Abstract
Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare liver malignancy in adolescents and young adults. Surgery is the mainstay of therapy for primary and metastatic disease. Most patients relapse, with development of both local and distant metastases. Brain metastases from solid tumors are rare in the pediatric and young adult population. Here we document three patients with brain metastases from FLHCC, confirmed by histology and molecular characterization of the chimeric fusion DNAJB1-PRKACA, each necessitating neurosurgical intervention. These observations highlight the ability of FLHCC to metastasize to the brain and suggest the need for surveillance neuroimaging for patients with advanced-stage disease.
Introduction
Fibrolamellar hepatocellular carcinoma (FLHCC) is a primary liver malignancy that occurs most commonly in adolescents and young adults, without underlying liver disease. FLHCC is a relatively rare tumor, with an estimated age-adjusted incidence rate of 0.02 per 100,000 in the United States.1,2 It has a peak age of detection of 21 years, and patients are usually diagnosed between the ages of 10 and 40 years. FLHCC appears to be slightly more common in women, but otherwise is not associated with any known risk factors4,5. While initially characterized as a morphologic variant of hepatocellular carcinoma (HCC),6 FLHCC is uniquely associated with a 400-kb deletion on one copy of chromosome 19 that produces a functional chimeric fusion between the heat shock protein, DNAJB1, and the catalytic subunit of protein kinase A, PRKACA.7 The transcriptomic and proteomic changes in FLHCC are consistent from patient-to-patient and distinct from HCC.8 Molecular assays detecting the DNAJB1-PRKACA fusion have improved diagnostic accuracy for FLHCC.7,9-13
Current treatment for FLHCC focuses on gross total resection,14-17 as systemic therapy remains unstandardized.16,18-20 However, FLHCC is frequently diagnosed at an advanced stage,12 and the majority relapse within 1 year despite resection.21 Only aggressive resection of recurrent disease is correlated with improved durable survival.22 FLHCC exhibits both local invasion into adjacent organs, including the stomach, diaphragm, and pancreas, and lymphovascular spread to celiac, gastric, and para-aortic lymph nodes.12 Distant lung, peritoneal, bone and adrenal metastases occur in approximately 30% of patients23,24 Reports of 2 patients with brain metastasis exist in the literature16,25 but neither provide neuroimaging, pathologic or molecular analysis of the lesions. Here, we report 3 cases of confirmed FLHCC brain metastasis.
Case Reports
Patient 1
An 18-year-old female presented with a symptomatic abdominal mass and underwent resection of her primary liver tumor. One year after surgery, she underwent a liver transplant for disease recurrence. Post-transplant, she developed metastases in the lungs, left ovary, and apex of the vagina, which necessitated multiple resections. Five years post-diagnosis, her disease progressed to include the transplanted liver and she underwent radiofrequency ablation and treatment with Oxaliplatin, Gemcitabine, and Bevacizumab. Seven years after diagnosis she had further disease progression with metastasis to the lungs. She underwent resection of the left upper lobe of the lung, stereotactic radiation of her pulmonary metastases, partial pericardiectomy, and sorafenib. Eight years post-diagnosis CT scan of the chest showed increased thoracic, pleural and pulmonary metastasis, and left vocal cord paralysis. She was treated with palliative radiation (3Gy X 10, 5 F/W) to the mediastinum, and received ENMD-2076 (NCT02234986), a small molecule multi-kinase inhibitor with activity against Aurora kinase A, VEGFRs, FGFRs, c-Kit, CSFR1 and Flt3. She then developed headache, altered mental status, and a generalized tonic-clonic seizure. Brain imaging revealed a hemorrhagic left frontal lobe mass with midline shift (Figure 1 A,B). She underwent a left frontotemporal craniotomy and removal of the mass. Histology was consistent with FLHCC (Figure 2 A,B). PCR for the DNAJB1-PRKACA mRNA fusion was positive (Figure 2G). MSK-IMPACT™ testing on the brain metastasis demonstrated BCOR, HGF and ROS1 somatic alterations. She died 3 months after surgery due to progressive extracranial disease.
Figure 1.
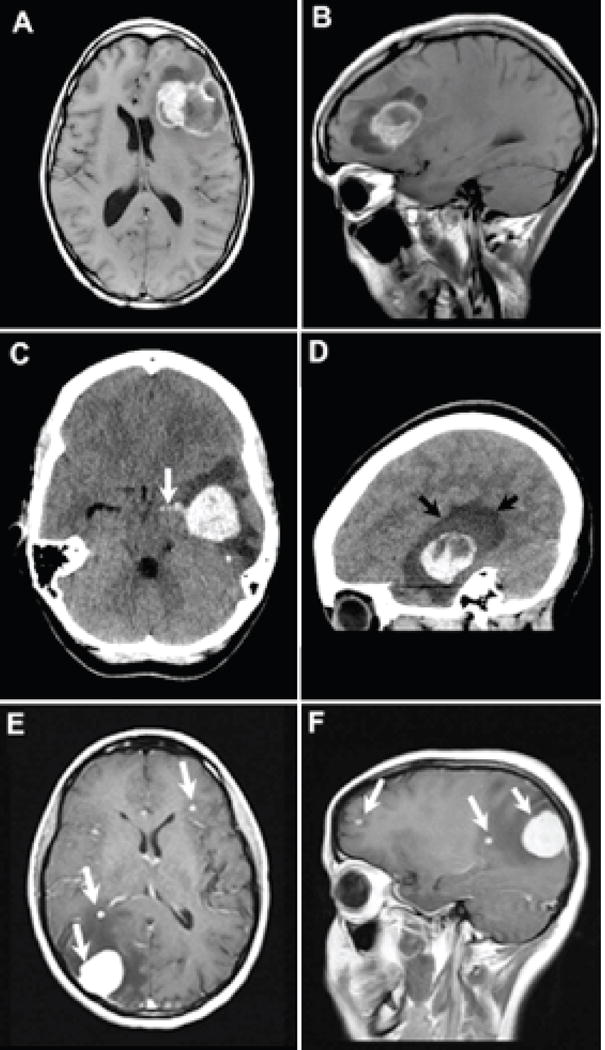
Patient 1: Axial (A) T1-weighted contrast-enhanced and sagittal (B) T1-weighted pre-contrast, demonstrate a cystic and solid metastasis within the left frontal lobe resulting in mid line shift. The metastasis contains T1 iso to hyperintense material that are iso to hyperintense on the T2-weighted images consistent with blood products (arrows). Sagittal contrast enhanced T1-weighted images depict the solid enhancing components surrounded by cystic parts of the metastasis. Patient 2: Axial (C) and sagittal (D) reformatted CT images demonstrate a hyperdense hemorrhagic metastasis within the left temporal lobe. Rupture into ventricular system with blood extending into temporal horn of the lateral ventricle [white arrow image (C)]. Edema surrounding the metastasis dark shade of gray designated by black arrows on image (D). Patient 3: Axial (E) and sagittal (F) T1-weighted contrast enhanced images reveal several enhancing metastases (white arrows).
Figure 2.
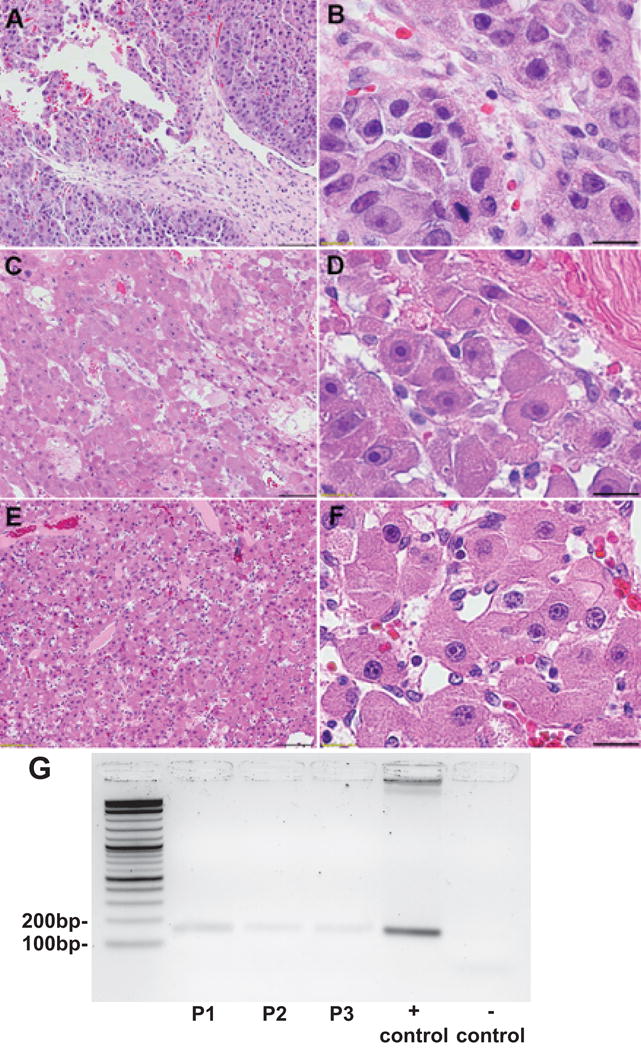
H&E of resected brain metastasis at 10x (A,C,E) and 60x (B,D,F) magnification from Patient 1 (A,B) and Patient 2 (C,D) and Patient 3 (E,F), demonstrating well-differentiated tumor cells with abundant eosinophilic cytoplasm and prominent nucleoli. Fibrous bands, classic for FLHCC are demonstrated in Patient 2 (D, top right corner) and were absent in Patients 1 and 3. Scale bars 100 microns (A,C,E) and 20 microns (B,D,F). RT-PCR agarose gel electrophoresis for the DNAJB1-PRKACA fusion mRNA, is positive for a 148bp amplicon in brain metastases from Patient 1 (lane 2), Patient 2 (lane 3), Patient 3 (lane 4), positive control for DNAJB1-PRKACA (lane 5) and a negative control (lane 6). Lanes are shown from left to right with a 10Kb ladder (lane 1) (G).
Patient 2
An 18-year-old female presented with a 1-year history of vague abdominal pain. Imaging revealed a large left hepatic mass with scattered lesions in the remaining liver and an enlarged peri-pancreatic lymph node. She underwent a left hepatectomy, resection of satellite hepatic lesions and peri-pancreatic lymph node. One month after surgery, disease at the hepatic resection margin was treated with arterial embolization. Post-operatively her disease progressed despite treatment with Fluorouracil and Interferon-alpha26, and 1 year later she underwent surgical debulking of her abdominal disease. Postoperative surveillance imaging again revealed a para-esophageal mass and new pulmonary metastasis in the lingula. She then developed acute mental status change and CT scan revealed a mass with acute intracranial hemorrhage (Figure 1 C,D). The patient underwent craniotomy and resection of the lesion. Histology was consistent with FLHCC (Figure 2 C,D). PCR on the primary tumor and metastasis confirmed the presence of the DNAJB1-PRKACA fusion transcript (Figure 2G). MSK-IMPACT™ testing on the brain metastasis additionally demonstrated loss of Chromosome 8q, and CTNNB1, ATR, SOX17, ZFH3 somatic alterations. One year after resection of her brain metastasis, she received palliative radiation to metastases in her pelvis and lower abdomen and Erlotinib. She is currently alive, without recurrence of her brain metastases and continuing therapy with Erlotinib for her extracranial disease.
Patient 3
An 18-year-old female presented with recurrent emesis and lethargy. Initial workup revealed hyperammonemia, a right hepatic mass, and a pulmonary nodule; and an extensive left leg deep vein thrombosis (DVT) requiring fasciotomy and anticoagulation. After management of her DVT, the patient underwent resection of the liver tumor and lung metastasis. Over the next 3 years she received multiple chemotherapeutic regimens including: imetelstat; tivatinib; cisplatin, gemcitabine and necitumumab; sorafenib, denosumab, everolimus, letrozole and leuprolide; all with inadequate response. Due to progressive pulmonary disease she received ENMD-2076 (NCT02234986). She then developed severe headache, left-sided blurry vision, nausea and vomiting. Neuroimaging demonstrated brain metastases (Figure 1 E,F). The patient underwent resection of the dominant lesion, and histology was compatible with FLHCC (Figure 2 E,F). PCR confirmed the presence of the DNAJB1-PRKACA fusion transcript (Figure 2G). MSK-IMPACT™ on the brain metastasis identified somatic alterations in WT1, SYK, SF3B1, NOTCH3, GRIN2A, BRD4, and AGO2. Postoperatively, stereotactic radiation was administered to the remaining intracranial metastases. Twelve months from resection, she had >10 foci of intracranial metastases, and developed a metastasis to the acetabulum. She is currently not receiving therapy.
3. Methods
Tissue specimens were either fresh frozen in optimal cutting temperature (OCT) compound or fixed in 10% formalin and embedded in paraffin (FFPE). The histology for each case was reviewed by a liver pathologist (M.S.T.). RNA extraction from FFPE or OCT embedded tissue was completed using the miRNeasy FFPE Kit or miRNeasy Kit (Qiagen; Venlo, The Netherlands), respectively. The presence of the DNAJB1-PRKACA chimera was confirmed by PCR for the fusion mRNA transcript DNAJB1-PRKACA as previously described.7 Samples underwent Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT™) testing.
4. Discussion
We describe the first 3 patients with confirmed FLHCC metastasis to the brain. On molecular analysis, each patient’s brain tumor demonstrated the DNAJB1-PRKACA fusion. All three developed multiple relapses at extrahepatic sites after undergoing initial resection of their primary disease, including pulmonary metastases. None underwent neuroimaging prior to developing neurological symptoms.
Solid tumor metastasis to the brain is rare in the pediatric population, with sarcomas comprising the majority of reported cases.27 Cursory reference to brain parenchymal metastasis in a patient with FLHCC exists twice in the literature.16,25 Clinical history is only available for one patient who was diagnosed at the age of 46, uncommon for FLHCC, and the diagnosis was not molecularly confirmed. A potential third case in the literature describes a hemorrhagic cerebellar stroke in a young patient with unresectable FLHCC and pulmonary metastasis receiving Sorafenib.28 It is unclear how the diagnosis of FLHCC was made and the authors conclude the hemorrhagic stroke was a rare adverse drug event, rather than a metastasis.
Assessment of the time course from disease onset to development of brain metastasis proves challenging. Intracranial metastasis was detected 9, 2, and 5 years post-diagnosis (Patients 1, 2, and 3, respectively). All patients had large asymptomatic intraparenchymal lesions prior to their acute presentation, suggesting a lengthy period of indolent intracranial disease. All underwent image-guided surgical resection of their intracranial lesions without operative complication.
Currently, no prescribed recommendations exist for surveillance neuroimaging in patients with FLHCC. Given the frequency of advanced stage at diagnosis, the lack of effective systemic therapies, and low progression-free survival among resected patients, we believe the at-risk cohort of patients for brain metastases is nontrivial. Additionally, with an absence of serum tumor markers, current surveillance relies on physical examination and imaging. The presence of lung metastasis, which occurs in approximately 30% of FLHCC patients, may be associated with the risk for developing brain metastasis. We recommend that patients with advanced stage FLHCC, particularly those with lung metastases, undergo neuroimaging at time of diagnosis, even in the absence of symptoms. For those without intracranial metastasis at time of diagnosis, there is currently no data to support the interval for surveillance neuroimaging, but it is our recommendation that it be incorporated into the assessment of extra-cranial disease.
Acknowledgments
Support: This study was supported in part by a Cancer Center Support Grant from the National Institutes of Health/National Cancer Institute to Memorial Sloan Kettering Cancer Center (#P30 CA008748) and NIH grant 1R56CA207929-01 to SMS
Footnotes
Authors Statement:
WJH, GL, JAS, and SMS contributed to the writing of this manuscript. WJH, GL, JAS, BAF, ED, TS, CB, JRA, SK, CWB, MST and SMS were involved in data collection. Data analysis was completed by WJH, GL, SK, JAS, JRA, SK, MST, MPL, SMS.
Declaration of Interests:
The authors of the manuscript have no conflicts of interest to disclose.
Role of Medical Writer or Editor:
Rosalind C. Simmons, Editor, Pediatric and Orthopaedic Services; Department of Surgery, Memorial Sloan Kettering Cancer Center, 1275 York Ave., New York, NY 10065; 1-212-639-2628; simmonr1@mskcc.org
In-Press Papers: None
References
- 1.Eggert T, McGlynn KA, Duffy A, et al. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J. 2013;1:351–7. doi: 10.1177/2050640613501507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004;39:798–803. doi: 10.1002/hep.20096. [DOI] [PubMed] [Google Scholar]
- 3.Eggert T, McGlynn KA, Duffy A, et al. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000-10. Gut. 2013;62:1667–1668. doi: 10.1136/gutjnl-2013-305164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica. 2012;2012:743790. doi: 10.6064/2012/743790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavros MN, Mayo SC, Hyder O, et al. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–30. doi: 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–86. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 7.Honeyman JN, Simon EP, Robine N, et al. Detection of a Recurrent DNAJB1-PRKACA Chimeric Transcript in Fibrolamellar Hepatocellular Carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon EP, Freije CA, Farber BA, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1424894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol. 2015;28:822–9. doi: 10.1038/modpathol.2015.4. [DOI] [PubMed] [Google Scholar]
- 10.Graham RP, Terracciano LM, Meves A, et al. Hepatic adenomas with synchronous or metachronous fibrolamellar carcinomas: both are characterized by LFABP loss. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.59. [DOI] [PubMed] [Google Scholar]
- 11.Ross HM, Daniel HD, Vivekanandan P, et al. Fibrolamellar carcinomas are positive for CD68. Mod Pathol. 2011;24:390–5. doi: 10.1038/modpathol.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torbenson M. Fibrolamellar Carcinoma: 2012 Update. Scientifica. 2012;2012:743790. doi: 10.6064/2012/743790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malouf G, Falissard B, Azoulay D, et al. Is histological diagnosis of primary liver carcinomas with fibrous stroma reproducible among experts? J Clin Pathol. 2009;62:519–24. doi: 10.1136/jcp.2008.062620. [DOI] [PubMed] [Google Scholar]
- 14.Darcy DG, Malek MM, Kobos R, et al. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg. 2015;50:153–6. doi: 10.1016/j.jpedsurg.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331–8. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 16.Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol. 2014;110:412–5. doi: 10.1002/jso.23658. [DOI] [PubMed] [Google Scholar]
- 18.Fakih M. A case of fibrolamellar cancer with a palliative response and minor radiographic regression with erlotinib and bevacizumab combination therapy. Am J Ther. 2014;21:e207–10. doi: 10.1097/MJT.0b013e3182840fa6. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca GM, Varella AD, Coelho FF, et al. Downstaging and resection after neoadjuvant therapy for fibrolamellar hepatocellular carcinoma. World J Gastrointest Surg. 2014;6:107–11. doi: 10.4240/wjgs.v6.i6.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gras P, Truant S, Boige V, et al. Prolonged Complete Response after GEMOX Chemotherapy in a Patient with Advanced Fibrolamellar Hepatocellular Carcinoma. Case Rep Oncol. 2012;5:169–72. doi: 10.1159/000338242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg. 2016;20:1725–31. doi: 10.1007/s11605-016-3216-x. [DOI] [PubMed] [Google Scholar]
- 22.Hemming AW, Langer B, Sheiner P, et al. Aggressive surgical management of fibrolamellar hepatocellular carcinoma. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 1997;1:342–346. doi: 10.1016/s1091-255x(97)80055-8. [DOI] [PubMed] [Google Scholar]
- 23.Do RK, McErlean A, Ang CS, et al. CT and MRI of primary and metastatic fibrolamellar carcinoma: a case series of 37 patients. Br J Radiol. 2014;87:20140024. doi: 10.1259/bjr.20140024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichikawa T, Federle MP, Grazioli L, et al. Fibrolamellar hepatocellular carcinoma: pre- and posttherapy evaluation with CT and MR imaging. Radiology. 2000;217:145–51. doi: 10.1148/radiology.217.1.r00se46145. [DOI] [PubMed] [Google Scholar]
- 25.Ordi Maja J, Sarasa Corral JL, Cortes JM, et al. Fibrolamellar hepatocarcinoma with a long clinical course and possible production of fibrinogen. Rev Clin Esp. 1986;179:252–5. [PubMed] [Google Scholar]
- 26.Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology. 2013;85:197–203. doi: 10.1159/000354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kebudi R, Ayan I, Gorgun O, et al. Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol. 2005;71:43–8. doi: 10.1007/s11060-004-4840-y. [DOI] [PubMed] [Google Scholar]
- 28.Vandewynckel YP, Geerts A, Verhelst X, et al. Cerebellar stroke in a low cardiovascular risk patient associated with sorafenib treatment for fibrolamellar hepatocellular carcinoma. Clin Case Rep. 2014;2:4–6. doi: 10.1002/ccr3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]


