Abstract
Objective
The decline in C-peptide in the five years after diagnosis of Type 1 diabetes has been well studied, but little is known about the longer-term trajectory. We aimed to examine the association between log-transformed C-peptide levels and duration of diabetes up to 40 years after diagnosis
Research Design and Methods
We assessed the pattern of association between urinary C-peptide creatinine ratio (UCPCR) and duration of diabetes in cross sectional data from 1549 individuals with Type 1 diabetes using non-linear regression approaches. Findings were replicated in longitudinal follow-up data in both UCPCR (n=161 individuals, 326 observations) and plasma C-peptide (n=93 individuals, 473 observations).
Results
We identified two clear phases of C-peptide decline: an initial exponential fall over 7 years (47% decrease per year [95%CI -50%,-43%]) followed by a stable period thereafter (+0.09% [-1.3,+1.5] per year). The two phases had similar duration and slope in patients above and below the median age at diagnosis (10.8 years) although levels were lower in the younger patients irrespective of duration. Patterns were consistent in both longitudinal UCPCR ((n=162) ≤7y duration: -48% per year [-55%,-38%]; >7y duration -0.1% [-4.1%,+3.9%]) and plasma C-peptide ((n=93) >7y duration only: -2.6% [-6.7%,+1.5%]).
Conclusions
These data support two clear phases of C-peptide decline: an initial exponential fall over a 7 year period, followed by a prolonged stabilization where C-peptide levels no longer decline. Understanding the pathophysiological and immunological differences between these two phases will give crucial insights into understanding beta-cell survival.
Background
Type 1 diabetes is a chronic disease characterized by autoimmune destruction of the beta cells in the pancreas. Traditionally, the autoimmunity has been considered as an ongoing destructive process, ultimately leading to absolute insulin deficiency. However, recent studies have challenged this view by revealing that 29-80% of individuals having type 1 diabetes with over 5 years duration still produce some C-peptide(1–5). Importantly, this is responsive to meal stimulation(1) suggesting that at least some of the residual beta cells are functional. These findings are consistent with histological studies of the pancreas in which residual insulin containing islets have been found in patients with longstanding type 1 diabetes (6–8). The presence of both C peptide and beta-cells in long-standing type 1 diabetes suggests an attenuation in the rate of beta-cell loss over time.
Studying the longer-term trajectory of beta cell decline will be a key step to understanding the preservation of C-peptide secretion in type 1 diabetes. Many studies have examined early C-peptide loss and these have revealed a rapid and continuing decline in the first 5 years after diagnosis(9–14). However, very little attention has been paid to the progression of C-peptide loss in longer duration of type 1 diabetes. For example, it is not known whether the rate of C-peptide loss slows or stabilizes, and if so, whether this is dependent on duration of diabetes or age of the patient.
Therefore, we aimed to examine the trajectory of C-peptide levels measured in a large cohort of patients up to 40 years after type 1 diabetes was diagnosed.
Research Design and Methods
We used both cross sectional and longitudinal datasets to explore the trajectory of C-peptide over time in patients with type 1 diabetes. Characteristics of the patients in these cohorts are in Supplemental Table S1.
Cross-sectional cohort
Initial analysis examined the association between C-peptide and duration of diabetes in a cross-sectional cohort of 1549 individuals with type 1 diabetes. Patients were recruited from two discrete geographic regions in the UK as part of the UNITED Study that aimed to recruit all patients diagnosed ≤30 years in these regions(15). For our study we only examined patients with a clinical diagnosis of type 1 diabetes who were insulin treated from diagnosis, To rule out Type 2 diabetes, patients were excluded if they had a BMI >30kg/m2 (or above the 80th percentile if aged under 22 at the time of recruitment) unless they were positive for GAD or IA2 autoantibodies. As part of the UNITED study, all patients with UCPCR>0.2nmol/mmol and negative islet antibody results(15; 16) were tested for 35 known monogenic diabetes subtypes(15; 16). Any patients with an identified monogenic cause for their diabetes were excluded from this analysis. All patients had a duration of diabetes less than or equal to 40 years.
Subjects had their endogenous insulin secretion tested by a post meal urinary C peptide creatinine ratio (UCPCR). This test has been validated against a formal assessment of C-peptide in a mixed meal tolerance test and shows a very high correlation with the stimulated C-peptide (r=0.91(17)). UCPCR results below the limit of detection were coded at 0.00072nmol/mmol (which is the limit of detection for the urinary C-peptide assay (0.03nmol/l) divided by the maximum urine creatinine seen in the study (41.6mmol/l)).
Longitudinal cohorts
We analysed changes over time of C-peptide using repeat samples from individuals to test findings in cross-sectional data. The patients were recruited from two different cohorts both from a single geographic region (Exeter, UK) and meet the used the same inclusion and exclusion criteria for Type 1 diabetes as the cross-sectional cohort
-
a)
UCPCR: A subset of patients who had UCPCR measured as part of the UNITED study (described above) or a UCPCR validation study(17) had repeat post meal UCPCR samples taken a median of (IQR) 4.3 (3.6, 5.1) years later ((n=221 patients in total, 2 repeat measurements except for 3 individuals with 3 measurements)).
-
b)
Plasma C-peptide: Repeat random non-fasting plasma C-peptide measurements were available on 105 patients with type 1 diabetes recruited to the Diabetes Alliance for Research in England (DARE) study. These patients consented for C-peptide to be measured at the same time as routine HbA1c testing using EDTA plasma. This enabled regular monitoring without specific research visits. C-peptide is stable for 24 hours at room temperature on EDTA plasma(18) and random non-fasting C-peptide has been shown to be highly correlated to 90 minute C-peptide in a mixed meal tolerance test (r=0.91) (19). All patients with at least 3 repeat measurements were included in the analysis. 529 C-peptide results were available from the 105 patients, with a median of 6 results available per patient, over a median (IQR) of 2.1 (1.3, 2.2) years.
The studies were approved by the National Research Ethics Service Committee South West Exeter and Bristol. All patients gave signed informed consent.
Laboratory analysis
Urinary C-peptide and plasma C-peptide were measured by electrochemiluminescence immunoassay (intra-assay coefficient of variation, 3.3%; interassay coefficient of variation, 4.5%) on a Roche Diagnostics (Mannheim, Germany) E170 analyzer by the Blood Sciences Department at the Royal Devon and Exeter National Health Service Foundation Trust, Exeter, U.K. The assay is a 2-site immunoassay employing monoclonal antibodies against human C-peptide, calibrated to WHO International Reference Reagent (IRR) for C-peptide of human. Urinary creatinine was analyzed on the Roche P800 platform using creatinine Jaffé reagent (standardized against isotope dilution mass spectrometry) to calculate UCPCR (nmol/mmol).
GAD and IA2 antibodies were measured as part of the UNITED study in those who had UCPCR>0.2nmol/mmol(15), and as part of the longitudinal plasma C-peptide studies. GAD and IA2 antibody analysis was performed using commercial ELISA assays (RSR Ltd., Cardiff, UK) and a Dynex DSX automated ELISA system (Launch Diagnostics, Longfield, UK). The laboratory participates in the Diabetes Autoantibody Standardization Program. Patients were considered positive for antibodies if their results were >97.5th centile (11 WHOunits/ml for GAD, 15 WHOunits/ml FOR IA2).
Statistical analysis
All C-peptide results, in both plasma and urine, were natural log transformed for analysis in line with previous studies (2; 10; 20), as the distribution of their values was heavily skewed.
Initial analysis of cross-sectional data used non-linear regression modelling to examine the association between duration and log UCPCR. Generalized additive models were used to explore the initial shape of the association. This revealed a pattern consistent with two phases that could be modelled with two lines of best fit. Segmented regression was used to determine the optimal breakpoints where the lines of best fit would meet, and to enable calculation of the intercept and slope of the two different phases, thereby modelling the starting point and rate of C-peptide decline.
To determine whether the association was similar in patients diagnosed both in childhood and in teenage years/young adulthood, the dataset was split by the median age at diagnosis and the analysis repeated in each group.
For the longitudinal analysis, data were split into two groups for the two phases: before and after the optimal breakpoints identified from cross sectional analysis. The intercept and slopes of the two different phases were determined using mixed effects models to model C-peptide against duration, with random effects at the patient level to allow each patient to contribute multiple C-peptide values at different time points. We used a random-intercept, random-slope model to allow for variability between individuals in terms of both C-peptide at diagnosis (the intercept) and in percentage change in C-peptide over time (the slope).
We repeated the analysis excluding those whose first value was below the lower limit of detection of the assay to ensure the finding did not represent a floor effect (i.e. that the results were not an artefact of those below the lower limit of the assay not being able to fall). Model assumptions were tested by examining normality of residuals and by plotting associations between residuals and fitted values and duration of diabetes.
The intercepts were back transformed (using the exponential) to estimate C-peptide at diagnosis from the models. As slopes were on a log scale they were interpreted in terms of percentage change per year (calculated from the exponential of the β coefficient minus 1). The half-life of C-peptide was calculated from loge(0.5)/β. The variability of individual slopes in the longitudinal models was determined using the standard deviation (SD) range (calculated by back transforming the β coefficient +/- 1 SD of the slopes)
All analysis was carried out in R version 3.3.2, including the mgcv package (for GAM models), lme4 package (for mixed effects models) and segmented package (for segmented regression).
Results
Cross-sectional analysis identifies two phases of C-peptide decline
Generalized additive modelling of cross-sectional data was used to explore the initial shape of the association and revealed a non-linear association between log UCPCR and disease duration (Figure 1a). This is suggestive of two phases: an initial log-linear (exponential) decline followed by a more stable period where the association flattens out. Characteristics of the 1549 individuals in the cross-sectional cohort are shown in Supplemental Table 1.
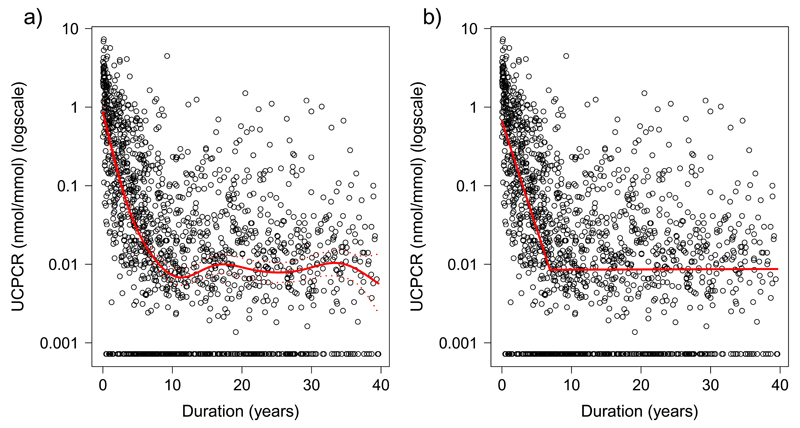
Figure 1.
Scatterplots of urinary C-peptide Creatinine Ratio (UCPCR) against duration of diabetes in 1549 individuals with Type 1 diabetes. Red lines show a) generalised additive modelling (non-linear) line of best fit, b) two straight lines of best fit meeting at the optimal breakpoint from segmented regression analysis
To model the slopes of these two phases, segmented regression was used. Figure 1b shows the two fitted slopes and the summary statistics, including the estimated UCPCR as modelled at diagnosis and at the breakpoint, are presented in Table 1. The optimal breakpoint (i.e. the point at which the slope changes) was modelled at 6.9 years from initial diagnosis (95% CI: 6.3, 7.5). Over this period, UCPCR declined by 47% (95% CI: 43%, 51%) per year (p<0.0001), equivalent to a half-life of 1.10 years (95% CI: 0.99, 1.25). Beyond 6.9 years the slope was flat, suggesting a more stable period with no further decline (+0.07% per year (95% CI: -1.3, +1.5), p=0.8).
Table 1.
Modelled C-peptide results and estimated percentage change per year of the two slopes from segmented regression analysis (Figure 1b) from the cross sectional data in 1549 patients with Type 1 diabetes. *No significant decrease in slope so half-life not calculated
| Whole group N=1549 | Age at diagnosis <=median (10.8y) N=782 | Age at diagnosis >median (10.8y) N=784 | P lower v higher age at diagnosis group | |
|---|---|---|---|---|
| Breakpoint (years) [95% CI] | 6.9 [6.3, 7.5] | 7.5 [6.6, 8.3] | 6.2 [5.4, 7.1] | 0.03 |
| Slope 1 (before breakpoint): | ||||
| Estimated UCPCR at diagnosis (nmol/mmol) * [95% CI] | 0.66 [0.50, 0.88] | 0.27 [0.18, 0.39] | 1.33 [0.91, 1.92] | <0.0001 |
| Percentage change in UCPCR per year† [95% CI] | -47% [-51, -43] | -42% [-47, -36] | -49% [-54, -43] | 0.13 |
| Half-life of UCPCR (years) ‡ [95% CI] | 1.1y [1.0, 1.3] | 1.3y [1.1, 1.5] | 1.0y [0.9, 1.2] | |
| Slope 2 (after breakpoint): | ||||
| UCPCR at breakpoint (nmol/mmol) § [95% CI] | 0.009 [0.006, 0.01] | 0.005 [0.003, 0.007] | 0.022 [0.014, 0.034] | <0.0001 |
| Percentage change in UCPCR per year† [95% CI] | +0.07% [-1.3, +1.5] | +1.6% [-0.3, +3.5] | -3.3% [-5.3, -1.3] | 0.0003 |
| Half-life of UCPCR(years) ‡ [95% CI] | Flat∥ | Flat∥ | 20y [13, 53] | |
exponential of intercept taken to show estimated C-peptide at diagnosis
calculated from the exponential of β (the regression slope) -1
calculated from log(0.5)/β
calculated from slope 1: (β * breakpoint) + intercept
No significant decrease in slope so half life not calculated
Plasma C-peptide (pmol/L) = UCPCR (nmol/mmol)*0.910(21)
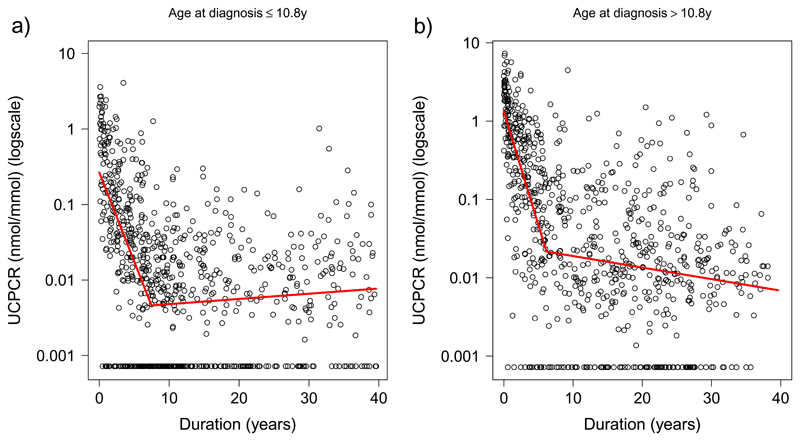
The rates of decline were similar for patients diagnosed at different ages but the overall UCPCR values were higher at all durations in those diagnosed at older ages
Figure 2 shows the patterns of association between disease duration and UCPCR when splitting the data by the median age at diagnosis (10.8y). In both ‘age at diagnosis’ groups, the pattern of beta cell decline was similar, showing an initial exponential fall followed by a more stable period. There was no significant difference in the slope of the first phase of decline in those diagnosed below 10.8y compared with those diagnosed above 10.8y (42% v 49% decrease per year, p=0.13; Table 2). The association between UCPCR and duration was much flatter in the second phase in both groups (Table 1). Although the initial slopes were similar in each age group, the absolute UCPCR value was higher across all time points in those who were older at diagnosis: the intercept was higher (indicating the UCPCR at diagnosis was higher) (1.32nmol/mmol v 0.27nmol/mmol, p<0.0001), as well as the modelled UCPCR at the breakpoint (i.e. the level for the start of the second more stable phase) (0.022 v 0.005 nmol/mmol, p<0.0001). Based on the initial UCPCR and half-life estimated from the slopes, we can calculate that it would take 0.6 years for those diagnosed <=10.8 years to reach the clinically important threshold of absolute insulin deficiency (0.2nmol/mmol (equivalent to 200 pmol/L))(21), compared with 2.7 years in the older group diagnosed >10.8y.
Figure 2.
Scatterplots of urinary C-peptide Creatinine Ratio (UCPCR) against duration of diabetes in 1549 individuals with Type 1 diabetes. Red lines show two lines of best fit from segmented regression analysis for a) individuals below the median age at diagnosis (<=10.8y) and b) individuals above the median age at diagnosis (>10.8y).
Table 2.
Results from mixed effects models of longitudinal repeated C-peptide measurements against duration of diabetes in a) urinary C-peptide creatinine ratio (UCPCR), and b) plasma, showing the pattern of decline in C-peptide over 40 years. Data presented for the two phases before and after 7 years duration. *No significant decrease in slope (i.e association flat) so half-life not calculated
| UCPCR | UCPCR (excluding values <lower limit of assay) | Serum C-peptide | Serum C-peptide (excluding values <lower limit) | |
|---|---|---|---|---|
| Total no. of individuals / observations | 162 ind / 326 obs | 117 ind / 236 obs | 93 ind / 473 obs | 63 ind / 335 obs |
| Median (IQR) years from 1st to last result | 4.3y (3.6, 5.1) | 4.5y (3.9, 5.4) | 2.1y (1.3, 2.2) | 2.0 (1.3, 2.2) |
| First phase (0-7y duration): | ||||
| No. of individuals / observations | 41 ind / 83 obs | 37 ind / 75 obs | - | - |
| C-peptide level at diagnosis* | 0.56 nmol/mmol | 1.2 nmol/mmol | ||
| Slope ≤7 years† (% change per year [95% CI]) | -48% [-55%, -38%] | -51% [-58%, -43%] | ||
| Half life‡ [95% CI] | 1.1y [0.9, 1.4] | 1.0y [0.8, 1.2] | ||
| Second phase (7-40y duration): | ||||
| No. of individuals / observations | 121 ind / 243 obs | 80 ind / 161 obs | 93 ind / 473 obs | 63 ind / 335 obs |
| C-peptide level at 7y breakpoint§ | 0.02 nmol/mmol | 0.12 nmol/mmol | 21.0 pmol/L | 50.8 pmol/L |
| Slope >7 years† (% decrease per year (95% CI)) | -0.1% [-4.1%, +4.0%] | -4.5% [-8.3%, -0.4%] | -2.6% [-6.7%, +1.5%] | -4.6% [-10%, +1.4%] |
| Half life‡ [95% CI] | Flat∥ | 15y [8, 164] | Flat∥ | Flat ∥ |
exponential of intercept of fixed effects from mixed effects model of observations ≤7y duration taken to show estimated C-peptide at diagnosis
calculated from the exponential of β (the regression slope) minus 1
calculated from log(0.5)/β
calculated from slope 1: (β * breakpoint) + intercept
No significant decrease in slope (i.e association flat) so half life not calculated
Plasma C-peptide (pmol/L) = UCPCR (nmol/mmol)*0.910(21)
Longitudinal cohorts validate the two phases of C-peptide decline in both plasma and urine
To validate the existence of two distinct phases, separate models were analyzed using longitudinal data obtained either in the first 7 years (individual patient data rounded to the nearest year), or after 7 years, in line with the estimated inflection point from cross sectional data. Of 221 patients with repeat UCPCR results, 41 had both initial and repeat results within 7 years of diagnosis and 121 had both initial and repeat results beyond 7 years after diagnosis.
The patterns were similar to those seen in the cross-sectional data, with an initial exponential fall in UCPCR during the first 7 years (48% decrease per year (SD range for variability of individual slopes: -67% to -18%), half-life = 1.1y; Table 2) and a more stable phase showing no further decline after that time (0.1% decrease per year (SD range -1.8% to +1.7%); Table 2). When excluding those whose first result was below the lower limit of the assay (to examine only those whose C-peptide levels could fall) there was a slight decline in the second phase, but this was far slower than that seen in the first 7 years (-4.5% per year (SD range -9.0 to +0.3%), half-life = 15y; Table 2).
In the 26 patients who had a UCPCR>0.2nmol/mmol (significant endogenous insulin(22)) after 7 years duration, there was no decrease in slope on repeat sampling (-0.7% [95% CI - 4%, +3%] per year, p=0.7). 15/26 of these patients were positive for either GAD or IA2 autoantibodies, and given the high positive predictive value for islet antibodies in this age group and our strict inclusion criteria this reinforces the conclusion that these individuals have Type 1 diabetes despite their high C-peptide levels.
Similar patterns were seen in the long duration patients when assessing the longitudinal plasma C-peptide data. Of the 105 patients with repeat plasma C-peptide results, only 5 had repeat samples in the initial 7 years, so analysis of the first phase was not carried out. Data were available from 93 patients who had initial and all repeat samples beyond 7 years duration and, again, there was no decline in slope over this second phase (-2.6% decrease per year, p=0.2, (SD range -12.6% to +8.5%); Table 2). Results were similar when excluding those whose first measurement was below the lower limit of the assay (Table 2).
Discussion
We have shown using both cross sectional data and longitudinal data, that there are two phases of C-peptide decline in the first 40 years after diagnosis of Type 1 diabetes. These comprise an initial exponential fall over the first 7 years, followed by a more stable period, where C-peptide levels either completely plateau or decline much more slowly.
The decline in C-peptide over the first few years after diagnosis has been studied in detail in a number of other studies(10–14; 20). The rate of decline we show of ˜47% per year up to 7 years is similar to that reported previously(10; 20). Some studies have not used log transformed data for analysis but, despite this, the patterns reported are consistent with an exponential fall(11; 13)
To our knowledge, this is the first study examining the continuous pattern of C-peptide concentrations over time in long duration Type 1 diabetes. Analysis of the T1D Exchange cohort investigated the prevalence of detectable C-peptide and found a decrease with increasing duration, but as the outcome was categorical this did not fully capture the changing association(4). The longest previous longitudinal study we have been able to identify modelled C-peptide over the first 7.4 years but used older less sensitive C-peptide assays, so was unable to evaluate the pattern of decline at lower levels(23). Our data suggest that there is a major change at around this point, with a dramatic decline in C-peptide secretion (half-life of approximately one year) over the first 7 years after diagnosis, followed by a relatively stable period beyond 7 years, where C-peptide levels either remain fairly constant or decline much more slowly (half-life estimated at 15 years from longitudinal plasma C-peptide data). This change is consistent with data showing HbA1c “tracks” over time stabilizing after around 6 years(24).
In our study, the absolute values of C-peptide differed according to age at diagnosis, with younger patients having lower levels on average, but the rate of decline and disease duration before the second stable phase was similar in individuals diagnosed below or above 10.8 years (the median age at diagnosis). The finding that younger patients have lower levels of C-peptide at diagnosis (and throughout the disease process) is well established(4; 10; 13; 20) and fits nicely with studies of the pancreas which show fewer residual insulin containing islets in patients having younger ages at disease onset(8). The similarity in the rate of decline between those diagnosed in childhood and in teenage years has also been seen previously(4; 10; 11). By contrast, the studies which have suggested that the rate of C-peptide decline is faster in patients diagnosed at younger ages have used other outcomes, such as time to a given low C-peptide threshold, to judge the rate and, as such, are not directly comparable with the present data (9; 12). Nevertheless, given that we find an exponential loss, and that younger patients with lower C-peptide levels at diagnosis would reach a low threshold more quickly, these previous results are not inconsistent with our data.
The finding of two phases suggests a change in the underlying biological processes leading to beta cell demise at around 7 years of disease duration. The fact that the pattern and inflection points were similar in those diagnosed at both younger and older ages, suggests this is a feature of disease progression, rather than being determined by the chronological age of the patient. This means that it is more likely to be a manifestation of changes occurring in the disease process in the pancreas rather than differences in puberty or in the maturity of the pancreas.
The nature of the biological changes that result in the stabilization of C-peptide are not revealed by our study so we see our findings as largely hypothesis generating. The stability of C-peptide around 7 years could reflect either a susceptible population of beta-cells that remain after an exponential decay or a change in the immune response at this time. Recent work has described a sub population of beta-cells and it may be that these “hub” cells are able to escape the immune destruction that affects all other beta cells(25). A change in the immune response is another possibility There is evidence that the immune attack may subside over time, given the recent finding that HLA Class I hyperexpression on the residual insulin-containing islets of individuals with type 1 diabetes (which is prominent at diagnosis) declines with disease duration (26). However, it could also reflect changes in antigen or antigen presentation.
Follow-up prospective studies involving repeated C-peptide measurements before and after the 7 year inflection point in larger numbers of people, would be of considerable value. These would allow the timing of the change in rate of C peptide decline to be examined in individual subjects and combined with simultaneous immune studies. However the considerable intra-individual variation in both C-peptide estimation and immune cell populations would mean that large numbers must be studied.
Understanding the mechanisms that mediate the change in C-peptide decline occurring at the inflection point will not only help elucidate the underlying biological mechanisms of beta-cell destruction over time in Type 1 diabetes, but may also lead to improved strategies for beta cell preservation. If the level of C-peptide attained in any given person at 7 years post diagnosis, is sustained, then this would also have implications for future intervention trials. The majority of trials currently focus on preserving beta cell function close to diagnosis of type 1 diabetes, but our new finding offers the potential for therapeutic trials to be undertaken later in the disease process
Our study has strengths and weaknesses. The major strengths are i) the large numbers studied (>1500), ii) that we combined both cross sectional and longitudinal studies, measuring both urine and plasma C-peptide, which show consistent support for both the stabilization of C peptide levels at 7 years and the rate of exponential deterioration before that, and iii) that we examined a large range of disease durations (up to 40 years). The major weaknesses are that the initial analysis was based on cross sectional data and the longitudinal studies based on repeat samples collected over a relatively short period (2-4 years) with the number of measurements limited to 2 for most individuals. Therefore larger, longer and more frequently sampled longitudinal replication would have considerable value, particularly prospective studies crossing the 7 year time point. Without longer follow-up time we cannot determine the extent to which our results reflect the pattern of C-peptide loss in all patients. However, given that the second phase, as modelled, is flat, this pattern would not occur if some patients were still declining at this point, without an equivalent number increasing their C-peptide to balance this. The fact that the second phase slope still remains relatively flat even when removing those individuals whose measured C-peptide is below the limit of the assay, suggests this is not an artefact caused by the inclusion of people with unrecordable values. Moreover, we used strict inclusion criteria to ensure that potential cases of Type 2 or monogenic diabetes were excluded. Given the rarity of other causes in those diagnosed young and the high proportion of positive islet autoantibodies in those with high C-peptide, we feel it is unlikely that the individuals studied had a form of diabetes other than type 1, and any potential misclassification will be minimal. It should also be emphasized that we have used home post-meal UCPCR and random non-fasting plasma C-peptide results rather than results from a gold standard mixed meal tolerance test for the present analysis. However, both of these C-peptide measurements have been validated against the MMTT and shown to be highly correlated(17; 19). Although, the measurements we used are potentially more prone to noise, we have used large sample sizes, and, importantly, the results were remarkably consistent in both plasma and urine. Finally, it is important to note that this study was carried out on predominantly White Caucasian cohorts. Further work is needed to determine whether the pattern is generalizable to other racial groups.
In conclusion, we have shown that there are two phases of C-peptide decline in Type 1 diabetes. The stabilization of C-peptide levels at around 7 years after diagnosis suggests there are important and previously unrecognized changes in immune function and/or beta cell viability around this time that may have critical implications for future pharmaceutical interventions.
Supplementary Material
Acknowledgements
Funding:
This work was principally supported by the Juvenile Diabetes Research Foundation (JDRF) (ref 3-SRA-2014-314-M-R), with additional funding from the Department of Health and Wellcome Trust Health Innovation Challenge Award (HICF-1009-041; WT-091985). ATH is an NIHR Senior investigator and Wellcome Trust senior investigator. BS is a core member of the NIHR Exeter Clinical Research facility. ERP holds a Wellcome Trust New Investigator award (102820/Z/13/Z). TJM is funded by an NIHR clinical senior lecturer fellowship.
RO holds a Diabetes UK Harry Keen Fellowship award. We acknowledge further funding from the JDRF Career Development award to SJR (5-CDA-2014-221-A-N) and project grants from Diabetes UK (16/0005480; to NGM & SJR).
This article/paper/report presents independent research supported by the JDRF, Diabetes UK, the Wellcome Trust and the National Institute for Health Research (NIHR) Exeter Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the JDRF, Diabetes UK, Wellcome Trust, NHS, the NIHR or the Department of Health.
We thank Bart Roep and Tim Tree of the TIGI steering group for their useful contributions to discussion around this paper and Rachel Besser and Angus Jones for the collection of samples for the UCPCR validation cohort used in the longitudinal analysis.
Footnotes
Conflicts of interest:
No potential conflicts of interest relevant to this article were reported
Author contributions:
BS carried out all statistical analysis, conceived the statistical aspects of study design, and wrote the manuscript. TM led the biochemical analysis, designed the serum follow up study, contributed to the study design and discussion, and reviewed/edited the manuscript. AVH is data manager for the longitudinal C-peptide studies and reviewed/edited the manuscript. MH is project manager for the UNITED study and reviewed/edited the manuscript. PL contributed to discussion and reviewed/edited the manuscript. EP was joint lead for the UNITED study and reviewed/edited the manuscript. SR contributed to discussion and reviewed/edited the manuscript. RO is Principal Investigator for the TIGI study (repeat urine C-peptide results), contributed to the study design and discussion, and reviewed/edited the manuscript. NM contributed to discussion and reviewed/edited the manuscript. ATH conceived of the idea, is joint lead for UNITED study and Chief Investigator for the DARE study, contributed to the study design and discussion, and reviewed/edited the manuscript.
BMS and ATH are the guarantors who take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
References
- 1.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oram RA, McDonald TJ, Shields BM, Hudson MM, Shepherd MH, Hammersley S, Pearson ER, Hattersley AT, Team U Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care. 2015;38:323–328. doi: 10.2337/dc14-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care. 2012;35:465–470. doi: 10.2337/dc11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, Goland RS, Greenberg EM, Liljenquist DR, Ahmann AJ, Marcovina SM, et al. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 5.Williams GM, Long AE, Wilson IV, Aitken RJ, Wyatt RC, McDonald TJ, Wong FS, Hattersley AT, Williams AJ, Bingley PJ, Gillespie KM. Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia. 2016;59:2722–2726. doi: 10.1007/s00125-016-4087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and beta-Cell Mass in the Natural History of Type 1 Diabetes. Diabetes. 2016;65:719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leete P, Willcox A, Krogvold L, Dahl-Jorgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential Insulitic Profiles Determine the Extent of beta-Cell Destruction and the Age at Onset of Type 1 Diabetes. Diabetes. 2016;65:1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 9.Besser RE, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care. 2013;36:195–201. doi: 10.2337/dc12-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabelea D, Mayer-Davis EJ, Andrews JS, Dolan LM, Pihoker C, Hamman RF, Greenbaum C, Marcovina S, Fujimoto W, Linder B, Imperatore G, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2012;55:3359–3368. doi: 10.1007/s00125-012-2719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD, Krause-Steinrauf H, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ, Type 1 Diabetes TrialNet Study G Fall in C-Peptide During First 4 Years From Diagnosis of Type 1 Diabetes: Variable Relation to Age, HbA1c, and Insulin Dose. Diabetes Care. 2016;39:1664–1670. doi: 10.2337/dc16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludvigsson J, Carlsson A, Deli A, Forsander G, Ivarsson SA, Kockum I, Lindblad B, Marcus C, Lernmark A, Samuelsson U. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013;100:203–209. doi: 10.1016/j.diabres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Nordwall M, Ludvigsson J. Clinical manifestations and beta cell function in Swedish diabetic children have remained unchanged during the last 25 years. Diabetes Metab Res Rev. 2008;24:472–479. doi: 10.1002/dmrr.871. [DOI] [PubMed] [Google Scholar]
- 15.Shields BM, Shepherd M, Hudson M, McDonald TJ, Colclough K, Peters J, Knight B, Hyde C, Ellard S, Pearson ER, Hattersley AT, et al. Population-Based Assessment of a Biomarker-Based Screening Pathway to Aid Diagnosis of Monogenic Diabetes in Young-Onset Patients. Diabetes Care. 2017;40:1017–1025. doi: 10.2337/dc17-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, et al. Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes. Diabetes Care. 2016;39:1879–1888. doi: 10.2337/dc16-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besser RE, Ludvigsson J, Jones AG, McDonald TJ, Shields BM, Knight BA, Hattersley AT. Urine C-peptide creatinine ratio is a noninvasive alternative to the mixed-meal tolerance test in children and adults with type 1 diabetes. Diabetes Care. 2011;34:607–609. doi: 10.2337/dc10-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald TJ, Perry MH, Peake RW, Pullan NJ, O'Connor J, Shields BM, Knight BA, Hattersley AT. EDTA improves stability of whole blood C-peptide and insulin to over 24 hours at room temperature. PLoS One. 2012;7:e42084. doi: 10.1371/journal.pone.0042084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hope SV, Knight BA, Shields BM, Hattersley AT, McDonald TJ, Jones AG. Random non-fasting C-peptide: bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med. 2016;33:1554–1558. doi: 10.1111/dme.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker A, Lauria A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, Mauricio D, Nordwall M, Van der Schueren B, Mandrup-Poulsen T, Scherbaum WA, et al. Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014;16:262–267. doi: 10.1111/dom.12216. [DOI] [PubMed] [Google Scholar]
- 21.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besser RE, Shepherd MH, McDonald TJ, Shields BM, Knight BA, Ellard S, Hattersley AT. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care. 2011;34:286–291. doi: 10.2337/dc10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snorgaard O, Lassen LH, Binder C. Homogeneity in pattern of decline of beta-cell function in IDDM. Prospective study of 204 consecutive cases followed for 7.4 yr. Diabetes Care. 1992;15:1009–1013. doi: 10.2337/diacare.15.8.1009. [DOI] [PubMed] [Google Scholar]
- 24.Nirantharakumar K, Mohammed N, Toulis KA, Thomas GN, Narendran P. Clinically meaningful and lasting HbA1c improvement rarely occurs after 5 years of type 1 diabetes: an argument for early, targeted and aggressive intervention following diagnosis. Diabetologia. 2018;61:1064–1070. doi: 10.1007/s00125-018-4574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016;24:389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jorgensen K, von Herrath M, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59:2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.