Abstract
A better understanding of intracellular lipoprotein assembly may help identify proteins with important roles in lipid disorders. apoB-containing lipoproteins (B-lps) are macromolecular lipid and protein micelles that act as specialized transport vehicles for hydrophobic lipids. They are assembled predominantly in enterocytes and hepatocytes to transport dietary and endogenous fat, respectively, to different tissues. Assembly occurs in the endoplasmic reticulum (ER) and is dependent on lipid resynthesis in the ER and on a chaperone, namely, microsomal triglyceride transfer protein (MTTP). Precursors for lipid synthesis are obtained from extracellular sources and from cytoplasmic lipid droplets. MTTP is the major and essential lipid transfer protein that transfers phospholipids and triacylglycerols to nascent apoB for the assembly of lipoproteins. Assembly is aided by cell death-inducing DFF45-like effector B and by phospholipid transfer protein, which may facilitate additional deposition of triacylglycerols and phospholipids, respectively, to apoB. Here, we summarize the current understanding of the different steps in the assembly of B-lps and discuss the role of lipid transfer proteins in these steps to help identify new clinical targets for lipid-associated disorders, such as heart disease.
Keywords: apolipoprotein B, MTP
Hydrophobic lipids serve as a source of energy, structural components for membranes, and precursors of hormones. Owing to their hydrophobicity, lipids require special means of transport in the aqueous milieu of the human body. Three different modes of lipid transport are known. First, micellar solubilization of lipids in the intestinal lumen facilitates hydrolysis of dietary lipids in the lumen and uptake by enterocytes. Second, transport of lipids bound to proteins, e.g., albumin, occurs in the circulation. Third, the most efficient process of transporting lipids in bulk through an aqueous milieu of the blood circulation is via special protein-lipid macromolecular micelles called lipoproteins, which consist of a hydrophilic surface and a hydrophobic core. The hydrophilic surface is a monolayer of phospholipids that also contains free cholesterol and other exchangeable proteins called apolipoproteins. The lipoprotein core is devoid of proteins and consists of hydrophobic lipids, such as triacylglycerols, cholesteryl esters, vitamin E, and vitamin A. A major protein constituent of these lipoproteins is apoB, which is a very hydrophobic scaffolding protein. The apoB-containing lipoproteins (B-lps) are large spherical particles and are classified based on their flotation densities from least to greatest, namely, chylomicrons, VLDLs, and LDLs. Chylomicrons and VLDLs are the predominant lipoproteins synthesized by the intestine and liver, respectively, to transport dietary and endogenous lipids. These two tissues assemble chylomicrons using apoB48 and VLDL using apoB100. These two differently sized polypeptides are derived from a single mRNA transcribed from the APOB gene. In the intestine, however, APOB mRNA undergoes posttranscriptional editing that generates a stop codon, and translation of the edited mRNA yields a single polypeptide that is only 48% of the full-length apoB100 peptide translated from unedited apoB100 mRNA in the liver (1, 2).
Lipoprotein assembly begins concurrently with apoB mRNA translation, and this cotranslational assembly is dependent on both lipid availability and the presence of the chaperone, microsomal triglyceride transfer protein (MTTP), and lipid availability (1, 3–10). B-lps cannot be synthesized in the absence of MTTP and/or lipids; rather, newly translated apoB is degraded (7, 11). The degradation process is assisted by several chaperones, such as Hsp90 and Hsp110 (7, 11). In addition to MTTP, B-lp assembly may be assisted by several other proteins, including phospholipid transfer protein (PLTP) and cell death-inducing DFF45-like effector B (CIDEB). The role of these and other proteins in lipoprotein assembly and secretion is usually inferred when their ablation leads to reduced production of B-lps. In this focused review, we first introduce four proteins and then discuss their known or hypothesized roles in the assembly and secretion of B-lps. This review does not cover proteins that affect lipoprotein secretion through modulation of intracellular degradation pathways or those involved in the intracellular trafficking of lipoproteins; this information can be gleaned in other reviews (7, 11–13).
SYNTHESIS AND MOBILIZATION OF LIPIDS FOR LIPOPROTEIN ASSEMBLY
Triacylglycerol synthesis and phospholipid synthesis in the smooth endoplasmic reticulum (ER) are critical for B-lp assembly because inhibition of their synthesis or ablation of genes involved in their biosynthesis adversely affects B-lp assembly (14–18). Fatty acids, glycerol, and monoacylglycerols are important precursors for their synthesis and are obtained from two sources. First, these precursors are taken up by cells from the extracellular environment via transporters present on the plasma membrane, such as CD36 and fatty acid transport proteins (10, 19–21), and are carried by cytoplasmic proteins, e.g., fatty acid-binding proteins (10, 22), from the plasma membrane to the ER. Second, these precursors can be generated intracellularly after the hydrolysis of lipids stored in cytoplasmic lipid droplets (cLDs).
Triacylglycerols are synthesized (de novo lipogenesis) in the smooth ER by two pathways, namely, the glycerol-3-phosphate and monoacylglycerol pathways (23–27). In the glycerol-3-phosphate pathway, which predominates in hepatocytes, glycerol and fatty acids are the main precursors. In the monoacylglycerol pathway, which predominates in enterocytes, monoacylglycerols and fatty acids are used as precursors. Phospholipid synthesis occurs via the Kennedy pathway in the ER using fatty acids and glycerol-3-phosphate as precursors (18). These precursors are also used for triacylglycerols. Thus, synthesis of these lipids depends on similar precursors and involves common intermediates. Cholesterol is taken up by cells via the transporter, NPC1L1 [and possibly other transporters (10)], and is converted to cholesteryl esters. Similar to triacylglycerols and phospholipids, cholesteryl esters are synthesized in the ER (23–26). Newly synthesized lipids are incorporated into the ER membrane and are hence available for B-lp assembly (28). Additionally, nonesterified cholesterol is incorporated into the surface of lipoproteins.
Lipids stored in cLDs are also an important source of lipids for lipoprotein assembly. The mobilization of fatty acids from cLDs is accomplished via the hydrolysis of triacylglycerols. Fatty acids released from the cLDs could theoretically reach the ER, in addition to other organelles such as mitochondria, and be utilized for lipid resynthesis and lipoprotein assembly. It is also known that cLDs interact with the ER, and thus fatty acids derived from the hydrolysis of triacylglycerols that reside in cLDs tethered to the ER can be preferentially delivered to the ER and utilized for lipid resynthesis and lipoprotein assembly. Recent evidence suggests that, during the fed state, cLDs are transported toward the plus end of microtubules by kinesin-1 near the smooth ER in liver cells (29). Rai et al. (29) observed that most cLDs in rat hepatoma McA-RH7777 cells were found at the extreme periphery where the microtubule plus end exists. Similar observations were made with human HHL-17 hepatocytes and liver sections from fed rats. Knockdown of kinesin-1 in McA-RH7777 cells scattered these cLDs throughout the cytoplasm away from the peripheral disposition. Moreover, knockdown of kinesin-1 in rats significantly reduced secretion of triacyglycerols without affecting apoB levels, resulting in secretion of less dense B-lps. Thus, kinesin-1 is predominantly involved in triacylglycerol supply to B-lps during assembly. Kinesin-1 may transport cLDs to the ER, and these cLDs may provide lipid precursors for VLDL assembly in the fed state.
Kinesin-1 is mainly found in the cytoplasm. Recruitment of kinesin-1 to cLDs involves activation of the small GTPase, ADP ribosylation factor 1 (ARF1), present on these cLDs (29). In the fed state, insulin signaling activates ARF1 to ARF1-GTP, leading to recruitment of kinesin-1 to cLDs. The amounts of ARF1 and kinsein-1 in cLDs obtained from the liver of fed rats were found to be higher compared with those measured in fasted rats. Kinesin-1 then transports cLDs on microtubules to the smooth ER. Once juxtaposed with the ER, fatty acids hydrolyzed from cLDs can be taken up by the ER for lipid resynthesis and lipoprotein assembly. It remains to be determined whether lipids diffuse from cLDs to the ER through contact sites and ultimately become incorporated in B-lps. In the fasted state, cLDs are less mobile and do not travel to the ER; instead, they remain in the cytoplasm and thus contribute less to hepatic lipoprotein assembly. It remains to be determined whether such a mechanism exists in the intestine. In the fasted state, triacylglycerols stored in adipose tissue are hydrolyzed, and free fatty acids bound to albumin are transported via the circulation to the liver. Resynthesis of triacylglycerols from adipose tissue-derived free fatty acids may be the major source of lipids for VLDL assembly during fasting.
PROTEINS INVOLVED IN LIPOPROTEIN ASSEMBLY
MTTP
MTTP is the master regulator of the intracellular assembly and secretion of B-lps (3, 4). MTTP was first identified in liver microsomes and was shown to transfer triacylglycerols, phospholipids, and cholesteryl esters in vitro between vesicles (30). MTTP is a cellular lipid transfer protein that differs from those present in the blood. Subsequently, the activity was purified from bovine liver and was shown to be mediated by a heterodimer of MTTP and protein disulfide isomerase (31). The importance of MTTP in B-lp assembly became obvious when it was shown that abetalipoproteinemia subjects, who do not have B-lps in their plasma, carry a mutation in MTTP (32). Initially it was thought that MTTP was mainly expressed in hepatocytes and enterocytes, i.e., the two major organs that synthesize B-lps. Later studies revealed that it is also expressed in several other tissues, such as heart, kidney, adipose tissue, eye, brain, and macrophages (4, 33). Its function in the liver, intestine, heart, and kidney is thought to be related to the assembly and secretion of B-lps mainly to export fat to other tissues and/or to avoid lipotoxicity in these tissues (4, 33). However, it is also expressed in adipose tissue, brain, and antigen-presenting cells, where apoB is not expressed. In antigen-presenting cells, it plays a role in the synthesis of CD1 proteins that are involved in lipid antigen presentation to specialized T cells known as natural killer cells (34, 35). MTTP function in adipose tissue is unknown, but deletion of mouse Mttp in adipose tissue results in a lean phenotype when mice are fed a high-fat diet (35% fat by weight or 60% calories), but not when fed a chow diet (5% fat or 13% calories) or Western diet (20% fat or 43% calories and 1.5% cholesterol) (36, 37). The role of MTTP in the brain is unknown. Because MTTP deficiency in humans is associated with neurological symptoms such as ataxia, dysmetria, and dysarthria, we speculate that MTTP may play a role in the control of walking, voluntary movement, and speaking.
MTTP was previously believed to be mainly involved in the transfer of triacylglycerols, diacylglycerols, phospholipids, cholesterol, and cholesteryl esters (30, 38). Now, it has been shown that MTTP also transfers sphingomyelin and ceramides, but not glycosylceramides (39). Thus, it is likely that MTTP is a nonspecific pattern-recognition and lipid transfer protein that can recognize hydrophobic motifs (39). This is supported by observations that transfer of lipids by MTTP is related to the hydrophobicity of the molecules. For example, MTTP does not transfer monoacylglycerols, but efficiently transfers diacylglycerols and triacylglycerols (38). Addition of one acyl chain to monoacylglycerols, diacylglycerols, lysophosphatidylcholine, or cholesterol increases their amounts of transfer by ∼10-fold. However, addition of a polar group, such as one of those present in phospholipids but not in triacylglycerols, reduces the amounts of transfer. Changing the zwitterionic head groups in phosphatidylcholine to yield a negative or positive charge, however, has no effect on transfer (38). Thus, different phospholipids with different head groups are transferred similarly by MTTP, albeit slowly compared with triacylglycerols.
Kinetic studies suggest that MTTP may have two lipid binding sites for phospholipids, one mainly for phospholipids and the other for all lipids, including phospholipids (3, 40, 41). The latter lipid binding site has high affinity for triacylglycerols, and inhibitors identified to date appear to competitively inhibit this transfer activity. Again, kinetic studies have suggested that MTTP interacts with membranes to pick up lipids and then interacts with other membranes to deposit them (40, 41). This lipid transfer activity does not require energy and perhaps depends on structural changes in the protein as well as in the membranes with which it interacts. In vitro, MTTP appears to facilitate lipid transfer down a concentration gradient and may transfer lipids from lipid-rich membranes to lipid-poor membranes (30, 38, 42, 43). When membranes have equal concentrations of any particular lipid, however, then MTTP may simply act as an exchange protein.
MTTP shares sequence homology with lipovitellin (3, 44–48). Therefore, attempts have been made to model MTTP structure (3, 45–48) based on the lipovitellin structure (49, 50). The predicted tertiary structure of MTTP consists of an N-terminal β-sheet, a central α-helical domain, and a C-terminal β-sheet. Based on three-dimensional structural comparison with lipovitellin and bactericidal/permeability-increasing protein, Read et al. (48) proposed that the C- and A-sheets within the C-terminal β-sheet of MTTP form a lipid binding cavity that is likely the main lipid transfer domain, as discussed earlier, that can transfer several lipids. This cavity is lined with several hydrophobic amino acid residues. Moreover, they identified two conserved α-helices (helix A and helix B) at the entrance of this lipid binding cavity. Helix A was shown to interact with membranes similarly to viral fusion peptides and thus was speculated to act as a membrane fusogen. Mutations in helix B abolished triacylglycerol binding. Based on these studies, they presented a model wherein helix A inserts into lipid membranes causing local thermodynamic instability, and thus allows interaction of helix B with acyl chains of lipids for extraction from the cavity and subsequent transfer. This model is supported by the existence of several missense mutations in this region that abolish MTTP’s lipid transfer activity (4).
In the absence of MTTP, membrane-associated apoB is degraded, indicating that MTTP prevents apoB degradation and favors its assembly into lipoproteins. MTTP may assist in lipoprotein assembly in two major ways. First, MTTP may transfer lipids to apoB. This process does not require protein-protein interactions with apoB, as purified MTTP can transfer lipids between synthetic vesicles that do not contain any protein. For proper lipoprotein assembly, lipid transfer should result in net deposition of lipids to apoB. Although the mechanism by which MTTP achieves net lipid deposition remains unclear, it is possible that association of MTTP with apoB and/or a phospholipid monolayer facilitates net deposition, whereas its interaction with a membrane bilayer is optimal for lipid acquisition. Second, ionic interactions between MTTP and the N-terminus of apoB are involved in lipoprotein assembly. These interactions are reduced as the length of apoB increases and as it becomes lipidated (51). These protein-protein interactions might be important for securing a proper conformation necessary for further lipidation of the nascent apoB (52).
MTTP deficiency in humans is associated with an absence of plasma B-lps. These abetalipoproteinemia subjects usually present with steatorrhea, diarrhea, abdominal pain, and stunted growth in infancy and have very low plasma triacylglycerol and cholesterol levels (4, 39, 53, 54). In mice, global MTTP deficiency is embryonically lethal (55). However, conditional deletion of hepatic and intestinal MTTP is not lethal in adult mice, but results in low levels of plasma B-lps, triacylglycerols, cholesterol, sphingomyelin, and ceramides, and therefore this genetic model may provide valuable information about abetalipoproteinemia (39).
PLTP
PLTP is a secreted protein that modulates lipoprotein metabolism in the circulation by transferring phospholipids from B-lps to HDLs. However, PLTP knockout mice unexpectedly exhibited lower plasma levels of B-lps (56), and PLTP overexpression increased VLDL production in mice (57). These and other studies suggest a role for PLTP in the second step of B-lp assembly, namely, core expansion (58). Studies have also shown that PLTP deficiency reduces the secretion of the N-terminal 1,000 residues of apoB (apoB:1,000). These studies suggest that PLTP may also play a role in the first step of lipoprotein assembly (59). It is likely that PLTP plays an auxiliary role in both the steps, as both steps depend on the availability of phospholipids.
The expression and activity of PLTP are similar in the intestine and liver (60). Lipid absorption studies have revealed that PLTP-deficient mice absorb similar amounts of triacylglycerols, but absorb significantly less cholesterol than wild-type mice (60). Biochemical studies demonstrated that PLTP-deficient enterocytes take up less cholesterol than wild-type cells, but in contrast, these enterocytes were as efficient as wild-type enterocytes with respect to oleic acid uptake; notably, secretion of cholesterol with both B-lps and HDLs was significantly reduced. Again, no defect in triacylglycerol secretion was observed in PLTP-deficient enterocytes. PLTP-deficient mice have low levels of mRNAs encoding NPC1L1, HMG-CoA reductase, MTTP, and ABCA1. These studies indicate that the effect of PLTP deficiency on cholesterol absorption is most likely secondary to low cellular uptake. Given that PLTP deficiency does not affect triacylglycerol absorption, PLTP likely plays a less significant role in lipoprotein assembly in the intestine than in the liver.
CIDEB
CIDEB is a membrane-associated protein that localizes to cLDs, smooth ER, and Golgi. CIDEB deficiency reduces triacylglycerol secretion in mice without affecting apoB secretion, resulting in the secretion of triacylglycerol-poor lipoproteins (61) and suggesting that CIDEB may assist triacylglycerol deposition during lipoprotein assembly. Further, CIDEB interacts with apoB (61, 62). Based on its localization and its ability to interact with apoB, CIDEB most likely affects lipoprotein assembly when apoB is posttranslationally associated with the ER membrane, that is, during the early stages of lipoprotein assembly. Because CIDEB deficiency mainly reduces the triacylglycerol concentration in B-lps, it may be involved in the addition of these lipids to apoB. We propose that CIDEB may facilitate lateral diffusion of triacylglycerols from cLDs to the ER membrane at contact sites and, within the ER membrane, from the site of lipid synthesis to apoB. In the absence of CIDEB, triacylglycerols are mainly diverted to cLDs, resulting in the production of triglyceride-poor lipoproteins and increased triacylglycerol mass in cLDs (61). In addition to its role in lipoprotein assembly, it has been suggested that CIDEB also contributes to the transport of VLDL via VLDL transport vesicles from the ER to the Golgi (13, 62).
CIDEB is also expressed in the intestine (63), with highest expression in the jejunum and decreased expression from the proximal to the distal end, i.e., similar to the expression of apoB and MTTP. A high-fat diet increases both the mRNA and protein levels of CIDEB in the intestine. CIDEB deficiency reduces the amount of triacylglycerol secreted by the small intestine and produces smaller-sized chylomicrons, but with no effect on apoB48 secretion. Further, its deficiency results in the accumulation of lipids in the intestine compared with wild-type controls. In differentiated human colon carcinoma Caco-2 cells, CIDEB is present in the ER and cLDs and appears to interact with apoB. Overexpression of CIDEB increases triacylglycerol secretion from these cells. Thus, as in the liver, these studies provide strong evidence that CIDEB contributes substantially to chylomicron assembly in the intestine.
Ancient ubiquitous protein 1
Like CIDEB, ancient ubiquitous protein 1 (AUP1) is also an ER membrane protein and a component of cLDs (64, 65). However, as opposed to CIDEB, AUP1 knockdown increases VLDL production and reduces the number of cLDs in human hepatoma HepG2 cells (65). AUP1 is involved in quality control of protein synthesis and cLDs via increasing the ubiquitination of ER- and cLD-associated proteins (64). Based on similar expression profiles and interaction with apoB and opposing effects on lipoprotein biosynthesis, we speculate that AUP1 may antagonize CIDEB function during the early stages of apoB lipidation. Thus, dynamic changes in the cellular levels of these two proteins may control lipoprotein assembly. It remains to be determined whether AUP1 plays any role in cLD formation and lipoprotein assembly in vivo and in intestinal cells.
LIPOPROTEIN ASSEMBLY
Initiation of lipoprotein assembly on the ER membrane
Based on an amino acid sequence analysis, it has been suggested that apoB100 consists of five structural domains, namely, NH2-βα1-β1-α2-β2-α3-COOH (66). Owing to the presence of long hydrophobic sequences in all of these domains, apoB has a propensity to associate with lipids and may initiate lipoprotein assembly on its own by interacting with the ER membrane. Although the βα1 domain is not very hydrophobic and can be secreted without the help of MTTP, it is essential for the assembly of B-lps, as its deletion abolishes lipoprotein assembly and secretion (67). Hence, substantial efforts have been made to understand its role in lipoprotein assembly. The βα1 domain can interact with lipids and, therefore, can act as a first point of contact with ER-membrane lipids and may form “nucleation sites” for lipoprotein assembly (Fig. 1). These nucleation sites may be stabilized when more hydrophobic sequences are translated. In addition to its interactions with membrane lipids, the βα1 domain may also interact with MTTP. These protein-protein interactions between apoB and MTTP appear essential for lipoprotein assembly (68). These interactions may generate a pocket that is more conducive to accepting a greater number of lipids (45, 52). Thus, the βα1 domain may play two important roles in the initiation of lipoprotein assembly, namely, interaction with membranes to form nucleation sites and interaction with MTTP to create a pocket for receiving more lipids.
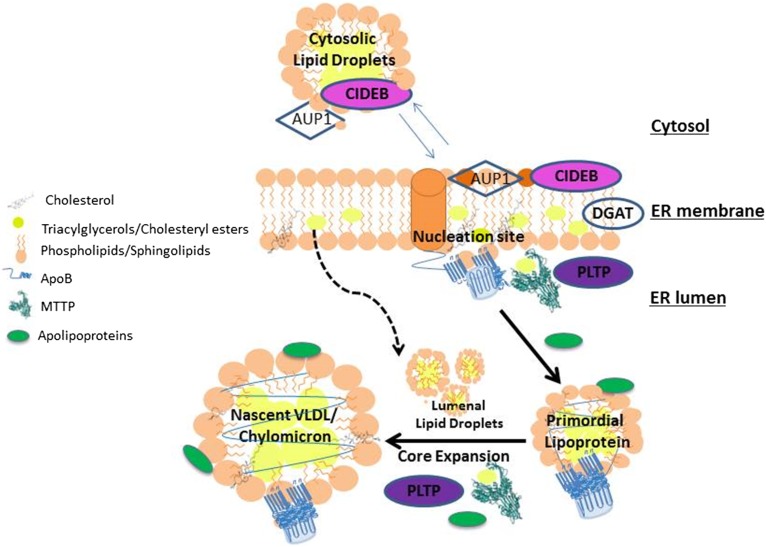
Fig. 1.
Schematic illustration of the role of lipid transfer proteins in the assembly of apoB-containing lipoproteins. apoB undergoes cotranslational lipidation during translocation through the ER membrane. Nascent apoB peptide interacts with the inner phospholipid monolayer, resulting in the formation of nucleation sites for the assembly of lipoproteins. MTTP is a critical chaperone for lipoprotein assembly and most likely helps by transferring lipids to nascent apoB and by physically interacting with apoB. PLTP is another ER lumenal lipid transfer protein that may assist the assembly of lipoproteins. As opposed to MTTP and PLTP, CIDEB and AUP1 are ER membrane proteins. CIDEB may facilitate addition of triacylglycerols to nascent apoB, whereas AUP1 appears to antagonize this function. Once a smaller primordial lipoprotein is formed, it undergoes a second step, namely, core expansion, which involves bulk addition of lipids from lumenal droplets to primordial lipoproteins, most likely involving membrane fusion. Several exchangeable apolipoproteins may stabilize the surface of these larger lipoproteins and assist in their assembly. All of these steps and proteins are critical for lipoprotein assembly and secretion in both the intestine and liver, except for PLTP that may not be as critical for intestinal lipoprotein assembly. The role of AUP1 has not been addressed in the intestine.
The nascent apoB at the nucleation sites may receive lipids via two mechanisms. Addition of phospholipids to these sites may be important and sufficient for the assembly of secretion-competent B-lps given that the phospholipid transfer activity of MTTP is sufficient for the assembly and secretion of primordial lipoproteins in cultured cells and in liver (69–71). PLTP may also transfer phospholipids to the nucleation sites and help prevent apoB degradation given that PLTP deficiency is associated with a reduced number of B-lps. However, PLTP is unable to support B-lp assembly in the absence of MTTP. These nucleation sites may receive triacylglycerols from their site of resynthesis via lateral diffusion within the ER membrane. Because this process depends on the concentration of triacyglycerols and can be affected by the membrane microenvironment, it is expected to proceed more slowly than a protein-mediated facilitated process. It is likely that certain proteins may facilitate this lateral diffusion. For example, deficiency of the membrane-associated protein, CIDEB, results in production of triacylglycerol-poor B-lps; hence, it is possible that CIDEB may facilitate the movement of triacylglycerols within the membrane bilayer. In contrast, MTTP can continuously transfer triacylglycerols to hasten the apoB lipidation process. Transfer of lipids by MTTP is critical for lipoprotein assembly because MTTP deficiency abrogates lipoprotein assembly and secretion. During the deposition of neutral lipids, the surface area of B-lps expands, requiring more phospholipids to cover and stabilize the surface. In this case, MTTP and PLTP can transfer more phospholipids from other sites to these sites to support lipoprotein assembly. Thus, several proteins participate in the lipidation of nascent apoB to yield primordial B-lps.
Dissociation of primordial lipoproteins from the ER membrane
At some point, B-lps detach from the ER membrane and become lumenal particles. The factors governing this detachment are not well understood. It is possible that the termination of apoB mRNA translation coincides with the release of B-lps from the ER membrane. Therefore, the size of the lipoproteins formed may depend on the time needed for mRNA translation and the coincident addition of lipids to the nascent apoB. Pausing during translation has been suggested to allow time for proper lipidation (72, 73). There is evidence to suggest that the size of the apoB polypeptide determines lipoprotein diameter (74). Additionally, there may be other mechanisms that sense completion of lipoprotein assembly and dislodge nascent lipoproteins from the membrane. Nevertheless, the “primordial lipoproteins” found in the ER lumen of hepatocytes and enterocytes are smaller (i.e., approximately the size of HDL particles) than those found in plasma (8, 75, 76). However, these smaller particles have been observed in cell culture media. The presence of these particles in the ER lumen gives credence to the hypothesis that HDL-sized apoB-containing particles may be the first primordial particles synthesized in both hepatocytes and enterocytes (8, 75, 76).
Formation of ER lumenal LDs
Besides B-lps, the ER lumen of both hepatocytes and enterocytes contains apoB-free lumenal LDs (75, 77). In the absence of apoB, these lumenal LDs accumulate in the ER (77), and in the presence of apoB, these lipids are transferred to B-lps and secreted. Like cLDs, the surface of the lumenal LDs is composed of a phospholipid monolayer that contains several proteins. The core consists of hydrophobic neutral lipids. The proteins found associated with ER lumenal LDs differ from those present in cLDs (78, 79). MTTP has been found on lumenal LDs from hepatocytes and enterocytes. Similar to the assembly of B-lps, the biogenesis of both cLDs and ER lumenal LDs begins at the ER membrane where lipid-synthesizing enzymes reside (28, 78, 79). Depending on the proteins that associate with triacylglycerol-rich membrane domains, the LDs can bud off to either the cytoplasm or the ER lumen (28). For example, perilipins may participate in the budding of cLDs as their overexpression increases the number of cLDs and decreases the secretion of B-lps (80). MTTP is critical for the formation of ER lumenal LDs in hepatocytes and enterocytes, as these ER lumenal LDs were found to be absent in MTP-deficient hepatocytes and enterocytes (81, 82), and inhibition of MTTP in liver-derived cells reduced the accumulation of apoB-free lumenal droplets (7, 78, 79, 81, 83, 84). Besides MTTP, apoCIII and apoE have also been implicated in the formation of ER lumenal LDs (57, 78). In short, in B-lp-producing cells, the biogenesis of cLDs and ER lumenal LDs and the assembly of B-lp may compete for the same source of triacylglycerols in the ER membrane.
Core expansion
The lumenal primordial B-lps undergo further modifications, the major modification being conversion of these smaller particles into larger particles found in plasma. This process is sometimes referred to as the second-step “core expansion”. In this step, HDL-sized B-lps are hypothesized to fuse with preformed ER lumenal LDs that do not contain apoB in both hepatocytes and enterocytes (8, 10, 85). Thus, this step involves two critical components, formation of lumenal LDs and fusion of these droplets with primordial lipoproteins. Although fusion among cLDs has been documented, convincing evidence for the fusion of B-lps and ER lumenal LDs is lacking. Further, no critical protein has been identified whose absence leads to exclusive synthesis and secretion of HDL-sized lipoproteins containing apoB100 or apoB48. Nevertheless, proteins known to participate in the first step of lipoprotein assembly have been implicated in the biosynthesis of larger lipoproteins during the second step. For example, besides its role in the formation of lumenal LDs (7, 78, 79, 81, 84), MTTP has been suggested to facilitate fusion (48). Thus, there is a need to know more about the molecular mechanisms and proteins involved in this second step.
Core expansion necessitates a substantial increase in the surface area of B-lps. Because each lipoprotein particle contains one apoB molecule, the surface area of larger lipoproteins perhaps requires stabilization by other proteins, and this can be achieved by the acquisition of several apolipoproteins. Indeed, both overexpression and knockdown approaches have demonstrated that exchangeable apolipoproteins, including apoE, apoCIII, and apoAIV, contribute to lipoprotein assembly (86). Besides surface stabilization, these exchangeable apolipoproteins may also play other yet unappreciated roles in the biogenesis of B-lps.
Exit of nascent lipoproteins from the ER lumen
Newly assembled B-lps are transported via specialized vesicles, referred to as prechylomicron or pre-VLDL transport vesicles, which differ from those involved in protein transport for secretion (12, 13). At least two determining factors may be involved in the exit of lipoproteins from the ER lumen. First, MTTP may dissociate from these particles, allowing their escape from the ER lumen. Second, apoB or any other determinant on these particles is recognized by coat-protein complex II (COPII) for selective transport. These COPII transport vesicles deliver lipoprotein particles to the cis-Golgi where they undergo further maturation via glycosylation and further lipidation (87). Other critical, yet less understood, control steps exist in the Golgi that monitor lipoprotein assembly. For example, abnormal lipoproteins are delivered from the trans-Golgi to lysosomes for degradation via autophagy (11). Normal, mature lipoproteins are transported from the Golgi to the cell surface for secretion by different types of transport vesicles (12, 13).
CONCLUSIONS AND FUTURE DIRECTIONS
Although we are starting to piece together the early events and identify proteins involved in the early stages of B-lp assembly, very little is known about the second step of lipoprotein core expansion. Therefore, novel approaches and methodologies are needed to understand this latter process. Identification of critical proteins in different steps of lipoprotein assembly may inform potential drug targets for lowering plasma lipoprotein levels, as the elevation of plasma lipids constitutes a risk factor for cardiovascular disease.
It is unclear why MTTP is essential for lipoprotein assembly. At least three important functions of MTTP have been identified: lipid transfer, apoB binding, and formation of lumenal LDs. Several proteins have been identified that have similar functions of MTTP. For example, CIDEB and PLTP can facilitate triglyceride mobilization and phospholipid transfer, respectively. Although PLTP has not been demonstrated to interact with apoB, CIDEB indeed interacts with apoB. Thus, MTTP is not the only apoB binding protein. The only specific function of MTTP is its ability to transfer triacylglycerols; however, it has been shown that the phospholipid transfer activity of MTTP is sufficient for lipoprotein assembly and secretion (71). But, PLTP is unable to substitute for MTTP for B-lp assembly. Therefore, a further understanding of MTTP function in lipoprotein assembly is required. An understanding of the structural basis for MTTP function could be achieved via X-ray crystallography. A cryo-structure of purified MTTP could also prove helpful.
Earlier studies concerning B-lp assembly concentrated mainly on the role of apoB and MTTP in this process, yet emerging evidence has highlighted the importance of additional proteins in B-lp assembly. Identification of other proteins involved in the lipidation of apoB during the first and second steps of lipoprotein assembly may provide novel targets for the treatment of lipid-associated disorders such as heart disease.
Footnotes
Abbreviations:
- ARF1
- ADP ribosylation factor 1
- AUP1
- ancient ubiquitous protein 1
- B-lp
- apoB-containing lipoprotein
- CIDEB
- cell death-inducing DFF45-like effector B
- cLD
- cytoplasmic lipid droplet
- COPII
- coat-protein complex II
- ER
- endoplasmic reticulum
- MTTP
- microsomal triglyceride transfer protein
- PLTP
- phospholipid transfer protein
This work was supported in part by National Institutes of Health Grants R56DK046900-17A1 and 1RO1HL137202-01A1, and Health Services Research and Development Grant BX001728 to M.M.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government. The authors declare that they have no conflicts of interest with respect to the content of this article.
REFERENCES
- 1.Davidson N. O., and Shelness G. S.. 2000. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20: 169–193. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., and Bakillah A.. 1996. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1300: 151–170. [DOI] [PubMed] [Google Scholar]
- 3.Hussain M. M., Shi J., and Dreizen P.. 2003. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 4.Walsh M. T., and Hussain M. M.. 2017. Targeting microsomal triglyceride transfer protein and lipoprotein assembly to treat homozygous familial hypercholesterolemia. Crit. Rev. Clin. Lab. Sci. 54: 26–48. [DOI] [PubMed] [Google Scholar]
- 5.Olofsson S. O., and Boren J.. 2005. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 258: 395–410. [DOI] [PubMed] [Google Scholar]
- 6.Fisher E. A., and Ginsberg H. N.. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380. [DOI] [PubMed] [Google Scholar]
- 7.Rutledge A. C., Su Q., and Adeli K.. 2010. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem. Cell Biol. 88: 251–267. [DOI] [PubMed] [Google Scholar]
- 8.Rustaeus S., Lindberg K., Stillemark P., Claesson C., Asp L., Larsson T., Boren J., and Olofsson S. O.. 1999. Assembly of very low density lipoprotein: a two-step process of apolipoprotein B core lipidation. J. Nutr. 129: 463S–466S. [DOI] [PubMed] [Google Scholar]
- 9.Sundaram M., and Yao Z.. 2010. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. (Lond.). 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abumrad N. A., and Davidson N. O.. 2012. Role of the gut in lipid homeostasis. Physiol. Rev. 92: 1061–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher E. A. 2012. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim. Biophys. Acta. 1821: 778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansbach C. M., and Siddiqi S. A.. 2010. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari S., and Siddiqi S. A.. 2012. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 32: 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luchoomun J., Zhou Z., Bakillah A., Jamil H., and Hussain M. M.. 1997. Assembly and secretion of VLDL in nondifferentiated Caco-2 cells stably transfected with human recombinant apolipoprotein B48 cDNA. Arterioscler. Thromb. Vasc. Biol. 17: 2955–2963. [DOI] [PubMed] [Google Scholar]
- 15.Cole L. K., Vance J. E., and Vance D. E.. 2012. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta. 1821: 754–761. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z., Luchoomun J., Bakillah A., and Hussain M. M.. 1998. Lysophosphatidylcholine increases apolipoprotein B secretion by enhancing lipid synthesis and decreasing its intracellular degradation in HepG2 cells. Biochim. Biophys. Acta. 1391: 13–24. [DOI] [PubMed] [Google Scholar]
- 17.Pan M., Liang Js J. S., Fisher E. A., and Ginsberg H. N.. 2002. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J. Biol. Chem. 277: 4413–4421. [DOI] [PubMed] [Google Scholar]
- 18.Fagone P., and Jackowski S.. 2009. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 50 (Suppl.): S311–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepino M. Y., Kuda O., Samovski D., and Abumrad N. A.. 2014. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 34: 281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazantzis M., and Stahl A.. 2012. Fatty acid transport proteins, implications in physiology and disease. Biochim. Biophys. Acta. 1821: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatz J. F., and Luiken J. J.. 2017. From fat to FAT (CD36/SR-B2): understanding the regulation of cellular fatty acid uptake. Biochimie. 136: 21–26. [DOI] [PubMed] [Google Scholar]
- 22.Storch J., and Thumser A. E.. 2010. Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 285: 32679–32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal J., and Hussain M. M.. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M. M., Fatma S., Pan X., and Iqbal J.. 2005. Intestinal lipoprotein assembly. Curr. Opin. Lipidol. 16: 281–285. [DOI] [PubMed] [Google Scholar]
- 25.Pan X., and Hussain M. M.. 2012. Gut triglyceride production. Biochim. Biophys. Acta. 1821: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain M. M., Leung T. M., Zhou L., and Abu-Merhi S.. 2013. Regulating intestinal function to reduce atherogenic lipoproteins. Clin. Lipidol. 8: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman R. A., Lewin T. M., and Muoio D. M.. 2000. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu. Rev. Nutr. 20: 77–103. [DOI] [PubMed] [Google Scholar]
- 28.Sturley S. L., and Hussain M. M.. 2012. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J. Lipid Res. 53: 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai P., Kumar M., Sharma G., Barak P., Das S., Kamat S. S., and Mallik R.. 2017. Kinesin-dependent mechanism for controlling triglyceride secretion from the liver. Proc. Natl. Acad. Sci. USA. 114: 12958–12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wetterau J. R., and Zilversmit D. B.. 1984. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J. Biol. Chem. 259: 10863–10866. [PubMed] [Google Scholar]
- 31.Wetterau J. R., and Zilversmit D. B.. 1985. Purification and characterization of microsomal triglyceride and cholesteryl ester transfer protein from bovine liver microsomes. Chem. Phys. Lipids. 38: 205–222. [DOI] [PubMed] [Google Scholar]
- 32.Wetterau J. R., Aggerbeck L. P., Bouma M., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., and Gregg R. E.. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 33.Hussain M. M., Rava P., Walsh M., Rana M., and Iqbal J.. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond.). 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brozovic S., Nagaishi T., Yoshida M., Betz S., Salas A., Chen D., Kaser A., Glickman J., Kuo T., Little A., et al. . 2004. CD1d function is regulated by microsomal triglyceride transfer protein. Nat. Med. 10: 535–539. [DOI] [PubMed] [Google Scholar]
- 35.Dougan S. K., Salas A., Rava P., Agyemang A., Kaser A., Morrison J., Khurana A., Kronenberg M., Johnson C., Exley M., et al. . 2005. Microsomal triglyceride transfer protein: lipidation and control of CD1d on antigen presenting cells. J. Exp. Med. 202: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakillah A., and Hussain M. M.. 2016. Mice subjected to aP2-Cre mediated ablation of microsomal triglyceride transfer protein are resistant to high fat diet induced obesity. Nutr. Metab. (Lond.). 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swift L. L., Love J. D., Harris C. M., Chang B. H., and Jerome W. G.. 2017. Microsomal triglyceride transfer protein contributes to lipid droplet maturation in adipocytes. PLoS One. 12: e0181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamil H., Dickson J. K. Jr., Chu C. H., Lago M. W., Rinehart J. K., Biller S. A., Gregg R. E., and Wetterau J. R.. 1995. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J. Biol. Chem. 270: 6549–6554. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal J., Walsh M. T., Hammad S. M., Cuchel M., Tarugi P., Hegele R. A., Davidson N. O., Rader D. J., Klein R. L., and Hussain M. M.. 2015. Microsomal triglycerdie transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 290: 25863–25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atzel A., and Wetterau J. R.. 1994. Identification of two classes of lipid molecule binding sites on the microsomal triglyceride transfer protein. Biochemistry. 33: 15382–15388. [DOI] [PubMed] [Google Scholar]
- 41.Atzel A., and Wetterau J. R.. 1993. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry. 32: 10444–10450. [DOI] [PubMed] [Google Scholar]
- 42.Rava P., Athar H., Johnson C., and Hussain M. M.. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 43.Athar H., Iqbal J., Jiang X. C., and Hussain M. M.. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 44.Shoulders C. C., Narcisi T. M., Read J., Chester A., Brett D. J., Scott J., Anderson T. A., Levitt D. G., and Banaszak L. J.. 1994. The abetalipoproteinemia gene is a member of the vitellogenin family and encodes an alpha-helical domain. Nat. Struct. Biol. 1: 285–286. [DOI] [PubMed] [Google Scholar]
- 45.Segrest J. P., Jones M. K., and Dashti N.. 1999. N-terminal domain of apolipoprotein B has structural homology to lipovitellin and microsomal triglyceride transfer protein: a “lipid pocket” model for self-assembly of apoB-containing lipoprotein particles. J. Lipid Res. 40: 1401–1416. [PubMed] [Google Scholar]
- 46.Mann C. J., Anderson T. A., Read J., Chester S. A., Harrison G. B., Köchl S., Ritchie P. J., Bradbury P., Hussain F. S., Amey J., et al. . 1999. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J. Mol. Biol. 285: 391–408. [DOI] [PubMed] [Google Scholar]
- 47.Rava P., and Hussain M. M.. 2007. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry. 46: 12263–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read J., Anderson T. A., Ritchie P. J., Vanloo B., Amey J., Levitt D., Rosseneu M., Scott J., and Shoulders C. C.. 2000. A mechanism of membrane neutral lipid acquisition by the microsomal triglyceride transfer protein. J. Biol. Chem. 275: 30372–30377. [DOI] [PubMed] [Google Scholar]
- 49.Anderson T. A., Levitt D. G., and Banaszak L. J.. 1998. The structural basis of lipid interactions in lipovitellin, a soluble lipoprotein. Structure. 6: 895–909. [DOI] [PubMed] [Google Scholar]
- 50.Banaszak L., Sharrock W., and Timmins P.. 1991. Structure and function of a lipoprotein: lipovitellin. Annu. Rev. Biophys. Biophys. Chem. 20: 221–246. [DOI] [PubMed] [Google Scholar]
- 51.Hussain M. M., Bakillah A., and Jamil H.. 1997. Apolipoprotein B binding to microsomal triglyceride transfer protein decreases with increases in length and lipidation: implications in lipoprotein biosynthesis. Biochemistry. 36: 13060–13067. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Z. G., Liu Y., Hussain M. M., Atkinson D., and McKnight C. J.. 2008. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J. Mol. Biol. 383: 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., and Wetterau J. R.. 2000. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20: 663–697. [DOI] [PubMed] [Google Scholar]
- 54.Lee J., and Hegele R. A.. 2014. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J. Inherit. Metab. Dis. 37: 333–339. [DOI] [PubMed] [Google Scholar]
- 55.Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Véniant M. M., Hamilton R. L., and Young S. G.. 1998. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang X. C., Qin S., Qiao C., Kawano K., Lin M., Skold A., Xiao X., and Tall A. R.. 2001. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat. Med. 7: 847–852. [DOI] [PubMed] [Google Scholar]
- 57.Okazaki H., Goldstein J. L., Brown M. S., and Liang G.. 2010. LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size. J. Biol. Chem. 285: 6801–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yazdanyar A., and Jiang X. C.. 2012. Liver phospholipid transfer protein (PLTP) expression with a PLTP-null background promotes very low-density lipoprotein production in mice. Hepatology. 56: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manchekar M., Liu Y., Sun Z., Richardson P. E., and Dashti N.. 2015. Phospholipid transfer protein plays a major role in the initiation of apolipoprotein B-containing lipoprotein assembly in mouse primary hepatocytes. J. Biol. Chem. 290: 8196–8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu R., Iqbal J., Yeang C. W. D. Q. H., Hussain M. M., and Jiang X. C.. 2007. Phospholipid transfer protein-deficient mice absorb less cholesterol. Arterioscler. Thromb. Vasc. Biol. 27: 2014–2021. [DOI] [PubMed] [Google Scholar]
- 61.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., and Li P.. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9: 177–190. [DOI] [PubMed] [Google Scholar]
- 62.Tiwari S., Siddiqi S., and Siddiqi S. A.. 2013. CideB is required for the biogenesis of VLDL transport vesicle. J. Biol. Chem. 288: 5157–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L. J., Wang C., Yuan Y., Wang H., Wu J., Liu F., Li L., Gao X., Zhao Y. L., Hu P. Z., et al. . 2014. Cideb facilitates the lipidation of chylomicrons in the small intestine. J. Lipid Res. 55: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klemm E. J., Spooner E., and Ploegh H. L.. 2011. Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control. J. Biol. Chem. 286: 37602–37614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J., Zamani M., Thiele C., Taher J., Amir A. M., Yao Z., and Adeli K.. 2017. AUP1 (ancient ubiquitous protein 1) is a key determinant of hepatic very-low-density lipoprotein assembly and secretion. Arterioscler. Thromb. Vasc. Biol. 37: 633–642. [DOI] [PubMed] [Google Scholar]
- 66.Segrest J. P., Jones M. K., Mishra V. K., Anantharamaiah G. M., and Garber D. W.. 1994. apoB-100 has a pentapartite structure composed of three amphipathic alpha-helical domains alternating with two amphipathic beta-strand domains. Detection by the computer program LOCATE. Arterioscler. Thromb. 14: 1674–1685. [DOI] [PubMed] [Google Scholar]
- 67.Gretch D. G., Sturley S. L., Wang L., Lipton B. A., Dunnin A., Grunwald K. A. A., Wetterau J. R., Yao Z., Talmud P., and Attie A. D.. 1996. The amino terminus of apolipoprotein B is necessary but not sufficient for microsomal triglyceride transfer protein responsiveness. J. Biol. Chem. 271: 8682–8691. [DOI] [PubMed] [Google Scholar]
- 68.Bakillah A., Nayak N., Saxena U., Medford R. M., and Hussain M. M.. 2000. Decreased secretion of apoB follows inhibition of apoB-MTP binding by a novel antagonist. Biochemistry. 39: 4892–4899. [DOI] [PubMed] [Google Scholar]
- 69.Sellers J. A., Hou L., Athar H., Hussain M. M., and Shelness G. S.. 2003. A drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B: Implications for human and insect lipid transport and metabolism. J. Biol. Chem. 278: 20367–20373. [DOI] [PubMed] [Google Scholar]
- 70.Rava P., Ojakian G. K., Shelness G. S., and Hussain M. M.. 2006. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J. Biol. Chem. 281: 11019–11027. [DOI] [PubMed] [Google Scholar]
- 71.Khatun I., Zeissig S., Iqbal J., Wang M., Curiel D., Shelness G. S., Blumberg R. S., and Hussain M. M.. 2012. Phospholipid transfer activity of MTP promotes assembly of phospholipid-rich apoB-containing lipoproteins and reduces plasma as well as hepatic lipids in mice. Hepatology. 55: 1356–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kivlen M. H., Dorsey C. A., Lingappa V. R., and Hegde R. S.. 1997. Asymmetric distribution of pause transfer sequences in apolipoprotein B-100. J. Lipid Res. 38: 1149–1162. [PubMed] [Google Scholar]
- 73.Rusiñol A. E., Hegde R. S., Chuck S. L., Lingappa V. R., and Vance J. E.. 1998. Translocational pausing of apolipoprotein B can be regulated by membrane lipid composition. J. Lipid Res. 39: 1287–1294. [PubMed] [Google Scholar]
- 74.Yao Z. M., Blackhart B. D., Linton M. F., Taylor S. M., Young S. G., and McCarthy B. J.. 1991. Expression of carboxyl-terminally truncated forms of human apolipoprotein B in rat hepatoma cells: Evidence that the length of apolipoprotein B has a major effect on the buoyant density of the secreted lipoproteins. J. Biol. Chem. 266: 3300–3308. [PubMed] [Google Scholar]
- 75.Alexander C. A., Hamilton R. L., and Havel R. J.. 1976. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J. Cell Biol. 69: 241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cartwright I. J., and Higgins J. A.. 2001. Direct evidence for a two-step assembly of ApoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J. Biol. Chem. 276: 48048–48057. [DOI] [PubMed] [Google Scholar]
- 77.Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., and Young S. G.. 1998. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39: 1543–1557. [PubMed] [Google Scholar]
- 78.Yao Z., Zhou H., Figeys D., Wang Y., and Sundaram M.. 2013. Microsome-associated lumenal lipid droplets in the regulation of lipoprotein secretion. Curr. Opin. Lipidol. 24: 160–170. [DOI] [PubMed] [Google Scholar]
- 79.Lehner R., Lian J., and Quiroga A. D.. 2012. Lumenal lipid metabolism: implications for lipoprotein assembly. Arterioscler. Thromb. Vasc. Biol. 32: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 80.Magnusson B., Asp L., Bostrom P., Ruiz M., Stillemark-Billton P., Linden D., Boren J., and Olofsson S. O.. 2006. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 26: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 81.Raabe M., Véniant M. M., Sullivan M. A., Zlot C. H., Björkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., and Young S. G.. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., and Davidson N. O.. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 83.Kulinski A., Rustaeus S., and Vance J. E.. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with Apo B, as well as for Apo B lipidation. J. Biol. Chem. 277: 31516–31525. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y., Tran K., and Yao Z.. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 85.Hussain M. M. 2014. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 25: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sundaram M., and Yao Z.. 2012. Intrahepatic role of exchangeable apolipoproteins in lipoprotein assembly and secretion. Arterioscler. Thromb. Vasc. Biol. 32: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 87.Siddiqi S. A., Gorelick F. S., Mahan J. T., and Mansbach C. M.. 2003. COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J. Cell Sci. 116: 415–427. [DOI] [PubMed] [Google Scholar]