Abstract
Introduction
The improvement in outcomes for children with acute lymphoblastic leukemia (ALL) is one of the greatest success stories of modern oncology however the prognosis for patients who relapse remains dismal. Recent discoveries by high resolution genomic technologies have characterized the biology of relapsed leukemia, most notably pathways leading to the drug resistant phenotype. These observations open the possibility of targeting such pathways to prevent and/or treat relapse. Likewise, early experiences with new immunotherapeutic approaches have shown great promise.
Areas Covered
We performed a literature search on PubMed and recent meeting abstracts using the keywords below. We focus on the biology and clonal evolution of relapsed disease and highlight potential new targets of therapy. We further summarize the results of early trials of the three most prominent immunotherapy agents currently under investigation.
Expert Commentary
Discovery of targetable pathways that lead to drug resistance and recent breakthroughs in immunotherapy show great promise towards treating this aggressive disease. The best way to treat relapse, however, is to prevent it which makes incorporation of these new approaches into frontline therapy the best approach. Challenges remain to balance efficacy with toxicity and to prevent the emergence of resistant subclones which is why combining these newer agents with conventional chemotherapy will likely become standard of care.
Keywords: Acute lymphoblastic leukemia, relapse, clonal evolution, targeted therapy, immunotherapy, chimeric antigen receptor T-cells, bispecific T-cell engagers, monoclonal antibodies
I. INTRODUCTION
a. Background of Relapsed ALL, Treatment Approaches and Current Outcomes
Acute lymphoblastic leukemia (ALL) is the most common malignancy affecting children, comprising 19% of cancers occurring before age 19 years [1]. Outcomes for children with ALL have drastically improved over the last fifty years due to the advent of multidrug, risk-adapted chemotherapy regimens, improved central nervous system (CNS) prophylaxis and the recognition of clinical, biological and treatment response characteristics that identify patients at risk for treatment failure. Cure rates are now approximately 90% however the prognosis for the 10–20% of children who relapse remains dismal and has not improved over the past two decades [2]. Despite efforts to intensify reinduction strategies including allogenic hematopoietic stem cell transplant (HSCT), the great majority of these patients will succumb to their disease [3]. Clinical (age, initial white blood cell (WBC) count and presence of extramedullary disease) and biological (immunophenotype, genotype and response to therapy as assessed by minimal residual disease (MRD)) variables can be used to risk stratify patients at initial diagnosis [4,5]. In contrast, the most important predictors of outcome following relapse are phenotype, length of initial remission, and the site of relapse (Table 1) [6]. Patients with B ALL (e.g. precursor B ALL, (pB-ALL)) who relapse early (< 36 months) in the bone marrow have a dismal outcome approaching 15% long term survival, while those who relapse late (> 36 months) have a better but still unsatisfactory 40–50% rate of salvage [7–10]. The best outcomes are seen in patients with late extramedullary relapse where survival rates approach 80% [3]. On the other hand, patients with T ALL who relapse have uniformly poor outcome, with a 5–10 year event-free survival (EFS) of only 15–20% [11,12].
Table 1.
Classification of Relapsed ALL – BFM versus COG
| Berlin-Frankfurt-Munster Group (BFM), Germany | Treatment | 5-year OS [6] | |
|---|---|---|---|
|
| |||
| S1 | Late IEM (>6 months from end of treatment) | Chemotherapy | 60–70% |
|
| |||
| S2 | Early and very early IEM (<6 months from end of treatment) | Chemotherapy Chemotherapy + HSCT |
40% 60% |
| B-ALL late BM (>6 months from end of treatment) | |||
| B-ALL combined (early or late) | |||
|
| |||
| S3 | B-ALL early BM (>18 months from diagnosis to < 6 months from end of treatment) | Chemotherapy Chemotherapy + HSCT |
< 5% 30% |
|
| |||
| S4 | Very early BM (<18 months from diagnosis) | Chemotherapy Chemotherapy + HSCT |
< 5% 30% |
| Very early combined (<18 months from diagnosis) | |||
| T-ALL marrow (early or late) | |||
|
| |||
| Children’s Oncology Group (COG), USA | Treatment | 5-year OS [11, 12] | |
|
| |||
| Low | Late IEM (CR1 duration ≥ 18 months) | Chemotherapy | 50–80% |
|
| |||
| Intermediate | B-ALL late BM (≥36 months from diagnosis) | Chemotherapy +/− HSCT | 50–60% (late marrow) 30–50% (early IEM) |
| B-ALL late combined (≥36 months from diagnosis) | |||
| Early IEM (CR1 duration < 18 months) | |||
|
| |||
| High | B-ALL early BM or combined (<36 months from diagnosis) | Chemotherapy + HSCT | 15% |
| T-ALL marrow or combined marrow/extramedullary (early or late) | |||
Definitions: OS: overall survival; IEM: isolated extramedullary; HSCT: hematopoietic stem cell transplantation; BM: bone marrow; combined: BM and extramedullary; CR1: first complete remission
Current approaches to treating relapsed ALL share many similarities to frontline treatment. All patients, regardless of site of relapse, require systemic reinduction chemotherapy as even extramedullary relapses will develop systemic disease if left untreated. Reinduction regimens often contain many of the same drugs used for newly diagnosed patients, however, they are frequently delivered with either increased dose intensity or alternative schedules. Upon achieving second complete remission (CR2), patients will continue intensive chemotherapy with or without radiation or allogenic HSCT depending on the timing and site of relapse, the immunophenotype and response to reinduction therapy as assessed by MRD [13]. Patients with early relapse and unsatisfactory MRD response benefit from allogenic HSCT [14,15].
Despite these approaches, the overall survival (OS) rates for relapsed ALL remain between 25 and 40% highlighting the need for alternative therapy [3]. These outcomes are remarkably similar among various groups despite differences in reinduction strategies and utilization of HSCT [16]. Furthermore, efforts to overcome poor prognostic factors such as early isolated bone marrow relapse by intensifying therapy have failed to improve outcomes [17]. Targeted therapy aimed at the biological pathways that drive relapse and novel immunotherapeutic approaches hold the most immediate promise in improving outcomes of patients with relapsed disease. In this review, we focus on the biology of relapsed ALL, emphasizing drivers of relapse and potential targets for therapy, as well as promising new immunotherapeutic approaches.
b. Biology of Relapse and “Driver” Mutations
A characteristic feature of recurrent disease and the strongest contributing factor to poor outcomes is the intrinsic “drug resistance” that blasts acquire at relapse. This has been demonstrated repeatedly in ex vivo studies where relapsed blasts display increased resistance to chemotherapy compared to blasts harvested at diagnosis [18]. The clinical complement to this is evidenced by lower remission reinduction rates and persistence of MRD at relapse despite intensive retreatment. [11,15,19]. To discover the underlying biological pathways that are responsible for the drug resistant phenotype acquired at relapse, we and others have deployed the strategy of utilizing matched diagnosis-relapse patient pairs to better understand the clonal evolution of relapsed ALL in response to the selective forces of chemotherapy [10,18,20–22].
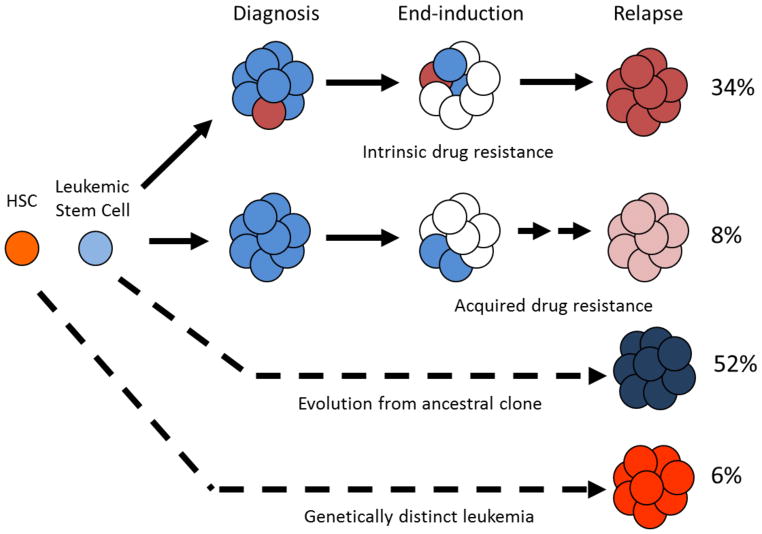
A variety of unbiased genomic approaches such as gene expression, copy number and methylation assays as well as high through-put sequencing has led to the discovery of several novel genetic alterations specific to relapsed blasts [21–23]. We and others have noted that distinct gene expression profiles characterize early vs. late relapse consistent with known differences in the clinical biology [21]. The comparison of diagnosis and relapse samples has also provided the vital opportunity to map the origin of the relapsed clone and study the evolution over time (Figure 1) [22,24]. The overwhelming majority of relapses are derived from the diagnosis clone (~94%) with a small minority (~6%) representing a new leukemia. The relapse clone emerges directly from a small subclone present at diagnosis approximately one third of the time whereas about half of relapses are derived from an ancestral clone [24].
Figure 1.
Clonal evolution of relapsed leukemia. 94% of relapsed clones exhibit a clear relationship to the clone seen at diagnosis. Intrinsically drug resistant clones can exist at low levels at diagnosis and survive treatment while other times, the drug resistance may be acquired. The majority of cases reveal a relapsed clone that has directly evolved from the leukemic stem cell. Rarely, the clone seen at relapse is genetically distinct from that at diagnosis and represents a new leukemia. Modified from Mullighan, Science (2008) and Bhatla, J Pediatr Hematol Oncol (2014).
Additionally, copy number analysis of matched diagnosis-relapse bone marrow pairs revealed that focal deletions were more common than amplifications. While most copy number abnormalities (CNAs) were shared between diagnosis and relapse, 12.5% of CNAs were unique to the relapse sample [25]. Copy number analysis and high through-put sequencing results have further revealed the presence of somatic alterations that are either relapse-specific or enriched at relapse compared to diagnosis. Such lesions are candidates for drivers of drug resistance. Genome-wide copy number profiling showed that deletions of IKZF1, EBF1, BTG1, TBL1XR1 and MSH6 were more common at relapse [22]. Deletions in IKZF1, the gene responsible for encoding the lymphoid transcription factor IKAROS, impart a poor prognosis and have been identified as strong predictors of relapse [26]. Likewise, deletions in MSH6, involved in mismatch repair, are also more common in relapse and have been implicated in resistance to thiopurines [21,22]. It is also not surprising that deletions in genes involved in glucocorticoid signaling, such as NR3C1, BTG1 and TBL1XR1, have also been identified in relapsed B ALL consistent with the fact that relapsed blasts notoriously harbor glucocorticoid resistance [18]. NR3C1 encodes the glucocorticoid receptor itself, BTG1 encodes a coactivator for the glucocorticoid receptor complex and TBL1XR1 prevents repression of the complex when bound to target genes [21,22,24,25] and each of these deletions has been shown in vitro to be relevant in steroid resistance [27–29].
In contrast to specific alterations that confer resistance to a single class of agents, a variety of relapse-specific genomic lesions may converge on distinct biological pathways conferring pan-resistance. For example, integrated genomic profiling (e.g. copy number, gene expression and methylation analysis) has revealed that activation of both the WNT and MAPK pathway are frequently seen at relapse and associated with pan-resistance to agents used in therapy [21]. Additionally, somatic mutations leading to activation of the Ras pathway (KRAS, NRAS, FLT3 and PTPN11) have also been identified at relapse. Indeed, preclinical models demonstrate that inhibition of these pathways restore chemosensitivity making MAPK and WNT pathway inhibitors promising strategies to prevent/treat relapsed disease [30–32].
High throughput next generation sequencing has provided further insights into the clonal architecture of ALL and the rise and fall of subclones from diagnosis to relapse. In general, deep sequencing reveals that each sample contains on average three subclones (range 1 to 5) with one clearly dominant [33]. The median number of mutations is 11 and while the mutation burden usually does not change between diagnosis and relapse, some samples show hypermutation at relapse. When coupled with copy number changes, six pathways contained a high frequency of mutations at both timepoints: Ras signaling, JAK-STAT signaling, transcriptional regulation of lymphoid development, nucleoside metabolism, epigenetic modification and cell cycle regulation. While the frequency of Ras, JAK-STAT, lymphoid development and cell cycle alterations are similar, there is enrichment for mutations in epigenetic regulators and nucleoside metabolism at relapse.
Overall, the most common mutations exclusively identified at relapse occur in NT5C2, which encodes a 5’-nucleotidase, a key player in nucleotide metabolism [34,35]. Mutations in NT5C2 lead to enhanced enzymatic activity that confers resistance to thiopurines, a major component of maintenance therapy in ALL. Interestingly, individuals with NT5C2 mutations at relapse almost always relapse within 36 months of initial diagnosis (i.e. early relapse) [34]. “Back-tracking” studies have revealed that these mutations sometimes exist in a small subclone at diagnosis and their emergence when therapy is heavily dependent on the selective pressures conferred by thiopurines (e.g. transition to maintenance) is consistent with a Darwinian model of clonal evolution. Likewise, relapse-specific mutations in PRPS1, also involved in purine biosynthesis, have also been discovered, underscoring the importance of purine nucleosides in the therapy of ALL [33]. Similar to clones harboring NT5C2 mutations, PRPS1 mutations lead to resistance to thiopurines and are associated with early relapse for the same reason as noted for NT5C2 [36]. Moreover, alterations in genes whose protein products modulate DNA mismatch repair (MSH6, MSH2 and PMS2) have been associated with thiopurine resistance and have been seen in most (but not all) cases associated with a high mutation burden at relapse [22,33,37,38]. The large number of genetic defects associated with resistance to thiopurines illustrates the great selective pressure applied to leukemic cells by these agents.
Epigenetic dysregulation also plays a major role in acquisition of drug resistance [39]. About two thirds of relapse samples contain somatic mutations in epigenetic regulators such as SETD2, CREBBP KDMA6A, WHSC1 (NSD2/MMSET) and KMT2D (MLL2) and these mutations are currently a major focus of study by our lab and others [33,39,40]. Note both WHSC1 and CREBBP mutations are specifically enriched at relapse [40,41]. CREBBP is a protein/histone acetyl transferase and CREBBP mutations have been associated with reduced expression of genes regulated by glucocorticoids thereby impacting glucocorticoid response [33,40,42] while mutations in WHSC1 lead to global increases in histone 3 lysine 36 dimethylation gene activation [41]. Data indicate also that CREBBP mutations may cooperate with Ras pathway mutations [43].
II. TARGETING UNDERLYING MECHANISMS OF RELAPSE
There are many promising agents currently being evaluated in ALL and their description has been summarized recently [44–47]. Identifying and targeting new biological subgroups such as “Ph-like” ALL with tyrosine kinase inhibitors is a prime example of ways to decrease relapse in high risk subgroups [48,49]. In this review, we will focus on specifically targeting the drivers of relapse that could be expected to subvert resistance thereby preventing relapse before it occurs. The early detection of low level clones carrying genetic alterations associated with relapse (e.g. NT5C2 or PRPS1) could predict eventual relapse and serve as a biomarker for a specific intervention such as altering maintenance therapy in these mutations that rely heavily on thiopurine resistance [50]. The introduction of pulses of non-cross resistant therapy might extinguish such clones. However, before such a strategy could be introduced, additional investigations are needed to determine if such clones exist in patients who never relapse because the clone does not gain the essential fitness to survive.
While specific mutations in apoptotic regulators that orchestrate cell death have not been identified, modulating the apoptotic pathway toward cell death is an attractive option. Members of the cellular inhibitor of apoptosis proteins (IAPs) family prevent cell death and are often overexpressed in cancer cells. For instance, expression of survivin (BIRC5), a member of the inhibitor of apoptosis family, has been found to be consistently upregulated in relapse as compared to diagnosis [51,52] and in vitro knockdown of survivin mRNA leads to reduced gene expression, induction of apoptosis and increased chemosensitivity in leukemia cells [53]. These findings led to a recent phase I trial using an antisense oligonucleotide targeted to survivin in children with second or greater bone marrow relapse of B ALL. While the trial closed due to significant off-target toxicity, it remains an attractive target for future agents [54]. Moreover, IAPs are counterbalanced by second mitochondrial-derived activator of caspases (SMAC) and SMAC mimetics promote cell death through inhibition of IAPs. Recent work indicates that resistant/relapsed ALL cells are particularly vulnerable to SMAC mimetics and cell death is orchestrated through routes distinct from conventional chemotherapeutic agents [55]. Finally, ex vivo profiling of resistant B ALL samples showed that a subset of B ALL samples were quite sensitive to Bcl-2 selective BH3 mimetic inhibitor venetoclax [56]. This has been validated in xenograft models particularly MLL rearranged leukemias although other BH3 mimetics may have broader activity [57].
As mentioned above, genetic alterations at relapse may converge on pathways, a prime example being the Wnt and MAPK pathways which appear to be activated at relapse in many cases. The MAPK pathway is activated by many extracellular signals and about one-third of cases of childhood ALL show mutations in genes that form part of this pathway (e.g. FLT3, NRAS, KRAS and PTPN11) [58]. Ras pathway mutations are particularly frequent in the high hyperdiploid subgroup. For instance, Irving at al showed that while the overall incidence of samples with mutations does not differ significantly at relapse, about half of the relapse samples with Ras pathway mutations were wild type (WT) at diagnosis [32]. Note multiple subclonal Ras mutations may exist either in the same gene or different Ras pathway genes [33]. Patients whose blasts carried Ras mutations are more likely to be associated with high risk features such as early relapse and failure to achieve complete remission [59]. In a comprehensive genomic evaluation of a large cohort of diagnosis/relapse pairs using gene expression, copy number and DNA methylation analysis, our laboratory also showed convergence on activation of the MAPK pathway [21]. The importance of this pathway in mediating glucocorticoid resistance was validated in a genome wide shRNA screen. Importantly phosphoflow analysis on samples also reveals activation of the pathway even in the absence of known genetic mutations. The availability of inhibitors of MEK (located at the terminal part of the Ras pathway) offers the potential for therapeutic intervention. Indeed, both selumetinib and trametinib have shown great promise in pre-clinical models and their integration into therapy to prevent and/or treat relapse is an attractive option for future investigation [31,32,60].
Finally, the Wnt pathway is upregulated in relapse due primarily to methylation and/or copy number alterations of inhibitory components of the pathway [21]. This finding was validated using phosphoflow cytometry to detect activated β-catenin (and its target, survivin) in relapsed patient samples [30]. Importantly, treatment of ALL cell lines and patient samples with a novel Wnt inhibitor had a synergistic impact on apoptosis when used in combination with conventional agents. Given the role of Wnt in many cancers (e.g. colon cancers and melanoma), a variety of Wnt inhibitors are in the early stages of clinical investigation.
The enrichment for mutations in epigenetic regulators and the availability of many new agents that target the epigenetic machinery offer great possibility to impact relapsed and refractory disease. We previously hypothesized that if gene expression changes between diagnosis and relapse could be pharmacologically “reversed,” chemosensitivity might be restored to diagnosis levels. Indeed, data mining of a large gene expression data base (the “connectivity” database) led to the identification of the histone deacetylase inhibitor vorinostat as such a candidate. Vorinostat was shown to specifically alter about 50% of the top relapse-specific gene expression changes in cell lines and clinical samples [61]. Importantly, pretreatment of both cell lines and patient samples with vorinostat increased sensitivity synergistically with conventional agents. Furthermore, global methylation profiles of diagnosis-relapse pairs demonstrated that within individuals, the relapse genome is hypermethylated compared to diagnosis [21]. Similar experiments showed that pretreatment with the DNA methyltransferase inhibitor decitabine also increased sensitivity to a panel of drugs used in routine therapy. The combination of a DNA methyltransferase inhibitor and histone deacetylase inhibitor yielded better results than either agent alone. These results led to a phase I study incorporating decitabine and vorinostat into standard reinduction chemotherapy for patients with relapsed ALL which demonstrated the combination was not only tolerated but also displayed some efficacy making this an attractive option for future treatment strategies [62].
III. IMMUNOTHERAPY
Though the above strategies show great potential, no agents have shown more promise towards improving outcomes for patients with relapsed B-ALL than immunotherapeutic approaches. In the next section, we will summarize the pharmacology and clinical data supporting the use of the three most prominent immunotherapy agents and the future direction of their use in the treatment of relapsed B ALL.
a. Blinatumomab
i. Pharmacology and Rationale for Use
Bispecific T-cell engaging antibodies (BiTE) are made up of the variable antigen-binding domains of two antibodies connected by a non-immunogenic linker peptide. Blinatumomab, derived from a B lineage-specific antitumor mouse monoclonal antibody, is the most clinically advanced BiTE to date. Blinatumomab is composed of an anti-CD3 arm (to engage CD3-expressing T-cells) and an anti-CD19 arm (to bind CD19+ B-cells). The B-cell lineage cell surface protein antigen CD19 is present on more than 90% of B-cell leukemias making it an attractive target for therapy [63]. The BiTE facilitates interaction (called an “immunological synapse”) between CD3+ T-cells and tumor cells leading to upregulation of T-cell activation markers and perforin-mediated cytotoxicity and subsequent initiation of caspase activated apoptosis [64]. The anti-tumor effect is further enhanced by increased T-cell proliferation also mediated by blinatumomab.
Blinatumomab was initially delivered as an intermittent infusion 2 to 3 times per week but led to serious toxicity without any clinical efficacy noted. It is now delivered as a 28-day continuous infusion in an effort to mitigate side effects and maintain prolonged exposure and activity of this rapidly-cleared protein [64].
ii. Clinical Experience in Adults
While the first clinical trial of blinatumomab showed efficacy in non-Hodgkin’s lymphomas [65], it has demonstrated the most activity against ALL, both in the relapsed/refractory disease setting and in patients with positive MRD after conventional treatment. Early trials in ALL investigated its efficacy in eradicating MRD, the first of which was a phase II open-label, multi-center, single arm clinical trial. The study enrolled 21-patients with B ALL in CR with either persistent MRD or an MRD positive relapse (initially MRD negative but subsequently demonstrated MRD positive disease). Patients received blinatumomab as a continuous, 28-day infusion at a dose of 15 μg/m2 per day with two weeks between cycles [66]. Twenty patients were evaluable at the end of the trial of which 80% (16 out of 20) achieved a negative MRD status at the end of the first month. Notably, 12 of the 16 responders had molecular refractory disease (defined as never having achieved an MRD-negative status) prior to treatment. Nine patients went on to receive HSCT. At a median follow-up of 33 months, 61% of patients remained in relapse free survival (RFS) with an impressive 50% of patients remaining in remission at 5-years [67,68]. The most common adverse effects included pyrexia, chills, hypokalemia and hypogammaglobulinemia with Grade III lymphopenia occurring in 33% of patients. A confirmatory phase II multicenter trial (BLAST) further evaluated the efficacy of blinatumomab in 116 newly diagnosed patients with persistent MRD or patients who relapsed with MRD+ status at end induction [68]. After the first cycle, 78% of patients achieved MRD negativity and 67% were able to proceed to HSCT.
Clinical trials were next conducted to assess the efficacy of blinatumomab in patients with relapsed/refractory B ALL. In a phase II open label, multicenter trial that enrolled 36 patients who received cycles of 28-day continuous infusions of blinatumomab [69], complete response was achieved in 25 patients (69%), with half of those patients proceeding to HSCT. RFS was 7.6 months, with an OS of 9.8 months. This was further confirmed in 189 patients with relapsed or refractory Philadelphia chromosome negative (Ph-negative) B ALL [70]. The most common adverse events (AEs) were pyrexia, febrile neutropenia, peripheral edema, hypokalemia and anemia. Approximately 50% of patients experienced neurological toxicity which were mainly low grade and reversible. Cytokine release syndrome (CRS) was also a significant AE but rates of this complication were significantly decreased with dexamethasone pretreatment. Three patients with Grade III CRS required treatment with tocilizumab, an anti-IL6 monoclonal antibody approved for use in the treatment of severe CRS.
Results of the multi-center phase III trial comparing blinatumomab versus physician’s choice chemotherapy were recently published [71]. The blinatumomab arm received up to two cycles of induction therapy with 6 weeks per cycle followed by up to 3 cycles of consolidation for patients in morphological remission (≤5% bone marrow blasts) and up to 12 months of maintenance therapy for patients in continued CR. A significantly higher proportion of patients in the blinatumomab group achieved remission within 12 weeks after treatment initiation whether with full (34% vs. 16%) or partial (44% vs. 25%) hematologic recovery. Furthermore, among patients who achieved CR, MRD negativity was achieved in 78% of patients in the blinatumomab arm versus only 48% in the chemotherapy arm. OS was also significantly better for patients in the blinatumomab group (7.7 months) compared to the chemotherapy group (4 months) (hazard ratio for death, 0.71) with a median duration of follow up of just under 12 months for each group. The most common AE observed were similar to those in prior studies.
iii. Clinical Experience in Children
A phase I/II clinical trial has been conducted in patients less than 18 years old with relapsed/refractory B ALL [72]. This open-label study was composed of a dose-escalating phase I part (49 patients) followed by a phase II part (44 patients) administering blinatumomab in 6 week cycles similar to those used in adults. For the phase I portion of the study, 4 patients experienced dose-limiting toxicity; three patients experienced grade 4 CRS (one with fatal cardiac failure) and one patient succumbed to respiratory failure. The maximum-tolerated dose was determined to be 15 μg/m2/day making the recommended blinatumomab dosing 5 μg/m2/day for the first 7 days followed by 15 μg/m2/day thereafter. Among the 70 patients who received the recommended dose in either phase I or phase II, 39% of patients achieved a CR within the first two cycles, of which 52% achieved MRD- status. Overall, response rates were greater in patients with lower tumor burden at initiation of treatment with a 56% CR rate in patients with < 50% bone marrow blasts versus 33% in patients with ≥ 50%. Of the 27 responders, three patients died in CR after HSCT and 15 had relapsed and died (four with a CD19 negative clone) at the end of the 2-year follow up. Of the nine remaining patients, four were still alive in CR, two were alive in relapse and three had withdrawn consent (one in CR and two in relapse). Median RFS was 4.4 months among patients who received the recommended dosing. The most common AEs reported were pyrexia, anemia, nausea and headache with Grade 3 and 4 AEs mostly limited to cytopenias. Most AEs occurred within the first few days of the first cycle. A total of six patients had fatal AEs and three patients died after HSCT. Eight patients experienced CRS of any grade with only one grade 4 CRS.
Data from a compassionate use protocol in children with post-transplant relapsed B ALL treated with blinatumomab have also been published [73]. Blinatumomab was administered per the above recommended dose. Nine patients received a total of 18 cycles, four of whom achieved a CR after the first cycle and two others after the second; four patients underwent haploidentical HSCT. They noted a 30% EFS at a median follow up of 398 days in this particularly high risk subset of patients. These studies both indicate that blinatumomab is effective in inducing molecular remission in pediatric patients even in the post-transplant setting.
iv. Future Direction
Due to the promising single agent activity of blinatumomab, trials in both children and adults with relapsed B ALL are currently investigating the safety and efficacy of blinatumomab in combination with chemotherapy. The Children’s Oncology Group study AALL1331 (Risk-Stratified Randomized Phase III Testing of Blinatumomab (IND#117467, NSC#765986) in First Relapse of Childhood B-Lymphoblastic Leukemia (B-ALL)) is comparing the substitution of blinatumomab for some reinduction chemotherapy blocks to chemotherapy alone in children and young adults with first relapsed B ALL. A similar trial is taking place in Europe.
b. Inotuzumab Ozogamicin
i Pharmacology and Rationale for Use
CD22 is a B-cell lineage restricted transmembrane protein expressed during intermediate steps in B cell development. The great majority of cases of B ALL (over 90%) display CD22 [74]. Therapeutic targeting of CD22, in contrast to the other two agents discussed in this section, is a form of passive immunotherapy. It has been deployed in several B cell malignancies including a single arm clinical trial in relapsed ALL conducted by the COG, ADVL04P2 where epratuzumab, a “naked” anti-CD22 monoclonal antibody, was added to a standard re-induction platform. While it did not improve the overall CR rate, it was quite tolerable when given with chemotherapy and showed a trend towards improvement in MRD response supporting the use of anti-CD22 agents in future trials [75]. Unlike epratuzumab, inotuzumab is conjugated to drug (calicheamicin) allowing localization and internalization of this toxin into the target cells thereby exploiting the rapid internalization of CD22 upon antibody binding with the aim to increase efficacy [76]. Calicheamicin is a potent DNA damaging agent initially isolated from Micromonospora echinospora and its derivative, N-acetyl-γ-calicheamicin, was conjugated to a humanized anti-CD22 monoclonal antibody (IgG4) to create the antibody drug conjugate inotuzumab ozogamicin (INO). The anti-CD22 antibody on INO binds to lymphoblasts and is internalized, after which calicheamicin exerts its effects and induces DNA double stranded breaks and apoptosis [77,78]. In contrast to traditional chemotherapeutic agents who target highly proliferative cells, INO is capable of targeting quiescent malignant cells giving the drug access not only to proliferating blasts, but also the leukemic stem cell.
ii. Clinical Experience in Adults
Initial phase I studies to determine the maximum-tolerated dose (MTD), safety, and preliminary efficacy of INO have been completed. A multi-center, open-label, dose-escalating study investigating the MTD and preliminary efficacy of INO in B-cell non-Hodgkin’s lymphoma patients established an MTD of 1.8 mg/m2 intravenously given every 3 to 4 weeks [79]. A total of 79 patients were enrolled with an objective response rate of 39%. The most frequent AEs reported was reversible thrombocytopenia followed by asthenia, nausea and neutropenia.
A follow up, single institution phase II trial performed at MD Anderson Cancer Center enrolled 49 patients with relapsed-refractory B-lymphoblastic leukemia [80]. Patients received single-dose INO from 1.3 to 1.8 mg/m2 as a short, intravenous infusion once every 3 to 4 weeks. Treatment with paracetamol, diphenhydramine and hydrocortisone was given prior to each dose of INO. Of the 49 patients treated, 73% were second or greater relapse. The overall response rate (ORR) was 57% with a median survival of 5.1 months. Fever, hypotension and liver-related toxic effects were the most frequently reported AEs. While liver related toxicities were generally reversible, a small subset of patients who underwent HSCT developed veno-occlusive disease (VOD).
To reduce toxicity, the INO dose was modified in a second cohort of patients to weekly doses of 0.8 mg/m2 on day 1 followed by 0.5 mg/m2 on days 8 and 15 every 3–4 weeks for a total of 1.8 mg/m2 per course [81]. Ninety patients were enrolled; 68% were in second or greater relapse. A complete response (CR) was achieved in 19% of patients while 30% had a CR without platelet recovery. Response rates were similar for single-dose and weekly dose regimens (57% vs. 59% respectively) however reversible hyperbilirubinemia, hepatotoxicity, fever and hypotension were observed less often on the weekly regimen.
The follow up phase III, randomized control trial comparing INO with standard of care treatment in adult patients with ALL, Study NCT01564784 (INO-VATE Trial), revealed the superiority of INO [82,83]. Two hundred and eighteen adults were randomized to receive either weekly doses of INO (0.8 mg/m2 on day 1 followed by 0.5 mg/m2 on days 8 and 15) or standard of care, intensive chemotherapy. The rate of CR was significantly higher in the INO group with 80.7% of patients achieving CR versus only 29.4% in the standard therapy arm. Additionally, of the patients who achieved a CR, those in the INO group were more likely to achieve MRD negativity (78.4% vs. 28.1%), have longer durations of remission and improved OS. It should be noted, however, that while improvement in OS was statistically significant, it only equated to an additional one month survival. As in prior studies, hepatotoxicity remained a significant AE in which VOD occurred in 11% of patients receiving INO versus only 1% in the standard arm. The incidence was higher in patients who underwent HSCT with regimens including dual alkylating agents. INO was granted Breakthrough Therapy designation by the U.S. Food and Drug Administration (FDA) in October 2015 based on the preliminary results of this study.
Clinical Experience in Children
While there are no completed randomized trials specifically in pediatrics, a retrospective review of the above-mentioned MD Anderson trial was performed on the 5 pediatric patients in that cohort [84]. The children ranged from 4–15 years of age, had relapsed B-lymphoblastic leukemia and were treated with either single-dose INO monotherapy (1.3 mg/m2 for the first course followed by 1.8 mg/m2 for subsequent courses; n=3) or the weekly dose regimen (0.8 mg/m2 on day 1 followed by 0.5 mg/m2 on days 8 and 15; n=2). All children received two cycles. Two patients had no response, two had a CR after the first cycle and one after two cycles. All responders went on to receive HSCT and at the time of publication, two had died and one was still undergoing treatment. This study did not evaluate MTD in children, a major limitation. However, the study established a possible minimum starting dose where activity can be anticipated. Fever was the most commonly reported adverse effect and no pediatric patient experienced hypotension or Grade 3–4 liver toxicity. Of the patients who proceeded to transplant, only one developed VOD.
Recently, retrospective data was collected from 43 patients with relapsed/refractory B ALL who were treated with INO obtained through compassionate use program through Pfizer at various institutions throughout United States (personal communication, Deepa Bhojwani). In this heavily pretreated cohort including 37 patients who were refractory to their preceding regimen, an impressive 62% of patients achieved a CR. The most common AEs were infection and hepatotoxicity. While no patients experienced VOD during INO therapy, 9/15 patients who went on to HSCT developed VOD, one of whom died. Additionally, a phase I, dose-finding trial of INO in combination with chemotherapy is planned by the Innovative Therapies for Children with Cancer (ITCC) European consortium.
iii. Future Direction
Current trials are investigating the safety and efficacy of incorporating INO in multidrug regimens and the results of two such studies were recently presented at the 2016 American Society of Hematology (AHS) Annual Meeting and Exhibition. A phase I trial investigating the safety of INO in combination with cyclophosphamide, vincristine and prednisone in patients with refractory CD22+ acute leukemia is ongoing [85]. Preliminary data demonstrated that of the 19 evaluable patients, Grade 3–4 hematologic toxicity was the most commonly reported AE, most often febrile neutropenia. Only one of the three patients who subsequently underwent HSCT developed VOD which resolved. A phase II trial in older adults investigating the safety and efficacy of addition of INO to mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, methotrexate at 75% dose reduction and cytarabine without anthracyclines) has also been conducted [86]. Forty of the forty-two evaluable patients (95%) achieved a CR or CR with incomplete platelet recovery with 93% of these patients also achieving MRD negative status. Most common side effects were again hematologic and of the four patients who developed VOD, two died. The 3-year OS rate was 52% which is notably higher than historical controls for this high-risk subset of patients making this a potentially safe and effective approach to treatment.
c. Chimeric Antigen Receptor T Cells
i. Pharmacology and Rationale for Use
Adoptive immunotherapeutic approaches rely on the transfer of effector cells to enforce the immune response. Chimeric antigen receptor T cells (CARTs) are an example of adoptive immunotherapy where a patient’s own engineered effector T cells are utilized to enforce an immune response. After T cells are harvested from the patient by leukapheresis, they are reengineered ex vivo to contain an antigen recognition domain specific for a target on the cell of interest. A monoclonal antibody recognition fragment is effectively linked to T cell signaling domains which in turn activates the T cell’s cytotoxic machinery upon binding the target cell [87]. Prior to reinfusion, the T cells are expanded via various ex vivo culture systems. In contrast to monoclonal antibodies, the engineered T cells may display long-lasting in vivo survival.
First-generation CARTs joined the single-chain variable fragment (scFv) of an antibody (the extracellular domain) to the CD3 zeta chain of the T cell receptor (intracellular signaling domain) [88] linking the two domains through a transmembrane spacer. Second- and third-generation CARTs were modified to contain one or two additional costimulatory domains, respectively (such as CD28, 4-1BB, or OX40) after initial preclinical trials showed poor proliferation of first generation CARTs after reinfusion. The majority of current clinical trials are investigating second-generation CARTs as they have shown the most clinical efficacy [89].
Once reinfused, CARTs bind to tumor antigens which not only activates the cytotoxic effect of the T cells but also leads to significant T cell proliferation in vivo, further contributing to their longevity. The CARTs diffuse in bone marrow and tissues, including the CNS, making them an attractive therapy for even extramedullary disease. Currently, the most clinically advanced CARTs for ALL and therefore the focus of the remainder of this discussion is on the anti-CD19 CARTs. CD19, as discussed above, is an attractive target due to its single lineage specificity (B cells) and its almost universal expression on B cell malignancies. The function of normal B cells is also replaceable with intravenous immunoglobulins making the resultant B cell aplasia due to CAR T cell therapy relatively manageable.
ii. Clinical Experience in Children and Adults
While the earliest report of clinical activity of CARTs was seen in chronic lymphocytic leukemia [90], the highest response rates have been observed in B ALL, specifically in children. After promising results in two children with relapsed B ALL [91], initial phase I trials of anti-CD19 CARTs conducted at the Children’s Hospital of Philadelphia (CHOP) and The University of Pennsylvania (Penn) (CTL019) showed profound response rates. They enrolled 25 pediatric and 5 adult patients with relapsed/refractory B ALL and demonstrated a 90% CR rate with 67% EFS and 78% OS at 6-months [91–93]. Notably, two of the responders had blinatumomab-refractory disease and 50% of patients had previously undergone HSCT. All patients on this trial experienced CRS, 27% of whom were classified as severe but all responded to tocilizumab. Severe CRS was associated with higher tumor burden at treatment initiation.
The follow up phase I/IIa trial of second-generation CAR T cells (CART19) also conducted at CHOP/Penn expanded the original cohort to contain 59 children and young adults with B ALL in second or greater relapse [94], two-thirds of which relapsed after HSCT. Of the 59 patients, 44 had detectable disease at study entry while 15 were MRD-. The results were again impressive 93% of patients in CR one month after infusion. MRD negative status as measured by flow cytometry was achieved in 52 of the 59 patients (88%). After a median follow-up of 12 months, 34 patients (58%) remained in remission with only five of those patients having received a subsequent HSCT and one receiving a subsequent donor lymphocyte infusion. OS at 12 months was 79% but 20 patients (34%) eventually relapsed, 13 of which relapsed with CD19 negative clones. CARTs persisted as did B-cell aplasia in patients with sustained CR. Eighty eight percent of patients experienced CRS however none were grade 5.
Memorial Sloan Kettering Cancer Center (MSKCC) has also developed a clinically active CART with an anti-CD19 antibody and CD28 co-stimulatory domain. Their initial trial of 16 adults with relapsed B ALL led to a CR rate of 88% (75% MRD negative) with seven of the 16 patients going on to receive HSCT [95]. This cohort included Ph-chromosome positive (Ph-positive) patients as well as patients who relapsed after transplant. OS at 6 months was higher for patients who proceeded to HSCT versus those who did not (70% versus 64%, respectively). CRS with reversible neurological complications were the most common AEs noted (7/16 patients, 44%). In updated results reported at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, CR rates were 91% in 21 patients with minimal disease (<5% bone marrow blasts at initial infusion) versus 75% in patients with morphologic disease (>5% bone marrow blasts at infusion). This translated to a 6-month OS of 73% and 57% for the minimal versus morphological cohorts, respectively [96].
A phase I dose-escalation trial conducted at the National Cancer Institute (NCI) has also been conducted [97]. Autologous T cells were engineered to express a CD19-CART with TCR zeta and CD28 costimulatory domains. They enrolled 20 children and young adults with. The MTD was determined to be 1x106 cells/kg due to grade 4 CRS, although all toxicities were reversible. They reported a 70% CR rate with an OS of 51.6% at a median follow up of 10 months. Updated results reported at the ASH Annual Meeting and Exhibition in 2015 showed a CR rate in 61% of 38 ALL patients. The best long-term survival rates were seen in patients who subsequently received HSCT.
iii. Future Direction
The remarkable results of phase I studies of CAR T cells in relapsed/refractory B ALL are unprecedented, but CAR T cell therapy continues to be optimized. There are various CARTs currently under investigation. Optimal duration of CART persistence, need for continued CAR T cell infusions and ways to prevent relapse remain to be determined. The two mechanisms by which post-CAR T cell patients relapse are short CAR T cell persistence (CD19+ relapses) or antigen escape (CD19- relapse) and methods to overcome these challenges will be needed. CRS also remains a challenge and current investigations are evaluating optimal prevention, identification and treatment of this potentially fatal complication. CARTs directed against other antigens, such as CD22, or CARTs with dual specificity are currently under investigation. Numerous clinical trials are being planned including integration of CARTs into frontline therapy for very high risk patients. Finally while the manufacture of CAR T cells now takes less than a month, breakthroughs in gene editing techniques has allowed disruption of the endogenous TCR (to prevent GVHD) and HLA class I and II antigens (to prevent rejection) so that “off the shelf” third party CAR T cells is a realistic possibility which is especially important in cases where harvesting autologous T-cells is not feasible (e.g. low T-cells, high tumor burden) [98,99].
IV. EXPERT COMMENTARY
There has been minimal progress in treating relapsed disease for the last two decades and for the most part few new drugs have been developed for the treatment of ALL. While the underlying biology of drug resistance is emerging, many of the responsible pathways have yet to be targeted. Importantly, additional research is needed to develop strong biomarkers of impending relapse. It is possible that the genetic lesions seen at relapse are present in some clones which never gain the growth advantage to lead to clinical relapse. Finally, tumor heterogeniety remains the biggest barrier to curing cancer regardless of the strategy (e.g. conventional, targeted and/or immunotherapy) with the eventual escape due to minor resistant subclones. Future strategies will undoubtedly continue to rely on combination therapy however careful consideration of sequence will be required for maximum benefit. Thus, better control over the evolutionary, selective forces that lead to clonal selection (e.g. rotating single agents or combination therapy) could extinguish emerging subclones and thereby prevent relapse from occurring.
V. FIVE YEAR VIEW
Many questions still remain regarding the use of these newer agents however they are likely to become standard of care in the next 5 years. Determining how to use them in a safe and effective manner therefore remains paramount. While blinatumomab and INO show great promise in eradicating relapsed or refractory disease, their duration of response makes them unlikely to serve as definitive, curative therapy. Their benefit will most likely be seen in preventing relapse (as part of upfront therapy) or as a bridge to transplant. This raises the concern of toxicity, specifically for INO, as the high rate of VOD is a major concern. Strict patient selection, appropriate conditioning regimens, careful monitoring and prompt initiation of treatment will be imperative to mitigate this risk. Also, as the risk of VOD increases with INO dose, limiting the INO exposure pre-transplant will also likely prove to be helpful. In contrast to blinatumomab and INO, CAR T cells show significant promise towards sustained, durable responses for patients not only with bone marrow relapse but also those patients who relapse in the CNS. They therefore have the potential to replace HSCT as the definitive cure of relapsed disease and while CRS remains a valid concern, it may be an attractive alternative to the complications of HSCT. Current studies are looking at potential biomarkers to identify patients at higher risk for CRS as well as improved treatment to further decrease the morbidity and mortality associated with this treatment. Finally, just as tumors have found ways to evade conventional treatment, relapse post-immunotherapy is a major hurdle to overcome. For instance, relapse post CARs has been reported to occur and mainly by two main mechanisms – failure of CARs to persist or antigen escape – however lineage switch is also possible. Additional studies in pediatric patients are also needed to not only determine their safety and efficacy in this subset of patients, but also to compare these agents head to head to ensure we are using the most effective, least toxic agents in the appropriate setting.
Key Issues.
Relapsed disease is clonally related to the disease at diagnosis in almost all cases.
Relapse is due to the emergence of drug resistant cells with genetic and epigenetic alterations in key pathways related to drug pharmacodynamics.
Risk stratification for relapsed B ALL is dependent on the timing, location and phenotype of relapsed disease.
Targeting recurrent relapse-specific biological pathways may prove to be an effective strategy to prevent and treat relapse.
The use of immunotherapies to directly target leukemia cell surface antigens with monoclonal antibody-drug conjugates, T-cell engagers and chimeric antigen receptor T-cells is a very promising and rapidly emerging strategy.
Resistance to immunotherapeutic agents is a major concern, therefore a combination of these agents with conventional chemotherapy will likely be the focus of new trials.
The ability of new treatment modalities such as CAR T cells to replace hematopoietic stem cell transplant for high risk disease remains to be determined.
Finally, the best way to treat relapse is to prevent it in the first place and incorporating these agents into frontline therapy holds the most promise to improve outcomes for these patients.
Acknowledgments
The authors would like to acknowledge support from the following agencies: National Cancer Institute National Institute of Health (R01 CA140729-05, WLC and TB), the Leukemia and Lymphoma Society Specialized Center for Research (SCOR, WLC), the Perlmutter Cancer Center (P30 CA016087) and the Three Strohm Sister’s Foundation (LH).
References
- 1.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics. 2014;134(4):e945–955. doi: 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–136. doi: 10.1182/asheducation-2012.1.129. [DOI] [PubMed] [Google Scholar]
- 4.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109(3):926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll WL, Raetz EA. Clinical and laboratory biology of childhood acute lymphoblastic leukemia. J Pediatr. 2012;160(1):10–18. doi: 10.1016/j.jpeds.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120(14):2807–2816. doi: 10.1182/blood-2012-02-265884. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Zhang MJ, Devidas M, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with acute lymphoblastic leukemia in a second remission after an isolated central nervous system relapse: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Leukemia. 2008;22(2):281–286. doi: 10.1038/sj.leu.2405037. [DOI] [PubMed] [Google Scholar]
- 8.Freyer DR, Devidas M, La M, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Group. Blood. 2011;117(11):3010–3015. doi: 10.1182/blood-2010-07-294678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulsipher MA, Langholz B, Wall DA, et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant. 2015;50(9):1173–1179. doi: 10.1038/bmt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatla T, Jones CL, Meyer JA, Vitanza NA, Raetz EA, Carroll WL. The biology of relapsed acute lymphoblastic leukemia: opportunities for therapeutic interventions. J Pediatr Hematol Oncol. 2014;36(6):413–418. doi: 10.1097/MPH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] J Clin Oncol. 2008;26(24):3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22(12):2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 14.Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol. 2006;24(19):3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 15.Eckert C, Henze G, Seeger K, et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31(21):2736–2742. doi: 10.1200/JCO.2012.48.5680. [DOI] [PubMed] [Google Scholar]
- 16.Bader P, Kreyenberg H, von Stackelberg A, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol. 2015;33(11):1275–1284. doi: 10.1200/JCO.2014.58.4631. [DOI] [PubMed] [Google Scholar]
- 17.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28(14):2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 18.Klumper E, Pieters R, Veerman AJ, et al. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995;86(10):3861–3868. [PubMed] [Google Scholar]
- 19.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hongo T, Fujii Y. In vitro chemosensitivity of lymphoblasts at relapse in childhood leukemia using the MTT assay. Int J Hematol. 1991;54(3):219–230. [PubMed] [Google Scholar]
- 21.Hogan LE, Meyer JA, Yang J, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118(19):5218–5226. doi: 10.1182/blood-2011-04-345595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JJ, Bhojwani D, Yang W, et al. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112(10):4178–4183. doi: 10.1182/blood-2008-06-165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhojwani D, Kang H, Moskowitz NP, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2006;108(2):711–717. doi: 10.1182/blood-2006-02-002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 26.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CL, Bhatla T, Blum R, et al. Loss of TBL1XR1 disrupts glucocorticoid receptor recruitment to chromatin and results in glucocorticoid resistance in a B-lymphoblastic leukemia model. J Biol Chem. 2014;289(30):20502–20515. doi: 10.1074/jbc.M114.569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Galen JC, Kuiper RP, van Emst L, et al. BTG1 regulates glucocorticoid receptor autoinduction in acute lymphoblastic leukemia. Blood. 2010;115(23):4810–4819. doi: 10.1182/blood-2009-05-223081. [DOI] [PubMed] [Google Scholar]
- 29.Irving JA, Minto L, Bailey S, Hall AG. Loss of heterozygosity and somatic mutations of the glucocorticoid receptor gene are rarely found at relapse in pediatric acute lymphoblastic leukemia but may occur in a subpopulation early in the disease course. Cancer Res. 2005;65(21):9712–9718. doi: 10.1158/0008-5472.CAN-05-1227. [DOI] [PubMed] [Google Scholar]
- 30.Dandekar S, Romanos-Sirakis E, Pais F, et al. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014;167(1):87–99. doi: 10.1111/bjh.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones CL, Gearheart CM, Fosmire S, et al. MAPK signaling cascades mediate distinct glucocorticoid resistance mechanisms in pediatric leukemia. Blood. 2015;126(19):2202–2212. doi: 10.1182/blood-2015-04-639138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irving J, Matheson E, Minto L, et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood. 2014;124(23):3420–3430. doi: 10.1182/blood-2014-04-531871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Edmonson M, Yergeau D, et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun. 2015;6:6604. doi: 10.1038/ncomms7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer JA, Wang J, Hogan LE, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet. 2013;45(3):290–294. doi: 10.1038/ng.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzoneva G, Perez-Garcia A, Carpenter Z, et al. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med. 2013;19(3):368–371. doi: 10.1038/nm.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Li H, Bai Y, et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med. 2015;21(6):563–571. doi: 10.1038/nm.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diouf B, Cheng Q, Krynetskaia NF, et al. Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat Med. 2011;17(10):1298–1303. doi: 10.1038/nm.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madhusoodhan PP, Evensen NA, Saliba J, et al. Measurement of Phosphorylated ERK as a Prognostic and Predictive Marker for MEK Inhibition in Pediatric B-Lmphoblastic Leukemia: A Pilot Study. Blood; American Society of Hematology Annual Meeting and Exhibition; San Diego, CA: 2016. p. 1739. [Google Scholar]
- 39.Mar BG, Bullinger LB, McLean KM, et al. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat Commun. 2014;5:3469. doi: 10.1038/ncomms4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullighan CG, Zhang J, Kasper LH, et al. CREBBP mutations in relapsed acute lymphoblastic leukae mia. Nature. 2011;471(7337):235–239. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaffe JD, Wang Y, Chan HM, et al. Global chromatin profiling reveals NSD2 mutations in pediatric acute lymphoblastic leukemia. Nat Genet. 2013;45(11):1386–1391. doi: 10.1038/ng.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inthal A, Zeitlhofer P, Zeginigg M, et al. CREBBP HAT domain mutations prevail in relapse cases of high hyperdiploid childhood acute lymphoblastic leukemia. Leukemia. 2012;26(8):1797–1803. doi: 10.1038/leu.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malinowska-Ozdowy K, Frech C, Schönegger A, et al. KRAS and CREBBP mutations: a relapse-linked malicious liaison in childhood high hyperdiploid acute lymphoblastic leukemia. Leukemia. 2015;29(8):1656–1667. doi: 10.1038/leu.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhojwani D, Yang JJ, Pui CH. Biology of childhood acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):47–60. doi: 10.1016/j.pcl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tasian SK, Loh ML, Hunger SP. Childhood acute lymphoblastic leukemia: Integrating genomics into therapy. Cancer. 2015;121(20):3577–3590. doi: 10.1002/cncr.29573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tasian SK, Hunger SP. Genomic characterization of paediatric acute lymphoblastic leukaemia: an opportunity for precision medicine therapeutics. Br J Haematol. 2016 doi: 10.1111/bjh.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts KG, Mullighan CG. How new advances in genetic analysis are influencing the understanding and treatment of childhood acute leukemia. Curr Opin Pediatr. 2011;23(1):34–40. doi: 10.1097/MOP.0b013e3283426260. [DOI] [PubMed] [Google Scholar]
- 48.Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high risk B-ALL: a study from the Children's Oncology Group. Blood. 2017 doi: 10.1182/blood-2016-12-758979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saliba J, Evensen NA, Meyer J, et al. Using Whole Exome Sequencing in Pediatric Acute Lymphoblastic Leukemia Germline, Diagnosis, and Relapse Trios to Discover Novel Relapse Enriched Mutations for Clonal Backtracking By Ddpcr. Blood; American Society of Hematology Annual Meeting and Exhibition; San Diego, CA. 2016. [Google Scholar]
- 51.Troeger A, Siepermann M, Escherich G, et al. Survivin and its prognostic significance in pediatric acute B-cell precursor lymphoblastic leukemia. Haematologica. 2007;92(8):1043–1050. doi: 10.3324/haematol.10675. [DOI] [PubMed] [Google Scholar]
- 52.Tyner JW, Jemal AM, Thayer M, Druker BJ, Chang BH. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia. 2012;26(4):623–632. doi: 10.1038/leu.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison DJ, Hogan LE, Condos G, et al. Endogenous knockdown of survivin improves chemotherapeutic response in ALL models. Leukemia. 2012;26(2):271–279. doi: 10.1038/leu.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raetz EA, Morrison D, Romanos-Sirakis E, et al. A phase I study of EZN-3042, a novel survivin messenger ribonucleic acid (mRNA) antagonist, administered in combination with chemotherapy in children with relapsed acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia and lymphoma (TACL) consortium. J Pediatr Hematol Oncol. 2014;36(6):458–463. doi: 10.1097/MPH.0b013e3182a8f58f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McComb S, Aguadé-Gorgorió J, Harder L, et al. Activation of concurrent apoptosis and necroptosis by SMAC mimetics for the treatment of refractory and relapsed ALL. Sci Transl Med. 2016;8(339):339ra370. doi: 10.1126/scitranslmed.aad2986. [DOI] [PubMed] [Google Scholar]
- 56.Frismantas V, Dobay MP, Rinaldi A, et al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 2017;129(11):e26–e37. doi: 10.1182/blood-2016-09-738070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khaw SL, Suryani S, Evans K, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. 2016;128(10):1382–1395. doi: 10.1182/blood-2016-03-707414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Case M, Matheson E, Minto L, et al. Mutation of genes affecting the RAS pathway is common in childhood acute lymphoblastic leukemia. Cancer Res. 2008;68(16):6803–6809. doi: 10.1158/0008-5472.CAN-08-0101. [DOI] [PubMed] [Google Scholar]
- 59.Irving JA, Enshaei A, Parker CA, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128(7):911–922. doi: 10.1182/blood-2016-03-704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polak A, Kiliszek P, Sewastianik T, et al. MEK Inhibition Sensitizes Precursor B-Cell Acute Lymphoblastic Leukemia (B-ALL) Cells to Dexamethasone through Modulation of mTOR Activity and Stimulation of Autophagy. PLoS One. 2016;11(5):e0155893. doi: 10.1371/journal.pone.0155893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhatla T, Wang J, Morrison DJ, et al. Epigenetic reprogramming reverses the relapse-specific gene expression signature and restores chemosensitivity in childhood B-lymphoblastic leukemia. Blood. 2012;119(22):5201–5210. doi: 10.1182/blood-2012-01-401687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burke MJ, Lamba JK, Pounds S, et al. A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2014;89(9):889–895. doi: 10.1002/ajh.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribera JM, Ferrer A, Ribera J, Genescà E. Profile of blinatumomab and its potential in the treatment of relapsed/refractory acute lymphoblastic leukemia. Onco Targets Ther. 2015;8:1567–1574. doi: 10.2147/OTT.S70524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buie LW, Pecoraro JJ, Horvat TZ, Daley RJ. Blinatumomab: A First-in-Class Bispecific T-Cell Engager for Precursor B-Cell Acute Lymphoblastic Leukemia. Ann Pharmacother. 2015;49(9):1057–1067. doi: 10.1177/1060028015588555. [DOI] [PubMed] [Google Scholar]
- 65.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 66.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 67.Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 68.Gökbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017 doi: 10.3324/haematol.2016.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 70.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 71.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 73.Schlegel P, Lang P, Zugmaier G, et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. 2014;99(7):1212–1219. doi: 10.3324/haematol.2013.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piccaluga PP, Arpinati M, Candoni A, et al. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52(2):325–327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 75.Raetz EA, Cairo MS, Borowitz MJ, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children's Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer. 2015;62(7):1171–1175. doi: 10.1002/pbc.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irving JA. Towards an understanding of the biology and targeted treatment of paediatric relapsed acute lymphoblastic leukaemia. Br J Haematol. 2016;172(5):655–666. doi: 10.1111/bjh.13852. [DOI] [PubMed] [Google Scholar]
- 77.Shor B, Gerber HP, Sapra P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015;67(2 Pt A):107–116. doi: 10.1016/j.molimm.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Thorson JS, Sievers EL, Ahlert J, et al. Understanding and exploiting nature's chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des. 2000;6(18):1841–1879. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 79.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol. 2010;28(12):2085–2093. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 80.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–411. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 81.Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeAngelo DJ. The use of novel monoclonal antibodies in the treatment of acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2015;2015:400–405. doi: 10.1182/asheducation-2015.1.400. [DOI] [PubMed] [Google Scholar]
- 83.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rytting M, Triche L, Thomas D, O'Brien S, Kantarjian H. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(2):369–372. doi: 10.1002/pbc.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Advani AS, Moseley A, Liedtke M, et al. A Phase 1 Trial of Inotuzumab in Combination with CVP (Cyclophosphamide, Vincristine, Prednisone) for Relapsed/Refractory CD22+ Acute Leukemia (SWOG 1312). Blood; American Society of Hematology Annual Meeting and Exposition; San Diego, CA. 2016. [Google Scholar]
- 86.Sasaki K, Jabbour EJ, O'Brien SM, et al. Inotuzumab Ozogamicin in Combination with Low-Intensity Chemotherapy (mini-hyper-CVD) As Frontline Therapy for Older Patients with Acute Lymphoblastic Leukemia (ALL): Interim Result of a Phase II Clinical Trial. Blood; American Society of Hematology Annual Meeting and Exposition; San Diego, CA. 2016. [Google Scholar]
- 87.Maude SL. Future directions in chimeric antigen receptor T cell therapy. Curr Opin Pediatr. 2017;29(1):27–33. doi: 10.1097/MOP.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 88.Huguet F, Tavitian S. Emerging biological therapies to treat acute lymphoblastic leukemia. Expert Opin Emerg Drugs. 2016:1–15. doi: 10.1080/14728214.2016.1257606. [DOI] [PubMed] [Google Scholar]
- 89.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol; 2016 ASCO Annual Meeting; Chicago, IL. 2016. [Google Scholar]
- 95.Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14(10):802–808. [PMC free article] [PubMed] [Google Scholar]
- 96.Park JH, Riviere I, Wang X, Purdon T, Sadelain M, Brentjens RJ. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. ASCO Annual Meeting; 2016. [Google Scholar]
- 97.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris EC, Stauss HJ. Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood. 2016;127(26):3305–3311. doi: 10.1182/blood-2015-11-629071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]