Abstract
Staphylococcus aureus causes a wide range of diseases that together embody a significant public health burden. Aided by metabolic flexibility and a large virulence repertoire, S. aureus has the remarkable ability to hematogenously disseminate and infect various tissues, including skin, lung, heart, and bone, among others. The hallmark lesions of invasive staphylococcal infections, abscesses, simultaneously denote the powerful innate immune responses to tissue invasion, as well as the ability of staphylococci to persist within these lesions. In this manuscript, we review the innate immune responses to S. aureus during infection of skin and bone, which serve as paradigms for soft tissue and bone disease, respectively.
Introduction
Staphylococcus aureus is a Gram-positive bacterium that colonizes approximately 30% of the population (1). Despite this relatively innocuous lifestyle, S. aureus is capable of breaching tissue barriers, circulating through the bloodstream, and infecting nearly every organ system in the body. S. aureus is the most common cause of bacterial skin and soft tissue infections in the United States (2, 3). Other infection sites include but are not limited to bone, lung, kidney, and heart. A critical tenant in the battle against staphylococcal infections is to understand host risk factors, including those that parse out individuals capable of local control of infection, versus those that progress to invasive disease. A better understanding of the innate immune responses to S. aureus will also aid the development of new adjunctive therapies to ameliorate the morbidity of staphylococcal disease.
Historical perspectives on anti-staphylococcal immunity
In the early 1880’s, Dr. Alexander Ogston examined purulent material from patients with soft tissue infection, noting microscopic “masses or clusters, like the roe of a fish, to which I gave the name ‘staphylococcus’” (4, 5). Following Ogston’s landmark discovery, it is clear that S. aureus is the preeminent bacterial pathogen causing purulent infections. Although much is known regarding the architecture of staphylococcal abscesses and the cellular contributors to pyogenic immune responses (6), many questions remain unanswered. In the sections that follow, we review the key events underlying effective recognition and microbiologic control of S. aureus skin and bone infection.
Immune responses to S. aureus in the skin
Skin is a complex organ that performs vital functions including immune responses, hormone and vitamin production, and formation of a protective mechanical and chemical barrier (7). Skin is composed of an outer epidermis overlying an inner dermis, separated by a basement membrane. The physical and biochemical barriers are derived from the association of keratinocytes (KCs) with the products of sweat, lipid and antimicrobial peptides (AMP) (7). The epidermis is formed by KCs in different maturation stages, Langerhans cells (LC) and T cells. The dermis contains extracellular matrix (ECM) components such as connective tissues, collagen, and elastin fibers (8). The fibers provide a structural framework to host blood vessels, adipocytes, fibroblasts, skin-resident macrophages, dermal dendritic cells, mast cells, T and B lymphocytes, plasma cells and NK cells (8). As such, resident immune cells are abundant in skin, and these cells are all involved in the control of S. aureus infection by influencing different arms of the immune response (9, 10).
Key players involved in bacterial recognition and inflammatory response
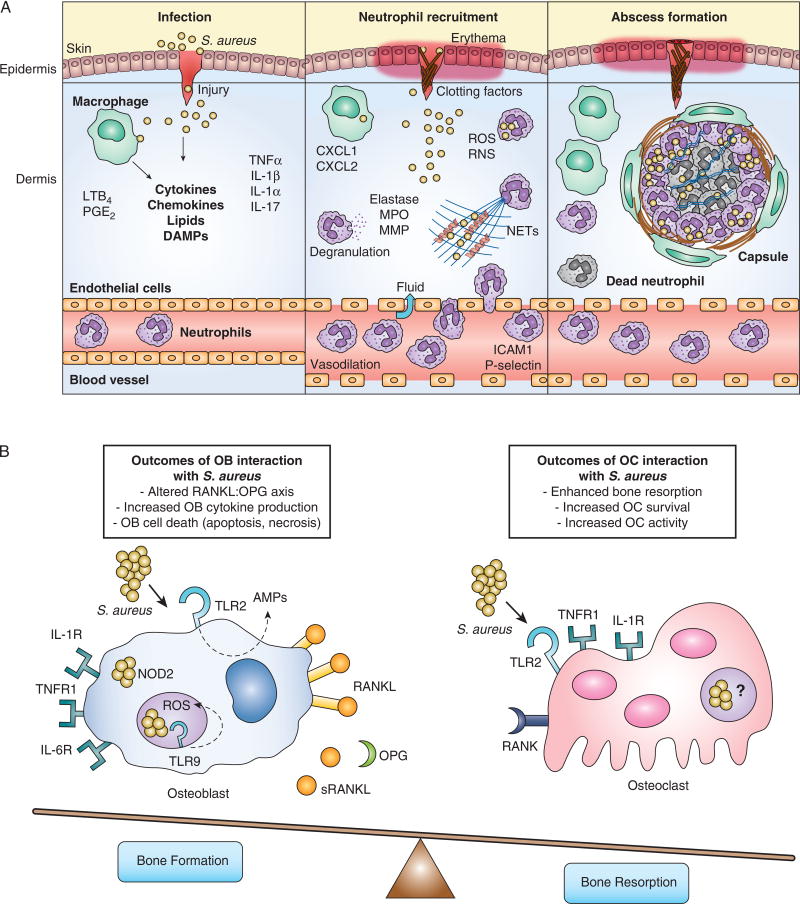
KCs, together with the other resident immune cells in the skin, participate in the recognition and response to invading pathogens (10, 11). KCs are typically the first cells that encounter pathogens, and recognize pathogen associated molecular patterns (PAMPs) via different pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-1 and −2, and the scavenger receptors CD36 and MARCO (10–12). Signaling through these receptors induces activation of transcription factors such as NFκB, AP1, and CREB to generate cytokines (IL-1α, IL-1β, IFN-γ, TNFα and IL-17A, IL-17F and IL-22), chemokines (CXCL1, CXCL2, CXCL9, CXCL10, CXCL11, CCL27, and CCL20) and antimicrobial effectors, such as antimicrobial peptides (AMPs) and inducible nitric oxide synthase (7, 12, 13) (Figure 1A; left).
Figure 1. Innate immune responses to S. aureus during skin and bone infection.
(A) Left panel: S. aureus infects skin after breaching the epithelial layers. Keratinocytes and skin-resident macrophages produce inflammatory mediators that promote neutrophil responses. Middle panel: Neutrophils are recruited to the skin where they phagocytose bacteria, undergo degranulation, and produce extracellular traps that aid in bacterial killing. Right panel: S. aureus infection is contained by abscess formation. Live and dead neutrophils and bacteria are found within the abscess. The abscess becomes encapsulated with fibrous material and surrounded by macrophages. (B) Bone remodeling activities of osteoblasts and osteoclasts are altered following interactions between innate immune receptors and S. aureus. In osteoblasts, TLR2 recognition of extracellular S. aureus leads to production of AMPs, TLR9 detection of bacterial CpG DNA in the endosome induces an antibacterial ROS response, and NOD2 sensing of cytoplasmic S. aureus occurs following escape from the endosome. The culmination of osteoblastic innate recognition results in production of pro-inflammatory cytokines, such as TNFα, IL-1, and IL-6. These cytokines allow osteoblasts to favor increased production of RANKL and decreased release of the RANKL inhibitory cytokine OPG. The increased RANKL:OPG ratio and pro-inflammatory cytokine production have a net effect to enhance osteoclast differentiation. However, OB activation and the effects of staphylococcal toxins may also result in osteoblast cell death through apoptosis and necrosis. RANKL production allows for enhanced differentiation of osteoclast precursors. Pro-inflammatory cytokines such as TNFα and IL-1 can signal directly onto osteoclast precursors to increase osteoclast survival and bone resorption activity. Osteoclast expression and ligation of TLR2 has been shown to allow for the further differentiation down the osteoclast lineage, however this occurs only in cells that have first been stimulated with RANKL. Whether or not S. aureus can invade osteoclasts or activate endosomal or cytoplasmic PRRs remains to be determined.
TLR1, −2, and- 6 recognizes the S. aureus cell wall components, specifically lipopeptides and peptidoglycan. These TLRs utilize the signaling adapter MyD88 to induce robust and efficient transcriptional programs that lead to inflammatory responses. TLR1, −2, and −6 are involved in many stages of S. aureus infection. Initially, TLR2 on KCs recognize bacteria to produce neutrophil chemoattractants and antimicrobial peptides, such as the cathelicidin LL-37 and defensins, which form pores in bacterial membranes (13). TLR2 is highly expressed on resident macrophages and recruited neutrophils and monocytes, which promptly respond to S. aureus and further stimulate cytokine production and phagocytosis. Therefore, it is expected that TLR2 is critical for both systemic and localized S. aureus infection. Mice deficient in TLR1, −2, −6 and MyD88 are highly susceptible to S. aureus infection in intranasal and intravenous infection as evidenced by increased bacterial load, poor inflammatory response and enhanced mortality or morbidity in various models of disease (14–18). In skin, the role of TLR2 is controversial. This may be due to differences in virulence of bacterial strains, infectious dose, and measured endpoints. While Miller et al., have demonstrated that TLR2 is dispensable to control S. aureus infection (17), Hoebe et al., have shown that TLR2-/- mice are more susceptible to infection (19). The strain used in the Hoebe et al. manuscript (ALC2906) shows higher lesion sizes and dermonecrosis, while the Xen 8.1 parental strain 8325-4 is less virulent in vivo. Distinct bacterial strains express unique virulence factors and toxins that could underlie different TLR2 requirements. Furthermore, the infection inoculum varies between these studies (2.5×106 vs. 105 CFU). If TLR2 is required for fine-tuning the immune response, higher amounts of bacteria (as used by Miller et al.) could override the TLR2 requirement to induce an efficient response, while lower doses of the bacterial could require TLR2 to mount a robust immune response.
The intracellular PRRs NOD1 and NOD2 also detect bacterial peptidoglycan to induce inflammation, antimicrobial peptide production, and phagocytic effector functions. NOD2 recognizes muramyl-dipeptide derived from S. aureus peptidoglycan. NOD2-deficient mice are highly susceptible to S. aureus skin and systemic infections when compared to WT counterparts (20–24). Finally, scavenger receptors CD36, SRBII and MARCO are required for optimal S. aureus skin host defense (25–27). Consequently, CD36-/- mice show increased bacterial loads and develop severe alpha-toxin-induced dermonecrosis (25).
Skin resident macrophages assist in the initial clearance of S. aureus and in conjunction with, e.g., perivascular macrophages, they regulate the recruitment of neutrophils and monocytes to the site of infection (28, 29). Dermal macrophages can phagocytose and kill S. aureus efficiently by producing reactive oxygen and nitrogen species, AMPs, and chelating proteins that starve bacteria of essential nutrients (9, 30). Furthermore, dermal macrophages secrete different chemoattractants that provide signals for neutrophil recruitment in a manner dependent on IL-1R and MyD88 (17). These cells are also involved in the clearance of dead cells at the site of infection, which is essential for resolution of disease (9, 30).
Once neutrophils arrive to the site of infection, they ingest S. aureus and attempt to control microbial growth by producing different antimicrobial effectors (see below) (9, 31, 32). Neutrophils are short-lived cells that readily undergo apoptosis and need to be cleared from the site of infection. However, S. aureus produces several toxins, such as alpha-toxin, γ-hemolysin, Panton–Valentine leukocidin (PVL), and phenol soluble modulins (PSMs), that can hasten neutrophil cell death by inducing necrosis and leading to release of the danger associated molecular patterns (DAMPs) (6, 9, 31, 33–36). DAMPs released during S. aureus infection include IL-33, IL-1α, HGMB1, calprotectin and ATP (37–39). How these different modes of cell death lead to differential outcomes during infection is an active area of study.
Skin LCs and dermal DCs “sample” their surroundings, capturing antigens before traveling to skin-draining lymph nodes (28, 40). We and others have shown that during S. aureus infection of skin, LCs ingest the bacteria, are activated by PAMPs, and then migrate to draining lymph nodes, where LCs elicit S. aureus-specific adaptive responses (41, 42). Although there are several distinct DC subsets in the skin, their roles in S. aureus skin infection are not well understood (28).
Effector mechanisms of bacterial control
S. aureus can be ingested using receptors that recognize both opsonized and non-opsonized bacteria (9, 33, 43). When coated with opsonins (e.g. C3b and IgG), S. aureus elicits various antimicrobial effector functions (44). Reactive oxygen species (ROS) (such as O2−, H2O2 and HOCl) are produced following phagocytosis through the actions of NADPH oxidase and myeloperoxidase, and can directly kill bacteria or facilitate further killing by other mechanisms (45, 46). Nitric oxide (NO) is a major reactive nitrogen species (RNS) that is produced from nitric oxide synthase and has antimicrobial and immunomodulatory activity (47). Both genetic deletion and pharmacologic inhibition of NO formation render mice highly susceptible to S. aureus infection (48, 49). However, high concentrations of NO can exert anti-inflammatory effects. High NO levels may therefore predispose to infection by inhibiting cell proliferation, inducing host cell death, and preventing phagocyte-induced TNFα production and antigen presentation. Furthermore, S. aureus utilizes NO to proliferate and precludes induction of the stress regulon via lactic acid fermentation (50, 51).
Neutrophils kill pathogens by degranulation of toxic components (52). Degranulation induces the secretion of specific granules containing AMPs, including LL37 (human) and cathelicidin-related antimicrobial peptide (CRAMP, mouse homolog), and defensins. Degranulation also releases azurocidin, cathepsins, lactoferrin, lysozymes, proteinase-3, and elastase (53, 54).
As an additional effector mechanism to control S. aureus infection, neutrophils secrete DNA rich structures, termed neutrophil extracellular traps (NETs). NETs are produced in a MyD88- and TLR2-dependent mechanism and are necessary for containing S. aureus in the skin to prevent bacteremia (55) (Figure 1A, middle). NETs limit the spread of pathogens, since they are rich in antimicrobial molecules such as AMPs, cathepsins, elastase, histones, and proteases (56). However, S. aureus can destroy NETs, and the degradation product 2'-deoxy-adenosine induces apoptosis in macrophages which increases bacterial survival in the abscess (57).
Immune mechanisms of abscess formation
Abscesses are the hallmark inflammatory lesions during S. aureus infection, and function to restrain and eliminate the pathogen (6, 9, 58). The abscess core contains fibrin, viable and necrotic neutrophils, tissue debris, and live bacteria. Abscess maturation is accompanied by formation of a fibrous capsule at the periphery; however, if the abscess is not tightly organized, systemic spread of infection may occur via the bloodstream (6, 9, 58). Interestingly, macrophages are localized to the periphery of the abscess in areas near the fibrous capsule, which may suggest a role in neutrophil chemotaxis toward and egress from the abscess (6, 9).
The immune mechanisms involved in abscess formation are beginning to be uncovered. Cho et al. have shown that neutrophil-derived IL-1β is required for S. aureus-induced abscess formation (59). Recently, Feuerstein et al. suggested that resident macrophages expressing MyD88 contribute to abscess maturation (14). Our unpublished data show that the lipid mediator leukotriene B4 (LTB4) is essential for neutrophil direction to the infectious focus, microbial killing, and fibrous capsule formation (manuscript under review). Furthermore, an ointment containing LTB4 increases S. aureus clearance and decreases lesion size. These findings correlate with neutrophil recruitment, abscess formation, ROS production, and IL-1β generation. Although there is much more to learn regarding the host-derived products that contribute to formation of abscess, a considerable amount of research has focused on the staphylococcal factors that promote survival within abscesses.
Among the S. aureus virulence factors involved in abscess formation, staphylocoagulase (Coa), von Willebrand factor binding protein (vWbp) and clumping factor A (ClfA) are all required for abscess formation. These proteins promote coagulation leading to fibrin generation and the formation of a pseudocapsule surrounding “staphylococcal abscess communities” within individual abscess lesions (6, 60).
Taken together, understanding the immune responses to S. aureus in skin, as well as host and bacterial mechanisms of abscess formation and survival, will aid in understanding the dynamics of staphylococcal pathogenesis and could lead to effective therapeutic strategies to prevent deeper infection (Figure 1A, right).
Immune responses to S. aureus during skeletal infection
Osteomyelitis as a paradigm for invasive staphylococcal infection
Beyond skin infections, S. aureus has a remarkable ability to invade and proliferate within nearly every organ system. Of the many tissues that S. aureus is capable of colonizing, bone is one of the most frequently infected, and unfortunately, one of the most debilitating manifestations of disease.
S. aureus is by far the most common cause of osteomyelitis (61, 62). Treatment regimens include prolonged antimicrobial therapy in conjunction with surgery to remove infected or devitalized bone. These surgical procedures are necessary given that S. aureus triggers profound bone destruction, which is accompanied by a loss of vascular architecture, and thus decrease delivery of antimicrobials to the site of infection. S. aureus is also the most common cause of septic arthritis, which can trigger subchondral bone destruction or even frank osteomyelitis if contiguous spread occurs (63, 64). Osteomyelitis is therefore paradigmatic for invasive staphylococcal infections that are recalcitrant to treatment and carry considerable morbidity. In the following sections, we detail advances in our understanding of the innate immune responses to S. aureus infection of bone.
Bone as a target tissue for S. aureus infection
Bone is a complex tissue consisting of a mineralized organic matrix that is constantly remodeled by the coordinated actions of osteoblasts, bone-forming cells, and osteoclasts, bone-resorbing cells. While osteoblasts differentiate from mesenchymal stem cells, osteoclasts develop from monocytic progenitors, providing an inherent link between innate immunity and bone remodeling. S. aureus is capable of colonizing skeletal tissues following hematogenous dissemination, via direct inoculation following trauma, or by spread of a contiguous infection. Upon colonization of bone, S. aureus is capable of establishing chronic infection, often surviving within traditional abscess lesions in the bone marrow, or invading directly into damaged bone through the network of osteocytic canaliculi (65). In addition to invading into healthy bone tissue, S. aureus can also invade and adhere to pieces of devitalized bone known as ‘sequestra’, creating a niche for chronic infection (Figure 2) (65). The mechanisms utilized by staphylococci to persist within bone are an area of ongoing investigation and are outside the scope of this review (66–70). However, the events leading to detection of invading staphylococci by the immune system in bone are poorly understood in comparison to studies in skin. Moreover, innate immune responses to bacterial pathogens in bone lead to profound effects on bone remodeling, which in turn influence the outcome of infection (66, 71–75).
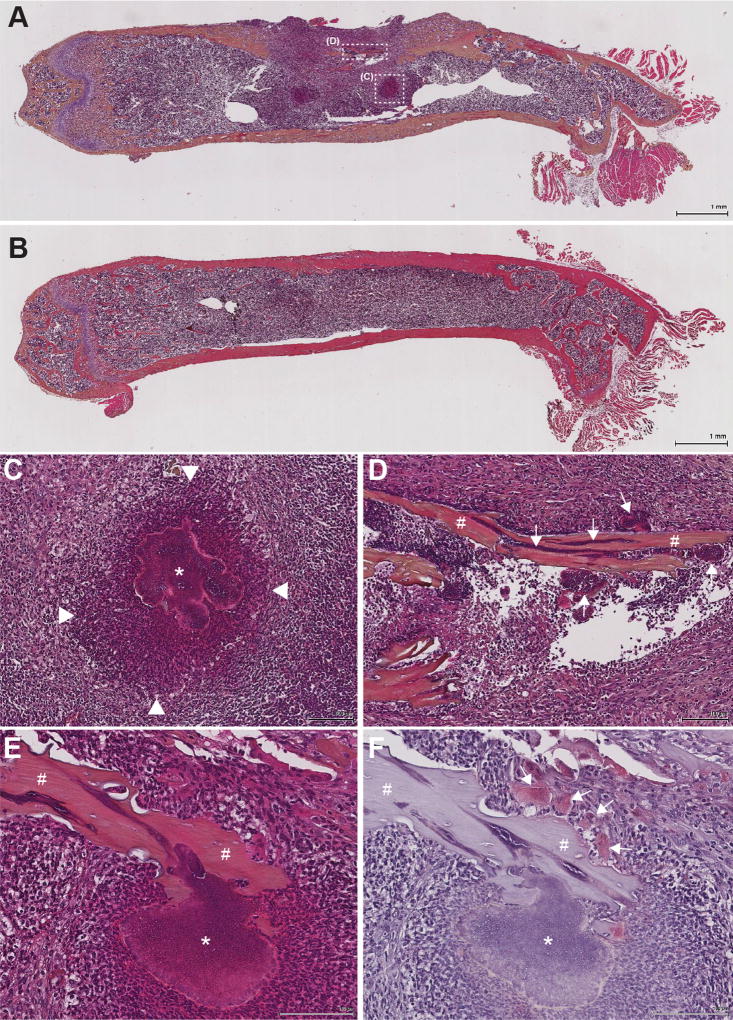
Figure 2. S. aureus forms traditional abscesses in bone marrow, but also grow directly on and invade into, living and dead bone fragments.
Murine femurs were extracted, fixed in neutral buffered formalin, and dehydrated in 70% ethanol. Following decalcification in 20% EDTA pH 7.4, femurs were processed and embedded in paraffin. Femurs infected with S. aureus (A) or mock-infected with PBS (B) were sectioned and stained with a modified hematoxylin and eosin (H&E) stain prior to imaging at 1× magnification. Different abscess morphologies, including a traditional abscess (white box “C”) in the bone marrow (C), and sequestra (white box “D”) along cortical bone fragments (D) were observed in the S. aureus infected femurs upon imaging at 10× magnification. Arrowheads in C denote the boundaries of the abscess’ neutrophilic infiltrate. * denotes the staphylococcal abscess community surrounded by an eosinophilic pseudocapsule in the center of the abscess. # in D denotes a nonviable piece of cortical bone (sequestrum) with tightly adherent clusters of staphylococci (arrows) both on the surface of and within the sequestrum. (E–F) A second murine osteomyelitis sample was stained with both modified H&E (E) and tartrate-resistant acid phosphatase (mature osteoclast marker) (F) to demonstrate that S. aureus can also adhere to segments of living cortical bone (denoted by #), as osteoclasts (arrows) are visualized remodeling the same fragment of cortical bone. * denotes a large cluster of staphylococci directly adherent to the bone segment.
Osteoimmunology: Reciprocal interactions between the skeleton and the immune system
The intricate cellular interactions that lead to bone remodeling took many decades to delineate and are still an active area of research. In the late 1980’s, osteoblasts were linked to the regulation of osteoclastogenesis, even before the primary signals for osteoclastogenesis had been identified (76–78). M-CSF was identified as a critical factor supporting osteoclastogenesis, which was in keeping with the observation that osteoclasts arise from myeloid cells during co-culture experiments (79, 80). These early discoveries paved the way for the identification of a TNF-family cytokine, receptor activator of NFκB-ligand (RANKL), as the canonical osteoclast differentiation factor (81, 82), as well as the discovery of a related inhibitory molecule known as osteoprotegrin (OPG) (83, 84). Osteoblast-lineage cells produce both RANKL and OPG to maintain the balance between bone formation and resorption (81, 82, 85).
The field of “osteoimmunology” emerged from decades of work dating back to the 1970s, in which the effects of various immune cell-derived factors and cytokines on bone homeostasis were examined (86, 87). TNFα, IL-1, and IL-6 favor bone resorption by promoting osteoclast differentiation and function. Indeed, IL-1 was initially described as “osteoclast activating factor” due to its effects on bone (88, 89). IL-1, IL-6, and TNFα trigger osteoblast lineage cells to upregulate RANKL (90), while IL-1 and TNFα can also act on osteoclasts to promote differentiation, survival, and bone resorbing activity (91–93). However, both TNFα and IL-1 can only affect osteoclast precursors that have first been primed with RANKL (94, 95). Interestingly, bone remodeling mediated by TNFα is in part driven by its ability to alter osteoblastic expression of IL-1 and the IL-1R (96). In addition to these cytokines, TH17 cells contribute to bone loss during arthritis, as IL-17 triggers RANKL production and osteoclastogenesis (97, 98). In contrast to IL-1, IL-6, TNFα, and IL-17, anti-inflammatory and TH2 cytokines are largely anti-osteoclastogenic. IL-10 can signal directly onto pre-osteoclasts to suppress RANKL-induced transcription factors (99, 100). Similarly, IL-4 and IL-13 inhibit osteoblast proliferation, favor production of OPG, and decrease RANK expression on osteoclasts (101–104). Therefore, pro-inflammatory and anti-inflammatory cytokines have major impacts on osteoclastogenesis, with the major common mechanism being modulation of osteoblast-lineage RANKL production.
Bone cells as innate sensors of bacterial pathogens
S.aureus has an extraordinary virulence repertoire that facilitates binding to host tissues, subsequent tissue invasion, host cell death, and bacterial dissemination (105–108). Yet these virulence factors also serve as potent stimuli for activation of innate immune responses.
Staphylococcal adhesins allow binding to ECM components found in bone, including fibronectin and collagen (109). Select adhesins also promote endocytic uptake into non-professional phagocytic cells such as osteoblasts (109, 110). Once internalized, S. aureus can escape into the cytoplasm by lysing the endosome (111–114). This close association with bone cells triggers immune responses, as osteoblasts, osteoclasts, and their precursor cells express a repertoire of PRRs (115–120).
Depending on the cell type, PRR ligation has variable outcomes. PRR stimulation prevents myeloid precursor cells from subsequently becoming osteoclasts, but enhances RANKL-primed, pre-osteoclast differentiation (115). Additionally, osteoblast PRR activation leads to production of pro-osteoclastogenic cytokines, such as TNFα and RANKL, as well as other cytokines and AMPs (115, 121, 122). RANKL signaling on myeloid cells induces signaling cascades through TRAF6, NIK, IKK, p38, ERK, and JNK, activating non-canonical and canonical NFκB, AP-1, MITF, and NFATc1 transcription factors (123). These differentiation pathways overlap with immune-mediated signaling and provide potential for crosstalk downstream of immune activation. IL-1 cytokines also signal through TRAF6 to activate p38 MAPK, leading to enhanced osteoclastogenesis (96, 124). Taken together, the effect of TLR/IL-1R ligation on osteoclast differentiation is complex, but once cells are primed with RANKL, these stimuli appear to enhance osteoclastogenesis.
Specific PRRs on bone cells that sense S. aureus include TLR2 recognition of peptidoglycan and lipoteichoic acid (118, 125, 126), TLR9 endosomal recognition of bacterial DNA, and NOD-mediated recognition of cytoplasmic bacteria following escape from the endosome. Similar to the interactions with resident skin cells, S. aureus activates TLR2 on osteoblasts in vitro, leading to release of AMPs and cell death (121, 127). Once internalized, S. aureus in osteoblasts can be killed in the endosome through TLR9-mediated induction of oxidative stress, though not as robustly as professional phagocytes (128, 129). S. aureus also triggers expression of NOD2 by osteoblasts (130, 131), and cooperation between TLR2 and NOD2 induces RANKL production (116, 117, 132). Finally, the NLRP3 inflammasome can be activated by S. aureus peptidoglycan and bone particles in myeloid cells (133, 134). Consequently, recognition of S. aureus by multiple PRRs on bone cells induces a robust inflammatory response and alters bone remodeling (Figure 1B). S. aureus recognition by PRRs such as TLR2 and NOD2 allows for shared innate mechanisms between resident skin and bone cells, emphasizing the importance of response to general bacterial motifs.
Deconvolution of the innate immune responses to S. aureus osteomyelitis using animal models
Animal models of osteomyelitis can be used to define critical immune responses leading to inflammation and alterations in bone remodeling (66, 68, 70, 71, 135–139). In a murine model of post-traumatic S. aureus osteomyelitis, Yoshii et al. found high levels of IL-1, IL-6, and TNFα in bone early after infection, with TNFα remaining elevated for the 28-day course of infection (140). The chemokines CCL3, CXCL2, and CCL2 have also been detected at high levels during osteomyelitis, and importantly, CCL3 and CXCL2 can trigger osteoclastogenesis and enhance bone loss (141, 142).
Downstream of PRRs, signaling through MyD88 is critical for osteoclastogenesis enhanced by PAMPs and IL-1 (115, 143). Just as MyD88/IL-1R are important in neutrophil recruitment and S. aureus clearance in skin infection models (17), these signaling pathways are also crucial for bacterial control on implants in a post-arthroplasty model of infection (144). Furthermore, IL-1R–deficient mice were found to have a higher frequency and severity of septic arthritis in a systemic S. aureus model (145). The role of TLR2 in S. aureus infection is largely dependent on the model system employed and the target tissue examined (see above). TLR2 enhances bone resorption in response to injection of heat-killed S. aureus, but not a lipoprotein-deficient strain (146). This supports a mechanism whereby TLR2 senses systemic bacterial components and can mediate changes in bone homeostasis. These studies corroborate that MyD88-dependent PRRs and cytokines are critical for bone remodeling and control of S. aureus infection.
S. aureus secreted virulence factors induce bone cell death and contribute to the pathogenesis of osteomyelitis
S. aureus pathogenesis is partially dependent on secreted virulence factors, including cytolytic toxins and proteins that modify immune functions. In experimental models of osteomyelitis, several S. aureus proteins impact bone architecture and contribute to comorbidities such as sepsis. Abscess formation in the bone marrow and around devitalized bone leads to a hypoxic environment, which subsequently alters quorum sensing and toxin production (67). PSMs mediate approximately 30% of the cortical bone loss observed in a murine model of osteomyelitis, with direct cytolytic effects on osteoblasts (66, 67). Bone destruction can also be triggered by the superantigen TSST-1 and staphylococcal protein A (Spa), which both activate osteoclast signaling to enhance bone resorption (75, 147, 148). PVL enhances early bacterial survival in bone and promotes bacterial spread to nearby muscles and joints in a rabbit model of osteomyelitis (149). Furthermore, alpha-hemolysin (Hla) contributes to severe sepsis-related mortality following osteomyelitis in rabbits (150).
In addition to their role in osteomyelitis, staphylococcal toxins significantly contribute to the pathogenesis of infection in other organ systems. For example, PSMs are small, amphipathic pore-forming toxins that are relatively promiscuous in their ability to induce toxicity among several cell types and species (67, 151). In the skin, PSMs stimulate keratinocytes to release proinflammatory cytokines (152). PVL contributes to staphylococcal skin disease by facilitating spread to neighboring muscle during skin infection (153). Taken together, these findings highlight the essential role of staphylococcal secreted virulence factors in disease pathogenesis, and highlight the broad tissue tropism of cytolytic toxins.
Limited but compelling evidence implicates the S. aureus toxin repertoire in disease severity during human infection. S. aureus strains expressing PVL are associated with more severe local disease and a greater systemic inflammatory response in children with osteomyelitis (154). Additionally, PVL has been shown to be mediate lysis of human myeloid cells, including osteoclasts, after binding the C5a receptor (147). Yet, the contribution of staphylococcal toxins regarding disease severity and pathogenesis varies based on the infection site and the repertoire of virulence factors expressed by the infecting S. aureus strains, which may not be fully assessed in experimental models.
Putting it all together: Staphylococcal immune response in humans
Individuals with diseases that impact innate immunity are at enhanced risk of staphylococcal infection. Genetic diseases that predispose individuals to S. aureus infections include chronic granulomatous disease (CGD) (155), deficiencies in MyD88 (156), IRAK-4 (157), TIRAP (158), and RAC2 (159), Wiskott-Aldrich Syndrome (159), leukocyte adhesion deficiency (160), severe congenital neutropenia (160), and allelic variants of cytokines IL-1α, IL-4, and IL-6 (161), among others. Increased risk of S. aureus infection has also been associated with co-morbidities such as diabetes (162, 163), malnutrition (164), bone marrow transplantation (165), and HIV infection (166). In general, these conditions are associated with extreme dysregulation of the immune response. While people with malnutrition (164, 167), newborns (168, 169) and bone-marrow transplant recipients (170) are functionally immunocompromised, subjects with uncontrolled diabetes (171–173), obesity (174, 175) and advancing age (176, 177) exhibit chronic low-grade inflammation and are also susceptible to infection. However, the common ground that favors S. aureus infection remains to be determined.
Remaining questions and future research
The innate immune response to S. aureus mediates infection outcomes and is dependent on host genetics and comorbidities, the tissue environment, and mechanisms of immune evasion by bacterial pathogens. Skin and bone cells participate in the induction of innate immunity and subsequent tissue remodeling events. Future research should therefore investigate how tissue resident cells instigate immune responses through the elaboration of cytokines, the recruitment of phagocytes, and the production of antimicrobial compounds. At the same time, these studies must address the consequences of immune activation on tissue homeostasis and remodeling, factors which play a large role in the morbidity of infectious diseases and the eventual recovery of a functional organ system. Specific questions remain about the contribution of individual cell lineages to immunity in both skin and bone. Targeted inactivation of innate pathways in tissue resident cells using genetic tools such as CRISPR-Cas or Cre-lox technology will be necessary to study their contribution to anti-staphylococcal immunity in vivo. Additional areas of future research include the redundancy or compensation between PRRs, crosstalk downstream of common PRR and tissue-specific signaling pathways, and mechanisms of adaptive immunity that limit morbidity from primary innate immunodeficiency. Furthermore, the cellular and species tropism of secreted S. aureus virulence factors is worthy of ongoing investigation (178). The contribution of individual toxins to disease pathogenesis is controversial when considering data from different animal models. For example, PVL activity is restricted to the human and rabbit C5a receptor, thus the effects of this toxin cannot be elucidated using murine models (179). Similarly, other staphylococcal bi-component toxins have species-specific interactions with receptors, therefore not all animal models are appropriate to measure toxin effects (178). While innate immune responses are the first line of defense to prevent dissemination of S. aureus, these early events influence subsequent adaptive responses. A thorough understanding of immune protection from staphylococcal disease will therefore only result from study of both arms of the immune system.
Conclusions
In conclusion, innate immunity to S. aureus infection is multi-faceted and tissue specific. Decades of research on staphylococcal pathogenesis have elucidated important roles for key PRRs such as TLR2 and NOD2, as well as for specific cytokine signaling pathways such as IL-1. The roles of tissue resident cells in these signaling processes are beginning to be explored, and will be facilitated by new mammalian genetic tools. Understanding how innate immune responses impact tissue homeostasis is a critical future direction, given that tissue pathology is a significant driver of morbidity, mortality, and treatment failure. New therapies aimed at boosting innate immunity or blocking immunoevasive factors produced by S. aureus hold considerable progress as adjunctive therapies for the treatment of invasive infection (180–182).
Acknowledgments
This work was supported by National Institutes of Health grants HL10377701 (CHS), T32AI060519 (SLB), R01AI132560 (JEC), K08AI113107 (JEC), and 1F31AI133926-01 (NEP). JEC is also supported by a Burroughs Wellcome Fund Career Award for Medical Scientists.
Footnotes
Competing interests. The authors have no conflict of interest.
References
- 1.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, Group MINS. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 3.Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13:252. doi: 10.1186/1471-2334-13-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newsom SW. Ogston's coccus. J Hosp Infect. 2008;70:369–372. doi: 10.1016/j.jhin.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Ogston A British Medical Association. Scientific Grants Committee. Report upon micro-organisms in surgical diseases presented to the Scientific Grants Committee of the British Medical Association. British Medical Association; London: 1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends in microbiology. 2011;19:225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 8.Matejuk A. Skin Immunity. Arch Immunol Ther Exp (Warsz) 2017 doi: 10.1007/s00005-017-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim F, Khan T, Pujalte GG. Bacterial Skin Infections. Prim Care. 2015;42:485–499. doi: 10.1016/j.pop.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Mistry RD. Skin and soft tissue infections. Pediatr Clin North Am. 2013;60:1063–1082. doi: 10.1016/j.pcl.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Miller LS. Toll-like receptors in skin. Adv Dermatol. 2008;24:71–87. doi: 10.1016/j.yadr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitschar K, Wolz C, Krismer B, Peschel A, Schittek B. Keratinocytes as sensors and central players in the immune defense against Staphylococcus aureus in the skin. J Dermatol Sci. 2017;87:215–220. doi: 10.1016/j.jdermsci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Feuerstein R, Seidl M, Prinz M, Henneke P. MyD88 in macrophages is critical for abscess resolution in staphylococcal skin infection. J Immunol. 2015;194:2735–2745. doi: 10.4049/jimmunol.1402566. [DOI] [PubMed] [Google Scholar]
- 15.Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J Invest Dermatol. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Pietrocola G, Arciola CR, Rindi S, Di Poto A, Missineo A, Montanaro L, Speziale P. Toll-like receptors (TLRs) in innate immune defense against Staphylococcus aureus. Int J Artif Organs. 2011;34:799–810. doi: 10.5301/ijao.5000030. [DOI] [PubMed] [Google Scholar]
- 19.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 20.Schaffler H, Demircioglu DD, Kuhner D, Menz S, Bender A, Autenrieth IB, Bodammer P, Lamprecht G, Gotz F, Frick JS. NOD2 stimulation by Staphylococcus aureus-derived peptidoglycan is boosted by Toll-like receptor 2 costimulation with lipoproteins in dendritic cells. Infect Immun. 2014;82:4681–4688. doi: 10.1128/IAI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker D, Planet PJ, Soong G, Narechania A, Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog. 2014;10:e1003951. doi: 10.1371/journal.ppat.1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M, Cavaillon JM, Adib-Conquy M. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect. 2010;12:759–767. doi: 10.1016/j.micinf.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci U S A. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG., Jr Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun. 2009;77:1376–1382. doi: 10.1128/IAI.00940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castleman MJ, Febbraio M, Hall PR. CD36 Is Essential for Regulation of the Host Innate Response to Staphylococcus aureus alpha-Toxin-Mediated Dermonecrosis. J Immunol. 2015;195:2294–2302. doi: 10.4049/jimmunol.1500500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchet C, Jouvion G, Fitting C, Cavaillon JM, Adib-Conquy M. Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on the site of infection. PLoS One. 2014;9:e87927. doi: 10.1371/journal.pone.0087927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 28.Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 29.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, Weninger W. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuerstein R, Kolter J, Henneke P. Dynamic interactions between dermal macrophages and Staphylococcus aureus. J Leukoc Biol. 2017;101:99–106. doi: 10.1189/jlb.3MR0316-097RR. [DOI] [PubMed] [Google Scholar]
- 31.Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012;34:261–280. doi: 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol. 2017;7:286. doi: 10.3389/fcimb.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anwar S, Prince LR, Foster SJ, Whyte MK, Sabroe I. The rise and rise of Staphylococcus aureus: laughing in the face of granulocytes. Clin Exp Immunol. 2009;157:216–224. doi: 10.1111/j.1365-2249.2009.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevost G, Colin DA, Staali L, Baba Moussa L, Gravet A, Werner S, Sanni A, Meunier O, Monteil H. [Pore-forming leukotoxins from Staphylococcus aureus: variability of the target cells and 2 pharmacological processes] Pathol Biol (Paris) 1998;46:435–441. [PubMed] [Google Scholar]
- 36.Antonelou M, Knowles J, Siddiqi S, Sharma P. Recurrent cutaneous abscesses caused by PVL-MRSA. BMJ Case Rep. 2011:2011. doi: 10.1136/bcr.01.2011.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachet M, Liang YY, Oehler R. The immune response to secondary necrotic cells. Apoptosis. 2017 doi: 10.1007/s10495-017-1413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn D, Prince A. Participation of Necroptosis in the Host Response to Acute Bacterial Pneumonia. J Innate Immun. 2017;9:262–270. doi: 10.1159/000455100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker D, Prince A. Immunoregulatory effects of necroptosis in bacterial infections. Cytokine. 2016;88:274–275. doi: 10.1016/j.cyto.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol. 2017;18:1068–1075. doi: 10.1038/ni.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejani NN, Brandt SL, Pineros A, Glosson-Byers NL, Wang S, Son YM, Medeiros AI, Serezani CH. Topical Prostaglandin E Analog Restores Defective Dendritic Cell-Mediated Th17 Host Defense Against Methicillin-Resistant Staphylococcus Aureus in the Skin of Diabetic Mice. Diabetes. 2016;65:3718–3729. doi: 10.2337/db16-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouchi T, Kubo A, Yokouchi M, Adachi T, Kobayashi T, Kitashima DY, Fujii H, Clausen BE, Koyasu S, Amagai M, Nagao K. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. The Journal of experimental medicine. 2011;208:2607–2613. doi: 10.1084/jem.20111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison CJ. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva pediatrica. 2009;61:503–514. [PubMed] [Google Scholar]
- 44.Okumura CY, Nizet V. Subterfuge and sabotage: evasion of host innate defenses by invasive gram-positive bacterial pathogens. Annu Rev Microbiol. 2014;68:439–458. doi: 10.1146/annurev-micro-092412-155711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buvelot H, Posfay-Barbe KM, Linder P, Schrenzel J, Krause KH. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev. 2017;41:139–157. doi: 10.1093/femsre/fuw042. [DOI] [PubMed] [Google Scholar]
- 46.Beavers WN, Skaar EP. Neutrophil-generated oxidative stress and protein damage in Staphylococcus aureus. Pathog Dis. 2016:74. doi: 10.1093/femspd/ftw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Li H, Jiang Z, Zhang T, Wang Y, Li Z, Wu Y, Ji S, Xiao S, Ryffel B, Radek KA, Xia Z, Lai Y. Interleukin-33 increases antibacterial defense by activation of inducible nitric oxide synthase in skin. PLoS Pathog. 2014;10:e1003918. doi: 10.1371/journal.ppat.1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett. 2008;119:84–90. doi: 10.1016/j.imlet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Grosser MR, Weiss A, Shaw LN, Richardson AR. Regulatory Requirements for Staphylococcus aureus Nitric Oxide Resistance. J Bacteriol. 2016;198:2043–2055. doi: 10.1128/JB.00229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitko NP, Spahich NA, Richardson AR. Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. MBio. 2015:6. doi: 10.1128/mBio.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi Y. Neutrophil biology: an update. EXCLI J. 2015;14:220–227. doi: 10.17179/excli2015-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenlee-Wacker M, DeLeo FR, Nauseef WM. How methicillin-resistant Staphylococcus aureus evade neutrophil killing. Curr Opin Hematol. 2015;22:30–35. doi: 10.1097/MOH.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Kessel KP, Bestebroer J, van Strijp JA. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus. Front Immunol. 2014;5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 57.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370:1039–1047. doi: 10.1056/NEJMra1212788. [DOI] [PubMed] [Google Scholar]
- 59.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 62.Copley LA. Pediatric musculoskeletal infection: trends and antibiotic recommendations. The Journal of the American Academy of Orthopaedic Surgeons. 2009;17:618–626. doi: 10.5435/00124635-200910000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verdrengh M, Carlsten H, Ohlsson C, Tarkowski A. Rapid systemic bone resorption during the course of Staphylococcus aureus-induced arthritis. J Infect Dis. 2006;194:1597–1600. doi: 10.1086/508751. [DOI] [PubMed] [Google Scholar]
- 65.de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J Bone Miner Res. 2017;32:985–990. doi: 10.1002/jbmr.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell host & microbe. 2013;13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilde AD, Snyder DJ, Putnam NE, Valentino MD, Hammer ND, Lonergan ZR, Hinger SA, Aysanoa EE, Blanchard C, Dunman PM, Wasserman GA, Chen J, Shopsin B, Gilmore MS, Skaar EP, Cassat JE. Bacterial Hypoxic Responses Revealed as Critical Determinants of the Host-Pathogen Outcome by TnSeq Analysis of Staphylococcus aureus Invasive Infection. PLoS Pathog. 2015;11:e1005341. doi: 10.1371/journal.ppat.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loughran AJ, Gaddy D, Beenken KE, Meeker DG, Morello R, Zhao H, Byrum SD, Tackett AJ, Cassat JE, Smeltzer MS. Impact of sarA and Phenol-Soluble Modulins on the Pathogenesis of Osteomyelitis in Diverse Clinical Isolates of Staphylococcus aureus. Infect Immun. 2016;84:2586–2594. doi: 10.1128/IAI.00152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pförtner H, Niemann S, Geraci J, Van de, Vyver H, Fraunholz MJ, Cheung AL, Herrmann M, Völker U, Sordelli DO, Peters G, Löffler B. Sigma Factor SigB Is Crucial to Mediate Staphylococcus aureus Adaptation during Chronic Infections. PLoS Pathog. 2015;11:e1004870. doi: 10.1371/journal.ppat.1004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Cheng LI, Helfer DR, Ashbaugh AG, Miller RJ, Tzomides AJ, Thompson JM, Ortines RV, Tsai AS, Liu H, Dillen CA, Archer NK, Cohen TS, Tkaczyk C, Stover CK, Sellman BR, Miller LS. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A. 2017;114:E5094–E5102. doi: 10.1073/pnas.1703427114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horst SA, Hoerr V, Beineke A, Kreis C, Tuchscherr L, Kalinka J, Lehne S, Schleicher I, Kohler G, Fuchs T, Raschke MJ, Rohde M, Peters G, Faber C, Loffler B, Medina E. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: an integrated view of disease pathogenesis. American Journal of Pathology. 2012;181:1206–1214. doi: 10.1016/j.ajpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Mbalaviele G, Novack DV, Schett G, Teitelbaum SL. Inflammatory osteolysis: a conspiracy against bone. J Clin Invest. 2017;127:2030–2039. doi: 10.1172/JCI93356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner JM, Jaurich H, Wallner C, Abraham S, Becerikli M, Dadras M, Harati K, Duhan V, Khairnar V, Lehnhardt M, Behr B. Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity, and increased osteoclast activity. J Orthop Res. 2017;35:2425–2434. doi: 10.1002/jor.23555. [DOI] [PubMed] [Google Scholar]
- 74.Widaa A, Claro T, Foster TJ, O'Brien FJ, Kerrigan SW. Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS One. 2012;7:e40586. doi: 10.1371/journal.pone.0040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendoza Bertelli A, Delpino MV, Lattar S, Giai C, Llana MN, Sanjuan N, Cassat JE, Sordelli D, Gómez MI. Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochim Biophys Acta. 2016;1862:1975–1983. doi: 10.1016/j.bbadis.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 77.Perry HM, Skogen W, Chappel J, Kahn AJ, Wilner G, Teitelbaum SL. Partial characterization of a parathyroid hormone-stimulated resorption factor(s) from osteoblast-like cells. Endocrinology. 1989;125:2075–2082. doi: 10.1210/endo-125-4-2075. [DOI] [PubMed] [Google Scholar]
- 78.Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 79.Hofstetter W, Wetterwald A, Cecchini MC, Felix R, Fleisch H, Mueller C. Detection of transcripts for the receptor for macrophage colony-stimulating factor, c-fms, in murine osteoclasts. Proc Natl Acad Sci U S A. 1992;89:9637–9641. doi: 10.1073/pnas.89.20.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burger EH, van der Meer JW, Nijweide PJ. Osteoclast formation from mononuclear phagocytes: role of bone-forming cells. J Cell Biol. 1984;99:1901–1906. doi: 10.1083/jcb.99.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 84.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 85.Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–3484. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- 86.Horton JE, Raisz LG, Simmons HA, Oppenheim JJ, Mergenhagen SE. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972;177:793–795. doi: 10.1126/science.177.4051.793. [DOI] [PubMed] [Google Scholar]
- 87.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 88.Dewhirst FE, Stashenko PP, Mole JE, Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985;135:2562–2568. [PubMed] [Google Scholar]
- 89.Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 90.Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 91.Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010;22:805–816. doi: 10.1093/intimm/dxq431. [DOI] [PubMed] [Google Scholar]
- 92.Jimi E, Shuto T, Koga T. Macrophage colony-stimulating factor and interleukin-1 alpha maintain the survival of osteoclast-like cells. Endocrinology. 1995;136:808–811. doi: 10.1210/endo.136.2.7835314. [DOI] [PubMed] [Google Scholar]
- 93.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 94.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N. The mechanism of osteoclast differentiation induced by IL-1. J Immunol. 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 96.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. The Journal of clinical investigation. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;8:4. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohamed SG, Sugiyama E, Shinoda K, Taki H, Hounoki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Ogawa H, Miyahara T. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW264.7 cells and mouse bone marrow cells. Bone. 2007;41:592–602. doi: 10.1016/j.bone.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Frost A, Jonsson KB, Brändström H, Ljunghall S, Nilsson O, Ljunggren O. Interleukin (IL)-13 and IL-4 inhibit proliferation and stimulate IL-6 formation in human osteoblasts: evidence for involvement of receptor subunits IL-13R, IL-13Ralpha, and IL-4Ralpha. Bone. 2001;28:268–274. doi: 10.1016/s8756-3282(00)00449-x. [DOI] [PubMed] [Google Scholar]
- 102.Palmqvist P, Lundberg P, Persson E, Johansson A, Lundgren I, Lie A, Conaway HH, Lerner UH. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J Biol Chem. 2006;281:2414–2429. doi: 10.1074/jbc.M510160200. [DOI] [PubMed] [Google Scholar]
- 103.Yamada A, Takami M, Kawawa T, Yasuhara R, Zhao B, Mochizuki A, Miyamoto Y, Eto T, Yasuda H, Nakamichi Y, Kim N, Katagiri T, Suda T, Kamijo R. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei S, Wang MW, Teitelbaum SL, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J Biol Chem. 2002;277:6622–6630. doi: 10.1074/jbc.M104957200. [DOI] [PubMed] [Google Scholar]
- 105.Thomer L, Schneewind O, Missiakas D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu Rev Pathol. 2016;11:343–364. doi: 10.1146/annurev-pathol-012615-044351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alonzo F, 3rd, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Powers ME, Bubeck Wardenburg J. Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS pathogens. 2014;10:e1003871. doi: 10.1371/journal.ppat.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis. Frontiers in cellular and infection microbiology. 2015;5:85. doi: 10.3389/fcimb.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heilmann C. Adhesion mechanisms of staphylococci. Adv Exp Med Biol. 2011;715:105–123. doi: 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- 111.Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, Trumble WR. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giese B, Glowinski F, Paprotka K, Dittmann S, Steiner T, Sinha B, Fraunholz MJ. Expression of δ-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of β-toxin. Cell Microbiol. 2011;13:316–329. doi: 10.1111/j.1462-5822.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 113.Qazi SN, Counil E, Morrissey J, Rees CE, Cockayne A, Winzer K, Chan WC, Williams P, Hill PJ. agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infect Immun. 2001;69:7074–7082. doi: 10.1128/IAI.69.11.7074-7082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grosz M, Kolter J, Paprotka K, Winkler AC, Schäfer D, Chatterjee SS, Geiger T, Wolz C, Ohlsen K, Otto M, Rudel T, Sinha B, Fraunholz M. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell Microbiol. 2014;16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bar-Shavit Z. Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity. 2008;41:195–203. doi: 10.1080/08916930701694469. [DOI] [PubMed] [Google Scholar]
- 116.Kassem A, Henning P, Lundberg P, Souza PP, Lindholm C, Lerner UH. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-κB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. J Biol Chem. 2015;290:20147–20158. doi: 10.1074/jbc.M115.655787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kassem A, Lindholm C, Lerner UH. Toll-Like Receptor 2 Stimulation of Osteoblasts Mediates Staphylococcus Aureus Induced Bone Resorption and Osteoclastogenesis through Enhanced RANKL. PLoS One. 2016;11:e0156708. doi: 10.1371/journal.pone.0156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang P, Liu J, Xu Q, Harber G, Feng X, Michalek SM, Katz J. TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-kappaB. J Biol Chem. 2011;286:24159–24169. doi: 10.1074/jbc.M110.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krisher T, Bar-Shavit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem. 2014;115:2146–2154. doi: 10.1002/jcb.24891. [DOI] [PubMed] [Google Scholar]
- 120.Kim PD, Xia-Juan X, Crump KE, Abe T, Hajishengallis G, Sahingur SE. Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect Immun. 2015;83:2992–3002. doi: 10.1128/IAI.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Varoga D, Tohidnezhad M, Paulsen F, Wruck CJ, Brandenburg L, Mentlein R, Lippross S, Hassenpflug J, Besch L, Müller M, Jürgens C, Seekamp A, Schmitt L, Pufe T. The role of human beta-defensin-2 in bone. J Anat. 2008;213:749–757. doi: 10.1111/j.1469-7580.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warnke PH, Springer IN, Russo PA, Wiltfang J, Essig H, Kosmahl M, Sherry E, Acil Y. Innate immunity in human bone. Bone. 2006;38:400–408. doi: 10.1016/j.bone.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 124.Kadono Y, Okada F, Perchonock C, Jang HD, Lee SY, Kim N, Choi Y. Strength of TRAF6 signalling determines osteoclastogenesis. EMBO Rep. 2005;6:171–176. doi: 10.1038/sj.embor.7400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Q, Dziarski R, Kirschning CJ, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2-->MyD88-->IRAK-->TRAF-->NIK-->IKK-->NF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270–2276. doi: 10.1128/IAI.69.4.2270-2276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang J, Ryu YH, Yun CH, Han SH. Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J Leukoc Biol. 2009;86:823–831. doi: 10.1189/jlb.0309206. [DOI] [PubMed] [Google Scholar]
- 127.Chen Q, Hou T, Luo F, Wu X, Xie Z, Xu J. Involvement of toll-like receptor 2 and pro-apoptotic signaling pathways in bone remodeling in osteomyelitis. Cell Physiol Biochem. 2014;34:1890–1900. doi: 10.1159/000366387. [DOI] [PubMed] [Google Scholar]
- 128.Mohamed W, Domann E, Chakraborty T, Mannala G, Lips KS, Heiss C, Schnettler R, Alt V. TLR9 mediates S. aureus killing inside osteoblasts via induction of oxidative stress. BMC Microbiol. 2016;16:230. doi: 10.1186/s12866-016-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamza T, Li B. Differential responses of osteoblasts and macrophages upon Staphylococcus aureus infection. BMC Microbiol. 2014;14:207. doi: 10.1186/s12866-014-0207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marriott I, Rati DM, McCall SH, Tranguch SL. Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun. 2005;73:2967–2973. doi: 10.1128/IAI.73.5.2967-2973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chauhan VS, Marriott I. Differential roles for NOD2 in osteoblast inflammatory immune responses to bacterial pathogens of bone tissue. J Med Microbiol. 2010;59:755–762. doi: 10.1099/jmm.0.015859-0. [DOI] [PubMed] [Google Scholar]
- 132.Yang S, Takahashi N, Yamashita T, Sato N, Takahashi M, Mogi M, Uematsu T, Kobayashi Y, Nakamichi Y, Takeda K, Akira S, Takada H, Udagawa N, Furusawa K. Muramyl dipeptide enhances osteoclast formation induced by lipopolysaccharide, IL-1 alpha, and TNF-alpha through nucleotide-binding oligomerization domain 2-mediated signaling in osteoblasts. J Immunol. 2005;175:1956–1964. doi: 10.4049/jimmunol.175.3.1956. [DOI] [PubMed] [Google Scholar]
- 133.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Götz F, Liu GY, Underhill DM. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe. 2010;7:38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alippe Y, Wang C, Ricci B, Xiao J, Qu C, Zou W, Novack DV, Abu-Amer Y, Civitelli R, Mbalaviele G. Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci Rep. 2017;7:6630. doi: 10.1038/s41598-017-07014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoong P, Torres VJ. Animal models and imaging technologies: paving the way towards insights into Staphylococcus aureus-induced osteomyelitis. Future Microbiol. 2013;8:1515–1518. doi: 10.2217/fmb.13.136. [DOI] [PubMed] [Google Scholar]
- 137.Cassat JE, Skaar EP. Recent advances in experimental models of osteomyelitis. Expert Rev Anti Infect Ther. 2013;11:1263–1265. doi: 10.1586/14787210.2013.858600. [DOI] [PubMed] [Google Scholar]
- 138.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infection & Immunity. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niska JA, Meganck JA, Pribaz JR, Shahbazian JH, Lim E, Zhang N, Rice BW, Akin A, Ramos RI, Bernthal NM, Francis KP, Miller LS. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and muCT imaging in an orthopaedic implant infection in mice. PLoS ONE [Electronic Resource] 2012;7:e47397. doi: 10.1371/journal.pone.0047397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshii T, Magara S, Miyai D, Nishimura H, Kuroki E, Furudoi S, Komori T, Ohbayashi C. Local levels of interleukin-1beta, −4, −6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to staphylococcus aureus. Cytokine. 2002;19:59–65. doi: 10.1006/cyto.2002.1039. [DOI] [PubMed] [Google Scholar]
- 141.Dapunt U, Maurer S, Giese T, Gaida MM, Hänsch GM. The macrophage inflammatory proteins MIP1α (CCL3) and MIP2α (CXCL2) in implant-associated osteomyelitis: linking inflammation to bone degradation. Mediators Inflamm. 2014;2014:728619. doi: 10.1155/2014/728619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bost KL, Bento JL, Petty CC, Schrum LW, Hudson MC, Marriott I. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. J Interferon Cytokine Res. 2001;21:297–304. doi: 10.1089/107999001300177484. [DOI] [PubMed] [Google Scholar]
- 143.Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, Kobayashi Y, Takada H, Shibata K, Yamamoto M, Takeda K, Akira S, Noguchi T, Udagawa N. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. The Journal of experimental medicine. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection. J Orthop Res. 2011;29:1621–1626. doi: 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hultgren OH, Svensson L, Tarkowski A. Critical role of signaling through IL-1 receptor for development of arthritis and sepsis during Staphylococcus aureus infection. J Immunol. 2002;168:5207–5212. doi: 10.4049/jimmunol.168.10.5207. [DOI] [PubMed] [Google Scholar]
- 146.Kim J, Yang J, Park OJ, Kang SS, Kim WS, Kurokawa K, Yun CH, Kim HH, Lee BL, Han SH. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J Bone Miner Res. 2013;28:2381–2391. doi: 10.1002/jbmr.1973. [DOI] [PubMed] [Google Scholar]
- 147.Flammier S, Rasigade JP, Badiou C, Henry T, Vandenesch F, Laurent F, Trouillet-Assant S. Human Monocyte-Derived Osteoclasts Are Targeted by Staphylococcal Pore-Forming Toxins and Superantigens. PLoS One. 2016;11:e0150693. doi: 10.1371/journal.pone.0150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Claro T, Widaa A, McDonnell C, Foster TJ, O'Brien FJ, Kerrigan SW. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology. 2013;159:147–154. doi: 10.1099/mic.0.063016-0. [DOI] [PubMed] [Google Scholar]
- 149.Crémieux AC, Dumitrescu O, Lina G, Vallee C, Côté JF, Muffat-Joly M, Lilin T, Etienne J, Vandenesch F, Saleh-Mghir A. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One. 2009;4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crémieux AC, Saleh-Mghir A, Danel C, Couzon F, Dumitrescu O, Lilin T, Perronne C, Etienne J, Lina G, Vandenesch F. α-Hemolysin, not Panton-Valentine leukocidin, impacts rabbit mortality from severe sepsis with methicillin-resistant Staphylococcus aureus osteomyelitis. J Infect Dis. 2014;209:1773–1780. doi: 10.1093/infdis/jit840. [DOI] [PubMed] [Google Scholar]
- 151.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 152.Syed AK, Reed TJ, Clark KL, Boles BR, Kahlenberg JM. Staphlyococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infect Immun. 2015;83:3428–3437. doi: 10.1128/IAI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bocchini CE, Hulten KG, Mason EO, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–440. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 155.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, Yockey L, Darnell DN, Barnhart L, Daub J, Boris L, Rump AP, Anderson VL, Haney C, Kuhns DB, Rosenzweig SD, Kelly C, Zelazny A, Mason T, DeRavin SS, Kang E, Gallin JI, Malech HL, Olivier KN, Uzel G, Freeman AF, Heller T, Zerbe CS, Holland SM. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60:1176–1183. doi: 10.1093/cid/ciu1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al-Ghonaium A, Al-Rayes H, Al-Jumaah S, Al-Hajjar S, Al-Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 158.Israel L, Wang Y, Bulek K, Della Mina E, Zhang Z, Pedergnana V, Chrabieh M, Lemmens NA, Sancho-Shimizu V, Descatoire M, Lasseau T, Israelsson E, Lorenzo L, Yun L, Belkadi A, Moran A, Weisman LE, Vandenesch F, Batteux F, Weller S, Levin M, Herberg J, Abhyankar A, Prando C, Itan Y, van Wamel WJ, Picard C, Abel L, Chaussabel D, Li X, Beutler B, Arkwright PD, Casanova JL, Puel A. Human Adaptive Immunity Rescues an Inborn Error of Innate Immunity. Cell. 2017;168:789–800. doi: 10.1016/j.cell.2017.01.039. e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bonilla FA, Geha RS. 12. Primary immunodeficiency diseases. J Allergy Clin Immunol. 2003;111:S571–581. doi: 10.1067/mai.2003.86. [DOI] [PubMed] [Google Scholar]
- 160.Andrews T, Sullivan KE. Infections in patients with inherited defects in phagocytic function. Clin Microbiol Rev. 2003;16:597–621. doi: 10.1128/CMR.16.4.597-621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tsezou A, Poultsides L, Kostopoulou F, Zintzaras E, Satra M, Kitsiou-Tzeli S, Malizos KN. Influence of interleukin 1alpha (IL-1alpha), IL-4, and IL-6 polymorphisms on genetic susceptibility to chronic osteomyelitis. Clin Vaccine Immunol. 2008;15:1888–1890. doi: 10.1128/CVI.00209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Menne EN, Sonabend RY, Mason EO, Lamberth LB, Hammerman WA, Minard CG, Kaplan SL, Hulten KG. Staphylococcus aureus infections in pediatric patients with diabetes mellitus. J Infect. 2012;65:135–141. doi: 10.1016/j.jinf.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 163.Rich J, Lee JC. The pathogenesis of Staphylococcus aureus infection in the diabetic NOD mouse. Diabetes. 2005;54:2904–2910. doi: 10.2337/diabetes.54.10.2904. [DOI] [PubMed] [Google Scholar]
- 164.Bourke CD, Berkley JA, Prendergast AJ. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016 doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mihu CN, Schaub J, Kesh S, Jakubowski A, Sepkowitz K, Pamer EG, Papanicolaou GA. Risk factors for late Staphylococcus aureus bacteremia after allogeneic hematopoietic stem cell transplantation: a single-institution, nested case-controlled study. Biol Blood Marrow Transplant. 2008;14:1429–1433. doi: 10.1016/j.bbmt.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Drapeau CM, Angeletti C, Festa A, Petrosillo N. Role of previous hospitalization in clinically-significant MRSA infection among HIV-infected inpatients: results of a case-control study. BMC Infect Dis. 2007;7:36. doi: 10.1186/1471-2334-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jones KD, Berkley JA. Severe acute malnutrition and infection. Paediatr Int Child Health. 2014;34(Suppl 1):S1–S29. doi: 10.1179/2046904714Z.000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maraqa NF, Aigbivbalu L, Masnita-Iusan C, Wludyka P, Shareef Z, Bailey C, Rathore MH. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization and infection among infants at a level III neonatal intensive care unit. Am J Infect Control. 2011;39:35–41. doi: 10.1016/j.ajic.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 169.Rathore MH, Kline MW. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 1989;8:645–647. doi: 10.1097/00006454-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 170.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006;27:297–309. doi: 10.1055/s-2006-945530. [DOI] [PubMed] [Google Scholar]
- 171.Fulop T, Tessier D, Carpentier A. The metabolic syndrome. Pathol Biol (Paris) 2006;54:375–386. doi: 10.1016/j.patbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 172.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, Howlett S, Hunter K, Rainbow D, Rosa RL, Smink LJ, Todd JA, Peterson LB. Type 1 diabetes genes and pathways shared by humans and NOD mice. Journal of autoimmunity. 2005;25(Suppl):29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 173.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 174.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 175.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. Journal of the American Society of Nephrology : JASN. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 176.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Molecular oral microbiology. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 178.Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spaan AN, Schiepers A, de Haas CJ, van Hooijdonk DD, Badiou C, Contamin H, Vandenesch F, Lina G, Gerard NP, Gerard C, van Kessel KP, Henry T, van Strijp JA. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J Immunol. 2015;195:1034–1043. doi: 10.4049/jimmunol.1500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thomsen IP, Sapparapu G, James DBA, Cassat JE, Nagarsheth M, Kose N, Putnam N, Boguslawski KM, Jones LS, Wood JB, Creech CB, Torres VJ, Crowe JE., Jr Monoclonal Antibodies Against the Staphylococcus aureus Bicomponent Leukotoxin AB Isolated Following Invasive Human Infection Reveal Diverse Binding and Modes of Action. Journal of Infectious Diseases. 2017;215:1124–1131. doi: 10.1093/infdis/jix071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hendrix AS, Spoonmore TJ, Wilde AD, Putnam NE, Hammer ND, Snyder DJ, Guelcher SA, Skaar EP, Cassat JE. Repurposing the Nonsteroidal Anti-inflammatory Drug Diflunisal as an Osteoprotective, Antivirulence Therapy for Staphylococcus aureus Osteomyelitis. Antimicrobial agents and chemotherapy. 2016;60:5322–5330. doi: 10.1128/AAC.00834-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis. 2008;197:214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]