The recent topical review by Rice and colleagues on sensory profiling in animal models of neuropathic pain rightly makes a call for back-translation [24]. The authors state “… here, we focus on aligning sensory measurements made in animal models with current methods of clinical sensory assessment”. Sadly, calls may fall on deaf ears, but many basic scientists in pain do aim for translation. So, we are a little surprised that other than a series of question marks in the table, electrophysiology is not discussed as an endpoint. Perhaps we are nerds and have grown rather attached to our neurones, but neuronal activity is the currency of pain and can be measured in the periphery, spinal cord and brain. Action potentials build the pain experience. It is an old technique – in 1975, Besson and colleagues showed a suppressive effect of morphine on spinal neurones [17], a year later, Tony Yaksh showed the behavioural consequences in rats [29], and in 1978, spinal morphine was shown to be analgesic in patients [28]. An example of fast and accurate translation.
A few years later, diffuse noxious inhibitory controls (DNIC), acting on spinal neurones, whereby one pain inhibits another via descending inhibitions were first reported [16], and now form the basis for conditioned pain modulation (CPM), able to predict the response to duloxetine and tapentadol [20,30], in perfect alignment with the pharmacological basis for DNIC [2]. Logically it should follow that novel drugs or strategies that target descending modulation can be tested using a DNIC paradigm, and then subsequently examined in stratified patient groups exhibiting low CPM.
Temporal summation of pain is often measured as a proxy for central sensitisation; wind-up potentiation of spinal neurones represents a neural substrate of this phenomenon [15]. Both processes exhibit similar pharmacological dependencies e.g. sensitivity to ketamine [1,11] and these potentiated neuronal responses appear to be important in patients [4].
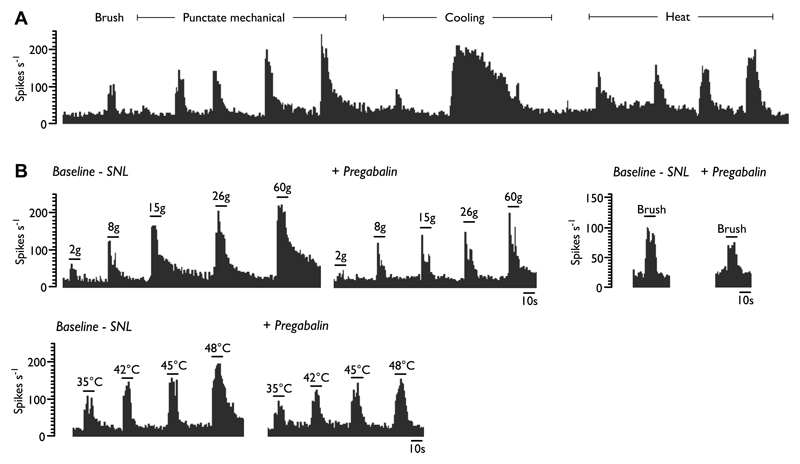
There are inherent difficulties in comparing perceptual outcomes determined with QST to endpoints in animals; by definition pain in animal models is inferred. However, the classical and elegant studies by Ron Dubner and colleagues highlighted the relationship between the fine-tuned intensity dependent coding of dorsal horn neurones and perceptual outcomes [12,19]. Importantly, human electrophysiological and micro-stimulation data from lateral thalamic pathways are available for comparison [6,7,18,22]. Here we show one example - a thalamic neurone where both stimulus-evoked and ongoing activity can be quantified in the same population of neurones (Figure 1A). These applied stimuli are very similar to those modalities used in clinical sensory assessment, and have been used in animals by many groups studying peripheral and central neuronal activity. Electrophysiological approaches are able to measure responses of sensory neurones to a wide range of stimuli, many of which are not used in behavioural studies. Using laser stimuli [26], and perturbing the system with UVB/heat [21], we have reported that spinal neurones in rats code various stimuli in a very similar manner to human psychophysics including QST.
Figure 1.
Example single unit histogram trace of a ventral posterolateral wide dynamic range neurone in a neuropathic rat. Various modalities of increasing intensity can be applied and the responses quantified (A). These responses can be pharmacologically modulated and pregabalin exerts modality selective inhibitory effects in SNL rats (B) (adapted from [23]).
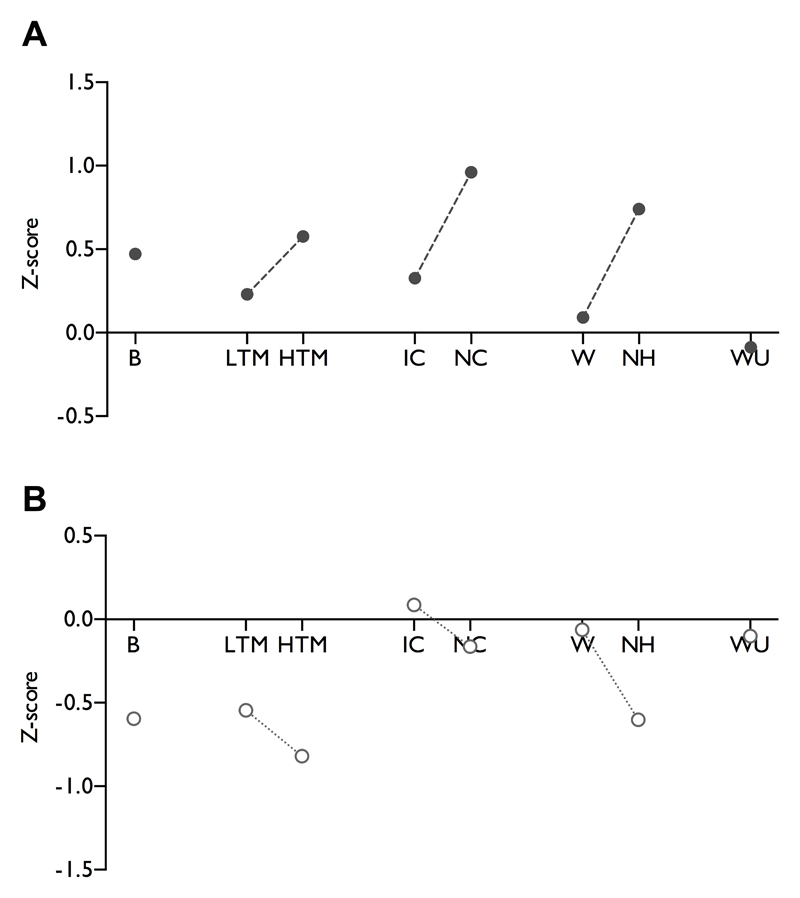
Electrophysiology has also been successfully used to decipher the neuronal effects of inherited human channelopathies in vitro [10]. Pharmacology can be done on neuronal responses, looking at the effects of clinically used drugs, novel agents or if both are lacking, electrophysiology can be done in transgenic animals. The example we give is from our attempts to back-translate from the effects of pregabalin in patients, allied to the work by Ralf Baron and colleagues in particular, to use sensory phenotypes as surrogates of mechanisms and move to a personalised treatment of their pain [14]. As can be seen (Figure 1B), there are selective effects of the drug on mechanical and heat stimuli, but no effect on ongoing firing. Notably, the largest inhibitory effect is against noxious punctate mechanical stimulation, so let’s see if this can translate. In an overall negative clinical trial, there is evidence that HIV patients with pinprick hyperalgesia had relief from the drug [27]. One proposal in the review is that animal models, like the patients in this clinical trial, could be classified according to their sensory profile. We provide an example of how this can be applied to the spinal nerve ligation model based on neuronal recordings, and other appropriate measures could be added (Figure 2A).
Figure 2.
A standardised representation of sensory gain in the spinal nerve ligation model in rats (A). Historical neuronal data from the ventral posterolateral thalamus was compiled from sham/naïve (n=84) and SNL rats (n=111) to determine ‘normal’ levels of sensory coding and the degree of sensory gain in SNL rats. Windup was determined by collating historical data from dorsal horn recordings from sham/naïve (n=66) and SNL rats (n=61). (B) Post hoc analysis of the standardised inhibitory effects of pregabalin on thalamic neuronal responses (n=15) [23], and dorsal horn neurones (WU only, n=9) [5] in SNL rats. Measures represent: B – brush, LTM – low threshold punctate mechanical, HTM – high threshold punctate mechanical, IC – innocuous cooling, NC – noxious cooling, W- warm temperature, NH – noxious heat, WU – windup.
The authors go on to recommend that we should ‘avoid anthropomorphising human emotions to rodents’; surely the converse extrapolation should also be true? The review includes 16 references on thigmotaxis and burrowing, but only one on conditioned place preference. Without convincing evidence that these former assays will forward translate, we would caution against widespread adoption. The assays might be sensitive to analgesics, but is it clear which neurological process is being studied and what is the relatable endpoint in humans? Using the power of CPP and neuronal recordings to study evoked responses some rather interesting findings with gabapentin on evoked and ongoing pain have emerged [3].
The authors rightly note that reflexive endpoints do not always lead to clinical efficacy and their role requires re-evaluation. One final point that those carrying out behavioural approaches have to consider. Pain is most often measured in humans by an analogue scale (i.e. a rating scale of 0–10). Behavioural responses to painful stimuli should arise at the pain threshold – so around 1- 2 on the scale and this is what is measured by reflex responses in animals. Electrophysiology in anaesthetised animals can continue to study responses up to their maximum – 10 on the scale and so has the potential to study the ‘neuronal load’ of pain levels of 6–7 that patients in trials often have. Here, we have a preclinical investigation of drug efficacy where neurones have their responses only modulated by pregabalin in an intensity and modality dependent manner (see figure 1B and 2B), whereas the drug ‘normalises’ withdrawal thresholds in a behavioural study [13]. The behaviour, only gauging responses to low intensities, is not translating to the partial effectiveness of the drug in some neuropathic patients, but the neuronal characterisations may provide more insight.
Aligning sensory testing in animals and humans is a logical and progressive step towards a mechanism-based rationale for identifying suitable treatments. However, it is imperative to align the most appropriate endpoints, otherwise we risk further failed outcomes for patients and undermine translational research. We passionately feel that neurones provide a powerful insight into pain processing. We wished to be brief here and have commented upon several other related points already [25]. We are finishing a back-translational study based on a sensory profiling investigation of oxcarbazepine in neuropathy (for some bedtime reading see [8] and [9]). Thank you for holding, your call is very important to us and we’ll get back to you shortly…
Acknowledgments
This work was supported by the Wellcome Trust Pain Consortium [102645 – Defining pain circuitry in health and disease].
Footnotes
Conflict of interest
AHD has received research funding and honoraria for lectures from Grünenthal GmbH.
References
- [1].Arendt-Nielsen L, Mansikka H, Staahl C, Rees H, Tan K, Smart TS, Monhemius R, Suzuki R, Drewes AM. A translational study of the effects of ketamine and pregabalin on temporal summation of experimental pain. Reg Anesth Pain Med. 2011;36(6):585–591. doi: 10.1097/AAP.0b013e31822b0db0. [DOI] [PubMed] [Google Scholar]
- [2].Bannister K, Patel R, Goncalves L, Townson L, Dickenson AH. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain. 2015;156(9):1803–1811. doi: 10.1097/j.pain.0000000000000240. [DOI] [PubMed] [Google Scholar]
- [3].Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain. 2017;158(12):2386–2395. doi: 10.1097/j.pain.0000000000001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Annals of neurology. 2013;74(5):630–636. doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- [5].Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140(1):209–223. doi: 10.1016/j.pain.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [6].Davis KD, Kiss ZH, Tasker RR, Dostrovsky JO. Thalamic stimulation-evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. J Neurophysiol. 1996;75(3):1026–1037. doi: 10.1152/jn.1996.75.3.1026. [DOI] [PubMed] [Google Scholar]
- [7].Davis KD, Lozano RM, Manduch M, Tasker RR, Kiss ZH, Dostrovsky JO. Thalamic relay site for cold perception in humans. J Neurophysiol. 1999;81(4):1970–1973. doi: 10.1152/jn.1999.81.4.1970. [DOI] [PubMed] [Google Scholar]
- [8].Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, Jensen TS, Sindrup SH. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015;156(11):2234–2244. doi: 10.1097/j.pain.0000000000000266. [DOI] [PubMed] [Google Scholar]
- [9].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- [10].Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nature reviews Neuroscience. 2013;14(1):49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- [11].Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- [12].Dubner R, Kenshalo DR, Jr, Maixner W, Bushnell MC, Oliveras JL. The correlation of monkey medullary dorsal horn neuronal activity and the perceived intensity of noxious heat stimuli. Journal of Neurophysiology. 1989;62(2):450–457. doi: 10.1152/jn.1989.62.2.450. [DOI] [PubMed] [Google Scholar]
- [13].Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su T-Z, Bramwell S, Corradini L, England S, Winks J. Identification of the α 2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proceedings of the National Academy of Sciences. 2006;103(46):17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Forstenpointner J, Otto J, Baron R. Individualized neuropathic pain therapy based on phenotyping: are we there yet? Pain. 2018;159(3):569–575. doi: 10.1097/j.pain.0000000000001088. [DOI] [PubMed] [Google Scholar]
- [15].Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- [16].Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- [17].Le Bars D, Menetrey D, Conseiller C, Besson JM. Depressive effects of morphine upon lamina V cells activities in the dorsal horn of the spinal cat. Brain Res. 1975;98(2):261–277. doi: 10.1016/0006-8993(75)90005-0. [DOI] [PubMed] [Google Scholar]
- [18].Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol. 1993;70(1):200–212. doi: 10.1152/jn.1993.70.1.200. [DOI] [PubMed] [Google Scholar]
- [19].Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr, Oliveras J-L. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Research. 1986;374(2):385–388. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- [20].Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. British journal of anaesthesia. 2014;113(1):148–156. doi: 10.1093/bja/aeu056. [DOI] [PubMed] [Google Scholar]
- [21].O'Neill J, Sikandar S, McMahon SB, Dickenson AH. Human psychophysics and rodent spinal neurones exhibit peripheral and central mechanisms of inflammatory pain in the UVB and UVB heat rekindling models. J Physiol. 2015;593(17):4029–4042. doi: 10.1113/JP270294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohara S, Weiss N, Lenz FA. Microstimulation in the region of the human thalamic principal somatic sensory nucleus evokes sensations like those of mechanical stimulation and movement. J Neurophysiol. 2004;91(2):736–745. doi: 10.1152/jn.00648.2003. [DOI] [PubMed] [Google Scholar]
- [23].Patel R, Dickenson AH. Neuronal hyperexcitability in the ventral posterior thalamus of neuropathic rats: modality selective effects of pregabalin. J Neurophysiol. 2016;116(1):159–170. doi: 10.1152/jn.00237.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rice ASC, Finnerup NB, Kemp HI, Currie GL, Baron R. Sensory profiling in animal models of neuropathic pain: a call for back-translation. Pain. 2017 doi: 10.1097/j.pain.0000000000001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sikandar S, Dickenson AH. II. No need for translation when the same language is spoken. British journal of anaesthesia. 2013;111(1):3–6. doi: 10.1093/bja/aet210. [DOI] [PubMed] [Google Scholar]
- [26].Sikandar S, Ronga I, Iannetti GD, Dickenson AH. Neural coding of nociceptive stimuli-from rat spinal neurones to human perception. Pain. 2013;154(8):1263–1273. doi: 10.1016/j.pain.2013.03.041. [DOI] [PubMed] [Google Scholar]
- [27].Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang JK. Pain relief by intrathecal injection of serotonin or morphine. Annales de l'anesthesiologie francaise. 1978;19(5):371–372. [PubMed] [Google Scholar]
- [29].Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192(4246):1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- [30].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]