Abstract
Biomaterials hold great promise in helping the adult brain regenerate and rebuild after trauma. Peptide amphiphiles (PA) are highly versatile biomaterials, gelling and forming macromolecular structures when exposed to physiological levels of electrolytes. We are here reporting on the first ever in vivo use of self-assembling peptide amphiphile carrying a Tenascin-C signal (E2Ten-C PA) for the re-direction of endogenous neuroblasts in the rodent brain. The PA forms highly aligned nanofibers, displaying the migratory sequence of Tenascin-C glycoprotein as epitope. In this in vivo work, we have formed in situ a gel of aligned peptide amphiphile nanofibers presenting a migratory Tenascin-C signal sequence in the ventral horn of the rostral migratory stream, creating a track reaching the neocortex. Seven days post-transplant, doublecortin positive cells were observed migrating inside and alongside the injected biomaterial, reaching the cortex. We observed a 24-fold increase in number of redirected neuroblasts for the E2Ten-C PA injected animals compared to control. We also found injecting the E2Ten-C PA to cause minimal neuroinflammatory response. Analyzing GFAP+ astrocytes and Iba1+ microglia activation, the PA does not elicit a stronger neuroinflammatory response than would be expected from a small needle stab wound. Re-directing endogenous neuroblasts and increasing the number of cells reaching a site of injury using peptide amphiphiles may open up new avenues for utilizing the pool of neuroblasts and neural stem cells within the adult brain for regenerating damaged brain tissue and replacing neurons lost to injury.
Keywords: Peptide Amphiphile Nanofiber Hydrogel, Tenascin-C, Rostral Migratory Stream, Cell Migration, Self Assembly
1. INTRODUCTION
The regenerative capacity of the adult brain is insufficient. This becomes especially apparent when stroke or brain trauma occurs, even though a population of proliferating stem cells exist in the adult brain capable of generating new neurons. Neural stem/progenitor cells (NSPCs) reside in two neurogenic niches of the adult brain, the subventricular zone (SVZ) of the lateral ventricles (Alvarez-Buylla & Garcia-Verdugo, 2002) and the hippocampus (Altman & Das, 1965; Cameron, Woolley, McEwen, & Gould, 1993). In rodents, NSPC’s migrate from the wall of the lateral ventricle, through the rostral migratory stream (RMS) to the olfactory bulb where they will eventually differentiate into mature neurons. The migration of neural progenitor cells through the RMS is largely guided by a migratory scaffold consisting of blood vessels and glial cells physically enveloping the cells in the RMS (Lois, Garcia-Verdugo, & Alvarez-Buylla, 1996; Whitman, Fan, Rela, Rodriguez-Gil, & Greer, 2009) and it is further enhanced by the expression of extracellular matrix (ECM) molecules, such as laminins, fibronectin and tenascins. When an injury such as cortical stroke or stab wound occurs, a subset of these NSPCs can be seen altering their normal migratory path and instead migrate to the site of injury (Saha, Peron, Murray, Jaber, & Gaillard, 2013), a process lasting up to one year post-ischemia (Osman, Porritt, Nilsson, & Kuhn, 2011). While a majority of the NSPCs reaching the site of injury become quiescent, a subset can differentiate into the phenotype of the resident neurons (Arvidsson, Collin, Kirik, Kokaia, & Lindvall, 2002).
In order to enhance the regenerative response of the adult brain and to provide new avenues for treatment, the brain’s endogenous resources could be combined with biomaterials. We report here on the use of a biomaterial to create an artificial migratory path in vivo in order to redirect neuroblasts to new brain areas. We have used an injectable self-assembling peptide amphiphile (PA) carrying a migratory bioactive Tenascin-C derived peptide sequence as epitope (Berns et al., 2016) for the re-direction of neuroblasts from the RMS. Tenascin-C (Ten-C) is an oligomeric extracellular glycoprotein highly expressed in the SVZ (Jankovski & Sotelo, 1996) and the RMS (Thomas, Gates, & Steindler, 1996). Ten-C protein is linked to various functions, ranging from neural precursor cell migration and proliferation (Garcion, Faissner, & ffrench-Constant, 2001) to promoting spinal cord regeneration (Yu et al., 2011). Studies have shown Tenascin-C to induce various responses during neuroinflammation and CNS injury, from increased recovery in astrocyte scratch assays (Johnson, Milner, & Crocker, 2015) to promoting axonal regeneration and functional recovery of spinal cord tissue after transection (Pan et al., 2014; Yu et al., 2011; Y. Zhang, Winterbottom, Schachner, Lieberman, & Anderson, 1997).
The type of PA used in this work consists of four domains (Fig. 1B.): a long hydrophobic alkyl tail, a sequence of β-sheet forming amino acids, a sequence of charged region and a peptide epitope. The amphiphilic design enables PAs to self-assemble into nanofibers by hydrophobic collapse of alkyl tail to a central core, with the beta-sheet forming region governing the one-dimensional assembly of the PA into cylindrical nanofibers as self-assembly occurs, while displaying the signaling peptide epitopes on its surface (Cui, Webber, & Stupp, 2010). Annealing a PA solution results in the formation of domains of highly aligned nanofibers, which can be drawn into a monodomain gel when shear is applied under physiological conditions, i.e. when coming in contact with divalent cations such as Ca2+ present in the blood and extracellular fluid (S. Zhang et al., 2010). Gels formed from aligned bioactive PA nanofibers have been used to direct cell migration in vitro using embryonic dorsal root ganglia (Berns et al., 2014).
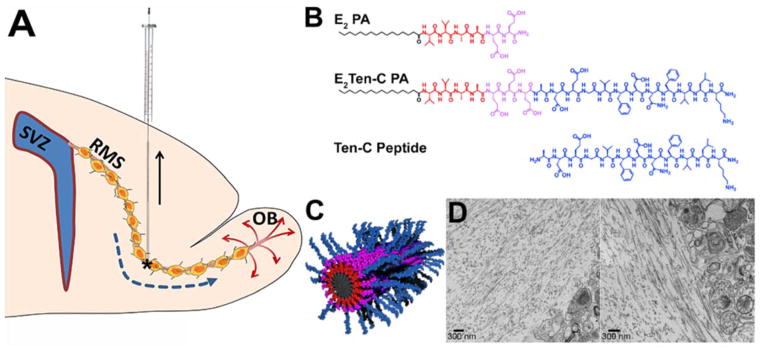
Fig 1.
(A) Schematic sagittal overview of injection to the ventral horn of the RMS, creating a track by retracting the syringe whilst injecting. Asterisk marks ventral horn of the RMS. (B) Chemical structure of the biologically inactive E2 PA (top) and bioactive E2Ten-C PA (middle) with a hydrophobic tail (black), β-sheet region (red), charged region (purple) and the Ten-C sequence epitope region (blue). The Ten-C peptide (bottom) is also used as a non-polymerizing control. (C) Schematic illustration showing nanofiber structure of co-assembled backbone E2 PA with bioactive E2Ten-C PA. (D) TEM micrographs of PA gel injected into rodent brain. On the left sides in both micrographs are PA nanofibers aligned parallel to the interface between the PA gel region and the brain tissue region (on the right in both images). Scale bar in (D) = 300μm.
Here we demonstrate the first ever in vivo use of a bioactive peptide amphiphile scaffold as an artificial matrix for redirecting migrating neuroblasts in the adult brain. By injecting PA into the adult rat brain, targeting the ventral horn of the RMS and creating a track reaching the cortex (Fig. 1A) we examined the ability of the functionalized PA to redirect neuroblasts. Additionally, we also investigated the neuroinflammatory response of astrocytes and microglia to the PA track.
2. MATERIALS AND METHODS
2.1 PA synthesis and functionalization
Peptide amphiphiles were synthesized as previously described (Goldberger, Berns, Bitton, Newcomb, & Stupp, 2011) and stored as lyophilized powder. The injectable PA solution was prepared about 12–18 hrs before surgery, by dissolving PA powder in aqueous solution of 150 mM NaCl/3 mM KCl (referred hereafter as dissolving solution) to obtain a 1 wt % solution. pH was adjusted to 7–7.4 using NaOH and HCl. Equal amounts of backbone PA (E2 PA) and backbone Ten-C conjugated PA (E2Ten-C PA) were mixed before sonication for 15 min followed by heating in water bath at 80°C for 30 minutes, and left to slowly cool down overnight (E2 PA sequence: palmitoyl-VVAAEE-NH2; E2Ten-C PA sequence: palmitoyl-VVAAEEEADEGVFDNFVLK-NH2, see Fig. 1B). E2Ten-C PA is mixed with inert E2 PA, since E2 PA enables formation of gels with aligned nanofibers (Berns et al., 2014). Before injection, the PA was further diluted in the dissolving solution to 0.25 wt % PA. A non-polymerizing, non-fibrous Ten-C peptide lacking the alkyl tail, β-sheet region, and charged region of the E2Ten-C PA was also synthesized (Ten-C peptide sequence: ADEGVFDNFVLK-NH2, see Fig. 1B). A schematic illustration of the final co-assembled nanofiber can be seen in Fig. 1C. Materials prepared from these PAs were compared at equal weight percentage to keep total PA mass equivalent between groups. The E2 PA control group provides information about the effect of the E2 PA so that the impact of adding the Ten-C PA to E2 PA (resulting in the E2Ten-C PA mixture) can be determined.
2.2 Evaluation of PA gelation and alignment in brain tissue using transmission electron microscopy
For the study of PA gelation brains from sacrificed mice were removed from the skull and placed in a petri dish containing PBS at 4 °C. A 25-gauge needle was attached to a 10 μL Hamilton syringe, which was mounted on a stereotactic apparatus. The needle was inserted 3.5 mm into the brain, perpendicular to the brain surface. As the needle was retracted at a rate of 1 mm/min, 3.5 μL of 1 wt% E2 PA solution was injected into the brain tissue. The brains were fixed in phosphate-buffered (0.1M, pH 7.4) 4% paraformaldehyde for 24 h. The brain regions of interest were dissected and transferred to modified Karnovsky’s fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4) for 24 h. Samples were post-fixed in osmium tetroxide and stained en-bloc by uranyl acetate solution. Stained samples were dehydrated by incubation in a graded series of ethanol. Following dehydration, ethanol was replaced with propylene oxide, and finally the samples were embedded in resin (Embed 812, Electron Microscopy Sciences) through intermediate exchange steps. 70 nm sections of resin-embedded brain were prepared (sectioned parallel to the direction of scaffold long-axis) using a Leica Ultracut UCT ultramicrotome. Sections were post stained with lead citrate and uranyl acetate for enhanced contrast and micrographs were obtained on Tecnai Spirit G2 microscope (FEI) operating at 120 kV.
2.3 Animals and Anesthesia
All experiments were approved by the Gothenburg Committee of the Swedish Animal Welfare Agency (application no. 101/2013 and 103/2010). Male Sprague Dawley rats (Charles River, Germany), 6–7 weeks old, were used in the study. Animals were housed in a barrier facility with ad libitum access to food and water with a 12 hr light/dark cycle. Animals were sedated either with an intraperitoneal injection of ketamine (33 μg/ml Ketanol, Pfizer) / xylazine (6.6μg/ml Rompun, Bayer Healthcare AG) cocktail at a dose of 2 ml/kg or with 5% isoflurane and maintained at 2.5% in a mixture of air and oxygen delivered via nose cone when animals were placed into a stereotaxic frame (Kopf instruments, Germany).
2.4 Surgery and Tissue Preparation
All animals received an injection of E2Ten-C PA in one hemisphere and a control injection in the opposite hemisphere containing either; E2 PA (lacking the biologically active Ten-C sequence, see Fig. 1B), or saline, for the migration studies, and vehicle (i.e. dissolving solution) for the neuroinflammatory cell response study. Timepoint to end experiments was set at seven days after surgery, allowing enough time for migratory cells to reach cortex from the RMS (see section 3.3). Animals were sedated using an overdose of pentobarbital and perfused transcardially with sterile 0.9% NaCl followed by phosphate-buffered (0.1 M [pH 7.4]) 4% paraformaldehyde (PFA). Following perfusion, brains were removed and post-fixed in 4% PFA for 24–48 hrs and prepared for cryosectioning by incubation in phosphate-buffered (0.1 M [pH 7.4]) 30% sucrose for at least 3 days. All brains were divided into left and right hemisphere (E2 PA/saline/vehicle vs. bioactive E2Ten-C PA) before cryosectioned coronally in 25 μm thick serial sections using a sliding microtome (Leica Microsystems) and stored at 4 °C in tissue cryoprotective solution (glycerol, ethylene glycol and 0.1 M PO4).
2.5 PA Injection
All surgeries were done using stereotactic instruments (Kopf Instruments and Stoelting Co). A 10 μl Hamilton syringe with 26G beveled needle tip attached to the stereotaxic frame was used for injections. Injections were done at the following coordinates from bregma: anteroposterior +2.7 mm and lateral ±1.2 mm, hitting the ventral horn of the RMS (Fig. 1A). Injections were done with a flow rate of 1 μl/min using an automated syringe pump at 6.5 mm below the skull while retracting the needle at 1 mm/min, stopping after five minutes for five minutes to minimize PA leakage from the track while polymerizing, injecting a total volume of 5 μl. Injections not reaching the neuroblast-containing RMS where omitted from the analysis for progenitor cell migration.
2.6 Histology
For immunoperoxidase staining, free floating sections were washed three times in tris-buffered saline (TBS), pre-treated with NaCl (10mM, pH 6) at 80 °C for 30 min and let to cool down to room temperature for 10 min. Sections were washed 3 times in TBS and incubated for 30 min in 0.6% H2O2, followed by incubation for 1 hour at room temperature in blocking solution (TBS with 0.1% Triton-X-100 and 3% normal donkey serum - Jackson ImmunoResearch, West Grove, PA). Doublecortin (DCX) was used as marker for migrating neuroblasts (Brown et al., 2003). Sections were incubated with goat anti-DCX antibody (Santa Cruz Biotechnology, USA) diluted 1:1500 in blocking solution at 4 °C overnight. Sections were washed 3 times in TBS, incubated with biotinylated donkey anti-goat 1:1000 (Jackson ImmunoResearch Lab, USA) in blocking solution for 1 h, followed by amplification with avidin-biotin complex (Vectastain ABC Elite, Vector Laboratories) and then visualized using a DAB/H2O2/NiCl2 (3,3′ diaminobenzidine tetrahydrochloride/hydrogen peroxide/nickel (II) chloride) in TBS solution for 5–10 minutes (0.25 mg/mL DAB, Saveen Biotech, 0.04% NiCl2). Sections were mounted on glass slides and coverslipped using NeoClear® (Merck) and NeoMount® (Merck).
For immunofluorescent staining, sections were washed three times in TBS before blocking 30 minutes. To detect astrocytes and microglia, sections were incubated overnight at 4°C with 1:500 rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (Dako, A/S, Denmark) and 1:500 dilution of goat anti- Iba1 polyclonal antibody (Abcam, Cambridge, UK), respectively. Sections were then washed three times in TBS and incubated for two hours at room temperature with secondary antibodies, Alexa Fluor 488 donkey anti-rabbit IgG (1:500, Molecular Probes, Leiden, The Netherlands) and Alexa Fluor 546 donkey anti-goat IgG (1:500, Molecular Probes, Oregon, USA) for fluorescence detection. The sections were then washed five times with TBS and mounted on glass slides and coverslipped with Prolong Gold antifade reagent with DAPI (Molecular Probes, Oregon, USA).
2.7 Quantification, image acquisition and analysis
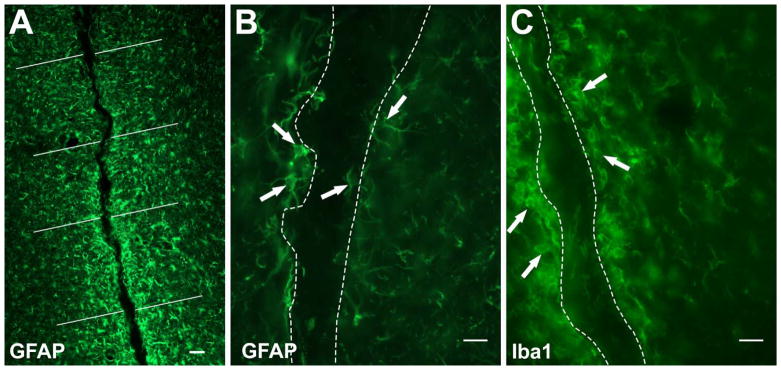
All image acquisition and quantification was done using Stereo Investigator 10 software (MicroBrightField Inc., USA) coupled to a Leica DM6000B microscope (Leica Microsystems, Germany). For quantification and analysis, all tissue sections were immunostained and analyzed. For migration studies, all coronal sections showing trace of injection were included in the analysis. All animals without detectable PA or with a track not reaching the RMS were omitted from the study. Location of the PA or needle injection was detected by changes in the tissue texture visible under the differential interference contrast filter in brightfield microscopy. The border of injection track was traced and all doublecortin positive cells quantified. DCX+ cells showing migratory morphology alongside the trace were included as “parenchymal tissue” migration. Distance migrated by doublecortin positive cells was quantified using the (X,Y) coordinates for each individual cell traced with the border of the RMS as origin. For neuroinflammatory response analysis, cell density was quantified using Stereo Investigator 10 fractionator function. Counting frame size was set at 100*100 μm with a grid size of 500*500 μm. GFAP and Iba1 positive cells were quantified up to 250 μm from the border of the lesion. For fluorescence intensity analysis, approximately eight evenly spaced 150 μm lines were drawn per coronal image from either border or midline of injection site (Fig. 3A), and fluorescent intensity for each individual underlying pixel was measured using ImageJ Version 1.49v (NIH, USA). Fluorescent intensity data was compiled across all lines and sections for each animal and averaged according to the three intervals for each animal, 1–50 μm, 51–100 μm and 101–150 μm. All settings during image acquisition were kept constant throughout the study to ensure proper intensity acquisition.
Fig 3.
(A) Fluorescent image of GFAP stained tissue illustrating 8 evenly drawn white lines for pixel intensity measurements. Representative images of quantified GFAP+ (B) and Iba1+ cells (C) parenchymal to the injection track. Dotted lines in (B) and (C) outline injection track. Scale bar in (A) = 50 μm, (B) and (C) = 25 μm.
2.8 Statistics
All statistical analysis was performed using Prism 6 software (Graphpad Inc.). One-way rank Anova (Kruskal-Wallis test) using Dunn’s multiple comparison test was used for doublecortin cell density analysis. GFAP+ and Iba1+ cell density were analyzed using Students t-test. Two-way Anova with Sidak’s multiple comparisons test was used for immunofluorescence intensity analysis. P values < 0.05 were considered statistically significant (*). All error bars represent standard error of the mean (SEM). For analysis of proportion of doublecortin positive cells in migration we used a Cochran-Armitage test for trend using the Epitools calculator (Sergeant, ESG, 2016, available through Ausvet Pty Ltd at: http://epitools.ausvet.com.au/content.php?page=trend).
3. RESULTS
3.1 Gelation and alignment of PA in vivo
To verify that the interstitial fluid in brain tissue contained a sufficiently high concentration of divalent cation to induce gelation of the PAs and that the injection procedure would result in a gel with aligned nanofibers, we tested the injection procedure in excised mouse brains. A needle was inserted dorso-ventrally from the cerebral cortical surface, and as the needle was retracted, an annealed 1 wt% solution of E2 PA was injected into the tissue. The brain was fixed and prepared for examination of the gel and tissue. TEM micrographs of ultrathin sections cut in the direction of the needle track revealed that the gel domains could be easily identified by the dense regions of PA nanofibers. We observed nanofibers aligned in the direction of the needle track throughout the gel and in higher abundance near the gel-tissue interface, likely resulting from shear stresses resulting from the injection procedure (Fig. 1D).
3.2 E2Ten-C peptide amphiphiles are able to re-direct migrating DCX positive NPSC’s
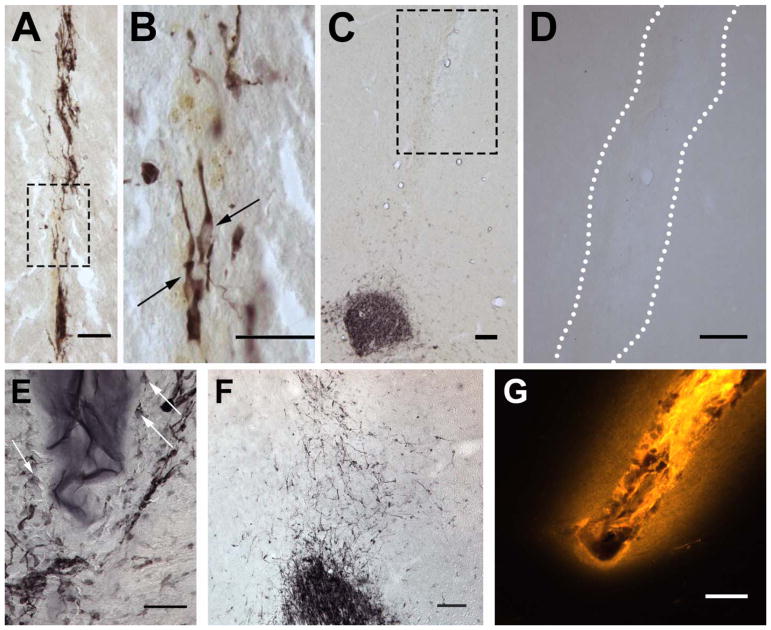
Neural progenitor cells continually migrate long distances within the adult brain through the rostral migratory stream. To investigate if the E2Ten-C PA is able to re-route migrating neural progenitor cells from the rostral migratory stream into the neocortex, biomaterial was stereotactically injected creating a migratory path from the ventral horn of the RMS into the neocortex (Fig. 1A). The site of injection was chosen since the NSPCs are already in a migratory state. In order to find the optimal stiffness of the gel for cell migration after injection, we tested different PA concentrations and studied the ability of the progenitor cells to migrate into and along the various biomaterials. Injecting the 0.25 wt% E2Ten-C PA, we observed DCX+-cells within the E2Ten-C PA track with the elongated morphology typical of migrating neuroblasts (Fig. 2A–B). In contrast, at 1 wt% E2Ten-C PA DCX+-cells can be seen bordering the biomaterial but no cells were detected inside the PA (Fig. 2E). Consequently, we did not observe any long distance migration of cells in animals receiving the 1 wt% PA. The higher concentration E2Ten-C PA also had a tendency to gel within the needle during injection clogging the needle and leading to incomplete injections. We also injected 0.5 %wt PA (data not shown), but due to higher incidences of syringe clogging we opted to continue with the lower 0.25 %wt PA concentration. During initial testing we detected little to no trace 2 weeks post-injection using the 0.25 %wt PA (data not shown), hence lower concentrations of PA were not considered due to rapid biodegradation of the PA.
Fig 2.
Detection of neuroblasts in brain tissue by doublecortin (DCX) immunohistochemistry. Neuroblast found inside the E2Ten-C PA track (A) show elongated morphology (B) typical of migrating cells. (C) No redirected cell migration was observed with E2Ten-C PA injection (white outline) when the injection track does not reach the RMS, evident by lack of DCX+ cells along the PA track. (D) When E2Ten-C PA was injected at 1 wt% concentration cells surrounded the polymer (arrows) but none infiltrated the biomaterial due to the high density. (E) When non-polymerizing Ten-C peptide was injected cells migrated out from the RMS a short distance in a random and dispersed fashion. (F) Fluorescent image showing diffusion of PA in tissue using a fluorescent TAMRA derived molecule covalently attached to the PA. Scale bars in (A–B) and (D–F) = 50 μm. Scale bar in (C) and (G) = 100 μm.
Next, we investigated if the Ten-C peptide epitope, which lacks the alkyl tail, β-sheet region, and charged region of the E2Ten-C PA, and thus does not self-assemble into nanofibers (Berns et al., 2016), can induce migration of neuroblasts in the RMS. When injecting the Ten-C peptide without the PA regions, neuroblasts displayed the tendency to move in a more random and dispersed pattern (Fig. 2F) compared to the directed migration observed in the E2Ten-C PA injected animals. We did also not observe any long-distance migration of doublecortin positive neuroblasts as all cells stayed in close proximity to the RMS when using the Ten-C peptide lacking the alkyl tail, β-sheet region, and charged region.
To investigate the in vivo gelling and diffusion of the PA to the surrounding parenchymal tissue, a polymerizing E2 PA with a covalently attached fluorescent TAMRA-derived molecule (E2TAMRA PA) was injected at 0.25 wt% and analyzed 7 days post-injection. As we observed a gradually declining fluorescent signal outside the needle track (Fig. 2G), we conclude that injected E2TAMRA creates a gradient of biomaterial from the border of the injection track into the tissue, that is still detectable one week after injection. We hypothesize this being either an effect of the pressure from the injection pushing the PA into the surrounding tissue or diffusion of the PA before gelling occurs.
3.3 Cell Migration in the PA matrix
Next we investigated to what extent the E2Ten-C PA can re-direct neuroblasts from the RMS. One week post-injection was chosen as the analysis timepoint since during this time interval cells should have migrated into the cortex assuming normal migratory speed in the RMS of about 5–7 mm per week (Lois & Alvarez-Buylla, 1994; Mejia-Gervacio, Murray, & Lledo, 2011). Cell migration was evaluated by analyzing DCX+-cell density along the injection tracks. Cell density was measured by outlining and quantifying the track area corresponding to the PA injections and quantifying the number of DCX+-cells associated with the needle track. A clear migratory effect was observed when the peptide amphiphiles were injected. Inside the track, substantially more cells were present in the E2Ten-C PA group compared to the saline injected control group (Fig. 4A). Our results indicate a 24-fold increase in total number of neuroblasts being re-directed from the RMS in the E2Ten-C PA group compared to the saline injected control group (Fig. 4C, p=0.0057). While we also observed a large number of cells migrating in tracks containing the E2 PA but lacking the bioactive Ten-C epitope, the increase in the density of migrating cells compared to saline control was not statistically significant (Fig. 4C, p=0.0746). Furthermore, neuroblasts were also observed migrating adjacent (≤250 μm) to the track for both the E2 PA and the E2Ten-C PA (Fig. 4B), resulting from the partial diffusion of the PA material into the surrounding tissue (Fig. 2G). E2Ten-C PA injected animals also exhibited a substantially larger migration of DCX+ cells in the parenchyma surrounding the injection track versus the saline control (Fig. 4B, p=0.0051). Nevertheless, the density of migrating cells was twice as high inside the E2Ten-C PA track compared to the adjacent parenchyma, with the E2 PA showing the same trend.
Fig 4.
Quantification of DCX*-cells re-directed from the RMS using saline (n=4 animals), E2 PA (n=8 animals) and E2Ten-C PA (n=8 animals). Substantial migration was seen for the E2Ten-C PA both inside the track (A) and in the surrounding parenchyma (B). 24-fold increase in total number (C) of re-directed doublecortin positive cells was seen for the E2Ten-C PA injected animals compared to saline injected animals. All data presented as mean ± SEM. **P < 0.01. (D) Proportion of cells in migration as a function of distance migrated. All cells from 8 animals per group were combined in distribution curves sorted according to their distance from the RMS.
3.4 More neural progenitor cells migrate longer distances using E2Ten-C PA gel
A significant increase in neuroblast cell density within and adjacent to the PA track indicates a migratory response for the E2Ten-C PA. To further characterize the migratory effect, we compared the bioactive and epitope free PAs in terms of distance traveled within the PA scaffold (Fig. 4D). When the percentage of cells in migration is plotted against distance it becomes apparent that a larger amount of cells migrated further in E2Ten-C PA compared to the control E2 PA. While a similar proportion of cells in both groups (about 40%) migrated less than 700 μm, 14% of the cells in E2Ten-C PA went beyond a migration distance of 2000 μm, while only less than 2% were able to migrate this far in the control E2 PA.
For a statistical evaluation of the longer distance migrated by DCX+ neuroblasts in the E2Ten-C PA compared to the control E2 PA, we performed a Cochran-Armitage test for trend (Armitage, 1955; Cochran, 1954) using six consecutive migration intervals of 500 μm from the RMS to the neocortex. We detected a significant Pearson’s Chi-square of 107.8, df=5, p<0.001, a Chi-square for slope (linear trend)=5.61, df=1, p=0.0179 and a Chi-square for non-linearity=102.1, df=4, p<0.001. This indicates a highly significant non-linear trend of more cells migrating further in E2Ten-C PA compared to the control E2 PA.
3.5 Neuroinflammatory response of GFAP and Iba1 positive cells to E2Ten-C PA gel
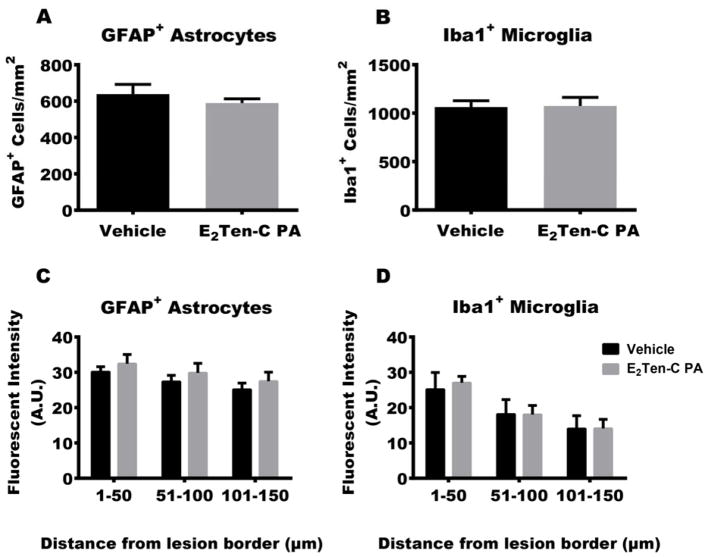
When foreign material is introduced in vivo, host immune cells are activated and respond to the lesion and the foreign material by accumulating around it, forming scar tissue. In the brain this scar consists mainly of astrocytes, with microglia being the second most prominent cell type (Buffo, Rolando, & Ceruti, 2010; Ridet, Malhotra, Privat, & Gage, 1997). As we are injecting a foreign material into the brain, we wanted to investigate the neuroinflammatory cell response to E2Ten-C PA gel, compared to what would be expected from a needle stab wound, where no additional space is occupied post-injection. We therefore determined the neuroinflammatory response of the resident astrocytes and microglia to the E2Ten-C PA implant using immunofluorescence staining of GFAP and Iba1 as respective markers for these cell types (Fig. 3B–C). Here we analyzed E2Ten-C PA injection compared to vehicle injections (sterile 150 mM NaCl, 3 mM KCl salt solution). Gliosis surrounding the injections was evaluated by quantifying reactive astrocytes and microglia using GFAP and Iba1 respectively. We quantified GFAP and Iba1 positive cells up to 250 μm from the border of the injection track. Cell density analysis showed no increase in the number of astrocytes and microglia observed in E2Ten-C PA injections versus the vehicle control (Fig. 5A–B).
Fig 5.
Glial response to PA injection. Cell density was quantified in the parenchymal tissue up to 250 μm from the border of the track. E2Ten-C PA injection had no effect on astrogliosis (A) determined by GFAP+ cell counts or microgliosis (B) determined by Iba1+ cell counts compared to the vehicle control (n=6 animals per group). Fluorescent intensity analysis of (C) GFAP+ and (D) Iba1+ cells up to 150 μm bordering the lesion. No difference observed in upregulation of GFAP (C) or Iba1 (D) immunofluorescence in E2Ten-C PA compared to vehicle group (n=6 animals per group). All data presented as mean ± SEM. P-value < 0.05 considered significant.
Since expression of GFAP and Iba1 are upregulated during reactive gliosis, fluorescent intensity of the marker proteins GFAP and Iba1 was determined for the E2Ten-C PA vs vehicle groups. The fluorescent intensity of individual pixels at evenly spaced intervals up to 150 μm around the track was measured (see Materials and Methods). As the neuroinflammatory response of the astrocytes and the microglia decline gradually extending out from the lesion, fluorescent intensity measurements were divided into three intervals to get a proper estimate of the neuroinflammatory response. Measurement of fluorescent signal intensities for GFAP+ astrocytes did not reveal any difference for E2Ten-C PA across the three intervals compared to vehicle control (Fig. 5C). In the Iba1+-microglia intensities analysis we also did not observe any difference (Fig. 5D), thus indicating that the E2Ten-C PA itself does not aggravate astrogliosis or microgliosis any further than the initial needle track lesion with vehicle injection.
4. Discussion
To our knowledge, this is the first study reporting the use of self-assembling peptide amphiphiles in vivo for the re-direction of endogenous neuroblasts in the adult brain. We created an artificial migratory path in vivo using peptide amphiphile nanofibers that display on their surfaces a biologically active Tenascin-C sequence as epitope. DCX+ neuroblasts were successfully re-directed from the ventral part of the rostral migratory stream into the neocortex, showing that self-assembling peptide amphiphiles can indeed act as a biocompatible migratory scaffold.
Significant neuroblast migration was observed in the E2Ten-C PA groups while barely any migration was detected in the saline group (Fig. 4A–C), with about half of the saline injections showing no migratory cells at all within the cannula track or in the parenchyma. Interestingly, we detected DCX+ neuroblasts within the PA track as well as near the track within the parenchyma even at significant distances from the RMS. This indicates that cells either migrated within the PA and exited the material at a distance, or that the cells migrated alongside the PA track within the parenchyma. Although we cannot distinguish between the two alternatives at this time, it is crucial that cells can be found outside the material in the target tissue, as it shows the migrating neuroblasts not being restricted to only inside the PA scaffold, allowing for cell-tissue integration. The fact that the PA forms a gradient into the tissue (Fig. 2G) rather than a sharp border, may allow some form of directed migratory behavior alongside the track.
The natural migration of neuroblasts within the RMS to the olfactory bulb is highly dependent on the migratory scaffold provided by the surrounding glial tubing and blood vessels (Thored et al., 2007). Although statistically not significant, there seems to be a trend of the E2 PA group showing clear potential in being able to stimulate and re-direct the migrating DCX+ neuroblasts from the RMS, both inside the track and the parenchyma (Fig. 4A–C). But even though we observe migrating cells in both PA groups, only the E2Ten-C PA significantly increased the number of migrating cells compared to saline controls. This is in agreement with in vitro data where mixing the E2Ten-C PA with the E2 PA increased migration by 41% (Berns et al., 2016). To investigate if a non-self-assembling Ten-C peptide lacking the PA structure, i.e. the alkyl tail, β-sheet region, and charged region of the E2Ten-C PA (Berns et al., 2016), (Fig. 1B) could also induce neuroblast migration in our in vivo model, we injected the Ten-C peptide lacking the PA structure in our in vivo model. We observed that neuroblasts did in fact move a short distance, although in a more random and dispersed pattern in comparison to the E2Ten-C PA injected animals where the polymerized PA provided supramolecular structural support along the injection track. By removing the polymerization capability of the PA, the cells appear to lose directional cues, which in the E2 PA and E2Ten-C PA injected animals were provided by the internal alignment of the nanofibers (Fig. 1D) (S. Zhang et al., 2010). And hence no long distance migration was observed in the Ten-C peptide injected animals. Since E2Ten-C PA was superior to E2 PA, we can argue that the combination of the PA scaffold support together with the biological Ten-C signal sequence is responsible for the extensive long-distance migration observed in the E2Ten-C PA group.
Cell behavior is highly regulated by extracellular matrix components and studies have shown that neurite outgrowth and axon elongation from NSPCs is promoted by specific domains on the Ten-C glycoprotein (Andrews et al., 2009; Gotz et al., 1996; Mercado et al., 2004). Neuroblast migration has striking similarities to neurite outgrowth, where the future axon of a developing neuron extends in order to find new synaptic connections. The same molecular machinery is used during migration to make contact to and extend plasma membrane along the underlying substrate and to contract, the difference between neurite outgrowth and cell migration is that the cell body and nucleus are moved in the latter case (da Silva & Dotti, 2002; Gupton & Gertler, 2007). Several studies have also shown correlation between progenitor cell migration and neurite outgrowth under different conditions (Berns et al., 2016; Berns et al., 2014; Husmann, Faissner, & Schachner, 1992; Stewart, Anderson, Kobayashi, & Young, 2008) with the two processes also sharing some molecular cues for migration and neurite projection, such as tenascins, slits and netrin proteins (Berns et al., 2016; Berns et al., 2014; Husmann et al., 1992; Stewart et al., 2008). The role of Ten-C in neurite outgrowth is context driven, as it can act both as an inhibitor and promoter. The VFDNFVLK peptide sequence used in our E2Ten-C PA has been shown to increase neurite length in vitro (Meiners, Nur-e-Kamal, & Mercado, 2001) by interacting with the α7β1 integrin (Mercado et al., 2004) which is in agreement with recent in vitro observation that E2Ten-C PA promotes neurite outgrowth and cell migration in the neurosphere cell culture model (Berns et al., 2016).
In the non-lesioned adult rodent brain, neuroblasts are primarily restricted to the RMS and the SVZ, not found in the cortex of the healthy brain. Insults to the brain can induce ectopic migration of DCX+ neuroblast from the RMS and SVZ to the site of injury (Arvidsson et al., 2002; Goings, Sahni, & Szele, 2004; Osman et al., 2011; Parent, Vexler, Gong, Derugin, & Ferriero, 2002; Ramaswamy, Goings, Soderstrom, Szele, & Kozlowski, 2005; Thored et al., 2007). We therefore considered the observed migration a possible reaction to the lesion caused by the needle track when injecting PA. As no difference in gliosis was not observed for the E2Ten-C PA group compared to the vehicle control group, we can safely assume that the increase in neuroblast migration was in fact an effect of the injected E2Ten-C PA biomaterial and not a neuroinflammatory response to a lesion caused by the injections. This is further strengthened by the fact that no migrating DCX+ cells were observed at the cortex in the saline injected animals (Fig. 2C–D).
Ten-C is upregulated in activated astrocytes, macrophages and fibroblasts at sites of neuroinflammation both in the spinal cord (Y. Zhang et al., 1997) and the tissue surrounding brain lesions (Fok-Seang et al., 1995; Laywell et al., 1992; Smith & Hale, 1997). Although Ten-C has been shown having pro-inflammatory properties (Machino-Ohtsuka et al., 2014; Midwood et al., 2009), our results do not indicate that the peptide signal sequence used in our E2Ten-C PA causes a neuroinflammatory response. To investigate if the E2Ten-C PA biomaterial caused a stronger neuroinflammatory response than the stab wound lesion from injecting the PA, vehicle was injected as control. Opting to inject vehicle instead of the inert E2 PA as control allows us to not only see any eventual upregulation in gliosis caused by the Ten-C sequence, but also the response to the PA taking up space. The injected biomaterial did not aggravate the response from the surrounding tissue more than would be expected from an initial needle track injury. We did not observe a higher number of GFAP+ astrocytes, nor an upregulation in signal strength for GFAP. Also, microglia, the resident immune cells of the brain, did not show any increase in activation in terms of cell density or Iba1+ immunofluorescence intensity for the E2Ten-C PA injected animals compared to the vehicle injected controls. This could in fact be explained by the ambiguous nature of the Ten-C molecule. Elucidating the role of naturally occurring tenascin-C is quite difficult due to its multimodular structure, enabling the existence of various isoforms of tenascin-C with a large range of function. The human tenascin-C can result in potentially 511 different isoforms. This complex nature of Ten-C allows the protein to have seemingly contradictory functions. While we in our study have used a specific Ten-C coding sequence known to have an effect on cell migration, nothing has been published on its inflammatory role, with the majority of studies published on Ten-C not distinguishing between the various occurring isoforms. Nevertheless, the E2Ten-C PA signal peptide sequence used in our study does not appear to elicit a stronger neuroinflammatory response. Further research will have to establish the fate of the neuroblasts migrating inside the E2Ten-C PA. While studies have reported that immature progenitors migrating after cortical lesion still have the capacity to form mature neurons (Saha et al., 2013), spontaneous neuronal differentiation of progenitor cells within the neocortex is rather limited (Arvidsson et al., 2002; Osman et al., 2011). Further studies are needed to investigate the fate of the re-directed neuroblasts as differentiation and survival of new neurons may require additional signals.
Over the past two decades, various strategies have been described using biomaterials for brain regeneration after injury or disease. Current ideas range from drug-releasing biomaterials (Nof & Shea, 2002; Song et al., 2012), scaffolds for cell transplantation (Campioni, Nobrega, & Sefton, 1998; Sarnowska et al., 2013), or strategies to further stimulate the brain’s endogenous regenerative potential (Cook et al., 2017; Ma et al., 2007). An ideal biomaterial should be injectable to minimize the invasiveness and injury to the brain tissue, it needs to be biocompatible and cause little to no neuroimmune response. It should deliver the appropriate bioactive molecules capable of creating the proper environmental signals needed for the required cellular responses. The biomaterial should also be biodegradable, removing the need for further interventions. The self-assembling peptide amphiphiles used here fulfill the above-mentioned criteria. Being liquid before gelation allows the possibility if easily injecting the biomaterial into brain tissue injection, forming an artificial extracellular matrix upon contact with physiological electrolytes, thus making the procedure minimally invasive. As shown here, our E2Ten-C PA does not aggravate the tissue any further than would be expected from a needle track inserted during surgery. Furthermore, the possibility of further tuning the bioactivity through gel and epitope density on the nanofibers, potential release of drugs from their hydrophobic cores (Soukasene et al., 2011), and biodegradable nature, makes these biomaterials highly versatile as artificial bioactive matrices in brain repair.
5. Conclusions
In this work, we have demonstrated that peptide amphiphiles with a conjugated Ten-C peptide sequence are able to redirect migrating cells from the RMS, evident by neuroblasts reaching the cortex instead of the olfactory bulb. We further show that the biomaterial itself does not seem to elicit a neuroinflammatory response, as evident by the astrocyte and microglia response to the E2Ten-C PA compared to control without the PA. Further in vivo experiments should be carried out to optimize the PA to increase cell migration even further. Also, other PA conjugates need to be investigated to find the optimum composition for re-directing neuroblasts.
Acknowledgments
This project was supported by Swedish Medical Research Council (Vetenskapsrådet) grant K2015-63X-20117-10-4, the Swedish Federal Government under the LUA/ALF agreement ALFGBG-450961, Swedish Brain Foundation (Hjärnfonden), Stroke-Riksförbundet, National Institutes of Health/National Institute of Biomedical Imaging And Bioengineering Award Number 5R01EB003806-07 and by the Center for Regenerative Nanomedicine at the Simpson Querrey Institute for BioNanotechnology at Northwestern. Peptide synthesis was performed in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Materiel Command, the Department of Energy, the Frederick S. Upton Foundation and Northwestern University provided funding to develop this facility.
References
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207(5000):953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, … Fawcett JW. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci. 2009;29(17):5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11(3):375–386. doi: 10.2307/3001775. [DOI] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Berns EJ, Alvarez Z, Goldberger JE, Boekhoven J, Kessler JA, Kuhn HG, Stupp SI. A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells. Acta Biomater. 2016;37:50–58. doi: 10.1016/j.actbio.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, … Stupp SI. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials. 2014;35(1):185–195. doi: 10.1016/j.biomaterials.2013.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010;79(2):77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56(2):337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Campioni EG, Nobrega JN, Sefton MV. HEMA/MMMA microcapsule implants in hemiparkinsonian rat brain: biocompatibility assessment using [3H]PK11195 as a marker for gliosis. Biomaterials. 1998;19(7–9):829–837. doi: 10.1016/S0142-9612(97)00241-X. [DOI] [PubMed] [Google Scholar]
- Cochran WG. Some Methods for Strengthening the Common X2 Tests. Biometrics. 1954;10(4):417–451. doi: 10.2307/3001616. [DOI] [Google Scholar]
- Cook DJ, Nguyen C, Chun HN, Llorente IL, Chiu AS, Machnicki M, … Carmichael ST. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37(3):1030–1045. doi: 10.1177/0271678X16649964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3(9):694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Fok-Seang J, Smith-Thomas LC, Meiners S, Muir E, Du JS, … Housden E, et al. An analysis of astrocytic cell lines with different abilities to promote axon growth. Brain Res. 1995;689(2):207–223. doi: 10.1016/0006-8993(95)00575-B. [DOI] [PubMed] [Google Scholar]
- Garcion E, Faissner A, ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development. 2001;128(13):2485–2496. doi: 10.1242/dev.128.13.2485. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996(2):213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Goldberger JE, Berns EJ, Bitton R, Newcomb CJ, Stupp SI. Electrostatic control of bioactivity. Angew Chem Int Ed Engl. 2011;50(28):6292–6295. doi: 10.1002/anie.201100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz B, Scholze A, Clement A, Joester A, Schutte K, Wigger F, … Faissner A. Tenascin-C contains distinct adhesive, anti-adhesive, and neurite outgrowth promoting sites for neurons. J Cell Biol. 1996;132(4):681–699. doi: 10.1083/jcb.132.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007(400):re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Husmann K, Faissner A, Schachner M. Tenascin promotes cerebellar granule cell migration and neurite outgrowth by different domains in the fibronectin type III repeats. J Cell Biol. 1992;116(6):1475–1486. doi: 10.1083/jcb.116.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371(3):376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Milner R, Crocker SJ. Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli. Neurosci Lett. 2015;600:104–109. doi: 10.1016/j.neulet.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Dorries U, Bartsch U, Faissner A, Schachner M, Steindler DA. Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc Natl Acad Sci U S A. 1992;89(7):2634–2638. doi: 10.1073/pnas.89.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ma J, Tian WM, Hou SP, Xu QY, Spector M, Cui FZ. An experimental test of stroke recovery by implanting a hyaluronic acid hydrogel carrying a Nogo receptor antibody in a rat model. Biomed Mater. 2007;2(4):233–240. doi: 10.1088/1748-6041/2/4/005. [DOI] [PubMed] [Google Scholar]
- Machino-Ohtsuka T, Tajiri K, Kimura T, Sakai S, Sato A, Yoshida T, … Imanaka-Yoshida K. Tenascin-C aggravates autoimmune myocarditis via dendritic cell activation and Th17 cell differentiation. J Am Heart Assoc. 2014;3(6):e001052. doi: 10.1161/JAHA.114.001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S, Nur-e-Kamal MS, Mercado ML. Identification of a neurite outgrowth-promoting motif within the alternatively spliced region of human tenascin-C. J Neurosci. 2001;21(18):7215–7225. doi: 10.1523/JNEUROSCI.21-18-07215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Gervacio S, Murray K, Lledo PM. NKCC1 controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev. 2011;6:4. doi: 10.1186/1749-8104-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado ML, Nur-e-Kamal A, Liu HY, Gross SR, Movahed R, Meiners S. Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal alpha7beta1 integrin. J Neurosci. 2004;24(1):238–247. doi: 10.1523/JNEUROSCI.4519-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, … Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- Nof M, Shea LD. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res. 2002;59(2):349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- Osman AM, Porritt MJ, Nilsson M, Kuhn HG. Long-term stimulation of neural progenitor cell migration after cortical ischemia in mice. Stroke. 2011;42(12):3559–3565. doi: 10.1161/STROKEAHA.111.627802. [DOI] [PubMed] [Google Scholar]
- Pan L, North HA, Sahni V, Jeong SJ, McGuire TL, Berns EJ, … Kessler JA. beta1-Integrin and integrin linked kinase regulate astrocytic differentiation of neural stem cells. PLoS One. 2014;9(8):e104335. doi: 10.1371/journal.pone.0104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053(1–2):38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Saha B, Peron S, Murray K, Jaber M, Gaillard A. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013;11(3):965–977. doi: 10.1016/j.scr.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Sarnowska A, Jablonska A, Jurga M, Dainiak M, Strojek L, Drela K, … Domanska-Janik K. Encapsulation of mesenchymal stem cells by bioscaffolds protects cell survival and attenuates neuroinflammatory reaction in injured brain tissue after transplantation. Cell Transplant. 2013;22(Suppl 1):S67–82. doi: 10.3727/096368913X672172. [DOI] [PubMed] [Google Scholar]
- Smith GM, Hale JH. Macrophage/Microglia regulation of astrocytic tenascin: synergistic action of transforming growth factor-beta and basic fibroblast growth factor. J Neurosci. 1997;17(24):9624–9633. doi: 10.1523/JNEUROSCI.17-24-09624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Song J, Zhang S, Anderson MA, Ao Y, Yang CY, … Sofroniew MV. Sustained local delivery of bioactive nerve growth factor in the central nervous system via tunable diblock copolypeptide hydrogel depots. Biomaterials. 2012;33(35):9105–9116. doi: 10.1016/j.biomaterials.2012.08.060. [DOI] [PubMed] [Google Scholar]
- Soukasene S, Toft DJ, Moyer TJ, Lu H, Lee HK, Standley SM, … Stupp SI. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano. 2011;5(11):9113–9121. doi: 10.1021/nn203343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Anderson RB, Kobayashi K, Young HM. Effects of NGF, NT-3 and GDNF family members on neurite outgrowth and migration from pelvic ganglia from embryonic and newborn mice. BMC Dev Biol. 2008;8:73. doi: 10.1186/1471-213X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LB, Gates MA, Steindler DA. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17(1):1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516(2):94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Cristofanilli M, Valiveti A, Ma L, Yoo M, Morellini F, Schachner M. The extracellular matrix glycoprotein tenascin-C promotes locomotor recovery after spinal cord injury in adult zebrafish. Neuroscience. 2011;183:238–250. doi: 10.1016/j.neuroscience.2011.03.043. [DOI] [PubMed] [Google Scholar]
- Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, … Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat Mater. 2010;9(7):594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Winterbottom JK, Schachner M, Lieberman AR, Anderson PN. Tenascin-C expression and axonal sprouting following injury to the spinal dorsal columns in the adult rat. J Neurosci Res. 1997;49(4):433–450. doi: 10.1002/(SICI)1097-4547(19970815)49:4<433::AID-JNR5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]