Abstract
The elucidation of the molecular basis of the rare disease, sitosterolemia, has revolutionized our mechanistic understanding of how dietary sterols are excreted and how cholesterol is eliminated from the body. Two proteins, ABCG5 and ABCG8, encoded by the sitosterolemia locus, work as obligate dimers to pump sterols out of hepatocytes and enterocytes. ABCG5/ABCG8 are key in regulating whole-body sterol trafficking, by eliminating sterols via the biliary tree as well as the intestinal tract. Importantly, these transporters keep xenosterols from accumulating in the body. The sitosterolemia locus has been genetically associated with lipid levels and downstream atherosclerotic disease, as well as formation of gallstones and the risk of gallbladder cancer. While polymorphic variants raise or lower the risks of these phenotypes, loss of function of this locus leads to more dramatic phenotypes, such as premature atherosclerosis, platelet dysfunction, and thrombocytopenia, and, perhaps, increased endocrine disruption and liver dysfunction. Whether small amounts of xenosterol exposure over a lifetime cause pathology in normal humans with polymorphic variants at the sitosterolemia locus remains largely unexplored. The purpose of this review will be to summarize the current state of knowledge, but also highlight key conceptual and mechanistic issues that remain to be explored.
Keywords: ATP binding cassette transporter G5, ATP binding cassette transporter G8, atherosclerosis, macrothrombocytopenia, platelets, bile, cholesterol, phytosterols, sitosterolemia
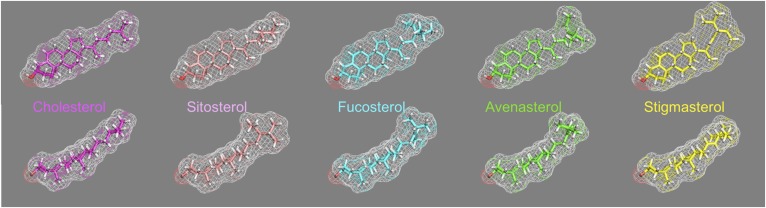
The description of the disease sitosterolemia by Bhattacharyya and Connor initiated a fundamental rewrite of how dietary sterols traffic and are eliminated by the body (1). And while sitosterolemia, named after the most abundant xenosterol detected in the plasma of the two affected sisters, was described in 1974, the disease is ancient, with mutant alleles that are several thousand years old (2). We know that the genetic locus whose dysfunction leads to sitosterolemia encodes two genes, ABCG5 and ABCG8, whose proteins function as obligate heterodimers (3, 4). ABCG5 and ABCG8 (also known as sterolins) are expressed only in hepatocytes, gallbladder epithelium, and enterocytes, and are responsible for excretion of sterols, with xenosterols preferred over cholesterol. Arguably, the naming of the disease has led to a bias in appreciating the fact that sterolins keep a vast range of xenosterols, not just sitosterol, from accumulating in the body (5). A better name should be xenosterolemia to reflect appreciation of this biology. Although the spectrum of xenosterols our diets contain is extensive, we show a few sterol structures that highlight the differences between cholesterol and these xenosterols (Fig. 1). In general, the major differences reside in the R tail of the sterol structure, with change in shape that will likely impact their organization in membranes (6–8).
Fig. 1.
Sterol structures of cholesterol as well as some xenosterols. The structure of cholesterol in two views viewed “en face” and from a side (top and bottom, respectively) is shown in the far right. The mesh indicates the overall surface-based shape of the sterol, based upon the carbons and hydrogens, and the 3-hydroxyl oxygen is shown in red in all molecules at the bottom left of each molecule for orientation. The “R” tail at the right end of each molecule shows the greatest difference between cholesterol and the xenosterols. Note in the bottom profile that stigmasterol (yellow) looks very similar to cholesterol (magenta), but in the top row profile shows the bulkiness of its R tail compared with cholesterol. Thus the packing of these sterols in a lipid bilayer will be affected by the differences in the R tail of each of these molecules.
While the importance of cholesterol trafficking is well-recognized for its roles in the pathogenesis of endothelial dysfunction, foam-cell formation, and atherosclerosis, ensuring that xenosterols are kept out and why [an evolutionary mechanism, conserved from fish to man (9)], has garnered less attention. The loss of function in humans, as well as in animal models, shows that accumulation of xenosterols leads to dramatic phenotypes, such as macrothrombocytopenia and platelet dysfunction, liver disease, and cholesterol accumulation with xanthoma formation and atherosclerosis (10), and in mouse models (but not humans), infertility, immune dysfunction, and cardiomyopathy have been reported (11–13). This begs the question whether a lifetime of low level exposure to dietary bioactive xenosterols, whose levels of entry and retention may be altered by polymorphisms in ABCG5 and ABCG8, may have biological consequences. This review will aim to highlight areas that, therefore, need further exploration.
GENETIC VARIATION AT THE SITOSTEROLEMIA LOCUS AND CLINICAL IMPLICATIONS
ABCG5 and ABCG8 were key suspects in regulating cholesterol homeostasis in atherosclerotic CVD (ASCVD). This was supported by the clinical observations of severe, and sometimes fatal, atherosclerosis in humans with sitosterolemia (though see below). Support for this contention came from the genome-wide association studies, which showed a link not only between genetic variations at sitosterolemia locus (previously referred to as STSL) and lipid levels, but also for coronary artery disease (14–16). And not surprisingly, because the ABCG5/ABCG8 heterodimer pumps cholesterol into bile (17–21), this locus was shown to predispose to gallstone disease (22–28). What is surprising, and the mechanistic connection remains to be addressed, was the link between a presumed hypermorphic variant, D19H, in ABCG8 and gallbladder cancer risk (25, 29). This locus is one of the strongest known genetic risk factors for this very rare cancer (though the genetic risk remains small). One pathway of how this may occur is that this variant leads to increased gall stone formation, subsequent inflammation, and consequently a predisposition to gallbladder cancer; this explanation remains unsubstantiated. Cancer, by its nature, requires multiple genetic changes to occur and how this would be caused or accelerated by the sitosterolemia locus remains to be explored. It is also not clear whether the variant alters the kinds of xenosterols that are concentrated in the bile leading to the formation of gallstones, prolonged inflammation, generation of genotoxic agents, and then oncogenesis.
Studies of these genetic variants have been association studies (30) and none have demonstrated a specific mechanistic pathway altered by these variants; we assume that cholesterol pumping into the bile or intestine would be altered. However, the liver has a predilection for excretion of xenosterols into bile (31), and the hypothesis that one of these dietary xenosterols is mechanistically involved has not been explored.
CLINICAL MANIFESTATIONS
The many clinical manifestations of sitosterolemia have been well-documented in a number of reviews (10, 32, 33). In summary, asymptomatic subjects (typically identified by cascade screening once a proband has been identified) can manifest no symptoms or signs to varying levels of affectations. There is no question whether affected individuals can have severe ASCVD, sometimes with fatal presentations (34–38), but with increased knowledge and case identification, many affected subjects have now been identified, who in the untreated state, seem to have very little evidence of ASCVD. Two new studies have now highlighted this. In five subjects who were diagnosed after investigation of hyperlipidemia (ages 19–32 years), no clinical evidence of ASCVD was identified (39). More recently, and provocatively, cascade screening in the Hutterites (where all of the cases have the same mutations and share a similar lifestyle), many subjects were identified with no overt clinical manifestation of ASCVD or any blood dyscrasia (40). The latter study is also remarkable, as it may shed light on an old observation by Salen and his colleagues who reported that hepatic LDL-receptor expression was increased, in contrast to the paradoxical suppression of cholesterol biosynthesis enzymes (41, 42). We had shown that children with sitosterolemia can manifest very high levels of cholesterol and had previously been misdiagnosed as having pseudo-homozygous familial hypercholesterolemia (43, 44), yet it is not clear why they have such very high levels of plasma cholesterol, a manifest defect in hepatic clearance of LDL particles. Mymin et al. (40) reported a nonsignificant inverse association between age and plasma cholesterol; when subjects were grouped by pre- or postpuberty, both plasma cholesterol and sitosterol levels were significantly higher in prepubertal compared with postpubertal subjects. In one subject, in the absence of treatment, these sterol levels also fell progressively with time, though never normalized (40). This suggests that hepatic expression of lipoprotein receptors is dramatically altered by the progressive accumulation of xenosterols, and this process is influenced by puberty.
Macrothrombocytopenia
Macrothrombocytopenia is another phenotype that has been observed in humans with sitosterolemia (45–47). Remarkably, this phenotype can be the only manifestation of sitosterolemia. The first description of this entity, Mediterranean macrothrombocytopenia, was reported in Australia (48), and we suspect it was a cohort of subjects who had sitosterolemia; all of these subjects have been lost to follow-up to allow confirmation. However, Rees et al. (45) showed that not only were plant sterols elevated in their cohort in Europe, they also confirmed mutations at the sitosterolemia locus as causative, a finding that has been now reported from many other sites around the world. This platelet phenotype is also observed in the murine models of sitosterolemia (46, 49, 50). Mutations seen in macrothrombocytopenia are also seen in sitosterolemia without the platelet phenotype, suggesting that these manifestations may be dependent upon the type of xenosterol exposure. While there is no doubt that xenosterol accumulation leads to platelet dysfunction (50), biogenesis of the platelet is also abnormal. The mechanism of how xenosterolemia affects platelet biogenesis remains to be explored. Additionally, it is assumed that these effects are from sitosterol accumulation (the most abundant accumulating xenosterol), but more bioactive less abundant xenosterols could be responsible. Other possibilities are bulk xenosterols altering raft membrane structures, or as a result of displacing cholesterol from key active sites (51).
Liver disease
Although the first case of liver failure associated with sitosterolemia (52) was regarded as an outlier or coincidence (and was only interesting as liver transplantation cured the sitosterolemia), a second case with a fatal outcome has now been reported. Bazerbachi et al. (53) reported a case of idiopathic cirrhosis, denied heart transplant from severe coronary artery disease, only to discover that the patient had sitosterolemia with the implication that both organ failures were caused by his one underlying condition. This raises the issues of the underlying mechanism for liver dysfunction and failure, and how many idiopathic cirrhosis cases are undiagnosed sitosterolemia. Mutational reviews of the two cases do not indicate any uniqueness when compared with others with sitosterolemia, and accumulation of misfolded proteins in the ER, akin to α1-antitrypsin deficiency, seems less likely (53). The Finnish case was heterozygous for mutations in ABCG8 (W361X and E423D) (52), but the US case was homozygous for premature stop codon in ABCG5 (R446X) (53). If so, then the hypothesis of toxic xenosterol accumulation is again raised. And if it is a toxic xenosterol species, it may be hard to detect as most sterol profiles have, to date, been limited to reporting sitosterol, campesterol, and stigmasterol. Other lesser known sterols are rarely reported. Expression of these mutants in vivo may be required to explore these possibilities.
Transintestinal cholesterol excretion in humans
Until recently, it was assumed that an elevation of plant sterols in the blood was diagnostic of sitosterolemia. Even in cases of liver dysfunction and failure, plant sterols remain low and the only clinical scenario where they are elevated is during administration of total parenteral nutrition, which contains large amounts of plant sterols (54, 55). This has now been challenged by a report of a case of primary biliary cirrhosis (PBC), where plant sterols were dramatically elevated (56). It should be noted that plant sterols are not normally elevated in PBC, thus this is a unique case. While the speculation was that the liver sterolin function was affected by this autoimmune process (56), it should be noted that ABCG5/ABCG8 should remain functionally active in the intestine, and they are key to transintestinal cholesterol excretion (TICE). Thus, TICE should have resulted in maintaining lower plant sterol levels [akin to the restoration of intestinal sterolin function by transgenes in the Abcg8 knockout mice (12), or in liver-only knockouts (57)], but it did not. In PBC, the secretion of bile into the biliary tree is dramatically reduced and perhaps in this case there may have been very little bile secreted; this would mean that for TICE to be effective, loss of all bile in the intestine may also impair TICE. Groen and colleagues have shown that the phospholipid content, rather than bile salts, was a major regulator of TICE (58), opening the avenue for therapeutic use of dietary phospholipids in cholestatic diseases to improve lipids via TICE and ABCG5/ABCG8. And in passing, it should be noted that TICE should be TISE, because sterols are excreted and continue to bias interpretation in a cholesterol-centric manner.
REGULATION OF ABCG5/G8
The secretion of cholesterol into bile is coupled to the secretion of phospholipids and bile acids that forms the mixed micelle acceptors for cholesterol within the aqueous lumen of the canaliculus. Mouse models of ABCG5/G8 deficiency suggest that the complex accounts for 70–90% of biliary cholesterol secretion (17, 20, 21), and in mouse models with a gain or loss of function, there is an approximate gene dosage effect on biliary sterol excretion (17, 18, 20, 59). Factors that increase or decrease the coupling of biliary cholesterol secretion to bile acids and phospholipids are likely to be ABCG5/G8 dependent and reflect changes in either ABCG5/G8 abundance or activity, although it should be noted that ABCG5/G8-independent biliary cholesterol secretion has been reported (60, 61).
Transcriptional regulation of ABCG5/G8
Expression of sterolins is restricted to the liver, small intestine, and gallbladder epithelium, but the mechanisms responsible for this limited tissue distribution have not been elucidated (3, 62, 63). Within these tissues, ABCG5 and ABCG8 are co-regulated at the transcriptional level, sharing a common bidirectional promoter of 374 base pairs that separates their initiation codons (3, 62, 64). Regulatory elements within the intergenic region have been identified for hepatocyte nuclear factor 4α (NR2A1), liver receptor homolog-1 (NR5A2), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), and Forkhead box protein O1 (64–68). Liver X receptor (LXR) response elements have been mapped to two conserved regions roughly 100 kB distal to the intergenic promoter, shown to bind both LXRα (NR1H3) and LXRβ (NR1H2) and synergistically activate the ABCG5/G8 promoter in combination with retinoid X receptor α (NR2B1), GATA4, hepatocyte nuclear factor 4α, and liver receptor homolog-1 (69). These findings corroborated earlier data in animal models demonstrating that ABCG5/G8 mRNAs were responsive to dietary cholesterol as well as LXR and retinoid X receptor agonists in a LXRα- and LXRβ-dependent fashion (70). Farnesoid X receptor (FXR; NR1H4) receptor agonists have also been shown to regulate ABCG5/G8 mRNAs in cultured hepatocytes, suggesting a direct mechanism of action. However, FXR response elements have yet to be mapped for ABCG5/G8 (70, 71). Thus, while a number of transcriptional factors are implicated in regulating the sitosterolemia locus, there remains a paucity of direct evidence of their roles.
In vivo, sterolin mRNAs and proteins can be modulated by a number of experimental conditions. Disruptions of bile acid metabolism alter hepatic ABCG5/G8 expression, presumably due to changes in bile acid signaling through FXR (72–74). Similarly, intestinal expression of ABCG5/G8 is modulated by bile acids (75, 76). Tissue-specific deletion of hepatic insulin receptors revealed a role for insulin signaling in the regulation of ABCG5/G8 through Forkhead box protein O1 (67). Alterations in ABCG5/G8 mRNAs have also been observed in experimental models of type 1 and type 2 diabetes, as well as in human diabetic subjects (77–81). Additional factors shown to alter hepatic, intestinal, or gallbladder ABCG5/G8 expression include thyroid hormone signaling, dietary calcium, iron depletion, constitutive androstane receptor agonists, and gallbladder disease (82–86). However, the molecular mechanisms underlying these effects are unclear and the extent to which they reflect novel regulatory nodes for ABCG5/G8 or are secondary to disruptions in established regulators is not known.
CpG islands have been identified near the transcriptional start sites for ABCG5 and ABCG8 and were hypomethylated in the liver and hypermethylated in kidney and cerebrum (87). This same study revealed differential histone H3 acetylation between liver and these non-ABCG5/G8-expressing tissues, suggesting that epigenetic mechanisms may control tissue-specific expression of the complex. A highly intriguing mechanism by which p53 promotes gene expression is by recognition of a structural-specific motif called triplex DNA (88). The ABCG5 proximal promoter contains a predicted T·A·T triplex. Expression of ABCG5 mRNA increased following transient transfection of p53 in cultured cells, as well as treatment with p53-stabilizing agents in p53-positive cells, but not in p53-negative cells (88). However, expression of ABCG8 was not examined, the cultured cells were not of hepatic, intestinal, or gallbladder epithelial origin, and the extent to which this mechanism influences ABCG5/G8 expression or sterol metabolism is not known. Interestingly, the insulin receptor was also identified as a T·A·T triplex-responsive gene. Whether this is coincidental or mechanistically links alterations in insulin signaling and ABCG5/G8 expression is unclear.
Regulation of complex formation and trafficking
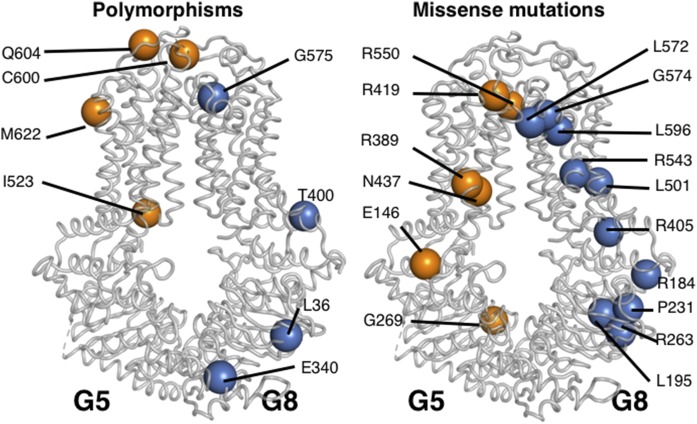
ABCG5 and ABCG8 are required to form a functional sterol transport complex. This marriage is formed as the complex folds in the ER and is dependent on the presence of N-linked glycans and the calnexin/calreticulin chaperone system (89). Using a series of chimeras comprised of the N- and C-terminal domains of ABCG1, ABCG2, ABCG5, and ABCG8, evidence for a an ER-retrieval signal within the N-terminal cytosolic domains of ABCG5 and ABCG8 that prevents the monomers from exiting the ER prematurely was identified (90). The heterodimer complex formation has now been supported by a high-resolution crystal structure (91) that will allow for future structure-function studies to be conducted (Fig. 2, graciously provided by Dr. Jyh-Yeung Lee, University of Ottawa). Although the N-terminal structures of ABCG5 and ABCG8 could not be established within the crystal structure (91), many of the nonsynonymous polymorphic changes, as well as the missense mutations can be mapped onto a structure (Fig. 2). The missense mutations (R419 and R550 in ABCG5 and L572, G574, L596, R573, L501, and L405 in ABCG8) are very close to the putative sterol-binding pocket [see also the putative sterol-binding pocket for a model of ABCG4 (92) based upon the ABCG5/ABCG8 crystal structure]. Interestingly, the polymorphic residues, I523 in ABCG5 and G575 in ABCG8, would also be close to this site and form good future candidates to explore their effect on sterol-specificity of binding. In polarized cultured hepatocytes, recombinant ABCG5/G8 was shown to reside at the canalicular membrane (93). Within the small intestine, endogenous mouse, as well as a human, ABCG8 transgenes were localized almost exclusively to the apical surface (19). However, biochemical fractionation and immunolocalization approaches suggest a broader intracellular distribution of ABCG5 and ABCG8 (94). More recently, a recombinant fluorescently tagged ABCG5/G8 complex was shown to reside in an intracellular pool that is recruited to the canalicular surface in response to a lithogenic diet (95). In isolated rat liver canalicular membranes, ABCG5 was shown to reside in Triton-soluble Lubrol-resistant microdomains (96). Similar results were obtained using bile acids as detergents in which ABCG5, ABCB4, and ABCB11 are colocalized, suggesting the complete biliary lipid secretion apparatus resides within microdomains at the canalicular surface (97).
Fig. 2.
Localization of nonsynonymous and missense amino acid changes on a model of ABCG5 (G5)-ABCG8 (G8) heterodimer crystal structure. Although the structures of the N termini of ABCG5 and ABCG8 could not be determined in the crystals subjected to X-ray diffraction, many of the known mutations and polymorphic variants could be mapped (91). This figure was kindly provided by Dr. Jyh-Yeung Lee, University of Ottawa.
Regulation of ABCG5/G8 activity
The mechanism by which ABCG5/G8 promotes (chole)sterol efflux is not fully understood. It was originally suggested that ABCG5/G8 function as a floppase, promoting the movement of cholesterol to the exofacial leaflet of the canalicular membrane based on the observation that there was less cholesterol in isolated canalicular membranes of ABCG8-deficient mice (98). In cell-based models, coexpression of ABCG5 and ABCG8 will promote cholesterol efflux to bile-acid micelles, but not other traditional acceptors, such as ApoAI, HDL, or cyclodextrins, indicating that the molecular mechanism by which ABCG5/G8 promotes sterol efflux at the apical membrane is distinct from other cholesterol efflux pumps, such as ABCA1 and ABCG1 (99, 100). Using purified native mouse ABCG5/G8, sterol transport between donor and acceptor liposomes suggests a 1:1 stoichiometry for ATP hydrolysis and cholesterol transport, and bile acids could stimulate this ATP hydrolysis (101). Among the tested bile acids, cholate was the most potent at stimulating ATPase activity, whereas its taurine or glycine conjugates were approximately 50% less effective. Using various lipid extracts and defined preparations, the presence of cholesterol stimulated ATPase activity to varying degrees. Thus, the presence of both bile acids and cholesterol appear to act cooperatively to activate the pump and promote cholesterol secretion. This is an attractive model for the coupling of bile acid and cholesterol secretion and supported by the publication of the ABCG5 and ABCG8 crystal structures that show a sterol-binding pocket, which may undergo structural changes upon ATP binding to make this more available at the luminal surface for phospholipid:bile acid acceptors to allow for excretion (91). What remains to be established is whether this mechanism is constantly active or is regulated by translocation of these heterodimers from an intracellular location to the apical surface. The crystal structure should also allow for exploration of how missense mutations disrupt function, as well as how the nonsynonymous polymorphic variants seen in humans may alter function.
Under a number of conditions, cholesterol and bile secretion can be uncoupled, resulting in reduced cholesterol excretion or the supersaturation of bile (102). The extent to which these conditions are due to alterations in ABCG5/G8 activity awaits further investigation.
MOUSE MODELS USED TO ELUCIDATE THE IMPACT OF STEROLINS IN PHYSIOLOGY AND DISEASE
The genes encoding ABCG5 and ABCG8 have been disrupted, as well as overexpressed, in mice in order to perform detailed physiological studies of sterolin function. Mice lacking functional sterolins in both liver and intestine have been created by standard gene targeting of Abcg5 (21), Abcg8 (20), or both Abcg5 and Abcg8 (17). Recently, mice were created with liver- and intestine-specific knockouts of Abcg5 and Abcg8 (57). The integration of the human ABCG5 and ABCG8 genes into the genome of mice resulted in mouse models with increased sterolin function in both liver and intestine (18) or liver only (103). In addition, adenoviral vectors have been employed in mice to increase or restore hepatic ABCG5 and ABCG8 function (19, 104, 105). These various mouse models have been employed to study the impact of ABCG5/G8 on sterol absorption and secretion, sitosterolemia, reverse cholesterol transport (RCT), TICE, metabolic disease, and atherosclerosis.
Xenosterol exclusion from the body
Like patients with sitosterolemia, mice lacking ABCG5/G8 function are unable to preferentially excrete xenosterols into bile or the intestinal lumen. When fed a diet that contains phytosterols, mice with homozygous disruption of Abcg5, Abcg8, or both Abcg5 and Abcg8 consistently developed sitosterolemia (12, 17, 20, 21, 46, 49, 72, 106). Plasma and tissue phytosterol levels in mice with intestinal- or liver-specific knockout of Abcg5 and Abcg8 were significantly less than those of mice with whole-body ABCG5/G8 deficiency (57). The rescue of intestinal ABCG5/G8 function in Abcg8−/− mice also significantly reduced, but did not normalize, plasma phytosterol concentrations (12). It can be concluded that intestinal ABCG5/G8 excretes the majority of xenosterols back into the lumen, acting as a first-pass gate. Hepatic ABCG5/G8 then pumps into the bile those xenosterols that had evaded the intestinal checkpoint.
In the absence of ABCG5/G8 function, xenosterols can accumulate at high levels in mice causing several pathological conditions. Infertility was observed in male and female Abcg5−/− and Abcg8−/− mice consuming a phytosterol-rich chow diet, but when phytosterol accumulation in the body was blocked with a meat-based (xenosterol free) diet, or the sterol absorption inhibitor, ezetimibe, fertility was restored (11, 12, 107). Several studies have shown that mice lacking functional ABCG5/G8 developed macrothrombocytopenia, a disorder characterized by significantly reduced platelet levels and increased platelet size. Abcg5−/−, Abcg8−/−, and Abcg5−/−,Abcg8−/− mice also displayed hemolytic anemia, prolonged bleeding times, and decreased platelet activation and aggregation (11, 49, 50). The macrothrombocytopenia and abnormal bleeding was the result of phytosterol accumulation in platelet membranes resulting in dysfunctional platelets (50). Mice deficient in ABCG5 and/or ABCG8 also developed cardiomyopathy characterized by phytosterol accumulation, histiocytic infiltration, multifocal fibrosis, and tissue calcification (11, 13, 49, 50). Interestingly, the development of cardiomyopathy is dependent both on the presence of high levels of xenosterols, as well as intact B and T cell function (13).
Cholesterol absorption
Action of ABCG5/G8 in the enterocytes is believed to reduce cholesterol absorption. Fractional cholesterol absorption (FCA) decreased and fecal neutral sterol excretion (FNSE) increased in transgenic mice with both intestine and liver (INTLIV-Tg) overexpression of human ABCG5/G8 (18, 108, 109). In contrast, FCA and FNSE were similar for wild-type controls and transgenic mice with liver-specific overexpression of human ABCG5/G8 (103). Because both transgenic lines had increased biliary cholesterol secretion, the difference in FCA and FNSE between the models could have been due to the human and mouse ABCG5/G8 pumping greater amounts of biliary and dietary cholesterol out of the enterocytes and back into the intestinal lumen of the INTLIV-Tg mice. However, when considering that the INTLIV-Tg mice versus the wild-type controls had 6- to 7-fold more cholesterol in bile, the absolute amount of cholesterol absorbed by the INTLIV-Tg mice versus the wild-type controls would have been significantly greater even with the 50% reduction in FCA. It is also possible that the increased FNSE was due to the bile of the INTLIV-Tg mice becoming supersaturated with cholesterol, thus reducing luminal cholesterol bioavailability to the enterocytes.
If overexpression of ABCG5/G8 decreases FCA and increases FNSE, then it would be anticipated that the absence of ABCG5/G8 would have the opposite effects. FNSE was reduced, but FCA, when measured using the fecal dual isotope or plasma dual isotope methods, was unchanged in chow-fed Abcg5−/−, Abcg8−/−, and Abcg5−/−,Abcg8−/− mice (17, 21, 72, 110, 111). Cholesterol absorption, as assessed by the movement of luminal-infused cholesterol into lymph, was found to be decreased in two studies using Abcg5−/−,Abcg8−/− mice and increased in another study using Abcg8−/− mice (112–114). Compared with wild-type controls, mice with intestinal-specific ABCG5/G8 deficiency had no changes in FCA or FNSE (57). These studies indicate that the absence of intestinal ABCG5/G8 does not lead to increased cholesterol absorption and, consequently, the reduced FNSE observed in whole-body ABCG5/G8-deficient mice could be driven by dramatically decreased biliary cholesterol secretion. Because most of the above studies were conducted in mice fed a chow diet (low in fat and cholesterol, but high in phytosterols), it will be important to determine the impact of liver and/or intestinal ABCG5/G8 deficiency on cholesterol absorption in mice consuming a Western-type diet (WTD) with a higher fat and cholesterol content. It will also be essential to assess whether cholesterol absorption in these models is affected by the inclusion in the diet of animal- or plant-derived fats that contain cholesterol and phytosterols, respectively.
Biliary cholesterol secretion
ABCG5/G8 are critical to the secretion of cholesterol into the bile. Abcg5−/−, Abcg8−/−, whole-body, and liver-specific Abcg5−/−,Abcg8−/− mice fed a chow diet displayed a 70–90% reduction in biliary cholesterol concentration or secretion (17, 20, 21, 57). In contrast, transgenic overexpression of human ABCG5/G8 in liver and intestine increased biliary cholesterol concentrations by 5-fold and >7-fold in chow-fed male and female mice, respectively (18). Biliary cholesterol concentration and secretion by hepatic overexpression of human ABCG5/G8 was unchanged on a chow diet, but was increased 1.5- to 2-fold on a high-cholesterol diet (103). In the absence of the biliary phospholipid transporter, Abcb4, biliary cholesterol concentration in mice was very low and was not increased by the hepatic expression of a human ABCG5/G8 transgene, indicating that biliary phospholipid secretion is required for ABCG5/G8-mediated cholesterol secretion (108). In addition, studies in which wild-type or Abcg8−/− mice were infused with hydrophilic and hydrophobic bile salts indicated that ABCG5/G8 drives biliary cholesterol secretion by flopping cholesterol from the inner to outer leaflet of the canalicular membrane (98). When fed a lithogenic diet, Abcg5−/− and Abcg5−/−,Abcg8−/− mice, compared with control C57BL/6J mice, had decreased incidence of gallstone development (115).
Macrophage RCT
Macrophage RCT (mRCT) is an anti-atherogenic pathway through which excess cholesterol is effluxed from plaque macrophages, transported on lipoproteins to the liver, secreted into bile, and excreted from the body. The primary method used to assess mRCT quantifies the movement of 3H-cholesterol from intraperitoneally injected macrophage foam cells to feces over 48 h (116). C57BL/6J mice with adenoviral-mediated overexpression of murine ABCG5/G8 (AdABCG5/G8) displayed increased fecal macrophage-derived 3H-neutral sterol (i.e., cholesterol), consistent with an increase in biliary cholesterol secretion. However, because the sum of 3H-neutral sterol and 3H-bile acid in feces was similar for AdABCG5/G8 and AdNull controls, it was concluded that increased hepatic ABCG5/G8 function alone does not impact macrophage-to-feces RCT. Studies in sterolin-deficient mice found that, in spite of significant reductions in biliary cholesterol secretion, mice lacking ABCG5/G8 function had no deficits in mRCT (105, 117, 118). However, treatment with LXR agonist increased 3H-neutral sterol excretion from wild-type mice, but not Abcg5−/−,Abcg8−/− mice, leading to the conclusion that stimulation of mRCT by LXR activation requires ABCG5/G8 (117).
TICE
TICE is the elimination of plasma cholesterol into the intestinal lumen by enterocytes. Several studies have indicated that intestinal ABCG5/G8 play an important role in TICE. Cholesterol fluxes, measured with tracer techniques, showed that the contribution of TICE to fractional neutral sterol excretion (FNSE) was 25% in Abcg5+/+ mice, but 15% in Abcg5−/− mice (110). Blocking cholesterol absorption with ezetimibe in wild-type mice elevated FNSE by 3.8-fold and TICE was responsible for 74% of this increase (111). Treatment of wild-type mice with the FXR agonist, PX20606, stimulated FNSE and TICE to a level similar to that observed with ezetimibe (119). In the absence of ABCG8, ezetimibe or PX20606 treatment blunted the increase in FNSE and TICE (111, 119). To gauge the importance of intestinal ABCG5/G8 in RCT, mice with intestinal-specific deficiency of ABCG5/G8 were injected with 3H-cholesterol and the levels of 3H-cholesterol in bile and feces were measured. In spite of similar biliary cholesterol and FCA, the intestinal-specific ABCG5/G8 knockouts compared with wild-type control mice had decreased fecal 3H-cholesterol excretion, likely due to a reduction in TICE (57). In contrast to the above studies, no difference in TICE was observed when measured in wild-type or sterolin mice by intestinal perfusion (120). The overall results indicate that ABCG5/G8 play a major role in TICE.
Atherosclerosis
Atherosclerosis development has been assessed in transgenic mice with both intestine and liver (IntLiv-Tg) and liver only (Liv-Tg) expression of human ABCG5/G8. Compared with controls lacking only the LDL receptor (Ldlr−/−), IntLiv-Tg;Ldlr−/− mice fed a WTD for 6 months had significantly less aortic atherosclerosis (109). The combination of increased biliary cholesterol secretion and decreased cholesterol absorption in the IntLiv-Tg;Ldlr−/− mice resulted in a significant reduction in the plasma cholesterol. In contrast, the Liv-Tg on either the LDLR or ApoE knockout background did not have reduced atherosclerosis development (103). Liv-Tg;ApoE−/− mice fed chow and Liv-Tg;Ldlr−/− mice fed WTD had significantly increased biliary cholesterol secretion, but compared with controls, no changes in cholesterol absorption or plasma cholesterol concentrations were noted. It was concluded that atherosclerosis was unaltered in IntLiv-Tg mice because the increased biliary cholesterol was being absorbed back into the body. When fed a WTD plus the cholesterol absorption inhibitor, ezetimibe, Liv-Tg;Ldlr−/− compared to Ldlr−/− mice displayed significant reductions in total plasma cholesterol and atherosclerosis development (121).
To assess the impact of ABCG5/G8 deficiency and consequently increased phytosterol levels on atherosclerosis development, whole-body ABCG5/G8-deficient mice expressing or lacking LDL receptor were studied (109). Feeding either a chow diet or WTD for 7 months caused a significant increase in plasma phytosterol concentration in Abcg5−/−,Abcg8−/− and Abcg5−/−,Abcg8−/−;Ldlr−/− mice compared with wild-type controls. Regardless of the genotype, atherosclerosis did not develop in mice fed chow. Consumption of the WTD by the Abcg5−/−,Abcg8−/−;Ldlr−/− and Abcg5+/+,Abcg8+/+;Ldlr−/− mice resulted in similar increases in total plasma sterols and atherosclerosis formation. Based upon these results, it was concluded that there was no association between phytosterol levels and atherosclerosis development in mice. The lack of difference in atherosclerosis formation between the Abcg5−/−,Abcg8−/−;Ldlr−/− and Abcg5+/+,Abcg8+/+;Ldlr−/− could have been the result of LXR activation by phytosterols offsetting the decreased biliary cholesterol secretion (122).
Metabolic disease
ABCG5/G8 function also appears to limit the development of metabolic disease. Sterolin-deficient mice fed a phytosterol-rich chow diet developed hypertriglyceridemia due to increases in intestinal triglyceride (TG) absorption and hepatic TG secretion and reductions in TG-rich lipoprotein clearance and lipoprotein lipase activity (123). When fed a phytosterol-free high-fat diet, these mice had increased hepatic TG and cholesterol accumulation leading to liver inflammation, loss of glycemic control, and insulin resistance (124). Obese db/db mice have reduced hepatic ABCG5/G8 protein and decreased biliary cholesterol (80). Adenoviral-mediated overexpression of ABCG5/G8 in db/db mice increased biliary cholesterol secretion and restored hepatic insulin sensitivity resulting in reduced fasting glucose and TGs and improved glucose tolerance (104). However, the relationship between biliary and intestinal sterol transport and the underlying mechanisms that contribute to metabolic disturbances remain unclear.
CONCLUSIONS
The human diet is very varied and exposes us to more than 60 different xenosterols, from shellfish, mushrooms, yeasts, seaweeds, plants, etc., and we need to have robust mechanisms to prevent toxic xenosterols from accumulation. The sitosterolemia locus is an evolutionarily conserved locus that has allowed organisms with a liver and biliary system to perform this function, from fish to man (9). Sitosterol may represent the most abundant of these dietary xenosterols, but this may have led to an error of scientific bias; all attention has focused on this sterol without critical questioning of whether other more potent and toxic sterols (stigmasterol, avenosterol, fucosterol, and methyl-cholesterol to name a few) are major endocrine disruptors, harmful to megakaryocyte biology, or even alter macrophage and immune function (125). In sitosterolemia (and we propose that the term xenosterolemia be used to increase this awareness), feeding a diet rich in shellfish sterols led to accumulation of these exotic sterols within the body (5). Blocking entry of these xenosterols by the drug ezetimibe has been shown to ameliorate this condition (126, 127). Control subjects who have normal ABCG5/ABCG8 function show trace plasma levels of these xenosterols, indicating that we have a daily exposure of these xenosterols to our tissues (5). Daily dietary intakes of these xenosterols are collectively greater than our daily dietary intake of cholesterol. ABCG5/G8 function as cholesterol regulators and in this role, because >99% of the mammalian body has cholesterol as its main sterol, cholesterol (and not xenosterol) trafficking remains as a key center piece. However, while the full spectrum of loss of sterolin function in humans may continue to evolve for this rare disease, the mammalian animal models have indicated that several biological steps can be disrupted by these xenosterols and that these processes are dependent on the continued presence of these xenosterols in their diets. We believe that it is time to contemplate mechanistic paths away from the cholesterol-centric view of pathophysiology, especially where diets are a key component, and consider the role of xenosterols. If so, our scientific rigor in measuring and reporting the sterol spectrum may be a first step in defining the problem.
Acknowledgments
The authors are grateful to Dr. Jyh-Yeuan (Eric) Lee for his generosity in sharing the mapping of the missense mutations and the non-synonymous polymorphic changes in ABCG5 and ABCG8 (Fig. 1).
Footnotes
Abbreviations:
- ASCVD
- atherosclerotic CVD
- FCA
- fractional cholesterol absorption
- FNSE
- fractional neutral sterol excretion
- FXR
- farnesoid X receptor
- LXR
- liver X receptor
- PBC
- primary biliary cirrhosis
- RCT
- reverse cholesterol transport
- TG
- triglyceride
- TICE
- transintestinal cholesterol excretion
- WTD
- Western-type diet
REFERENCES
- 1.Bhattacharyya A. K., and Connor W. E.. 1974. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Invest. 53: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandit B., Ahn G. S., Hazard S. E., Gordon D., and Patel S. B.. 2006. A detailed Hapmap of the sitosterolemia locus spanning 69 kb; differences between Caucasians and African-Americans. BMC Med. Genet. 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., and Hobbs H. H.. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 4.Lu K., Lee M. H., Hazard S., Brooks-Wilson A., Hidaka H., Kojima H., Ose L., Stanlenhoef A. F., Miettinen T., Bjorkhem I., et al. 2001. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am. J. Hum. Genet. 69: 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg R. E., Connor W. E., Lin D. S., and Brewer H. B. Jr. 1986. Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J. Clin. Invest. 77: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clejan S., Bittman R., and Rottem S.. 1981. Effects of sterol structure and exogenous lipids on the transbilayer distribution of sterols in the membrane of Mycoplasma capricolum. Biochemistry. 20: 2200–2204. [DOI] [PubMed] [Google Scholar]
- 7.Clejan S., and Bittman R.. 1984. Distribution and movement of sterols with different side chain structures between the two leaflets of the membrane bilayer of mycoplasma cells. J. Biol. Chem. 259: 449–455. [PubMed] [Google Scholar]
- 8.Kan C-C., and Bittman R.. 1991. Spontaneous rates of sitosterol and cholesterol exchange between phospholipid vesicles and between lysophospholipid dispersions: evidence that desorption rate is impeded by the 24a-ethyl group of sitosterol. J. Am. Chem. Soc. 113: 6650–6656. [Google Scholar]
- 9.Hazard S. E., and Patel S. B.. 2007. Sterolins ABCG5 and ABCG8: regulators of whole body dietary sterols. Pflugers Arch. 453: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S. B., and Salen G.. 2010. Sitosterolemia: xenophobia for the body. In Evidence-based Management of Lipid Disorders. M. N. Vissers, J. J. P. Kastelein, and E. S. Stroes, editors. TFM Publishing Ltd., Harley, Nr Shrewsbury, UK. 217–230. [Google Scholar]
- 11.McDaniel A. L., Alger H. M., Sawyer J. K., Kelley K. L., Kock N. D., Brown J. M., Temel R. E., and Rudel L. L.. 2013. Phytosterol feeding causes toxicity in ABCG5/G8 knockout mice. Am. J. Pathol. 182: 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solca C., Tint G. S., and Patel S. B.. 2013. Dietary xenosterols lead to infertility and loss of abdominal adipose tissue in sterolin-deficient mice. J. Lipid Res. 54: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson D. W., Oslund K. L., Lyons B., Foreman O., Burzenski L., Svenson K. L., Chase T. H., and Shultz L. D.. 2013. Inflammatory dilated cardiomyopathy in Abcg5-deficient mice. Toxicol. Pathol. 41: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatti C., Service S. K., Hartikainen A. L., Pouta A., Ripatti S., Brodsky J., Jones C. G., Zaitlen N. A., Varilo T., Kaakinen M., et al. 2009. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 41: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aulchenko Y. S., Ripatti S., Lindqvist I., Boomsma D., Heid I. M., Pramstaller P. P., Penninx B. W., Janssens A. C., Wilson J. F., Spector T., et al. 2009. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., and Hobbs H. H.. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., and Hobbs H. H.. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., and Hobbs H. H.. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282. [DOI] [PubMed] [Google Scholar]
- 20.Klett E. L., Lu K., Kosters A., Vink E., Lee M. H., Altenburg M., Shefer S., Batta A. K., Yu H., Chen J., et al. 2004. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plösch T., Bloks V. W., Terasawa Y., Berdy S., Siegler K., Van Der Sluijs F., Kema I. P., Groen A. K., Shan B., Kuipers F., et al. 2004. Sitosterolemia in ABC-Transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 126: 290–300. [Erratum. 2004. Gastroenterology. 126: 944.] [DOI] [PubMed] [Google Scholar]
- 22.Wittenburg H., Lyons M. A., Li R., Churchill G. A., Carey M. C., and Paigen B.. 2003. FXR and ABCG5/ABCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology. 125: 868–881. [DOI] [PubMed] [Google Scholar]
- 23.Wittenburg H., Lyons M. A., Li R., Kurtz U., Mossner J., Churchill G. A., Carey M. C., and Paigen B.. 2005. Association of a lithogenic Abcg5/Abcg8 allele on chromosome 17 (Lith9) with cholesterol gallstone formation in PERA/EiJ mice. Mamm. Genome. 16: 495–504. [DOI] [PubMed] [Google Scholar]
- 24.Acalovschi M., Ciocan A., Mostean O., Tirziu S., Chiorean E., Keppeler H., Schirin-Sokhan R., and Lammert F.. 2006. Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? A preliminary study in siblings with gallstones. Eur. J. Intern. Med. 17: 490–494. [DOI] [PubMed] [Google Scholar]
- 25.Buch S., Schafmayer C., Volzke H., Becker C., Franke A., von Eller-Eberstein H., Kluck C., Bassmann I., Brosch M., Lammert F., et al. 2007. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat. Genet. 39: 995–999. [DOI] [PubMed] [Google Scholar]
- 26.Grünhage F., Acalovschi M., Tirziu S., Walier M., Wienker T. F., Ciocan A., Mosteanu O., Sauerbruch T., and Lammert F.. 2007. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 46: 793–801. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Jiang Z. Y., Fei J., Xin L., Cai Q., Jiang Z. H., Zhu Z. G., Han T. Q., and Zhang S. D.. 2007. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin. Chim. Acta. 384: 80–85. [DOI] [PubMed] [Google Scholar]
- 28.Kuo K. K., Shin S. J., Chen Z. C., Yang Y. H., Yang J. F., and Hsiao P. J.. 2008. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br. J. Surg. 95: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava A., Tulsyan S., Pandey S. N., Choudhuri G., and Mittal B.. 2009. Single nucleotide polymorphism in the ABCG8 transporter gene is associated with gallbladder cancer susceptibility. Liver Int. 29: 831–837. [DOI] [PubMed] [Google Scholar]
- 30.Rudkowska I., and Jones P. J.. 2008. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr. Rev. 66: 343–348. [DOI] [PubMed] [Google Scholar]
- 31.Salen G., Tint G. S., Shefer S., Shore V., and Nguyen L.. 1992. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler. Thromb. 12: 563–568. [DOI] [PubMed] [Google Scholar]
- 32.Patel S. B. 2014. Recent advances in understanding the STSL locus and ABCG5/ABCG8 biology. Curr. Opin. Lipidol. 25: 169–175. [DOI] [PubMed] [Google Scholar]
- 33.Yoo E. G. 2016. Sitosterolemia: a review and update of pathophysiology, clinical spectrum, diagnosis, and management. Ann. Pediatr. Endocrinol. Metab. 21: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salen G., Horak I., Rothkopf M., Cohen J. L., Speck J., Tint G. S., Shore V., Dayal B., Chen T., and Shefer S.. 1985. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J. Lipid Res. 26: 1126–1133. [PubMed] [Google Scholar]
- 35.Kolovou G., Voudris V., Drogari E., Palatianos G., and Cokkinos D. V.. 1996. Coronary bypass grafts in a young girl with sitosterolemia. Eur. Heart J. 17: 965–966. [DOI] [PubMed] [Google Scholar]
- 36.Kawamura R., Saiki H., Tada H., and Hata A.. 2017. Acute myocardial infarction in a 25-year-old woman with sitosterolemia. J. Clin. Lipidol. 12: 246–249. [DOI] [PubMed] [Google Scholar]
- 37.Yagasaki H., Nakane T., Toda T., Kobayashi K., Aoyama K., Ichikawa T., and Sugita K.. 2017. Carotid intima media thickness in a girl with sitosterolemia carrying a homozygous mutation in the ABCG5 gene. J. Pediatr. Endocrinol. Metab. 30: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 38.Mymin D., Wang J., Frohlich J., and Hegele R. A.. 2003. Image in cardiovascular medicine. Aortic xanthomatosis with coronary ostial occlusion in a child homozygous for a nonsense mutation in ABCG8. Circulation. 107: 791. [DOI] [PubMed] [Google Scholar]
- 39.Hansel B., Carrie A., Brun-Druc N., Leclert G., Chantepie S., Coiffard A. S., Kahn J. F., Chapman M. J., and Bruckert E.. 2014. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis. 234: 162–168. [DOI] [PubMed] [Google Scholar]
- 40.Mymin D., Salen G., Triggs-Raine B., Waggoner D. J., Dembinski T., and Hatch G. M.. 2018. The natural history of phytosterolemia: Observations on its homeostasis. Atherosclerosis. 269: 122–128. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen L. B., Salen G., Shefer S., Tint G. S., Shore V., and Ness G. C.. 1990. Decreased cholesterol biosynthesis in sitosterolemia with xanthomatosis: diminished mononuclear leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and enzyme protein associated with increased low-density lipoprotein receptor function. Metabolism. 39: 436–443. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen L. B., Shefer S., Salen G., Ness G. C., Tint G. S., Zaki F. G., and Rani I.. 1990. A molecular defect in hepatic cholesterol biosynthesis in sitosterolemia with xanthomatosis. J. Clin. Invest. 86: 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel S. B., Salen G., Hidaka H., Kwiterovich P. O., Stalenhoef A. F., Miettinen T. A., Grundy S. M., Lee M. H., Rubenstein J. S., Polymeropoulos M. H., et al. 1998. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J. Clin. Invest. 102: 1041–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M. H., Lu K., and Patel S. B.. 2001. Genetic basis of sitosterolemia. Curr. Opin. Lipidol. 12: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rees D. C., Iolascon A., Carella M., O’marcaigh A. S., Kendra J. R., Jowitt S. N., Wales J. K., Vora A., Makris M., Manning N., et al. 2005. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br. J. Haematol. 130: 297–309. [DOI] [PubMed] [Google Scholar]
- 46.Kruit J. K., Drayer A. L., Bloks V. W., Blom N., Olthof S. G., Sauer P. J., de Haan G., Kema I. P., Vellenga E., and Kuipers F.. 2008. Plant sterols cause macrothrombocytopenia in a mouse model of sitosterolemia. J. Biol. Chem. 283: 6281–6287. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z., Cao L., Su Y., Wang G., Wang R., Yu Z., Bai X., and Ruan C.. 2014. Specific macrothrombocytopenia/hemolytic anemia associated with sitosterolemia. Am. J. Hematol. 89: 320–324. [DOI] [PubMed] [Google Scholar]
- 48.Ducrou W., and Kimber R. J.. 1969. Stomatocytes, haemolytic anaemia and abdominal pain in Mediterranean migrants. Some examples of a new syndrome? Med. J. Aust. 2: 1087–1091. [PubMed] [Google Scholar]
- 49.Chase T. H., Lyons B. L., Bronson R. T., Foreman O., Donahue L. R., Burzenski L. M., Gott B., Lane P., Harris B., Ceglarek U., et al. 2010. The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: a spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood. 115: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanaji T., Kanaji S., Montgomery R. R., Patel S. B., and Newman P. J.. 2013. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of Sitosterolemia. Blood. 122: 2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckstein J., Holzhutter H. G., and Berndt N.. 2018. The importance of membrane microdomains for bile salt-dependent biliary lipid secretion. J. Cell Sci. 131: 211524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miettinen T. A., Klett E. L., Gylling H., Isoniemi H., and Patel S. B.. 2006. Liver transplantation in a patient with sitosterolemia and cirrhosis. Gastroenterology. 130: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bazerbachi F., Conboy E. E., Mounajjed T., Watt K. D., Babovic-Vuksanovic D., Patel S. B., and Kamath P. S.. 2017. Cryptogenic Cirrhosis and Sitosterolemia: A Treatable Disease If Identified but Fatal If Missed. Ann. Hepatol. 16: 970–978. [DOI] [PubMed] [Google Scholar]
- 54.Clayton P. T., Bowron A., Mills K. A., Massoud A., Casteels M., and Milla P. J.. 1993. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 105: 1806–1813. [DOI] [PubMed] [Google Scholar]
- 55.Nghiem-Rao T. H., Tunc I., Mavis A. M., Cao Y., Polzin E. M., Firary M. F., Wang X., Simpson P. M., and Patel S. B.. 2015. Kinetics of phytosterol metabolism in neonates receiving parenteral nutrition. Pediatr. Res. 78: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baila-Rueda L., Mateo-Gallego R., Lamiquiz-Moneo I., Cenarro A., and Civeira F.. 2014. Severe hypercholesterolemia and phytosterolemia with extensive xanthomas in primary biliary cirrhosis: role of biliary excretion on sterol homeostasis. J. Clin. Lipidol. 8: 520–524. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Mitsche M. A., Lutjohann D., Cohen J. C., Xie X. S., and Hobbs H. H.. 2015. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 56: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velde A. E., Vrins C. L., van den Oever K., Seemann I., Oude Elferink R. P., van Eck M., Kuipers F., and Groen A. K.. 2008. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G203–G208. [DOI] [PubMed] [Google Scholar]
- 59.Kosters A., Frijters R. J., Schaap F. G., Vink E., Plosch T., Ottenhoff R., Jirsa M., De Cuyper I. M., Kuipers F., and Groen A. K.. 2003. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J. Hepatol. 38: 710–716. [DOI] [PubMed] [Google Scholar]
- 60.Coy D. J., Wooton-Kee C. R., Yan B., Sabeva N., Su K., Graf G., and Vore M.. 2010. ABCG5/ABCG8-independent biliary cholesterol excretion in lactating rats. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G228–G235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groen A., Kunne C., Jongsma G., van den Oever K., Mok K. S., Petruzzelli M., Vrins C. L., Bull L., Paulusma C. C., and Oude Elferink R. P.. 2008. Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology. 134: 2091–2100. [DOI] [PubMed] [Google Scholar]
- 62.Lee M. H., Lu K., Hazard S., Yu H., Shulenin S., Hidaka H., Kojima H., Allikmets R., Sakuma N., Pegoraro R., et al. 2001. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 27: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tauscher A., and Kuver R.. 2003. ABCG5 and ABCG8 are expressed in gallbladder epithelial cells. Biochem. Biophys. Res. Commun. 307: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 64.Remaley A. T., Bark S., Walts A. D., Freeman L., Shulenin S., Annilo T., Elgin E., Rhodes H. E., Joyce C., Dean M., et al. 2002. Comparative genome analysis of potential regulatory elements in the ABCG5-ABCG8 gene cluster. Biochem. Biophys. Res. Commun. 295: 276–282. [DOI] [PubMed] [Google Scholar]
- 65.Freeman L. A., Kennedy A., Wu J., Bark S., Remaley A. T., Santamarina-Fojo S., and Brewer H. B. Jr. 2004. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J. Lipid Res. 45: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 66.Sumi K., Tanaka T., Uchida A., Magoori K., Urashima Y., Ohashi R., Ohguchi H., Okamura M., Kudo H., Daigo K., et al. 2007. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol. Cell. Biol. 27: 4248–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biddinger S. B., Haas J. T., Yu B. B., Bezy O., Jing E., Zhang W., Unterman T. G., Carey M. C., and Kahn C. R.. 2008. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 14: 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balasubramaniyan N., Ananthanarayanan M., and Suchy F. J.. 2016. Nuclear factor-kappaB regulates the expression of multiple genes encoding liver transport proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 310: G618–G628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Back S. S., Kim J., Choi D., Lee E. S., Choi S. Y., and Han K.. 2013. Cooperative transcriptional activation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 genes by nuclear receptors including Liver-X-Receptor. BMB Rep. 46: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., and Mangelsdorf D. J.. 2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277: 18793–18800. [DOI] [PubMed] [Google Scholar]
- 71.Liu J., Lu H., Lu Y. F., Lei X., Cui J. Y., Ellis E., Strom S. C., and Klaassen C. D.. 2014. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol. Sci. 141: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu L., Gupta S., Xu F., Liverman A. D., Moschetta A., Mangelsdorf D. J., Repa J. J., Hobbs H. H., and Cohen J. C.. 2005. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 280: 8742–8747. [DOI] [PubMed] [Google Scholar]
- 73.Ratliff E. P., Gutierrez A., and Davis R. A.. 2006. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J. Lipid Res. 47: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 74.Wang J., Einarsson C., Murphy C., Parini P., Bjorkhem I., Gafvels M., and Eggertsen G.. 2006. Studies on LXR- and FXR-mediated effects on cholesterol homeostasis in normal and cholic acid-depleted mice. J. Lipid Res. 47: 421–430. [DOI] [PubMed] [Google Scholar]
- 75.Kamisako T., and Ogawa H.. 2007. Effect of bile duct obstruction on the expression of intestinal mRNA related to cholesterol and bile acid metabolism in the rat. J. Gastroenterol. Hepatol. 22: 125–131. [DOI] [PubMed] [Google Scholar]
- 76.Kamisako T., Ogawa H., and Yamamoto K.. 2007. Effect of cholesterol, cholic acid and cholestyramine administration on the intestinal mRNA expressions related to cholesterol and bile acid metabolism in the rat. J. Gastroenterol. Hepatol. 22: 1832–1837. [DOI] [PubMed] [Google Scholar]
- 77.Bloks V. W., Bakker-Van Waarde W. M., Verkade H. J., Kema I. P., Wolters H., Vink E., Groen A. K., and Kuipers F.. 2004. Down-regulation of hepatic and intestinal Abcg5 and Abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia. 47: 104–112. [DOI] [PubMed] [Google Scholar]
- 78.Lally S., Tan C. Y., Owens D., and Tomkin G. H.. 2006. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia. 49: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 79.Lally S., Owens D., and Tomkin G. H.. 2007. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism. 56: 430–438. [DOI] [PubMed] [Google Scholar]
- 80.Sabeva N. S., Rouse E. J., and Graf G. A.. 2007. Defects in the leptin axis reduce abundance of the ABCG5-ABCG8 sterol transporter in liver. J. Biol. Chem. 282: 22397–22405. [DOI] [PubMed] [Google Scholar]
- 81.Scoggan K. A., Gruber H., Chen Q., Plouffe L. J., Lefebvre J. M., Wang B., Bertinato J., L’Abbe M. R., Hayward S., and Ratnayake W. M.. 2009. Increased incorporation of dietary plant sterols and cholesterol correlates with decreased expression of hepatic and intestinal Abcg5 and Abcg8 in diabetic BB rats. J. Nutr. Biochem. 20: 177–186. [DOI] [PubMed] [Google Scholar]
- 82.Gälman C., Bonde Y., Matasconi M., Angelin B., and Rudling M.. 2008. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormone. Gastroenterology. 134: 1127–1136. [DOI] [PubMed] [Google Scholar]
- 83.Ma K. Y., Yang N., Jiao R., Peng C., Guan L., Huang Y., and Chen Z. Y.. 2011. Dietary calcium decreases plasma cholesterol by down-regulation of intestinal Niemann-Pick C1 like 1 and microsomal triacylglycerol transport protein and up-regulation of CYP7A1 and ABCG 5/8 in hamsters. Mol. Nutr. Food Res. 55: 247–258. [DOI] [PubMed] [Google Scholar]
- 84.Yoon J. H., Choi H. S., Jun D. W., Yoo K. S., Lee J., Yang S. Y., and Kuver R.. 2013. ATP-binding cassette sterol transporters are differentially expressed in normal and diseased human gallbladder. Dig. Dis. Sci. 58: 431–439. [DOI] [PubMed] [Google Scholar]
- 85.Cheng S., Zou M., Liu Q., Kuang J., Shen J., Pu S., Chen L., Li H., Wu T., Li R., et al. 2017. Activation of constitutive androstane receptor prevents cholesterol gallstone formation. Am. J. Pathol. 187: 808–818. [DOI] [PubMed] [Google Scholar]
- 86.Prasnicka A., Cermanova J., Hroch M., Dolezelova E., Rozkydalova L., Smutny T., Carazo A., Chladek J., Lenicek M., Nachtigal P., et al. 2017. Iron depletion induces hepatic secretion of biliary lipids and glutathione in rats. Biochim. Biophys. Acta. 1862: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 87.Imai S., Kikuchi R., Kusuhara H., Yagi S., Shiota K., and Sugiyama Y.. 2009. Analysis of DNA methylation and histone modification profiles of liver-specific transporters. Mol. Pharmacol. 75: 568–576. [DOI] [PubMed] [Google Scholar]
- 88.Brázdová M., Tichy V., Helma R., Bazantova P., Polaskova A., Krejci A., Petr M., Navratilova L., Ticha O., Nejedly K., et al. 2016. p53 Specifically binds triplex DNA in vitro and in cells. PLoS One. 11: e0167439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graf G. A., Cohen J. C., and Hobbs H. H.. 2004. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J. Biol. Chem. 279: 24881–24888. [DOI] [PubMed] [Google Scholar]
- 90.Hirata T., Okabe M., Kobayashi A., Ueda K., and Matsuo M.. 2009. Molecular mechanisms of subcellular localization of ABCG5 and ABCG8. Biosci. Biotechnol. Biochem. 73: 619–626. [DOI] [PubMed] [Google Scholar]
- 91.Lee J. Y., Kinch L. N., Borek D. M., Wang J., Wang J., Urbatsch I. L., Xie X. S., Grishin N. V., Cohen J. C., Otwinowski Z., et al. 2016. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature. 533: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dodacki A., Wortman M., Saubamea B., Chasseigneaux S., Nicolic S., Prince N., Lochus M., Raveu A. L., Decleves X., Scherrmann J. M., et al. 2017. Expression and function of Abcg4 in the mouse blood-brain barrier: role in restricting the brain entry of amyloid-beta peptide. Sci. Rep. 7: 13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Graf G. A., Li W. P., Gerard R. D., Gelissen I., White A., Cohen J. C., and Hobbs H. H.. 2002. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 110: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klett E. L., Lee M. H., Adams D. B., Chavin K. D., and Patel S. B.. 2004. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamazaki Y., Yasui K., Hashizume T., Suto A., Mori A., Murata Y., Yamaguchi M., Ikari A., and Sugatani J.. 2015. Involvement of a cyclic adenosine monophosphate-dependent signal in the diet-induced canalicular trafficking of adenosine triphosphate-binding cassette transporter g5/g8. Hepatology. 62: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 96.Ismair M. G., Hausler S., Stuermer C. A., Guyot C., Meier P. J., Roth J., and Stieger B.. 2009. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 49: 1673–1682. [DOI] [PubMed] [Google Scholar]
- 97.Guyot C., and Stieger B.. 2011. Interaction of bile salts with rat canalicular membrane vesicles: evidence for bile salt resistant microdomains. J. Hepatol. 55: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 98.Kosters A., Kunne C., Looije N., Patel S. B., Oude Elferink R. P., and Groen A. K.. 2006. The mechanism of ABCG5/ABCG8 in biliary cholesterol secretion in mice. J. Lipid Res. 47: 1959–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tachibana S., Hirano M., Hirata T., Matsuo M., Ikeda I., Ueda K., and Sato R.. 2007. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells is mediated by ATP-binding cassette transporters. Biosci. Biotechnol. Biochem. 71: 1886–1895. [DOI] [PubMed] [Google Scholar]
- 100.Vrins C., Vink E., Vandenberghe K. E., Frijters R., Seppen J., and Groen A. K.. 2007. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 581: 4616–4620. [DOI] [PubMed] [Google Scholar]
- 101.Johnson B. J., Lee J. Y., Pickert A., and Urbatsch I. L.. 2010. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry. 49: 3403–3411. [DOI] [PubMed] [Google Scholar]
- 102.Wang D. Q., Cohen D. E., and Carey M. C.. 2009. Biliary lipids and cholesterol gallstone disease. J. Lipid Res. 50 (Suppl.): S406–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J. E., Basso F., Shamburek R. D., Amar M. J., Vaisman B., Szakacs G., Joyce C., Tansey T., Freeman L., Paigen B. J., et al. 2004. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J. Biol. Chem. 279: 22913–22925. [DOI] [PubMed] [Google Scholar]
- 104.Su K., Sabeva N. S., Wang Y., Liu X., Lester J. D., Liu J., Liang S., and Graf G. A.. 2014. Acceleration of biliary cholesterol secretion restores glycemic control and alleviates hypertriglyceridemia in obese db/db mice. Arterioscler. Thromb. Vasc. Biol. 34: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dikkers A., de Boer J. F., Groen A. K., and Tietge U. J.. 2015. Hepatic ABCG5/G8 overexpression substantially increases biliary cholesterol secretion but does not impact in vivo macrophage-to-feces RCT. Atherosclerosis. 243: 402–406. [DOI] [PubMed] [Google Scholar]
- 106.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., and Cohen J. C.. 2004. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 45: 301–307. [DOI] [PubMed] [Google Scholar]
- 107.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., and Cohen J. C.. 2005. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J. Lipid Res. 46: 1739–1744. [DOI] [PubMed] [Google Scholar]
- 108.Langheim S., Yu L., von Bergmann K., Lutjohann D., Xu F., Hobbs H. H., and Cohen J. C.. 2005. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J. Lipid Res. 46: 1732–1738. [DOI] [PubMed] [Google Scholar]
- 109.Wilund K. R., Yu L., Xu F., Hobbs H. H., and Cohen J. C.. 2004. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr-/- mice. J. Lipid Res. 45: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 110.van der Veen J. N., van Dijk T. H., Vrins C. L., van Meer H., Havinga R., Bijsterveld K., Tietge U. J., Groen A. K., and Kuipers F.. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jakulj L., van Dijk T. H., Freark de Boer J., Kootte R. S., Schonewille M., Paalvast Y., Boer T., Bloks V. W., Boverhof R., Nieuwdorp M., et al. 2016. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 24: 783–794. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., and Rudel L. L.. 2012. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L. S., Xu M., Yang Q., Lou D., Howles P. N., and Tso P.. 2015. ABCG5/G8 deficiency in mice reduces dietary triacylglycerol and cholesterol transport into the lymph. Lipids. 50: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang H. H., Patel S. B., Carey M. C., and Wang D. Q.. 2007. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(-/-) mice. Hepatology. 45: 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H. H., Li X., Patel S. B., and Wang D. Q.. 2016. Evidence that the adenosine triphosphate-binding cassette G5/G8-independent pathway plays a determinant role in cholesterol gallstone formation in mice. Hepatology. 64: 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Zanotti I., Reilly M. P., Glick J. M., Rothblat G. H., and Rader D. J.. 2003. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 108: 661–663. [DOI] [PubMed] [Google Scholar]
- 117.Calpe-Berdiel L., Escola-Gil J. C., and Blanco-Vaca F.. 2009. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 203: 18–31. [DOI] [PubMed] [Google Scholar]
- 118.Altemus J. B., Patel S. B., and Sehayek E.. 2014. Liver-specific induction of Abcg5 and Abcg8 stimulates reverse cholesterol transport in response to ezetimibe treatment. Metab. 63: 1334–1341. [DOI] [PubMed] [Google Scholar]
- 119.de Boer J. F., Schonewille M., Boesjes M., Wolters H., Bloks V. W., Bos T., van Dijk T. H., Jurdzinski A., Boverhof R., Wolters J. C., et al. 2017. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 152: 1126–1138.e6. [DOI] [PubMed] [Google Scholar]
- 120.van der Velde A. E., Vrins C. L., van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., and Groen A. K.. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975. [DOI] [PubMed] [Google Scholar]
- 121.Basso F., Freeman L. A., Ko C., Joyce C., Amar M. J., Shamburek R. D., Tansey T., Thomas F., Wu J., Paigen B., et al. 2007. Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J. Lipid Res. 48: 114–126. [DOI] [PubMed] [Google Scholar]
- 122.Yang C., McDonald J. G., Patel A., Zhang Y., Umetani M., Xu F., Westover E. J., Covey D. F., Mangelsdorf D. J., Cohen J. C., et al. 2006. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281: 27816–27826. [DOI] [PubMed] [Google Scholar]
- 123.Méndez-González J., Julve J., Rotllan N., Llaverias G., Blanco-Vaca F., and Escola-Gil J. C.. 2011. ATP-binding cassette G5/G8 deficiency causes hypertriglyceridemia by affecting multiple metabolic pathways. Biochim. Biophys. Acta. 1811: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 124.Su K., Sabeva N. S., Liu J., Wang Y., Bhatnagar S., van der Westhuyzen D. R., and Graf G. A.. 2012. The ABCG5 ABCG8 sterol transporter opposes the development of fatty liver disease and loss of glycemic control independently of phytosterol accumulation. J. Biol. Chem. 287: 28564–28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weingärtner O., Teupser D., and Patel S. B.. 2015. The atherogenicity of plant sterols: the evidence from genetics to clinical trials. J. AOAC Int. 98: 742–749. [DOI] [PubMed] [Google Scholar]
- 126.Salen G., von Bergmann K., Lutjohann D., Kwiterovich P., Kane J., Patel S. B., Musliner T., Stein P., and Musser B.. 2004. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 109: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Musliner T., Cselovszky D., Sirah W., McCrary Sisk C., Sapre A., Salen G., Lutjohann D., and von Bergmann K.. 2008. Efficacy and safety of ezetimibe 40 mg vs. ezetimibe 10 mg in the treatment of patients with homozygous sitosterolaemia. Int. J. Clin. Pract. 62: 995–1000. [DOI] [PubMed] [Google Scholar]