Atherosclerosis (AS), a major etiology of cardiovascular disease, is considered to be a chronic inflammatory disease characterized by excessive inflammatory cells, such as macrophages, accumulated in the arterial wall (1). As the main effector cells of the immune/inflammatory system, macrophages engulf lipids and produce various inflammatory factors, thus participating in the progress of AS (1–3). Therefore, it is very important to clarify the mechanisms that regulate macrophage-related inflammatory response for the prevention of AS.
The report in the Journal of Lipid Research by Schneider et al. (4) shows that apolipoprotein A-I binding protein (AIBP), a secreted protein that avidly binds to apoA-I, the major component of HDL, plays a key role in regulating macrophage cholesterol efflux and inflammation in AS (5). Apoa1bp−/−Ldlr−/− mice fed a high-fat diet have shown exacerbated hypercholesterolemia, hypertriglyceridemia, and larger atherosclerotic lesions compared with Ldlr−/− mice. Conversely, overexpression or injection of AIBP reduced aortic inflammation and atherosclerotic plaques. In vitro, AIBP facilitated cholesterol efflux, the first step of reverse cholesterol transport, from cultured macrophages to HDL, reducing the cholesterol content in lipid rafts that inhibited the inflammatory responses to lipopolysaccharide (LPS), suggesting the crucial role of cholesterol efflux on AIBP-mediated anti-inflammatory and immunosuppressive functions.
Previous research demonstrated that AIBP promoted ABCA1-mediated cholesterol efflux from endothelial cells and macrophages via interaction with apoA-I (6–8). In zebrafish and mice, AIBP regulated cholesterol levels in endothelial cells that control angiogenesis via depleting lipid raft content in the cell membrane, therefore inhibiting vascular endothelial growth factor receptor 2 and upregulating Notch signaling (6, 8). Meanwhile, AIBP-mediated cholesterol efflux can also impair the lipid raft-containing Toll-like receptor 4 (TLR4), which has been shown to upregulate the MYD88-mediated activation of MAPK and NF-κB signaling as well as downstream inflammatory cytokines (9, 10). In addition, AIBP has been found to promote the binding of apoA-I to ABCA1 in macrophages and prevent ABCA1 protein from COP9 signalosome subunit 2-mediated degradation so as to prevent foam cell formation (7).
Several studies have revealed that ABCA1 can directly function as an anti-inflammatory receptor for apoA-I to suppress inflammation independently of its cholesterol efflux activity (11,12). The interaction of apoA-I and ABCA1 stimulated the JAK2/STAT3 signal pathway and tristetraprolin-dependent posttranscriptional regulation of pro-inflammatory cytokines mRNA decay (11). Knockout of ABCA1 in mice increases inflammatory cell infiltration in a number of tissues, including the vessel wall and peritoneal cavity, and blood circulation (13). These results suggest that secretory AIBP modulated the immune/inflammatory response through regulating lipid transport and lipid raft-related receptor activity.
Interestingly, there may be more mechanisms to explain the action of AIBP on inflammation and AS. The APOA1BP gene that codes AIBP was renamed as the NADHX epimerase (NAXE) by the Human Gene Organization Gene Nomenclature Committee. As an epimerase in mitochondria, AIBP converts R-NADHX, R-epimers of nicotinamide adenine dinucleotide hydration (NADHX), to biologically useful S-NADHX that rapidly reconverted to nicotinamide adenine dinucleotide (NADH) (14). NADHX has been shown to inhibit several dehydrogenases (15), which is necessary for mitochondria oxidative phosphorylation (OXPHOS) (16). OXPHOS, the major function of mitochondria, has recently emerged as a central organelle that integrates cellular metabolism and inflammatory responses (17). Yu et al. (18) have recently found that OXPHOS is reduced in AS, promoting mitochondrial dysfunction and necrotic core formation. The damaged mitochondria accumulated in macrophages results in the activation of the NLRP3 inflammasome and production of IL-1β (19). In addition, impaired mitochondrial OXPHOS has been found to prevent the repolarization of pro-inflammatory macrophages to anti-inflammatory macrophages (20). Recently, mutations of NAXE in children have been reported to result in acute-onset ataxia, cerebellar edema, spinal myelopathy, and skin lesions. Increased lactate in cerebrospinal fluid and R-NADHX in fibroblasts have been shown in these dieseases, indicating that NAXE is an unheeded target for controlling metabolism and the immune/inflammation system (21).
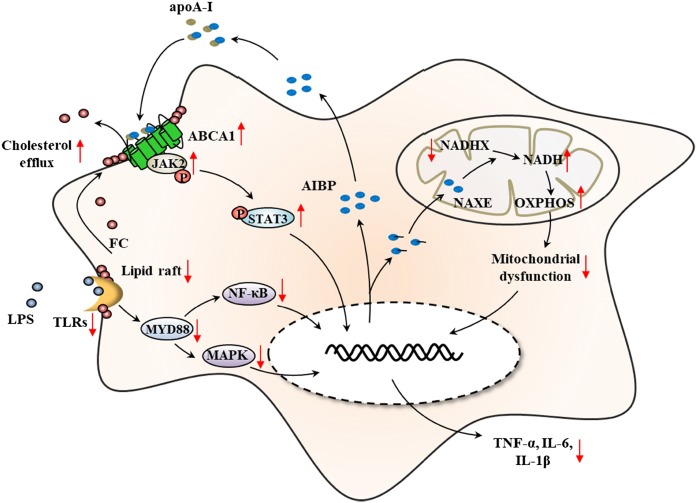
These studies improve our understanding of the potent anti-inflammatory properties of extracellular and intracellular AIBP (Fig. 1).Serum AIBP, mainly secreted from liver and kidney, inhibits inflammatory cytokine expression via TLR-4/ MyD88-mediated MAPK and NF-κB signaling as well as the ABCA1/JAK2/STAT3 pathway after binding with apoA-I. On the other hand, mitochondrial AIBP may function as an NADHX epimerase to prevent OXPHOS damage and mitochondrial dysfunction (Fig. 1). Moreover, lipid and energy metabolism may also be involved in the process of AIBP-mediated anti-inflammatory response, and this suggests that AIBP may be a novel therapeutic target for chronic metabolic inflammatory disease, such as AS.
Fig. 1.
The primary mechanisms by which AIBP protects against macrophage inflammatory response. In one way, AIBP/apoA-I disrupts lipid raft membrane micro-domains and decreases LPS-induced TLR4 activation via ABCA1-mediated cholesterol efflux, which activates MyD88-mediated NF-κB and MAPK signaling and then upregulates the expression of pro-inflammatory cytokines. In another way, AIBP may promote the direct anti-inflammatory function in an ABCA1-dependent manner via activation of JAK2/STAT3 signal pathway after interaction with apoA-I. In addition, AIBP can also function as an NADH epimerase involved in the reconversion of NADHX to NADH, which is critical for mitochondrial OXPHOS and plays a potential role in regulation of macrophage inflammation.
References
- 1.Parks B. W., and Lusis A. J.. 2013. Macrophage accumulation in atherosclerosis. N. Engl. J. Med. 369: 2352–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson G. K. 2017. Inflammation and atherosclerosis: the end of a controversy. Circulation. 136: 1875–1877. [DOI] [PubMed] [Google Scholar]
- 3.Chinetti-Gbaguidi G., Colin S., and Staels B.. 2015. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 12: 10–17. [DOI] [PubMed] [Google Scholar]
- 4.Schneider D. A., Choi S. H., Agatisa-Boyle C., Zhu L., Kim J., Pattison J., Sears D. D., Gordts P., Fang L., and Miller Y. I.. 2018. AIBP protects against metabolic abnormalities and atherosclerosis. J. Lipid Res. 59: 854–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter M., Buechler C., Boettcher A., Barlage S., Schmitz-Madry A., Orso E., Bared S. M., Schmiedeknecht G., Baehr C. H., Fricker G., et al. 2002. Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I. Genomics. 79: 693–702. [DOI] [PubMed] [Google Scholar]
- 6.Mao R., Meng S., Gu Q., Araujo-Gutierrez R., Kumar S., Yan Q., Almazan F., Youker K. A., Fu Y., Pownall H. J., et al. 2017. AIBP limits angiogenesis through gamma-secretase-mediated upregulation of notch signaling. Circ. Res. 120: 1727–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M., Li L., Xie W., Wu J. F., Yao F., Tan Y. L., Xia X. D., Liu X. Y., Liu D., Lan G., et al. 2016. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis. 248: 149–159. [DOI] [PubMed] [Google Scholar]
- 8.Fang L., Choi S. H., Baek J. S., Liu C., Almazan F., Ulrich F., Wiesner P., Taleb A., Deer E., Pattison J., et al. 2013. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 498: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., and Tall A. R.. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Jiao Y., and Xie M.. 2017. Paeoniflorin ameliorates atherosclerosis by suppressing TLR4-mediated NF-kappaB activation. Inflammation. 40: 2042–2051. [DOI] [PubMed] [Google Scholar]
- 11.Yin K., Deng X., Mo Z-C., Zhao G-J., Jiang J., Cui L-B., Tan C-Z., Wen G-B., Fu Y., and Tang C-K.. 2011. Tristetraprolin-dependent post-transcriptional regulation of inflammatory cytokine mRNA expression by apolipoprotein A-I. J. Biol. Chem. 286: 13834–13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin K., Liao D. F., and Tang C. K.. 2010. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol. Med. 16: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiello R. J., Brees D., and Francone O. L.. 2003. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 23: 972–980. [DOI] [PubMed] [Google Scholar]
- 14.Marbaix A. Y., Noel G., Detroux A. M., Vertommen D., Van Schaftingen E., and Linster C. L.. 2011. Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J. Biol. Chem. 286: 41246–41252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prabhakar P., Laboy J. I., Wang J., Budker T., Din Z. Z., Chobanian M., and Fahien L. A.. 1998. Effect of NADH-X on cytosolic glycerol-3-phosphate dehydrogenase. Arch. Biochem. Biophys. 360: 195–205. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed Yusoff, A. A., F. N. Zulfakhar, S. Z. N. Mohd Khair, W. S. Wan Abdullah, J. M. Abdullah, and Z. Idris. 2018. Mitochondrial 10398A>G NADH-dehydrogenase subunit 3 of complex I is frequently altered in intra-axial brain tumors in Malaysia. Brain Tumor Res. Treat. 6: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills E. L., Kelly B., and O’Neill L. A. J.. 2017. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18: 488–498. [DOI] [PubMed] [Google Scholar]
- 18.Yu E. P. K., Reinhold J., Yu H., Starks L., Uryga A. K., Foote K., Finigan A., Figg N., Pung Y. F., Logan A., et al. 2017. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler. Thromb. Vasc. Biol. 37: 2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ip W. K. E., Hoshi N., Shouval D. S., Snapper S., and Medzhitov R.. 2017. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 356: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Bossche J., Baardman J., Otto N. A., van der Velden S., Neele A. E., van den Berg S. M., Luque-Martin R., Chen H. J., Boshuizen M. C., Ahmed M., et al. 2016. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Reports. 17: 684–696. [DOI] [PubMed] [Google Scholar]
- 21.Kremer L. S., Danhauser K., Herebian D., Petkovic Ramadza D., Piekutowska-Abramczuk D., Seibt A., Muller-Felber W., Haack T. B., Ploski R., Lohmeier K., et al. 2016. NAXE mutations disrupt the cellular NAD(P)HX repair system and cause a lethal neurometabolic disorder of early childhood. Am. J. Hum. Genet. 99: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]