B. H., and Sparks L. M.. 2017. Metabolic flexibility in health and disease. Cell Metab. 25: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sindelar D. K., Chu C. A., Rohlie M., Neal D. W., Swift L. L., and Cherrington A. D.. 1997. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes. 46: 187–196. [DOI] [PubMed] [Google Scholar]
- 3.Kelley D. E., and Mandarino L. J.. 2000. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 49: 677–683. [DOI] [PubMed] [Google Scholar]
- 4.Consitt L. A., Bell J. A., and Houmard J. A.. 2009. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 61: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ertunc M. E., and Hotamisligil G. S.. 2016. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 57: 2099–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carobbio S., Pellegrinelli V., and Vidal-Puig A.. 2017. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Adv. Exp. Med. Biol. 960: 161–196. [DOI] [PubMed] [Google Scholar]
- 7.Ayala J. E., Bracy D. P., Malabanan C., James F. D., Ansari T., Fueger P. T., McGuinness O. P., and Wasserman D. H.. 2011. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J. Vis. Exp. (57): 3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speakman J. R. 2013. Measuring energy metabolism in the mouse—theoretical, practical, and analytical considerations. Front. Physiol. 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akie T. E., and Cooper M. P.. 2015. Determination of fatty acid oxidation and lipogenesis in mouse primary hepatocytes. J. Vis. Exp. (102): e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huynh F. K., Green M. F., Koves T. R., and Hirschey M. D.. 2014. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 542: 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miles J. M., Ellman M. G., McClean K. L., and Jensen M. D.. 1987. Validation of a new method for determination of free fatty acid turnover. Am. J. Physiol. 252: E431–E438. [DOI] [PubMed] [Google Scholar]
- 12.Issekutz B. Jr., Bortz W. M., Miller H. I., and Paul P.. 1967. Turnover rate of plasma FFA in humans and in dogs. Metabolism. 16: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 13.Havel R. J., Naimark A., and Borchgrevink C. F.. 1963. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1–C14. J. Clin. Invest. 42: 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillioja S., Foley J., Bogardus C., Mott D., and Howard B. V.. 1986. Free fatty acid metabolism and obesity in man: in vivo in vitro comparisons. Metabolism. 35: 505–514. [DOI] [PubMed] [Google Scholar]
- 15.Wisneski J. A., Gertz E. W., Neese R. A., and Mayr M.. 1987. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J. Clin. Invest. 79: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakes N. D., Kjellstedt A., Forsberg G. B., Clementz T., Camejo G., Furler S. M., Kraegen E. W., Olwegard-Halvarsson M., Jenkins A. B., and Ljung B.. 1999. Development and initial evaluation of a novel method for assessing tissue-specific plasma free fatty acid utilization in vivo using (R)-2-bromopalmitate tracer. J. Lipid Res. 40: 1155–1169. [PubMed] [Google Scholar]
- 17.Oakes N. D., Kjellstedt A., Thalen P., Ljung B., and Turner N.. 2013. Roles of fatty acid oversupply and impaired oxidation in lipid accumulation in tissues of obese rats. J. Lipids. 2013: 420754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carling D. 2017. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 45: 31–37. [DOI] [PubMed] [Google Scholar]
- 19.Winder W. W., and Hardie D. G.. 1996. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. 270: E299–E304. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., and Goodyear L. J.. 1998. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 47: 1369–1373. [DOI] [PubMed] [Google Scholar]
- 21.Koistinen H. A., Galuska D., Chibalin A. V., Yang J., Zierath J. R., Holman G. D., and Wallberg-Henriksson H.. 2003. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 52: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 22.Al-Khalili L., Krook A., Zierath J. R., and Cartee G. D.. 2004. Prior serum- and AICAR-induced AMPK activation in primary human myocytes does not lead to subsequent increase in insulin-stimulated glucose uptake. Am. J. Physiol. Endocrinol. Metab. 287: E553–E557. [DOI] [PubMed] [Google Scholar]
- 23.Kjøbsted R., Munk-Hansen N., Birk J. B., Foretz M., Viollet B., Björnholm M., Zierath J. R., Treebak J. T., and Wojtaszewski J. F.. 2017. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes. 66: 598–612. [DOI] [PubMed] [Google Scholar]
- 24.Oakhill J. S., Scott J. W., and Kemp B. E.. 2009. Structure and function of AMP-activated protein kinase. Acta Physiol. (Oxf.). 196: 3–14. [DOI] [PubMed] [Google Scholar]
- 25.Ross F. A., MacKintosh C., and Hardie D. G.. 2016. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 283: 2987–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson L. 2003. Identification and characterization of AMPK gamma 3 mutations in the pig. Biochem. Soc. Trans. 31: 232–235. [DOI] [PubMed] [Google Scholar]
- 27.Mahlapuu M., Johansson C., Lindgren K., Hjalm G., Barnes B. R., Krook A., Zierath J. R., Andersson L., and Marklund S.. 2004. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 286: E194–E200. [DOI] [PubMed] [Google Scholar]
- 28.Barnes B. R., Marklund S., Steiler T. L., Walter M., Hjalm G., Amarger V., Mahlapuu M., Leng Y., Johansson C., Galuska D., et al. 2004. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J. Biol. Chem. 279: 38441–38447. [DOI] [PubMed] [Google Scholar]
- 29.Barnes B. R., Glund S., Long Y. C., Hjalm G., Andersson L., and Zierath J. R.. 2005. 5′-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J. 19: 773–779. [DOI] [PubMed] [Google Scholar]
- 30.Massart J., Zierath J. R., and Chibalin A. V.. 2014. A simple and rapid method to characterize lipid fate in skeletal muscle. BMC Res. Notes. 7: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., and McGarry J. D.. 1987. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J. Biol. Chem. 262: 9812–9821. [PubMed] [Google Scholar]
- 32.Mott D. M., Hoyt C., Caspari R., Stone K., Pratley R., and Bogardus C.. 2000. Palmitate oxidation rate and action on glycogen synthase in myoblasts from insulin-resistant subjects. Am. J. Physiol. Endocrinol. Metab. 279: E561–E569. [DOI] [PubMed] [Google Scholar]
- 33.Ceccarelli S. M., Chomienne O., Gubler M., and Arduini A.. 2011. Carnitine palmitoyltransferase (CPT) modulators: a medicinal chemistry perspective on 35 years of research. J. Med. Chem. 54: 3109–3152. [DOI] [PubMed] [Google Scholar]
- 34.Kim T., Moore J. F., Sharer J. D., Yang K., Wood P. A., and Yang Q.. 2014. Carnitine palmitoyltransferase 1b deficient mice develop severe insulin resistance after prolonged high fat diet feeding. J. Diabetes Metab. 5: 1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrill G. F., Kurth E. J., Hardie D. G., and Winder W. W.. 1997. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 273: E1107–E1112. [DOI] [PubMed] [Google Scholar]
- 36.Winder W. W., Holmes B. F., Rubink D. S., Jensen E. B., Chen M., and Holloszy J. O.. 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88: 2219–2226. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Roves P. M., Osler M. E., Holmstrom M. H., and Zierath J. R.. 2008. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J. Biol. Chem. 283: 35724–35734. [DOI] [PubMed] [Google Scholar]
- 38.Estrade M., Ayoub S., Talmant A., and Monin G.. 1994. Enzyme activities of glycogen metabolism and mitochondrial characteristics in muscles of RN-carrier pigs (Sus scrofa domesticus). Comp Biochem Physiol Biochem Mol Biol. 108: 295–301. [DOI] [PubMed] [Google Scholar]
- 39.Bruss M. D., Khambatta C. F., Ruby M. A., Aggarwal I., and Hellerstein M. K.. 2010. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 298: E108–E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Khalili L., Chibalin A. V., Kannisto K., Zhang B. B., Permert J., Holman G. D., Ehrenborg E., Ding V. D., Zierath J. R., and Krook A.. 2003. Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell. Mol. Life Sci. 60: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi C. S., Savage D. B., Abu-Elheiga L., Liu Z. X., Kim S., Kulkarni A., Distefano A., Hwang Y. J., Reznick R. M., Codella R., et al. 2007. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc. Natl. Acad. Sci. USA. 104: 16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner N., Bruce C. R., Beale S. M., Hoehn K. L., So T., Rolph M. S., and Cooney G. J.. 2007. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 56: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 43.Trajcevski K. E., O’Neill H. M., Wang D. C., Thomas M. M., Al-Sajee D., Steinberg G. R., Ceddia R. B., and Hawke T. J.. 2013. Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model. PLoS One. 8: e71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. [DOI] [PubMed] [Google Scholar]
- 45.Shortreed K. E., Krause M. P., Huang J. H., Dhanani D., Moradi J., Ceddia R. B., and Hawke T. J.. 2009. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One. 4: e7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emken E. A. 2001. Stable isotope approaches, applications, and issues related to polyunsaturated fatty acid metabolism studies. Lipids. 36: 965–973. [DOI] [PubMed] [Google Scholar]
- 47.Tumanov S., Bulusu V., and Kamphorst J. J.. 2015. Analysis of fatty acid metabolism using stable isotope tracers and mass spectrometry. Methods Enzymol. 561: 197–217. [DOI] [PubMed] [Google Scholar]