Abstract
Understanding how sensory stimuli are processed in the brain to instruct appropriate behavior is a fundamental question in neuroscience. Drosophila has become a powerful model system to address this problem. Recent advances in characterizing the circuits underlying pheromone processing have put the field in a position to follow the transformation of these chemical signals all the way from the sensory periphery to decision making and motor output. Here we describe the latest advances, outline emerging principles of pheromone processing and discuss future questions.
Introduction
Pheromones are powerful chemosensory stimuli, released into the environment by individuals with the ultimate goal of altering the behavior or physiology of others members of their species. Since the first description of pheromones, tremendous advances have been made in our understanding of how these chemicals are detected by sensory systems. However, with one notable exception (see below) our understanding of how pheromones are processed in the brain is still relatively limited. Drosophila is an attractive model system for studying the logic of pheromone perception since (1) pheromone-receptor pairs have been identified, (2) most of its ~100,000 neurons are accessible to genetic manipulations and (3) pheromones modulate several, quantifiable behaviors. Since sensory pheromone detection in Drosophila has been extensively reviewed elsewhere (e.g. see [1]) we will only briefly outline its principles before discussing recent advances in understanding pheromone processing deeper in the fly brain (i.e. perception). Finally, we will address sex differences in pheromone processing and outline future challenges. Although putative larval pheromones have recently been described [2, 3], this review will focus on pheromone processing in the adult fly.
Pheromone detection in Drosophila
Pheromone-sensitive cells in Drosophila are incorporated into the chemosensory systems of smell and taste. Flies, like most insects, have distinct anatomical subsystems for detection of volatile (long-range) and non-volatile (contact) chemicals: olfactory receptor neurons (ORNs) on head appendages and gustatory receptor neurons (GRNs) on various body parts [4, 5].
Odors are detected by odorant receptor (OR) or ionotropic receptor (IR) -expressing ORNs housed in sensory bristles (sensilla) on the third antennal segment and maxillary palps [6, 7]. On each antenna, ~45 classes of ORNs [6] are arranged as clusters of 1–4 neurons in four morphologically distinct classes of sensilla (basiconic, coeloconic, intermediate and trichoid). Intriguingly, only trichoid ORNs seem to be responsive to fly odors [8]. Axons from 20–50 ORNs (i.e. first-order olfactory neurons) expressing the same OR / IR converge on each of ~50 glomeruli in the antennal lobe (AL), the first relay station for olfactory information. There they form synapses with 1–7 excitatory or inhibitory projection neurons (PNs, second-order olfatory neurons) and a complex network of excitatory and inhibitory local neurons (LNs) [9, 10, 11]. PNs then relay the olfactory information to two distinct higher brain centers, the mushroom body (MB, required for olfactory learning) and the lateral horn (LH) [12].
In contrast, non-volatile chemicals are detected via taste sensilla on the labellum, pharynx, legs, wing margins and ovipositor [4, 5, 13]. Most sensilla contain four GRNs that express one of either ~60 gustatory receptor (GR) or ~35 IR genes conferring sensitivity to sweet, bitter, salt, water, fatty acids or carbonation [14, 15, 16, 17]. Several of these GRs and IRs are candidate pheromone receptors; all are housed in labellar or tarsal taste sensilla. GRNs project to the gnathal ganglion (GNG, formerly the subesophageal ganglion), the putative first-order processing center for gustatory information and to specific thoracic ganglia in the ventral nerve cord. In the GNG, their projections are reported to segregate by gustatory organ and taste category, but not by receptor, as is the case for ORNs [18].
Drosophila pheromonal stimuli and their receptors
While the tuning of most Drosophila ORs, IRs and many GRs [5, 19] has been investigated using a wide range of stimuli, barely any pheromone receptor-ligand pairs have been identified. A handful of trichoid ORs were found to respond to volatile compounds in fly extracts [8], but the chemical identity of the ligands remains unknown, with one exception: 11-cis-vaccenyl acetate (cVA), produced in the male ejaculatory bulb and transferred to females during copulation, is detected by two narrowly tuned ORs in both sexes, OR67d and OR65a [8, 20, 21]. Similarly, only a small number of pheromone-responsive GRs has been identified. They are likely activated by cuticular hydrocarbons (CHCs) [22], long-chain fatty acids which are produced by oenocytes, specialized cells located on the inner surface of the abdominal cuticle [23]. Some of these CHCs have been shown to be volatile [24], suggesting that they might be detected through olfaction as well as taste. CHCs are an essential sensory component for courtship [23] and courtship-inhibiting CHCs are present on both males and females. At least three of these courtship-inhibiting CHCs, 7-T, 9-T and 11-P [22], which are secreted by conspecific males or flies of other species, are detected by Gr32a [25, 26] (and probably Gr33a [27]). Intriguingly, both Gr32a and Gr33a are also required for detection of bitter-tasting compounds [27], suggesting that reproductive dead ends have aversive valence [28]. Another receptor, Gr68a, which is expressed in chemosensory neurons of ~20 male-specific gustatory bristles in the forelegs, was proposed to detect female contact pheromones [29], although this finding has been called into question [30, 31]. Of these putative female CHC aphrodisiacs, two have been identified, 7,11-HD and 7,11-ND [22], but their receptors remain unknown. Two potential candidates are IR52c and IR52d, which are co-expressed in male-specific foreleg sensilla and activated by female stimuli [17]. Surprisingly, 7,11-HD acts both as an attractant for conspecific males and as a powerful anti-aphrodisiac for males of other Drosophilids [23], illustrating that contact pheromones can serve as species barriers. A separate population of GRNs, expressing ppk25, ppk23 and ppk29, members of the pickpocket/degenerin-epithelial (Ppk/DEG-ENaC) family of sodium channels, was found to be necessary for detection of contact pheromones involved in male courtship behavior [16, 31, 32, 33, 34, 35]. ppk23 marks a subset of paired neurons in male-specific chemosensory leg bristles. One of these neurons responds to male pheromones (7-T, cVA), whereas the other one, characterized by ppk25 co-expression, responds to female pheromones (7,11-ND, 7,11-HD) [16, 31, 32, 33, 35]. Interestingly, these ppk neurons express neither Gr32a nor Gr68a [16]. A picture emerges therefore in which parallel, but functionally complementary systems exist for contact pheromone detection in Drosophila.
Central circuits for pheromone perception
Olfactory information carried by PNs converges, apparently randomly, onto third-order neurons in the MB (Kenyon Cells) [36] which integrate inputs from different glomeruli linearly or sublinearly [37]. This probabilistic wiring is thought to reflect the MB’s role in learning and memory. In contrast, third-order lateral horn neurons (LHNs) exhibit stereotyped connectivity and odor responses [38, 39, 40], in accordance with the LH’s hypothesized role in mediating innate olfactory behaviors. Since most ORNs are broadly tuned, odorant identity is presumably encoded by combinatorial activity of ORN → PN ensembles. While this distributive model is appropriate for general odor coding (but see Mansourian & Stensmyr [this issue] for a discussion of combinatorial coding under natural odor concentrations), the only identified olfactory pheromone, cVA, seems be initially processed by a labeled line instead, i.e. a neural circuit dedicated to a specific sensory stimulus.
Following the cVA signal into the brain has given us unique insights into the logic of pheromone perception. The tuning of first-, second- and some third-order olfactory neurons to cVA is extremely narrow [39, 41, 42, 43] and all components of this circuit express male-specific transcripts of fruitless [21, 42, 43, 44], a transcription factor thought to instruct the formation of neural circuits underlying male courtship behavior [45]. Several features distinguish pheromone processing from that of general odors: cVA (as well as volatile fly extracts) elicits relatively weak responses in ORNs [8], a potential consequence of its relatively low volatility. However, these weak responses are strongly amplified in postsynaptic DA1 PNs [41] (of which there are 6–8, the highest reported for any glomerulus). In addition, Chou et al. [11] found that the innervation density of some LNs was significantly lower in the pheromone-processing glomeruli DA1, DL3 and VA1d, and that these “pheromone-avoiding” LNs fired a significantly higher percentage of their spikes during the first 100 ms of the odor response as compared to all other LNs. Apart from cholinergic PNs mediating excitatory, feedforward transmission, five classes of GABAergic, inhibitory PNs (iPNs) have been identified, three of which target the fru+ glomeruli DA1, VL2a and VA1lm [12]. While these iPNs potently inhibit LHN responses to food odors, responses to phero- and kairomonal stimuli (via DA1 and VL2a) are iPN-independent [38]. Interestingly, Hong and Wilson [46] recently found that ORNs vary dramatically in their sensitivity to inhibitory LN activation in the AL and that DA1 is particularly sensitive to this presynaptic inhibition [46]. Therefore, different types of inhibition within pheromone-processing circuits might operate according to different rules.
Taken together, these circuit characteristics (signal amplification, numerical robustness, exemption from some types of inhibition) might ensure high-fidelity transmission of pheromone information even in the presence of other olfactory stimuli. However, since cVA is the only identified volatile pheromone in Drosophila, it remains unclear whether these findings can be generalized.
Even less is known about central processing of contact pheromones (or any gustatory stimuli) in flies. As mentioned, GRNs projections in the GNG roughly segregate by organ location and taste category [18]. Indeed even though individual GRNs can detect multiple taste qualities, these tend to have the same valence [13]. Therefore, taste in Drosophila seems to be a “valence labeled line” modality [13]. Kain and Dahanukar [47] recently identified sweet-responsive gustatory PNs downstream of labellar Gr5a GRNs and traced their axonal projections to the antennal mechanosensory and motor center (AMMC) [47]. However, since this represents the only known class of of second-order gustatory neurons so far, it is unclear whether the ’valence labeled line’ organization is preserved in higher brain centers.
Integration of pheromone signals
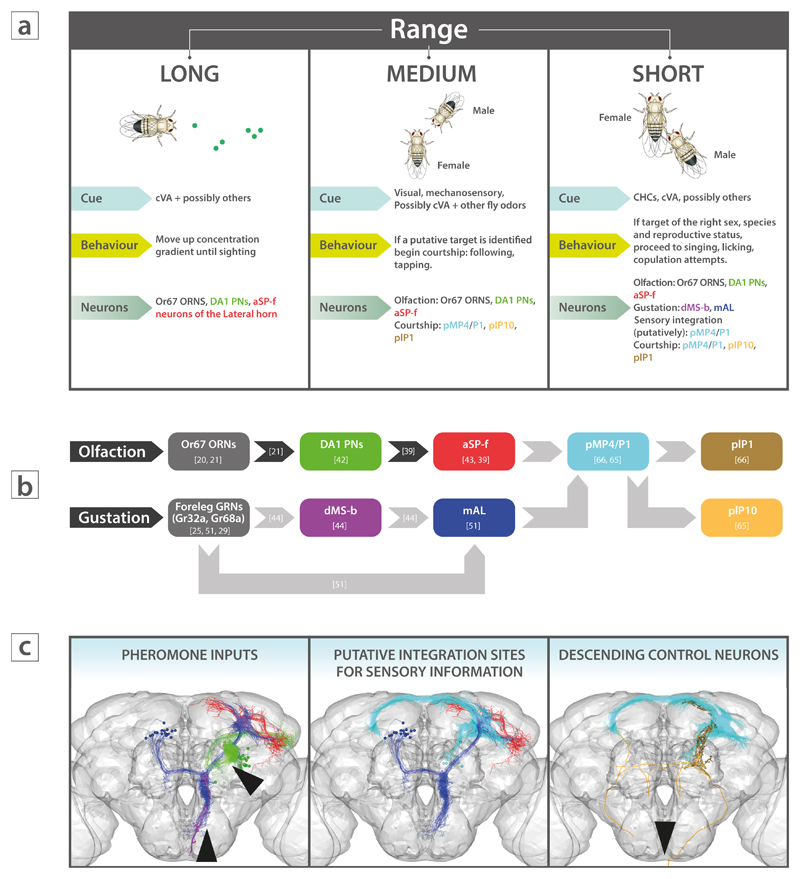
Why do flies use such a wide variety of receptors and structures to detect pheromone stimuli that may have similar meaning? Male courtship, a complex sequence of behaviors (Figure 1a), is uniquely suited to explore this question, since detection of both volatile and contact pheromones has been shown to coordinate this elaborate ritual, together with visual, auditory and mechanosensory cues [48].
Figure 1. Pheromone perception in Drosophila courtship.
(a) Proposed model for how pheromonal cues guide male behavior at different spatial ranges. Note that neuron names are color-coded to match the illustrations in (b) and (c).
(b) Hypothetical wiring diagram from chemosensory inputs to descending motor output. Darker arrows suggest strong experimental evidence for synaptic connections and the direction of information flow, whereas lighter arrows suggest probable connections based on neuroanatomical data, i.e. physical overlap (see (c)). The putative connection from mAL to pMP4/P1 may be inhibitory[26], suggesting that the wiring diagram above is incomplete, as pMP4/P1 neurons must get excitatory input from somewhere else.
(c) 3D renderings of neurons involved in pheromone processing (left), sensory integration (middle), and motor control (right). Black arrowheads illustrate sensory input (left) or motor output (right), respectively.
Fly cartoon images adapted with permission from Sokolowski, Nature Reviews Genetics 2:879-890 (2001).
Chemical cues vary widely in their volatility and the same cue may be detected by different sensory modalities at different ranges. Thus pheromones may be used to signal not just valence, but also reflect target distance, potentially acting sequentially to trigger the appropriate steps of courtship behavior. Initially, cVA in conjunction with food odors could act as a long- or medium-range aggregation pheromone to attract flies of both sexes (Figure 1a) [41, 49, 50] to food substrates suitable for mating. At closer range, and while interacting with other flies in a group, rapidly changing concentrations of cVA and other sex-specific volatile pheromones (as well as visual cues) could inform the selection of appropriate courtship targets (i.e. virgin females) worthy of following. Subsequently, detection of CHCs via direct contact (tapping) might represent a check point for deciding between continuation of courtship, i.e. singing, licking (female of correct species), fighting (male of correct species) or withdrawal (incorrect species). Finally, if a decision to continue with courtship is made, detection of secreted female pheromones via labellar receptors (licking) and auditory cues could gate the final step of the ritual, copulation. Although simplistic, this working model of the behavioral algorithm governing courtship behavior makes testable predictions:
(a) food and pheromone odors are integrated in the brain and (b) neural representations of volatile and contact pheromones interact, with perception of long-range signals possibly gating subsequent perception of short-range signals, either by modifying approach behavior or directly at the neural circuit level.
Some evidence exists in support of the first prediction, i.e. interactions between the neural representations of volatile food and pheromone stimuli. While PNs tuned to general odors target dorsal regions of the LH, PNs downstream of pheromone-responsive trichoid ORNs (DA1, VL2a, VA1lm, DL3) project to the anterioventral LH [12, 50]. This observation suggests that a spatially segregated representation of pheromones persists from the AL to the LH [12], but a few non-pheromonal chemicals that mediate innate attraction or aversion are also detected by second-order neurons that project to the vLH: farnesol (Or83c/DC3), geosmin (Or56a, DA2), PAA/PA (Ir84a, VL2a) (Table 1) and valencene (Or19a, DC1). Since processing of these odors stimuli shares several characteristics with pheromone processing (innate valence, narrowly tuned PNs with vLH projections), they can be described as kairomones, i.e. chemicals emitted by an organism, which attract exploiters of another species. Indeed, co-processing of pheromonal (e.g. cVA) and such kairomonal (e.g. PAA) stimuli has been suggested to serve the coordination of feeding and oviposition site selection with reproductive behaviors [50]. This is supported by the observations that cVA acts as an aggregation pheromone only in presence of attractive food odors [41, 49] and that the food odor PAA is only attractive when presented with fly odors [50]. The site of convergence of pheromonal and kairomonal signals with similar biological value might be found in third-order LHNs with dendrites in the vLH that integrate signals from both channels. As a consequence of such interactions, the labeled line model is probably only valid for the initial stages of pheromone processing.
Table 1. Drosophila pheromonal stimuli and their receptors.
| Pheromone | Range | emitter → detector | Receptor | PN | Behavior | Comments | Refs |
|---|---|---|---|---|---|---|---|
| cVA | volatile & contact | M → M | Or67d | DA1 | aggression | suppressed by chronic cVA via Or65a | [30, 53, 54] |
| F → M | Or67d | DA1 | male repulsion | transferred to female by prior mating | [21] | ||
| M → F | Or67d | DA1 | female receptivity | suppressed by chronic cVA via Or65a | [21, 56] | ||
| M/F → M/F | Or65a (?) | DL3 (?) | aggregation4 | [49] | |||
| M → M | ppk23/ppk291 | n/a | male-male repulsion | ppk channels pheromone receptors ? | [16] | ||
| 7-T | contact | M → F | Gr32a | n/a | female receptivity | increases female receptivity | [64] |
| M → M | Gr32a (Gr66a) |
n/a | male-male / interspecies repulsion | [26, 28] | |||
| M → M | ppk23/ppk291 | n/a | male-male repulsion | ppk channels pheromone receptors ? | [16, 31, 33, 34] | ||
| 7-P | contact | M → F | ? | n/a | female receptivity (?) | male-enriched | [22, 60] |
| 7,11-HD (7,11-ND)3 | contact | F → M | ppk23/ppk29/ppk25/nope1 | n/a | male courtship | ppk channels pheromone receptors ? | [16, 31, 33, 34] |
| 9-P | contact | F → M | ? | n/a | male copulation | role in courtship conditioning | [65] |
| CH503 | contact (?) | F → M | ? | ? | male repulsion | transferred to female by prior mating | [60] |
| 9-T, 11-P | contact | M → M | Gr32a / Gr33a5 | n/a | male-male / inter-species repulsion | no cVA-mediated aggression in Gr32a -/- males; contact fru+ neurons in GNG | [25, 26, 27, 28, 52, 66] |
| PAA, PA | volatile | food → M | Ir84a | VL2a | male courtship | kairomone aphrodisiac | [50] |
| SP | contact | M → F | SPR | n/a | postmating response | allohormone pheromone2, transferred with seminal fluid | [58] |
| M/F extract | contact | ? | Or47b | VA1v | ? | active compound(s) unidentified | [8] |
| M/F extract | contact | ? | Or88a | VA1d | ? | active compound(s) unidentified | [8] |
| ? | contact | F → M | Gr68a | n/a | male courtship | stimulate male courtship, doublesex-dependent | [29] |
| ? | contact | F → M | Gr39a | n/a | male courtship | role in sustaining male courtship behavior | [67] |
| ? | contact | F → M | IR52c / IR52d | n/a | male courtship | presumably contact fru+ neurons in prothoracic ganglia | [17] |
M, male, F, female, PAA, phenylacetic acid, PA, phenylacetaldehyde, 7-T, (z)-7-tricosene, 7-P, 7-pentacosene, 9-T, z-9-tricosene, 11-P, z-11-pentacosene, 7,11-HD, (7Z,11Z)-heptacosadiene, 7,11-ND, (7Z,11Z)-nonacosadiene, SP, sex peptide, fru+,1 unclear whether ppk29 neurons co-express fru, 2i.e. directly acting on target tissue, 3 detected by ppk23+ neurons [31], 4cVA acts as an aggregation pheromone only in conjunction with food odors (see [49]), 5Gr32a (but not Gr33a) required for inhibition of interspecies courtship [26].
The second prediction is that crosstalk between olfactory and contact pheromone signals exists. This notion is backed, amongst others, by the observations that Gr32a is required for the aggression-promoting effect of cVA [51], that 7,11-HD mitigates the deterrent effects of cVA [23] and that increased courtship caused by male CHC ablation is suppressed by a mutation in Or47b [51]. Furthermore, since cVA is detected by both olfactory and contact chemosensory neurons [8, 16, 20, 21] (Table 1) – interactions between both pheromone processing circuits are likely. This interaction probably does not occur between GRNs and central olfactory neurons, since neither DA1 PNs nor identified cVA-responsive LHNs project to the GNG. However, Gr32a neurons are anatomically poised to relay pheromone information to sexually dimorphic fru+ neurons in the GNG [52] and the ventrolateral protocerebrum [25], which in turn could connect to the fru+ cVA olfactory circuit.
Sexually dimorphic and state-dependent pheromone perception
How can a single pheromone, cVA, elicit aggression and male-male repulsion in males [21, 53, 54], increase receptivity in females [21] and act as aggregation signal in both sexes [49] (Figure 2)? Numerous differences exist between male and female fly brains, most controlled by fruitless [44] and doublesex [55]. Many of these differences are associated with sensory processing pathways [44]. Males have a higher number of trichoid sensilla [4] and three enlarged glomeruli, DA1, VA1v and VL2a, all of which express fru+ [45]. Deeper in the brain, DA1 PN axons show a male-specific ventral arborization [42], but it remains unclear if this has functional consequences. Surprisingly, cVA responses in first- and second-order olfactory neurons are non-dimorphic [8, 21, 42], but the dendrites of two groups of fru+ third-order olfactory neurons sex-specifically overlap with DA1 PN axon terminals, thereby forming a bidirectional circuit switch that reroutes the cVA signal between males and females [39]. Intriguingly, the position of this switch is controlled by fruitless acting cell-autonomously in third-order neurons [39]. Since only one candidate fourth-order neuron has been identified so far, the male specific fru+ DN1 neuron which projects to the VNC [43], it will now be critical to study how cVA processing after this circuit bifurcation instructs distinct behaviors. The circuit switch provides a simple conceptual explanation of how the same sensory stimulus can elicit sex-specific behaviors, but it does not address (1) how cVA can act as both repellent and aggregation signal in males and (2) why prolonged cVA exposure leads to response inhibition in both sexes [30, 54]. As mentioned above, cVA is processed by two parallel channels [8, 20, 21]. While elevated aggression and male-male repulsion are presumably mediated via acute perception of cVA through the high-affinity Or67d→DA1 channel [21, 53], chronic cVA exposure might recruit the low-affinity Or65a→DL3 channel and suppress aggression by lateral inhibition of DA1 in the AL [30, 54]. Activation of Or65a→DL3 (neither of which express fru) might therefore mediate the non-dimorphic aggregation effect of cVA. Lateral inhibition of DA1 by DL3 might also to be responsible for the abolished attraction of females to cVA after prolonged exposure [56], but this interglomerular interaction remains to be directly tested. Also, in the absence of functional recordings from DL3 PNs or their postsynaptic neurons, it remains unclear whether additional interactions exist between both cVA processing channels. Since DL3 PNs project to the vLH, there are likely to be third-order neurons postsynaptic to both DA1 and DL3 PNs.
Figure 2. Box 1 Open questions in Drosophila pheromone processing.
What similarities and differences are there in processing non-pheromone odors, especially those that have strong intrinsic valence?
How can we explain the different roles of cVA (i.e. courtship vs aggression vs aggregation)?
What are the ligands for OR47b?
What is the role of PAA/PA detection by IR84a in females? [50]
What is the identity and tuning of second-order gustatory neurons?
What is the role of ppk23+, ppk25+, ppk29+ neurons in females?
Where and how (i.e. sublinear/linear/supralinear) does integration of pheromone signals take place (e.g. see [37])?
Are cVA signals from tarsal ppk+ neurons and higher olfactory neurons integrated?
Does fruitless specify a dedicated circuit for pheromone perception?
Are there other labeled lines? If yes, how many are there and which genes specify them developmentally?
How plastic is pheromone processing and what mechanisms exist (e.g. receptor desensitization, plasticity of central circuits)
Where and how do circuits for innate (or: pheromone-driven) and learned behaviors interact? (e.g. see [57])
How do courtship promoting and inhibiting pheromonal cues interact to guide different stages of courtship behavior?
Sex differences in neuron number and morphology have also been identified in contact chemoreceptive structures (Table 1) [16, 29] – and these dimorphisms are also controlled by either fruitless or doublesex.
Since the fru isoforms sculpting these circuits are male-specific, female circuits remain drastically understudied. Since female-enlarged brain regions have been reported [44], it will be valuable to search for female-specific or -enriched transcripts which could provide a genetic entry point for studying female fly behaviors.
Finally, recent work from Keleman et al. [57] has illustrated that social experience can modulate hard-wired, pheromone-driven behaviors. Females decrease their receptivity after mating, one of several profound physiological changes mediated by transfer of sex peptide (SP) during copulation [58]. Since males also transfer cVA onto females during mating, this signal allows them to selectively court appropriate (i.e. receptive) targets. Keleman et al. [57] observed that courtship rejection drastically enhances the behavioral sensitivity of males to cVA and identified a group of fru+ dopaminergic neurons that might convey a rejection signal to the MB, thereby producing lasting changes in cVA processing [57].
Conclusions and future directions
In recent years, substantial progress has been made in understanding Drosophila pheromone processing, but several fundamental questions remain (Figure 2). At the sensory periphery, the most obvious frontier is the identification of monomolecular pheromone ligands and their receptors. Although several ORN and GRN populations were identified that are responsive to complex fly odors and cuticular extracts, respectively, the active compounds remain unknown. Importantly, the CHCs discovered so far represent only a small fraction of the complete cuticular profile of Drosophila [22, 59]. Only recently, a novel acetylated hydrocarbon, CH503, was identified which, like cVA, is transferred from males to females during mating and subsequently acts as a long-lived inhibitor of male courtship [60]. Approaches such as gas-chromatography-linked single-sensillum recordings [61] could be used to address these questions.
Deeper in the brain, most of our current knowledge about pheromone perception stems from following the cVA signal along a circuit hardwired by fruitless. It remains open whether additional labeled lines exist for other volatile or contact pheromones (Figure 2). Identification of corresponding genetic markers would allow us to investigate whether the circuit architecture underlying cVA processing can be generalized to other pheromones. Our understanding of contact pheromone processing faces an additional hurdle: we are only beginning to uncover the identity of second-order gustatory neurons. This is also the limiting factor for understanding how gustatory and olfactory (pheromone) signals might be integrated. However, with the availability of brain-wide neuron atlases and an ever-expanding palette of driver lines, these questions can certainly be addressed in the near future. We are therefore confident that Drosophila will remain an invaluable model system for studying the neural circuit logic of pheromone perception and, more generally, chemosensory processing.
Highlights.
Distinct sensory systems for volatile and contact pheromone detection
Identified Drosophila pheromones and their receptors
Central processing of pheromone signals
Sex-specific rerouting of pheromone signals
Acknowledgements
We thank J-C Billeter, S. Frechter, D. Mersch, M. Stensmyr and T. Wyatt for helpful comments on the manuscript, L. Hillier for help in graphical design of figure 1, and M. Sokolowski for allowing adapting parts of figure 1 from her review. We apologize to colleagues whose work could not be cited here due to space constraints. This work was supported by a European Research Council Starting Investigator grant, the European Molecular Biology Organization (EMBO) Young Investigator Programme, and Medical Research Council (MRC) Grant MC-U105188491 (to G.S.X.E.J.); and MRC Laboratory of Molecular Biology Graduate Scholarships (to J.K. and P.H.).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- [1].Sengupta S, S D. How Drosophila Detect Volatile Pheromones: Signaling, Circuits, and Behavior. Neurobiology of Chemical Communication. 2014 [PubMed] [Google Scholar]
- [2].Farine JP, Cortot J, Ferveur JF. Drosophila adult and larval pheromones modulate larval food choice. Proc Biol Sci. 2014;281:20140043. doi: 10.1098/rspb.2014.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mast JD, De Moraes CM, Alborn HT, Lavis LD, Stern DL. Evolved differences in larval social behavior mediated by novel pheromones. Elife. 2014;3 doi: 10.7554/eLife.04205. [* Combining behavioral experiments and gas chromatography-mass spectrometry this study identifies larval and adult chemicals that may act as pheromonal cues for aggregation and dispersal behavior for larvae of two Drosophila species.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- [5].Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–53. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- [7].Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–62. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–12. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–12. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chou YH, Spletter ML, Yaksi E, Leong JCS, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–49. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jefferis GSXE, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amrein H, Thorne N. Gustatory perception and behavior in Drosophila melanogaster. Curr Biol. 2005;15:R673–84. doi: 10.1016/j.cub.2005.08.021. [DOI] [PubMed] [Google Scholar]
- [15].Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–64. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- [16].Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–51. doi: 10.1016/j.cell.2012.03.045. [** The authors identify ppk23 and ppk29, expressed in fruitless-positive foreleg neurons as essential for male courtship behavior, and find that these neurons respond to either male or female cuticular hydrocarbons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 2014;83:850–65. doi: 10.1016/j.neuron.2014.07.012. [* The authors find that the ionotropic receptors IR52c and IR52d are coexpressed in neurons of the male foreleg which are activated by female contact. Intriguingly, these neurons seem to contact fruitless-positive neurons in the prothoracic ganglia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–91. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- [19].Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–60. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- [20].Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–33. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–6. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- [22].Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–95. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- [23].Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–91. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- [24].Farine JP, Ferveur JF, Everaerts C. Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS One. 2012;7:e40396. doi: 10.1371/journal.pone.0040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–6. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J, Renslo A, Baker BS, et al. Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell. 2013;154:89–102. doi: 10.1016/j.cell.2013.06.008. [* This study finds that activity of tarsal Gr32a neurons is necessary and sufficient to inhibit interspecies courtship.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–7. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, Manière G, Marion-Poll F, Ozaki M, Francke W, Cobb M, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–29. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- [30].Ejima A, Smith BPC, Lucas C, Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [** The authors demonstrate that ppk23+ foreleg neurons respond to the female aphrodisiac 7-11HD and that artificial activation of these neurons mimics courtship stimulation by 7-11HD.] [DOI] [PubMed] [Google Scholar]
- [32].Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci U S A. 2005;102:12831–6. doi: 10.1073/pnas.0506420102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, Pikielny CW. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J Neurosci. 2012;32:4665–74. doi: 10.1523/JNEUROSCI.6178-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu T, Starostina E, Vijayan V, Pikielny CW. Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci. 2012;32:11879–89. doi: 10.1523/JNEUROSCI.1376-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vijayan V, Thistle R, Liu T, Starostina E, Pikielny CW. Drosophila pheromone-sensing neurons expressing the ppk25 ion channel subunit stimulate male courtship and female receptivity. PLoS Genet. 2014;10:e1004238. doi: 10.1371/journal.pgen.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Caron SJC, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–7. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gruntman E, Turner GC. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat Neurosci. 2013;16:1821–9. doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, Luo L. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79:917–31. doi: 10.1016/j.neuron.2013.06.014. [* Excitatory and inhibitory PNs send parallel output to the lateral horn. Intriguingly, inhibitory PNs selectively suppress food-related odor responses while sparing signal transmission from pheromone channels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kohl J, Ostrovsky AD, Frechter S, Jefferis GSXE. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–23. doi: 10.1016/j.cell.2013.11.025. [** The authors find that cVA information is routed to sexually dimorphic third-order neurons in males and females by a changeover circuit switch and that fruitless controls this switch.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fişek M, Wilson RI. Stereotyped connectivity and computations in higher-order olfactory neurons. Nat Neurosci. 2014;17:280–8. doi: 10.1038/nn.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–30. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–7. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- [43].Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–90. doi: 10.1038/nature09554. [* Using photoactivatable GFP tracing, the authors delineate a cVA-responsive circuit in male flies.] [DOI] [PubMed] [Google Scholar]
- [44].Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–601. doi: 10.1016/j.cub.2010.07.045. [* By assembling an atlas of fruitless neurons in the fly brain, the authors were able to predict anatomical wiring differencs potentially underlying sexually dimorphic pheromone processing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- [46].Hong EJ, Wilson RI. Simultaneous encoding of odors by channels with diverse sensitivity to inhibition. Neuron. 2015;85:573–89. doi: 10.1016/j.neuron.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kain P, Dahanukar A. Secondary Taste Neurons that Convey Sweet Taste and Starvation in the Drosophila Brain. Neuron. 2015;85:819–32. doi: 10.1016/j.neuron.2015.01.005. [DOI] [PubMed] [Google Scholar]
- [48].Pavlou HJ, Goodwin SF. Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr Opin Neurobiol. 2013;23:76–83. doi: 10.1016/j.conb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone inDrosophila melanogaster. J Chem Ecol. 1985;11:1747–56. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- [50].Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GSXE, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–40. doi: 10.1038/nature10428. [** This study finds that IR84a-expressing olfactory neurons are activated by the aromatic plant odors phenylacetic acid and phenylacetaldehyde. IR84 ORNs are required for male courtship behavior and their postsynaptic PNs project to the ventral lateral horn, suggesting interactions between this food aphrodisiac and pheromones.] [DOI] [PubMed] [Google Scholar]
- [51].Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–62. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Koganezawa M, Haba D, Matsuo T, Yamamoto D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr Biol. 2010;20:1–8. doi: 10.1016/j.cub.2009.11.038. [DOI] [PubMed] [Google Scholar]
- [53].Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–31. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- [55].Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci. 2010;13:458–66. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lebreton S, Grabe V, Omondi AB, Ignell R, Becher PG, Hansson BS, Sachse S, Witzgall P. Love makes smell blind: mating suppresses pheromone attraction in Drosophila females via Or65a olfactory neurons. Sci Rep. 2014;4:7119. doi: 10.1038/srep07119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489:145–9. doi: 10.1038/nature11345. [* This study describes the neural and molecular mechanisms underlying courtship learning. A specific class of dopaminergic neurons together with some Kenyon cells of the mushroom body are responsible for the experience-dependent decrease in courtship towards non-virgin females.] [DOI] [PubMed] [Google Scholar]
- [58].Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–7. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- [59].Everaerts C, Farine JP, Cobb M, Ferveur JF. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS One. 2010;5:e9607. doi: 10.1371/journal.pone.0009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yew JY, Dreisewerd K, Luftmann H, Müthing J, Pohlentz G, Kravitz EA. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 2009;19:1245–54. doi: 10.1016/j.cub.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stensmyr MC, Dweck HKM, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–57. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- [62].Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–22. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [63].Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [** The authors develop a novel courtship assay using tethered males, enabling simultaneous in vivo recordings. Tarsal contact with a target female as sufficient to trigger initial, but not late, stages of courtship. Calcium imaging in male-specific P1 neurons indicates that these are activated by tarsal contact. Thermogenetic experiments indicated that these same neurons are sufficient to promote courtship (see also [61]).] [DOI] [PubMed] [Google Scholar]
- [64].Grillet M, Dartevelle L, Ferveur JF. A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci. 2006;273:315–23. doi: 10.1098/rspb.2005.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Siwicki KK, Riccio P, Ladewski L, Marcillac F, Dartevelle L, Cross SA, Ferveur JF. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn Mem. 2005;12:636–45. doi: 10.1101/lm.85605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J Exp Biol. 2005;208:891–8. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- [67].Watanabe K, Toba G, Koganezawa M, Yamamoto D. Gr39a, a highly diversified gustatory receptor in Drosophila, has a role in sexual behavior. Behav Genet. 2011;41:746–53. doi: 10.1007/s10519-011-9461-6. [DOI] [PubMed] [Google Scholar]