Abstract
X-rays and ventriculograms were the first imaging modalities used to localize intracranial lesions including brain tumors as far back as the 1880s. Subsequent advances in preoperative radiological localization included computed tomography (CT; 1971) and MRI (1977). Since then, other imaging modalities have been developed for clinical application although none as pivotal as CT and MRI. Intraoperative technological advances include the microscope, which has allowed precise surgery under magnification and improved lighting, and the endoscope, which has improved the treatment of hydrocephalus and allowed biopsy and complete resection of intraventricular, pituitary and pineal region tumors through a minimally invasive approach. Neuronavigation, intraoperative MRI, CT and ultrasound have increased the ability of the neurosurgeon to perform safe and maximal tumor resection. This may be facilitated by the use of fluorescing agents, which help define the tumor margin, and intraoperative neurophysiological monitoring, which helps identify and protect eloquent brain.
KEYWORDS : Brain tumor, endoscope, microscope, paediatric, technological advances
Practice points.
The operative microscope was first used in neurosurgery in 1957 and has since become an essential tool in tumor surgery allowing very precise tissue manipulation under high magnification and with improved lighting.
The endoscope was first introduced in neurosurgery in the early 1900s, although its use has only recently gained popularity. Its impact has been significant in the treatment of hydrocephalus associated with tumors, as well as for biopsy and/or resection of ventricular, pituitary and pineal region tumors. The applications of neuroendoscopy are steadily growing.
Neuronavigation, providing real-time visualization with respect to preoperative scans since the 1980s, is used for both tumor biopsy and resection. Neuronavigation techniques have refined the surgical approach and increased safety as well as the extent of tumor resection.
Intraoperative MRI, computed tomography and ultrasound have recently been employed as adjuncts to maximize the extent of tumor resection in a single sitting, thus avoiding return to theater to address residual tumors.
Intraoperative neurophysiological monitoring (including cortical and subcortical mapping) has been successfully used by many to assist in the safe removal of tumors in eloquent areas of the brain. It has enabled increased extent of resection while minimizing the risk of neurological injury in selected cases.
Intraoperative use of fluorescing agents such as 5-aminolevulinic acid (or Gliolan) is another adjunct to better define the tumor margin and further increase the extent of safe tumor resection. 5-aminolevulinic acid is, however, not currently licensed for use in children but has shown promise in case reports and small series around the world.
Sir William MacEwen (1848–1924) is credited with the first report of a successful operation in Glasgow in 1879 on a pediatric brain tumor (a probable meningioma in a 14-year-old girl) [1,2]. In his address on the surgery of the brain and spinal cord delivered at the annual meeting of the British Medical Association held in Glasgow on 9 August 1888, he spoke of two formidable barriers: “the inflammatory action which so often proved fatal as to shun active interference” and the fact that, at the time, the brain was “a dark continent in which surgeons could descry neither path nor guide capable of leading them to a particular diseased area, and, did they attempt to reach it, it could only be by groping in the dark”. He spoke of two advances current to his period that allowed him to undertake what he termed “cerebral surgery”: antisepsis, which he credited unsurprisingly to Joseph Lister (1827–1912), and the art of localization of cerebral lesions made possible by the works of Pierre Paul Broca, Alexander Robertson, Hughlings Jackson, Gustav Fritsch and Eduard Hitzig. These two advances, along with the development of anesthesia – which had been greatly contributed to by surgeons with an interest in the brain and spine – formed the building blocks of brain tumor surgery, both adult and pediatric.
Since the latter part of the 19th century, various challenges have been overcome with technological advances to transform neurosurgery into the specialty it is today and this is certainly evident in pediatric brain tumor surgery.
The aim of this review is to elaborate on the recent technological advances in brain tumor surgery which have been of most relevance in the pediatric setting.
Surgical advances
• Microscope
The surgical microscope, which was developed on the basis of the principles of optics and magnification, has been an essential tool in neurosurgery since the 1960s. The first recorded neurosurgical use of the microscope was by Theodore Kurze, who used it to remove a neurilemmoma of the facial nerve in 1957. Donaghy in 1957 established the first microneurosurgical training laboratory where several neurosurgeons trained, including the young Yasargil. Yasargil made several revolutionary improvements in the design of the operating microscope and was one of the major contributors to the field of microneurosurgery [3].
Over the last 50 years many advances have been made in microscope technology resulting in increased versatility. A key advance to the microscope has been the integration of intraoperative fluorescence. With the use of a microscope filter and fluorescing agents such as 5-aminolevulinic acid (5-ALA), indocyanine or sodium fluorescein, the surgeon can better identify tumors, their margins and their relation to vascular structures. 5-ALA is the most commonly employed fluorescing agent; the administration of exogenous 5-ALA results in increased accumulation of fluorescent protoporphyrin IX in malignant tissues (Figure 1). A randomized, controlled trial demonstrated that the use of 5-ALA significantly improved the extent of tumor resection in adults with high-grade gliomas, with a benefit in progression-free survival [4]. In recent years, there have been reports of 5-ALA use in pediatric neuro-oncology [5–7]. A single-center German study reported the successful use of 5-ALA to detect recurrent high-grade gliomas in children. Medulloblastomas, gangliogliomas and pilocytic astrocytomas appeared to have less consistent fluorescence [5]. A multicenter European survey on the use of 5-ALA in pediatric neuro-oncology reported data on a series of 78 children (the largest series reported so far). The use of 5-ALA appeared to be more useful in detecting high-grade gliomas and ependymomas, with varying degrees of ‘usefulness’ reported for other common pediatric tumors, including primitive neuroectodermal tumors, gangliogliomas, pilocytic astrocytomas and medulloblastomas [7]. It should be noted that the use of 5-ALA remains an ‘off-label’ application both in Europe and in the USA. In addition, clinical data remain confined to relatively small, nonrandomized case series, and further experience is needed to assess the impact of 5-ALA in pediatric brain tumors. Indocyanine green has proven useful in assessing flow in blood vessels intraoperatively and is used regularly in vascular neurosurgery. It is sometimes employed in pediatric neurosurgical oncology when tumors are in close proximity to vital vascular structures or when tumors are associated with abnormal vasculature (Figure 2).
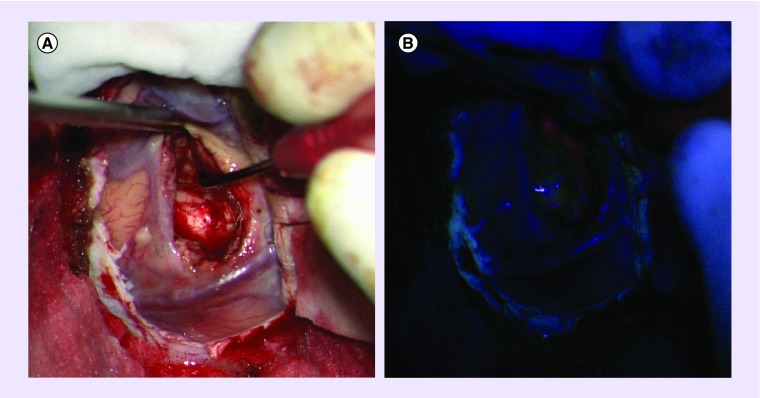
Figure 1. . Microscopic view of a high-grade glioma showing fluorescence.
(A) Standard operative light and (B) after 5-aminolevulinic acid injection.
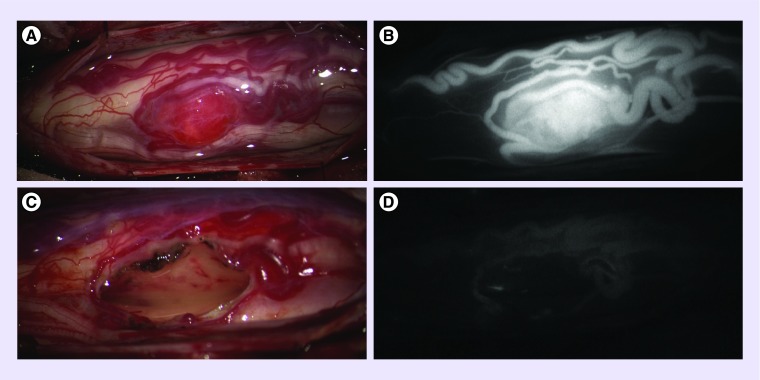
Figure 2. . Intraoperative images of an hemangioblastoma.
Intraoperative images of a hemangioblastoma in the thoracic spinal cord of a 16-year-old patient before (A & B) and after (C & D) resection – standard operative light (A & C) and after indocyanine green injection (B & D).
ICG: Indocyanine green.
Another advance has been the integration of the microscope and neuronavigation systems. This has allowed accurate and continuous correlation of the focal point of the microscope with preoperative imaging studies. The most recent incarnations of the microscope are motorized and have an automatic balancing system. This ensures that they do not drift in any direction and move seamlessly on command along any axis so that they may pivot around a lesion, hover in a set plane or rotate and point to a set target point [8]. These new possibilities can be combined with intraoperative imaging (MRI or computed tomography [CT]) to further augment surgical accuracy.
• Endoscope
The endoscope was introduced in neurosurgery in the early 1900s. Victor De L'Espinasse, a Chicago urologist, is credited with the first ventricular endoscopy in 1910 on a neonate with hydrocephalus where he used a cystoscope to resect choroid plexus. In the 1920s, William J Mixter, a neurosurgeon, performed the first endoscopic ventriculostomy in a child for the treatment of obstructive hydrocephalus. Walter Dandy, one of the fathers of neurosurgery, is also considered to be among the pioneers of neuroendoscopy having made significant contributions to the field [9].
Regarding the general indications for endoscopic tumor surgery, these are certainly evolving and dynamic, and dependent on the experience of the surgeon. In the beginning, the use of the endoscope was limited to the treatment of hydrocephalus (Figure 3), but the field has rapidly extended beyond this indication [10,11]. In 1973, Fukushima was the first to describe the use of a ventriculofiberscope for endoscopic biopsy of intraventricular tumors [12,13]. Since then, neuroendoscopic equipment has rapidly developed and neuroendoscopy has had an increasing role in the management of pediatric brain tumors [14–19]. Endoscopy can be used to obtain a biopsy or to debulk/resect intraventricular tumors (Figure 4). The decision between biopsy and resection depends on the experience of the endoscopic neurosurgeon. It is also usually based on factors such as tumor location, size and universus multifocality of the tumor [20]. An increasingly large variety of neuroendoscopes now exist, including single use scopes as well as multiuse ones, scopes as small as 1 mm in diameter with no working channels and scopes large enough to allow multiple instruments to be used through one or more working channels. It is the latter that are most suited for tumor biopsy and/or resection especially with the recent development of endoscopic monopolar and bipolar cauteries, laser as well as endoscopic ultrasonic aspirators. The coupling of the endoscope with image guidance has made it possible, in selected cases, to perform an endoscopic tumor biopsy or resection in children with intraventricular tumors even in the absence of ventriculomegaly [16].
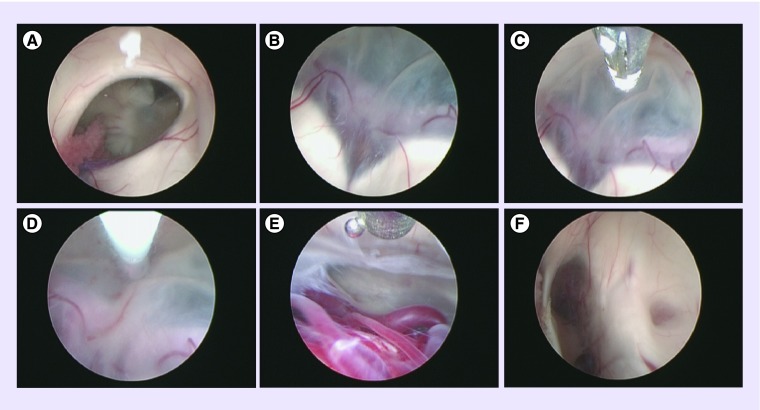
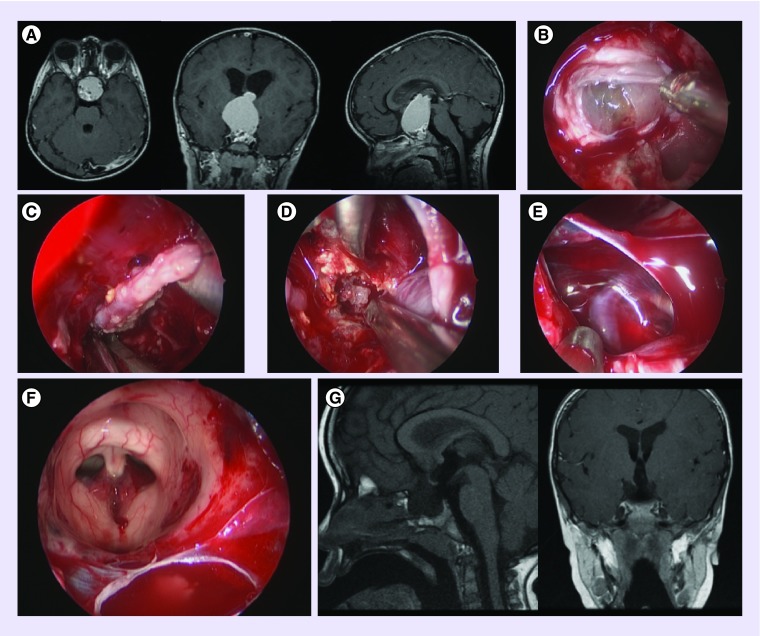
Figure 3. . Endoscopic third ventriculostomy in a child with a tectal plate/aqueductal glioma.
(A) Right foramen of Monro. (B) Floor of the third ventricle and the bifurcation of basilar artery. (C) Tip of the balloon approaching the floor of the third ventricle. (D) Ventrioculostomy using balloon. (E) Pontine perforators visible through the floor of the third ventricle. (F) Thickened tectal plate with stenosed aqueduct (on the right of the image) and pineal recess (on the left).
Figure 4. . Large colloid cyst in a 15-year-old child.
(A) Sagittal T2 CUBE demonstrating the cyst in the third ventricle at the level of the foramina of Monro. (B) Endoscopic view through the left lateral ventricle of the colloid cyst in the third ventricle. (C) Bipolar coagulation of surface of colloid cyst. (D) Mucinous material. (E) Suction of content of cyst. (F & G) Resection of cyst wall using biopsy forceps. (H) Sagittal T2 ventriculostomy sequence demonstrating complete removal of the lesion.
Numerous case series have demonstrated the efficacy of the endoscopic approach in the management of pineal region tumors. The main advantage is the possibility of obtaining a histological diagnosis as well as treating the associated hydrocephalus (through endoscopic third ventriculostomy) during the same operative intervention [21–23]. The majority of tumors in this region are sensitive to radiotherapy and chemotherapy, and, therefore, a combined biopsy and ventriculostomy is often the only surgical intervention required. In comparison to ‘closed’ biopsy procedures (e.g., stereotactic biopsy), the endoscopic technique has the advantage of providing direct visualization of the tumor and intraventricular anatomy allowing a safer approach (with the option of direct hemostasis in case of bleeding) with better yield from the biopsy.
Another relatively recent application of neuroendoscopy is in the management of pediatric anterior skull base tumors [24] with an increasing number of centers opting for the endoscopic transnasal approach to pituitary tumors as well as craniopharyngiomas (Figure 5).
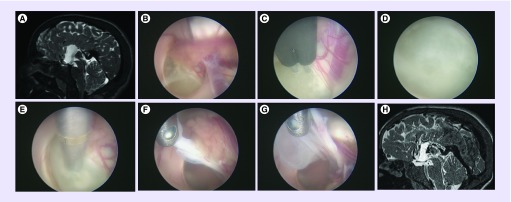
Figure 5. . Adamantinomatous craniopharyngioma in a 2-year-old patient.
(A) Scan demonstrating extensive invasion through the floor of third ventricle. (B) Transnasal transsphenoidal endoscopic approach to the sella with the dura opened and craniopharyngioma visible. (C) Capsule of the craniopharyngioma. (D) Calcified solid component of the craniopharyngioma. (E) Left carotid artery, oculomotor and optic nerves. (F) View of the roof of the third ventricle and foramina of Monro from below. (G) Postoperative scan demonstrating complete resection.
Furthermore, endoscope-assisted surgery is evolving rapidly and is becoming more commonplace in many minimally invasive approaches in conjunction with the microscope.
• Cavitating ultrasonic aspirators
Cavitating ultrasonic aspirators have been used in recent years by neurosurgeons to resect brain tumors while minimizing adverse effects on surrounding healthy tissue. They use ultrasonic energy to disrupt and fragment tissue in contact with the tip of the aspirator. The first ultrasonic aspirator for neurosurgical use was developed in 1976 in the USA. One advantage is that while tumor tissue usually disrupts easily owing to weak intracellular bonds and high fluid content, vessel walls and nerves are not easily fragmented because of stronger intracellular bonds due to their relatively higher content of elastin and collagen, and lower content of fluid [25,26].
Ultrasonic aspirators are particularly useful for the resection of hard and partially calcified tumors. They often have adjustable settings including irrigation, suction and amplitude of the ultrasonic energy as well as a variety of sizes and shapes of tips that can be tailored to the tumor's consistency and proximity of healthy tissue. They are extremely useful whether the tumor has a distinct margin or not. In the former, the ultrasonic aspirator is used to debulk the center of the tumor minimizing the extent of manipulation of the surrounding tissue; the periphery of the tumor can then be separated more easily using microsurgical techniques. In the latter, the ultrasonic aspirator can be used on a low setting as the indistinct tumor/healthy tissue interface is approached. The most recent advance in the field of ultrasonic aspirators is the development of tips that are variable in size and length, including tips that can be used down the channel of endoscopes allowing for increased versatility.
• Intraoperative neuronavigation
Frameless neuronavigation techniques have progressively emerged as an intraoperative adjunct during the 1990s, and currently represent an essential tool in both adult and pediatric neuro-oncology surgery [27]. Two systems are available for neuronavigation [28]. The most commonly employed one uses an optical tracking system, where dual infrared cameras track the position of a probe relative to a reference frame, usually fixed to the head support. The second has an electromagnetic tracking system that relies on the tracking of a probe within an electromagnetic field created by a fixed field generator. A coil attached to the head of the patient provides the reference. The main limitation of the optic system is the need to maintain an unobstructed field of view and a good alignment between the camera, the probe and the frame. Movements of the head relative to the reference frame are also not allowed, as this will result in a loss of accuracy. The electromagnetic system obviates this, since head movements are possible within the magnetic field, as long as the position of the reference coil is unchanged. Another aspect of the electromagnetic system is that it obviates the need for pin fixation, which is a particular advantage in young children with thin skulls and/or open sutures [29,30]. The main technical challenge of the electromagnetic tracking system is that the use of ferromagnetic surgical instruments can interfere with the electromagnetic field. This is overcome with the use of nonferromagnetic instruments. The accuracy of frameless techniques in the context of brain tumor surgery appears equivalent to conventional frame-based stereotaxy [31,32]. In addition, optical and electromagnetic systems provide similar accuracy [33].
Clinical applications of neuronavigation in brain tumor surgery include tumor biopsy as well as debulking/resection. The use of neuronavigation in tumor biopsies is increasingly popular in pediatric neurosurgery as it is less time consuming and – in the case of electromagnetic systems – does not require pin fixation or the use of stereotactic frames. The diagnostic yield has been reported as high as 99% [34]. Neuronavigation for tumor resection is used to tailor the craniotomy to gain the best access to the tumor and is especially useful when the tumor is close to eloquent brain or is deep seated [35]. Functional MRI and diffusion tensor imaging (DTI or tractography), when merged with the navigation imaging, provide additional information regarding specific functions (such as speech) and white matter tracts, respectively (Figures 6 & 7) [36].
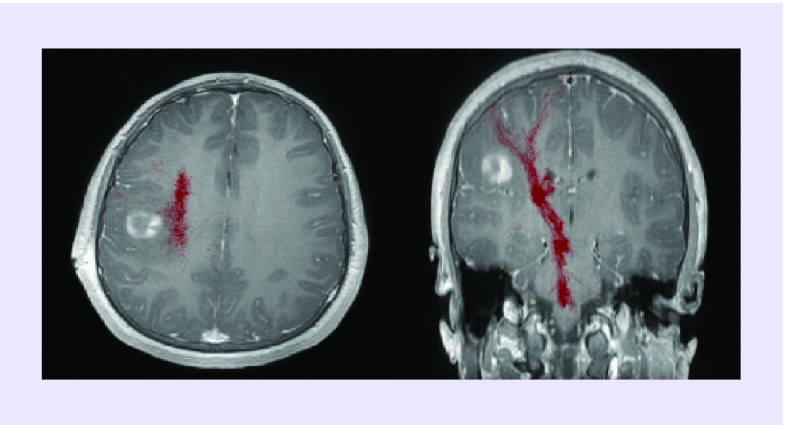
Figure 6. . Ganglioglioma grade I.
Tumor in a 14-year-old patient abutting the corticospinal tracts (red) as demonstrated by diffusion tensor imaging superimposed on T1 postcontrast magnetic resonance.
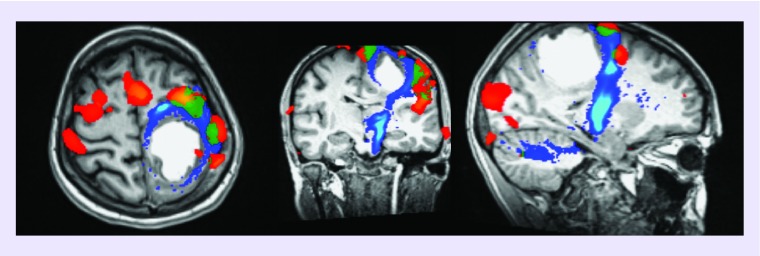
Figure 7. . Glioblastoma in a 17-year-old patient.
Relationship between the tumor, the motor areas (finger apposition and foot rocking) and the corticospinal tract. Functional MRI (red: finger apposition; green: foot rocking) and diffusion tensor imaging (corticospinal tracts in blue).
DTI: Diffusion tensor imaging; fMRI: Functional MRI.
A limitation in the use of neuronavigation is the phenomenon of brain shift, which can occur after significant loss of cerebrospinal fluid or extensive debulking of the tumor and reduces the accuracy of neuronavigation.
Randomized trials in adults to assess the impact of neuronavigation on the extent of tumor resection and patients’ outcome have provided conflicting results. Willems et al. [37] reported no benefit of neuronavigation-assisted versus standard surgery (i.e., without image guidance) for solitary enhancing brain tumors. The main caveat is that they only randomized patients in whom they felt neuronavigation would not be helpful. Wu et al. on the other hand conducted a prospective randomized controlled study in patients with gliomas (low and high grades) involving the pyramidal tract. The authors demonstrated that the use of DTI-based functional neuronavigation contributed to maximal safe resection while decreasing postoperative motor deficits and increasing survival [38]. Both these studies have been criticized in a recent Cochrane review for their small sample size and risk of allocation bias [39]. The authors concluded that there is low to very low quality evidence that image-guided surgery (using ioMRI, 5-ALA or DTI) increases the proportion of patients in whom complete tumor resection on MRI is achieved, with a theoretical concern that this may lead to increased adverse effects but that this has been poorly reported. Of note, no prospective or randomized trial evaluating the role of conventional or ‘functional’ (i.e., using functional MRI and/or DTI) neuronavigation has been reported in the pediatric population. Although this represents a potential field for future research, it is highly unlikely that such research will take place as neuronavigation is in routine use in many pediatric neurosurgical units, and this is exponentially increasing. Most surgeons would agree that the lack of evidence is not in itself evidence that neuronavigation is not beneficial.
• Intraoperative MRI
Intraoperative MRI (ioMRI) has emerged as an additional tool to assist in the removal of adult and pediatric brain tumors. Several groups have reported the use of ioMRI for the resection of intra-axial tumors [40–44]. The major advantages are the correction for brain shift, and identification of residual tumor and its margin. The main limitations are the reduced availability due to the high cost of installing an ioMRI solution, the need for MRI compatible equipment and the fact that the procedure is time consuming. A way to overcome some of these limitations is a ‘two-room’ solution ioMRI, with direct access between the operating theater and the MRI room. This also means that the MRI can be used routinely for diagnostic imaging providing a more cost-effective solution [45].
A randomized, controlled trial has shown the efficacy of ioMRI in increasing the extent of tumor resection in adults with high-grade gliomas. Senft et al. [41] randomized 58 glioma patients to ioMRI-guided resection or conventional surgery and found that ioMRI guidance was associated with a higher rate of complete resection when compared with the control group (96 vs 68%), and increased progression-free survival. Avula et al. [46] compared two cohorts of pediatric brain tumor patients: one group treated using conventional preoperative MRI only and the other group using ioMRI. About 14% of patients in the group that used only preoperative MRI required further surgery within 6 months. In the ioMRI group, 30% of patients had clear residual tumor on the intraoperative scan and had further resection under the same anesthetic. None of the patients in this group required further surgery at 6 months.
• Intraoperative ultrasound
Intraoperative ultrasound (ioUS) provides rapid real-time imaging. Thus ioUS-guided surgery prevents the loss of accuracy encountered due to brain shift when relying on image guidance based on preoperative CT or magnetic resonance (MR). The use of the Doppler mode can also help identify large blood vessels in proximity to or encased within tumors. Prada et al. [47,48] have also employed the use of contrast to further delineate vasculature in adults either in relation to tumors or vascular abnormalities but this is yet to be reported in children in the field of neurosurgery.
ioUS is considered by some authors an easily applicable and easily interpretable tool, after a short, albeit steep, learning curve, and is certainly a more cost-effective solution compared with ioMRI [49]. Also the use of intravenous contrast agents, other advances in the field of ultrasound (US) applied to neurosurgery include the development of 3D imaging and the concomitant use with CT- and/or MR-guided neuronavigation, where US can be used to correct for brain shift by superimposing the US image over that of the CT or MR (Figure 8) [50-53].
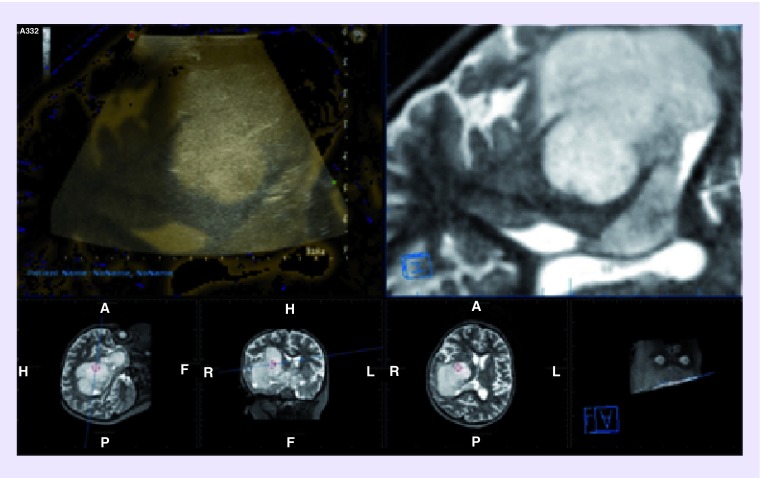
Figure 8. . Fusion of T2 MRI volumetric sequence for neuronavigation with intraoperative ultrasound.
A: anterior; H: head; F: foot; P: posterior; L: Left; R: right.
ioUS has been shown to be helpful not only in locating tumors, but also in identifying any residual tumor following resection. In pediatric brain tumors, this has been applied to the resection of posterior fossa lesions extending laterally in the fourth ventricle or upward toward the aqueduct [54]. A study of 60 patients found a 96.7% total excision rate using ioUS compared with 80% using conventional microsurgical techniques without US [54,55].
ioUS is not without limitations, however. It is an operator-dependant technique (and therefore, less reproducible). The walls of the resection cavity are usually hyper-echoic which makes it difficult to identify residual tumor, especially in the presence of edema/gliosis, with the risk of overestimating tumor volume. It is in this context that ioMRI may be superior in differentiating tumor from normal tissue [56,57].
• Intraoperative neuromonitoring
The goal of intraoperative neuromonitoring (IONM) is to provide real-time feedback to the surgical team to prevent injury to critical neural structures including eloquent brain (cortical and subcortical), spinal cord, cranial nerves, spinal nerve roots and peripheral nerves. Advances in IONM include the use of motor- and somatosensory-evoked potentials to assess the integrity of descending corticospinal and ascending somatosensory pathways, respectively, selective cortical and subcortical mapping to enable the resection of brain tumors from eloquent regions of the brain, electromyography to assess the integrity of cranial or spinal nerves. Visual evoked potentials as well as brainstem auditory-evoked potentials have also been used to enhance the safety of tumor resection when relevant [58–62].
In pediatric brain tumor surgery, cortical and subcortical brain mapping can be employed to identify eloquent cortex and white matter tracts and thus avoid injury to these areas.
There are specific anesthetic considerations that are essential for the successful use of IONM. Volatile anesthetic agents should be avoided as they can result in a dose-related reduction in the amplitude of motor-evoked potentials and an increase in the latency [63]. Total intravenous anesthesia is the method of choice with avoidance of neuromuscular blockade during the period of monitoring [64]). Care must be taken to reduce movement-induced injury through appropriately positioning and protecting the patient's extremities. Also care must be taken to reduce the risk of stimulation-induced seizures by avoiding overstimulation.
The major limitation of cortical mapping in children is the relative immaturity of the CNS in very young infants; as a result, very little data exist on efficacy of cortical mapping in children under 1 year of age. Another limitation is that speech cannot be tested with certainty unless the patient is awake and this is a major limiting factor in pediatric brain tumor surgery. The published pediatric series are small and have a bias toward older children who may tolerate awake surgery [65,66]. One solution is to implant subdural mat or grid electrodes under general anesthetic and perform the mapping by the bedside while the child is awake, and then perform the tumor surgery at a separate sitting.
• Evolving technologies
Less widely available technologies include robot-assisted surgery for tumor biopsy, ventriculoperitoneal shunt placement and endoscopic third ventriculostomy [67–70], lasers for thermal coagulation of deep-seated lesions including hamartomas (lasers are already commonly used by some centers for endoscopic cyst fenestration and endoscopic third ventriculostomies) [71–73] as well as convection-enhanced delivery of chemotherapy and immunotherapy [74,75]. In the latter, a drug delivery system is used with microcatheters inserted in deep tumors (diffuse pontine gliomas) delivering chemotherapy or immunotherapy. The results so far have not shown a survival benefit.
Conclusion & future perspective
Pediatric brain tumor surgery has reaped the benefits of many of the technological advances made in neurosurgery with a clear increase in survival rates. This is at least in part due to the improvement of surgical technique afforded to us by these advances, which have enabled us to perform a more extensive tumor resection while minimizing surgical morbidity. Together with major advances in the fields of neuroradiology, neuroanesthesia, intensive care, neuropathology, medical oncology and radiotherapy, we have made big strides in the multidisciplinary management of children with brain tumors.
The field of pediatric neuro-oncology is being transformed by advances in the molecular substratification of pediatric brain tumors reshaping the management of many tumors with new-targeted molecular therapies evolving.
In the meantime, surgical advances, in conjunction with advances in neuroimaging which are being brought into the operating room, along with an ever increasing ability to monitor brain function during surgery to reduce deficit and maximize resection, have allowed surgeons to achieve wider resections safely, using more minimally invasive approaches and in previously in-accessible areas.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Macewen W. An address on the surgery of the brain and spinal cord. Br. Med. J. 1888;2(1441):302–309. doi: 10.1136/bmj.2.1441.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pendleton C, Ahn ES, Quiñones-Hinojosa A. Harvey Cushing and pediatric brain tumors at Johns Hopkins: the early stages of development. J. Neurosurg. Pediatr. 2011;7(6):575–588. doi: 10.3171/2011.3.PEDS10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uluç K, Kujoth GC, Başkaya MK. Operating microscopes: past, present, and future. Neurosurg. Focus. 2009;27(3):E4. doi: 10.3171/2009.6.FOCUS09120. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre Phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 5.Preuß M, Renner C, Krupp W, et al. The use of 5-aminolevulinic acid fluorescence guidance in resection of pediatric brain tumors. Childs Nerv. Syst. 2013;29:1263–1267. doi: 10.1007/s00381-013-2159-8. [DOI] [PubMed] [Google Scholar]
- 6.Beez T, Sarikaya-Seiwert S, Steiger HJ, Hänggi D. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children-a technical report. Acta Neurochir. (Vienna) 2014;156(3):597–604. doi: 10.1007/s00701-014-1997-9. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, Rodrigues F, Schucht P, et al. European ALA Pediatric Brain Tumor Study Group. Predicting the ‘usefulness’ of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir. (Vienna) 2014;156(12):2315–2324. doi: 10.1007/s00701-014-2234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppenlander ME, Chowdhry SA, Merkl B, Hattendorf GM, Nakaji P, Spetzler RF. Robotic autopositioning of the operating microscope. Neurosurgery. 2014;10(Suppl. 2):214–219. doi: 10.1227/NEU.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 9.Dandy W. Cerebral ventriculoscopy. Bull. Johns Hopkins Hosp. 1922;33:189–190. [Google Scholar]
- 10.Decq P, Le Guerinel C, Palfi S, Djindjian M, Keravel Y, Nguyen JP. A new device for endoscopic third ventriculostomy. J. Neurosurg. 2000;93:509–512. doi: 10.3171/jns.2000.93.3.0509. [DOI] [PubMed] [Google Scholar]
- 11.Di Rocco C, Cinalli G, Massimi L, Spennato P, Cianciulli E, Tamburrini G. Endoscopic third ventriculostomy in the treatment of hydrocephalus in pediatric patients. Adv. Tech. Stand. Neurosurg. 2006;31:119–219. doi: 10.1007/3-211-32234-5_4. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima T, Ishijima B, Hirakawa K, Nakamura N, Sano K. Ventriculofiberscope: a new technique for endoscopic diagnosis and operation. Technical note. J. Neurosurg. 1973;38:251–256. doi: 10.3171/jns.1973.38.2.0251. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima T. Endoscopic biopsy of intraventricular tumors with the use of a ventriculofiberscope. Neurosurgery. 1978;2:110–111. doi: 10.1227/00006123-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Souweidane MM, Sandberg DI, Bilsky MH, Gutin PH. Endoscopic biopsy for tumors of the third ventricle. Pediatr. Neurosurg. 2000;33:132–137. doi: 10.1159/000028994. [DOI] [PubMed] [Google Scholar]
- 15.Badie B, Brooks N, Souweidane MM. Endoscopic and minimally invasive microsurgical approaches for treating brain tumor patients. J. Neurooncol. 2004;69:209–219. doi: 10.1023/b:neon.0000041884.93566.fb. [DOI] [PubMed] [Google Scholar]
- 16.Souweidane MM. Endoscopic management of pediatric brain tumors. Neurosurg. Focus. 2005;18:E1. doi: 10.3171/foc.2005.18.6.2. [DOI] [PubMed] [Google Scholar]
- 17.Souweidane MM. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2005;57:312–318. doi: 10.1227/01.neu.0000176641.17216.fb. discussion 312–318. [DOI] [PubMed] [Google Scholar]
- 18.Cinalli G, Spennato P, Cianciulli E, Fiorillo A, Di Maio S, Maggi G. The role of transventricular neuroendoscopy in the management of craniopharyngiomas: three patient reports and review of the literature. J. Pediatr. Endocrinol. Metab. 2006;19(Suppl. 1):341–354. [PubMed] [Google Scholar]
- 19.McLaughlin N, Ditzel Filho LF, Prevedello DM, Kelly DF, Carrau RL, Kassam AB. Side-cutting aspiration device for endoscopic and microscopic tumor removal. J. Neurol. Surg. B, Skull Base. 2012;73:11–20. doi: 10.1055/s-0032-1304834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J. Neurosurg. 1998;88:496–505. doi: 10.3171/jns.1998.88.3.0496. [DOI] [PubMed] [Google Scholar]
- 21.Oi S, Shibata M, Tominaga J, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J. Neurosurg. 2000;93:245–253. doi: 10.3171/jns.2000.93.2.0245. [DOI] [PubMed] [Google Scholar]
- 22.Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl. 2):575–597. doi: 10.1227/01.neu.0000316262.74843.dd. discussion 597–578. [DOI] [PubMed] [Google Scholar]
- 23.Morgenstern PF, Souweidane MM. Pineal region tumors: simultaneous endoscopic third ventriculostomy and tumor biopsy. World Neurosurg. 2013;79S18(Suppl. 2):e9–e13. doi: 10.1016/j.wneu.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Chivukula S, Koutourousiou M, Snyderman CH, Fernandez-Miranda JC, Gardner PA, Tyler-Kabara EC. Endoscopic endonasal skull base surgery in the pediatric population. J. Neurosurg. Pediatr. 2013;11:227–241. doi: 10.3171/2012.10.PEDS12160. [DOI] [PubMed] [Google Scholar]
- 25.Jallo GI. CUSA EXcel ultrasonic aspiration system. Neurosurgery. 2001;48:695–697. doi: 10.1097/00006123-200103000-00054. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS. Cavitron ultrasonic surgical aspirator. In: Feldman, Liane, Fuchshuber, Pascal, Jones, Daniel B, editors. The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE) Springer, NY, USA; 2012. pp. 133–138. [Google Scholar]
- 27.Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev. Med. Devices. 2012;9(5):491–500. doi: 10.1586/erd.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleary K, Peters TM. Image-guided interventions: technology review and clinical applications. Annu. Rev. Biomed. Eng. 2010;12:119–142. doi: 10.1146/annurev-bioeng-070909-105249. [DOI] [PubMed] [Google Scholar]
- 29.Sangra M, Clark S, Hayhurst C, Mallucci C. Electromagnetic-guided neuroendoscopy in the pediatric population. J. Neurosurg. Pediatr. 2009;3(4):325–330. doi: 10.3171/2008.12.PEDS0888. [DOI] [PubMed] [Google Scholar]
- 30.McMillen JL, Vonau M, Wood MJ. Pinless frameless electromagnetic image-guided neuroendoscopy in children. Childs Nerv. Syst. 2010;26(7):871–878. doi: 10.1007/s00381-009-1074-5. [DOI] [PubMed] [Google Scholar]
- 31.Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir. (Vienna) 2008;150(1):23–29. doi: 10.1007/s00701-007-1473-x. [DOI] [PubMed] [Google Scholar]
- 32.Widmann G, Schullian P, Ortler M, Bale R. Frameless stereotactic targeting devices: technical features, targeting errors and clinical results. Int. J. Med. Rob. 2012;8(1):1–16. doi: 10.1002/rcs.441. [DOI] [PubMed] [Google Scholar]
- 33.Mascott CR. Comparison of magnetic tracking and optical tracking by simultaneous use of two independent frameless stereotactic systems. Neurosurgery. 2005;57(Suppl. 4):295–301. doi: 10.1227/01.neu.0000176411.55324.1e. discussion 295. [DOI] [PubMed] [Google Scholar]
- 34.Shooman D, Belli A, Grundy PL. Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J. Neurosurg. 2010;113(2):170–178. doi: 10.3171/2009.12.JNS09573. [DOI] [PubMed] [Google Scholar]
- 35.Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C. Operative treatment of subcortical metastatic tumours in the central region. J. Neurooncol. 2011;103(3):567–573. doi: 10.1007/s11060-010-0420-5. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Chandra PS, Sharma BS, et al. The role of neuronavigation-guided functional MRI and diffusion tensor tractography along with cortical stimulation in patients with eloquent cortex lesions. Br. J. Neurosurg. 2014;28(2):226–233. doi: 10.3109/02688697.2013.835370. [DOI] [PubMed] [Google Scholar]
- 37.Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J. Neurosurg. 2006;104(3):360–368. doi: 10.3171/jns.2006.104.3.360. [DOI] [PubMed] [Google Scholar]
- 38.Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935–948. doi: 10.1227/01.neu.0000303189.80049.ab. [DOI] [PubMed] [Google Scholar]
- 39.Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst. Rev. 2014;1 doi: 10.1002/14651858.CD009685.pub2. CD009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatiboglu MA, Weinberg JS, Suki D, et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64(6):1073–1081. doi: 10.1227/01.NEU.0000345647.58219.07. [DOI] [PubMed] [Google Scholar]
- 41.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 42.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011;13(12):1339–1348. doi: 10.1093/neuonc/nor133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avula S, Mallucci CL, Pizer B, Garlick D, Crooks D, Abernethy LJ. Intraoperative 3-Tesla MRI in the management of paediatric cranial tumours – initial experience. Pediatr. Radiol. 2012;42(2):158–167. doi: 10.1007/s00247-011-2261-6. [DOI] [PubMed] [Google Scholar]
- 44.Yousaf J, Avula S, Abernethy LJ, Mallucci CL. Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg. Neurol. Int. 2012;3(Suppl. 2):S65–S72. doi: 10.4103/2152-7806.95417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parks C, Avula S, Abernethy L, Wright E, Mallucci CL. Intra-operative imaging for brain tumour resection in paediatric patients. Eur. Neurol. Rev. 2013;8(2):159–163. [Google Scholar]
- 46.Avula S, Pettorini B, Abernethy L, Pizer B, Williams D, Mallucci C. High field strength magnetic resonance imaging in paediatric brain tumour surgery – its role in prevention of early repeat resections. Childs Nerv. Syst. 2013;29:1843–1850. doi: 10.1007/s00381-013-2106-8. [DOI] [PubMed] [Google Scholar]
- 47.Prada F, Perin A, Martegani A, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery. 2014;74(5):542–552. doi: 10.1227/NEU.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 48.Prada F, Del Bene M, Saini M, Ferrali P, DiMeco F. Intraoperative cerebral angiosonography with ultrasound contrast agents: how I do it. Acta Neurochir. (Vienna) 2015;157(6):1025–1029. doi: 10.1007/s00701-015-2412-x. [DOI] [PubMed] [Google Scholar]
- 49.Unsgaard G, Rygh OM, Selbekk T, et al. Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir. (Vienna). 2006;148:235–253. doi: 10.1007/s00701-005-0688-y. [DOI] [PubMed] [Google Scholar]
- 50.Coenen VA, Krings T, Weidemann J, et al. Sequential visualization of brain and fiber tract deformation during intracranial surgery with three-dimensional ultrasound: an approach to evaluate the effect of brain shift. Neurosurgery. 2005;56(Suppl. 1):133–141. doi: 10.1227/01.neu.0000144315.35094.5f. discussion 133–141. [DOI] [PubMed] [Google Scholar]
- 51.Roth J, Biyani N, Beni-Adani L, Constantini S. Real-time neuronavigation with high-quality 3D ultrasound SonoWand in pediatric neurosurgery. Pediatr. Neurosurg. 2007;43(3):185–191. doi: 10.1159/000098830. [DOI] [PubMed] [Google Scholar]
- 52.Šteňo A, Karlík M, Mendel P, Čík M, Šteňo J. Navigated three-dimensional intraoperative ultrasound-guided awake resection of low-grade glioma partially infiltrating optic radiation. Acta Neurochir. (Vienna) 2012;154(7):1255–1262. doi: 10.1007/s00701-012-1357-6. [DOI] [PubMed] [Google Scholar]
- 53.Moiyadi AV, Shetty PM, Mahajan A, Udare A, Sridhar E. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir. (Vienna) 2013;155(12):2217–2225. doi: 10.1007/s00701-013-1881-z. [DOI] [PubMed] [Google Scholar]
- 54.El Beltagy MA, Aggag M, Kamal M. Role of intraoperative ultrasound in resection of pediatric brain tumors. Childs Nerv. Syst. 2010;26(9):1189–1193. doi: 10.1007/s00381-010-1091-4. [DOI] [PubMed] [Google Scholar]
- 55.El Beltagy MA, Atteya MM. The benefits of navigated intraoperative ultrasonography during resection of fourth ventricular tumors in children. Childs Nerv. Syst. 2013;29(7):1079–1088. doi: 10.1007/s00381-013-2103-y. [DOI] [PubMed] [Google Scholar]
- 56.LeRoux PD, Winter TC, Berger MS, Mack LA, Wang K, Elliott JP. A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J. Clin. Ultrasound. 1994;22:29–36. doi: 10.1002/jcu.1870220107. [DOI] [PubMed] [Google Scholar]
- 57.Shinoura N, Takahashi M, Yamada R. Delineation of brain tumor margins using intraoperative sononavigation: implications for tumor resection. J. Clin. Ultrasound. 2006;34:177–183. doi: 10.1002/jcu.20219. [DOI] [PubMed] [Google Scholar]
- 58.Sala F, Krzan MJ, Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv. Syst. 2002;18(6–7):264–287. doi: 10.1007/s00381-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 59.Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J. Neurosurg. 2003;98(4):764–778. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 60.Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J. Neurosurg. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- 61.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 62.Sloan TB, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr. Opin. Anaesthesiol. 2008;21:560–564. doi: 10.1097/ACO.0b013e32830f1fbd. [DOI] [PubMed] [Google Scholar]
- 63.Balvin MJ, Song KM, Slimp JC. Effects of anesthetic regimens and other confounding factors affecting the interpretation of motor evoked potentials during pediatric spine surgery. Am. J. Electroneurodiagnostic Technol. 2010;50(3):219–244. [PubMed] [Google Scholar]
- 64.Bercovici E, Pang EW, Sharma R, et al. Somatosensory-evoked fields on magnetoencephalography for epilepsy infants younger than 4 years with total intravenous anesthesia. Clin. Neurophysiol. 2008;119(6):1328–1334. doi: 10.1016/j.clinph.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 65.Balogun JA, Khan OH, Taylor M. Pediatric awake craniotomy and intra-operative stimulation mapping. J. Clin. Neurosci. 2014;21(11):1891–1894. doi: 10.1016/j.jocn.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Akay A, Rükşen M, Çetin HY, Seval HÖ, İşlekel S. Pediatric awake craniotomy for brain lesions. Pediatr. Neurosurg. 2016;51(2):103–108. doi: 10.1159/000442988. [DOI] [PubMed] [Google Scholar]
- 67.Lefranc M, Capel C, Pruvot-Occean AS, et al. Frameless robotic stereotactic biopsies: a consecutive series of 100 cases. J. Neurosurg. 2015;122(2):342–352. doi: 10.3171/2014.9.JNS14107. [DOI] [PubMed] [Google Scholar]
- 68.Calisto A, Dorfmüller G, Fohlen M, Bulteau C, Conti A, Delalande O. Endoscopic disconnection of hypothalamic hamartomas: safety and feasibility of robot-assisted, thulium laser-based procedures. J. Neurosurg. Pediatr. 2014;14(6):563–572. doi: 10.3171/2014.8.PEDS13586. [DOI] [PubMed] [Google Scholar]
- 69.Lollis SS, Roberts DW. Robotic catheter ventriculostomy: feasibility, efficacy, and implications. J. Neurosurg. 2008;108(2):269–274. doi: 10.3171/JNS/2008/108/2/0269. [DOI] [PubMed] [Google Scholar]
- 70.Niccolini M, Castelli V, Diversi C, Kang B, Mussa F, Sinibaldi E. Development and preliminary assessment of a robotic platform for neuroendoscopy based on a lightweight robot. Int. J. Med. Robot. 2016;12(1):4–17. doi: 10.1002/rcs.1638. [DOI] [PubMed] [Google Scholar]
- 71.Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir. 2012;(Suppl. 113):143–147. doi: 10.1007/978-3-7091-0923-6_29. [DOI] [PubMed] [Google Scholar]
- 72.Black PM, Guttmann C, Jolesz F. Present and future applications of lasers in neurosurgery. Keio J. Med. 1993;42(4):169–170. doi: 10.2302/kjm.42.169. [DOI] [PubMed] [Google Scholar]
- 73.Tovar-Spinoza Z, Choi H. Magnetic resonance-guided laser interstitial thermal therapy: report of a series of pediatric brain tumors. J. Neurosurg. Pediatr. 2016;17(6):723–733. doi: 10.3171/2015.11.PEDS15242. [DOI] [PubMed] [Google Scholar]
- 74.Hall WA, Rustamzadeh E, Asher AL. Convection-enhanced delivery in clinical trials. Neurosurg. Focus. 2003;14(2):e2. doi: 10.3171/foc.2003.14.2.3. [DOI] [PubMed] [Google Scholar]
- 75.Anderson RC, Kennedy B, Yanes CL, et al. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J. Neurosurg. Pediatr. 2013;11(3):289–295. doi: 10.3171/2012.10.PEDS12142. [DOI] [PMC free article] [PubMed] [Google Scholar]