Abstract
Mammalian spermatogenesis is a process whereby male germ-line stem cells (spermatogonial stem cells) divide and differentiate into sperm. Although a great deal of progress has been made in the isolation and characterization of spermatogonial stem cells (SSCs) in rodents, little is known about human SSCs. We have recently isolated human G protein-coupled receptor 125 (GPR125)-positive spermatogonia and GDNF family receptor alpha 1 (GFRA1)-positive spermatogonia using a 2-step enzymatic digestion and magnetic-activated cell sorting (MACS) from adult human testes. Cell purities of isolated human GPR125- and GFRA1-positive spermatogonia after MACS are greater than 95%, and cell viability is over 96%. The isolated GPR125- and GFRA1-positive spermatogonia coexpress GPR125, integrin, alpha 6 (ITGA6), THY1 (also known as CD90), GFRA1, and ubiquitin carboxyl-terminal esterase L1 (UCHL1), markers for rodent or pig SSCs/progenitors, suggesting that GPR125- and GFRA1-positive spermatogonia are phenotypically the SSCs in human testis. Human GPR125-positive spermatogonia can be cultured for 2 weeks with a remarkable increase in cell number. Immunocytochemistry further reveals that GPR125-positive spermatogonia can be maintained in an undifferentiated state in vitro. Collectively, the methods using enzymatic digestion and MACS can efficiently isolate and purify SSCs from adult human testis with consistent and high quality. The ability of isolating and characterizing human SSCs could provide a population of stem cells with high purity for mechanistic studies on human SSC self-renewal and differentiation as well as potential applications of human SSCs in regenerative medicine.
Keywords: Human male germ-line stem cells, Isolation, Enzymatic digestion, Magnetic-activated cell sorting, GPR125, GFRA1, Characterization
1. Introduction
Spermatogenesis is a process that involves the proliferation and differentiation of male germ-line stem cells (spermatogonial stem cells) into sperm in mammals. Studies on spermatogonial stem cells (SSCs) are of particular significance in view of their unique characteristics: (1) SSCs are the stem cells that transmit genetic information from one generation to subsequent generations (1); (2) SSCs may soon be differentiated into sperm in vitro to help infertility patients via the In Vitro Fertilization and Embryo Transfer (IVF-ET). Around 15% of couples are infertile around the world, and half can be attributed to male infertility. Clinicians often take a testicular biopsy and try to isolate cells that can be used for Intra Cytoplasmic Sperm Injection (ICSI). In vitro germ cell differentiation from SSCs has the potential to help infertile men father children. (3) There is the potential use of SSC transplantation (2, 3) to restore fertility in cancer patients after chemotherapy or irradiation therapy as reviewed in refs. 3, 4. (4) SSCs could be used as a target for a male contraceptive. It is possible to specifically target the SSCs with small molecules (e.g., microRNA inhibitors) to selectively interfere with SSC differentiation, thus leading to a novel male contraceptive. (5) SSCs are an excellent model to elucidate the mechanisms controlling stem cell renewal versus differentiation (5), since SSCs can self-renew throughout their lifetime and they can differentiate into spermatocytes, spermatids, and eventually sperm. Lastly and most significantly, numerous studies have recently demonstrated that mouse and human SSCs can acquire pluripotency to become embryonic stem (ES)-like cells that can differentiate into all cell lineages of the three germ cell layers (6–14). This provides new and promising therapeutic prospects for human SSCs to generate various mature cells for regenerative medicine and treatment of human diseases without ethical issues associated with human ES cells (15, 16).
There is a great deal of information available on the biochemical characterization and isolation of SSCs in rodents and other species. We and others have demonstrated that the GDNF family receptor alpha 1 (GFRA1) is a surface marker for SSCs and progenitor cells in the mouse testis (17–20), while others also showed integrin alpha 6 (ITGA6) and THY1 (also known as CD90) to be surface markers for mouse SSCs and progenitor cells (21, 22). Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) is regarded as a marker for porcine, bovine, and nonhuman primate spermatogonia (23). Of note, we have demonstrated, using immunohistochemistry, that UCHL1 is also expressed in human spermatogonia (24). Recently, G protein-coupled receptor 125 (GPR125) has been shown to be a marker for mouse SSCs and their progeny (8). In addition, SSCs can be separated efficiently from rodent testis and cultured for short and long periods (18, 25, 26), which makes it feasible to examine molecular mechanisms that regulate fate decisions of rodent SSCs.
In the human testis, Clermont first distinguished Adark and Apale spermatogonia and speculated that Adark spermatogonia were the reserve stem cells and Apale spermatogonia were the renewing stem cells (27–30). However, information on the isolation and characterization of human SSCs is limited (31). One major reason for little progress on human SSCs has been the difficulty in gaining access to sufficient quantities of normal human testis for research purposes. We have identified a novel source of human testicular material, namely, from recently (~1–2 h from death) deceased organ donors. Recently, we revealed that GPR125 and GFRA1 are expressed in a subset of human spermatogonia as shown by immunohistochemistry (24). Magnetic-activated cell sorting (MACS) technology has been used to effectively separate SSCs from mouse testis (32, 33). We were the first to report the isolation of GPR125-positive spermatogonia using a 2-step enzymatic digestion and MACS from adult human testes (24). In the sections below, we provide detailed descriptions of the procedures to effectively isolate and characterize human GPR125- and GFRA1-positive spermatogonia: (1) the 2-step enzymatic digestion (Fig. 1a, b), (2) MACS (Fig. 1c), and (3) immunocytochemical analysis of the freshly isolated GPR125- and GFRA1-positive spermatogonia (Figs. 1d–p and 2) as well as GFRA1-negative male germ cells (Fig. 3). Using these approaches, it is feasible to obtain human SSCs with the highest purity and viability for further studies on molecular mechanisms regulating human SSC self-renewal and differentiation as well as for potentially important implications of SSCs in regenerative medicine.
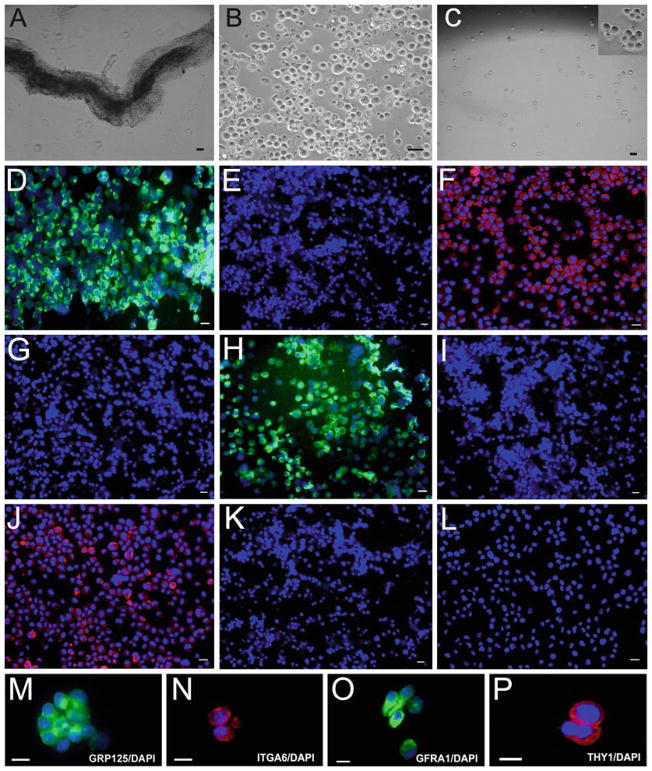
Fig. 1.
Isolation and phenotypic characterization of human GPR125-positive spermatogonia. (a) Seminiferous tubules are isolated from human testes by mechanical dissociation and the first enzymatic digestion. (b) Germ cells are obtained by a second enzymatic digestion and differential plating. (c) GPR125-positive spermatogonia are obtained by MACS with an antibody to GPR125. The photo insert in c is a high magnification view showing GPR125-positive spermatogonia. Immunocytochemistry shows the expression of GPR125 (d), ITGA6 (f), GFRA1 (h), and THY1 (j), in freshly isolated GPR125-positive spermatogonia. (m–p) High magnification image shows that freshly isolated GPR125-positive spermatogonia express GPR125 (m), ITGA6 (N), GFRA1 (o), and THY1 (p). No staining is observed in GPR125-negative male germ cells using antibody to GPR125 (e), ITGA6 (g), GFRA1 (i), or THY1 (k). (l) Replacement of primary antibody with PBS in the isolated GPR125-positive spermatogonia serves as a negative control. Scale bars in (a, c, d, f, h, j, l–o), and P = 10 μm; bars in (b, e, g, i), and K = 20 μm (Adapted from ref. 24. ©Society for the Study of Reproduction).
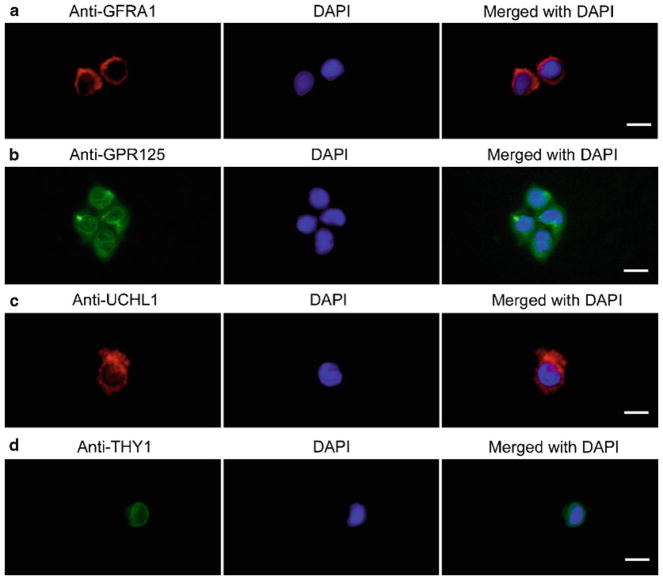
Fig. 2.
Phenotypic characterization of the freshly isolated GFRA1-positive spermatogonia from adult human testis. After MACS, immunocytochemical analysis showed the expression of GFRA1 (a), GPR125 (b), UCHL1 (c), and THY1 (d), in isolated GFRA1-positive spermatogonia. DAPI was used to stain cell nuclei. Scale bars in a–d = 10 μm.
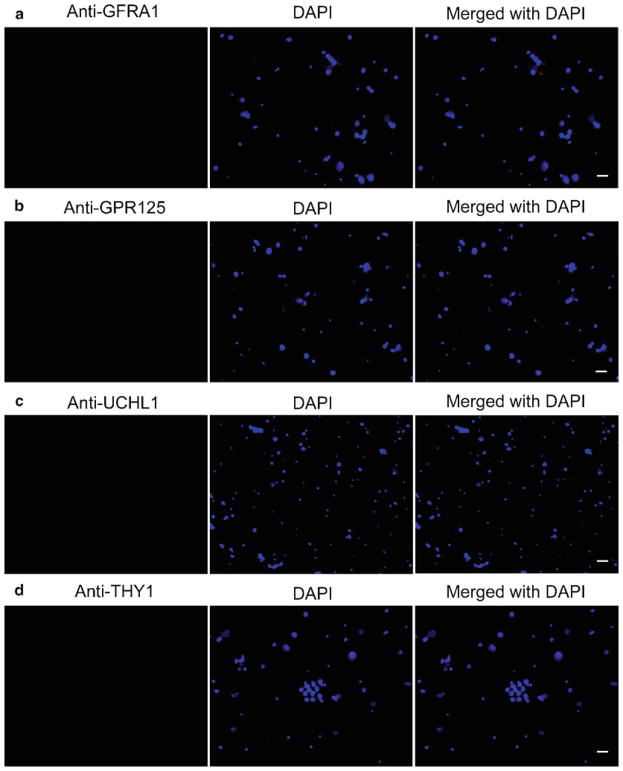
Fig. 3.
Phenotypic characterization of the freshly isolated GFRA1-negative male germ cells from adult human testis. After MACS, immunocytochemical analysis showed the expression of GFRA1 (a), GPR125 (b), UCHL1 (c), and THY1 (d), in the freshly isolated GFRA1-negative male germ cells. DAPI was used to stain cell nuclei. Scale bars in a–d = 10 μm.
2. Materials
2.1. Procurement of Testes from Organ Donors
Testes can be obtained from organ donors to the Washington Regional Transplant Consortium (Annandale, VA).
Heparin.
Viaspan™.
Dulbecco’s modified Eagle’s medium (DMEM) containing 1×antibiotic.
Freezing solution: 10% DMSO, 80% fetal bovine serum (FBS), and 10% DMEM.
2.2. Enzymatic Digestion of Human Testis Tissues
Enzymatic digestion solution I: 15 ml DMEM/F12 with 2 mg/ml collagenase IV or collagenase XI and 1 μg/μl DNase I.
Enzymatic digestion solution II: 15 ml DMEM/F12 with 4 mg/ml collagenase IV or collagenase XI, 2.5 mg/ml hyaluronidase, 2 mg/ml trypsin, and 1 μg/μl DNase I.
DMEM/F12 medium supplemented with 10% FBS.
NU serum.
Sterile acrodisc syringe filter (5 μm).
2.3. Differential Plating to Obtain Human Male Germ Cells
Culture medium: DMEM/F12 supplemented with 10% FBS; 0.1% gelatin.
2.4. Isolation of Human GPR125- and GFRA1-Positive Spermatogonia by MACS
DMEM supplemented with 10% NU serum and 1 μg/μl DNase I.
Rabbit polyclonal antibody to GPR125 (Abcam Inc.).
Rabbit polyclonal antibody to GFRA1 (Santa Cruz Biotechnology, Inc).
Trypan blue solution (0.4%).
BSA–EDTA–PBS buffer: ice-cold PBS containing 0.5% BSA and 2 mM EDTA.
MACS BSA stock solution (Miltenyi Biotec).
MACS Preseparation filters (Miltenyi Biotec).
MACS Separation Columns (Miltenyi Biotec).
MACS Separator (Miltenyi Biotec).
Goat anti-rabbit IgG Microbeads (Miltenyi Biotec).
2.5. Immunocytochemistry of Isolated Human GPR125- and GFRA1-Positive Spermatogonia
4% Paraformaldehyde (37% of paraformaldehyde is diluted with phosphate-buffered saline (PBS) to final concentration of 4%).
Blocking solution: 10% goat serum (Invitrogen).
Rabbit polyclonal antibody to GPR125 (2 μg/ml in PBS).
Rabbit polyclonal antibody to GFRA1 (2 μg/ml in PBS).
Mouse monoclonal antibody to THY1 (LifeSpan Biosciences) (1 μg/ml in PBS).
Rabbit polyclonal antibody to UCHL1 (AbD Serotec) (1 μg/ml in PBS).
Phycoerythrin (PE)-conjugated mouse monoclonal antibody to ITGA6 (BD Biosciences) (1 μg/ml in PBS).
Normal rabbit IgG (Santa Cruz Biotechnology Inc.) (2 μg/ml in PBS).
Normal mouse IgG (Santa Cruz Biotechnology Inc.) (2 μg/ml in PBS).
Fluorescein (FITC)-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories) at a 1:200 dilution in PBS.
Rhodamine-conjugated goat anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch Laboratories) at a 1:200 dilution in PBS.
DAPI (4′, 6′-diamidino-2-phenylindole).
3. Methods
3.1. Procurement of Testes from Organ Donors
During organ retrieval in a hospital operating suite, 30,000 U of heparin is injected intravenously prior to aortic “cross clamping” in the thorax and in the lower abdomen (24). After clamping, the aorta is immediately perfused with Viaspan™ (see Note 1), a standard organ preservation solution. The testes are retrieved after a 10-min perfusion, packed in cold Viaspan™, and sent to the laboratory within 1–2 h of removal from the donor. The testes are immediately placed aseptically in DMEM containing 1 × antibiotic. The testis tissue is frozen as ~1 g pieces in freezing solution, including 10% DMSO, 80% FBS, and 10% DMEM, in liquid nitrogen, and the integrity of the tissue should be excellent.
3.2. Enzymatic Digestion of Human Testis Tissue
The UV in the hood is turned on at least 30 min prior to use.
Enzymes including collagenase IV or collagenase XI (4°C), hyaluronidase, trypsin, and DNase I (kept at −20°C) are taken from the refrigerator.
Thirty milligrams of collagenase IV or collagenase XI is weighed, put in a 15-ml conical tube, and kept at 4°C.
Sixty milligrams of collagenase IV or collagenase XI, 37.5 mg of hyaluronidase, and 30 mg of trypsin are weighed, put in a 15-ml conical tube, and placed at 4°C.
The enzymes are returned to the refrigerator at 4°C immediately after the weighing. DNase I is thawed on wet ice.
The donor or frozen human testicular tissues are placed in 100 ml of DMEM/F12 in 120 ml container. All subsequent procedures are carried out in the sterile hood.
After weighing, testicular tissues (~1 or 2 g) are placed in 10 ml of DMEM/F12 and cut into small pieces measuring approximately 1 × 1 × 1 mm and washed three times in DMEM/F12 to eliminate any remaining blood cells.
Testicular tissues are minced using scissors until a semiliquid state has been achieved. After the addition of 20 ml of DMEM/F12, the tissues are transferred to a new 120-ml container.
20 ml of fresh DMEM/F12 is added to the container.
Enzyme I is taken out of the refrigerator (step 3) and dissolved in 15 ml of DMEM/F12 to yield 2 mg/ml collagenase IV or collagenase XI.
The dissolved collagenase IV or collagenase XI is sterilized in a 10-ml syringe mounted with a Sterile Acrodisc Syringe filter (5 μm). DNase I is added to enzyme I to yield 1 μg/μl DNase I and mixed gently with a pipette; this is enzyme solution I.
The minced testis tubules from step 8 are transferred to a 50-ml conical tube using a 10 ml glass pipette (plastic pipette is not suitable, see Note 2) and fresh DMEM/F12 is added to get a final volume of 50 ml. After a 5-min sedimentation, the supernatant is removed with a 25-ml pipette.
Enzyme solution I (from step 11) is added to the minced testis tissues (step 12) and incubated in a shaking water bath at 34°C for about 10 or 15 min.
The digested testis tissues are examined under a microscope to make sure that there are only seminiferous tubules with no gross lumps (see Note 3). If lumps remain, the testis tissue is pipetted up and down with a 5-ml glass pipette (plastic pipette is not suitable, see Note 2) for 1 min and incubated again for another 5 min until only seminiferous tubules are obtained. Almost all the interstitial tissue clumps should be removed after this procedure.
The solution containing seminiferous tubules is transferred to a new 50-ml conical tube and 30 ml of fresh DMEM/F12 containing 10% FBS is added to stop the digestion. After a 5-min sedimentation, the supernatant is removed with a 25-ml pipette and 50 ml of fresh DMEM/F12 is added to the pellet to wash the seminiferous tubules.
The seminiferous tubules are washed extensively to remove all Leydig cells and other interstitial cells: DMEM/F12 is added to the conical tube and sedimented for 5 min; the supernatant is removed and the wash is repeated another three times (see Note 4).
The enzyme mixture (from step 4) is taken out of the refrigerator and dissolved in 15 ml of DMEM/F12 to yield 4 mg/ml collagenase IV or collagenase XI, 2.5 mg/ml hyaluronidase, and 2 mg/ml trypsin.
The enzyme mixture is sterilized in a 10-ml syringe mounted with a Sterile Acrodisc Syringe filter (5 μm). DNase I is added to yield 1 μg/μl DNase I and mixed gently with 5-ml pipette. This is enzyme solution II.
The supernatant containing the seminiferous tubules (from step 16) is removed with a 25-ml pipette, and enzyme solution II is added to the tubules.
The mixture of tubules and enzyme solution II is transferred to a fresh container using a 10-ml glass pipette (plastic pipette is not suitable, see Note 2) and incubated in a shaking bath for 15 min at 100 cycles/min.
After a 15 min enzyme digestion, pipette the mixture gently 10–15 times using a 10-ml glass pipette (plastic pipette is not suitable, see Note 2) without producing air bubbles.
A drop of the mixture is placed on a microscope slide and examined with an inverted microscope to assess the effectiveness of the second enzyme digestion (see Note 5).
If some seminiferous tubules still exist, pipette them gently several times using a 10-ml glass pipette (plastic pipette is not suitable, see Note 2) without producing air bubbles and allow another 10 ~ 15 min for digestion by shaking at 34°C at 100 cycles/min.
The enzymatic digestion is checked again as step 22; if the seminiferous tubules are completely digested to single cells, 30 ml of fresh DMEM/F12 containing 10% FBS are added to stop the digestion; allow 10 min for sedimentation.
The supernatant containing germ cells and Sertoli cells is transferred to a new 50-ml conical tube and the sediment is discarded.
The supernatant is centrifuged at 112 × g for 5 min and the cell pellet is suspended in 50 ml of fresh DMEM/F12.
After 10 min of sedimentation, the supernatant containing germ cells and Sertoli cells is transferred to a new 50-ml conical tube and the sediment is discarded.
The supernatant is centrifuged at 112 × g for 5 min, and the cell pellet is suspended in fresh DMEM/F12 and filtered through a 40-μm nylon mesh to remove any cell aggregates.
The cell mixture is centrifuged at 112 × g for 5 min and the cell pellet is suspended in fresh DMEM/F12 supplemented with 10% FBS.
3.3. Differential Plating to Obtain Human Male Germ Cells
For differential plating, the cell mixture containing male germ cells and Sertoli cells is cultured in DMEM/F12 supplemented with 10% FBS in a 15-cm diameter tissue culture dish precoated with 0.1% gelatin (see Note 6) for 3 h at 34°C. Sertoli cells attach to the culture plates, and the germ cells remain in suspension and are collected by centrifugation at 112 × g for 5 min.
3.4. Isolation of Human GPR125- and GFRA1-Positive Spermatogonia by MACS
The germ cells are suspended in 1 ml of DMEM supplemented with 10% NU serum and 1 μg/μl DNase I (see Note 7).
A total of 25 μl of the antibody to GPR125 or GFRA1 is added to 1 ml cell solution and incubated at 4°C with rotation at a 50 cycles/min overnight.
After incubation with primary antibody, cell viability is checked with 0.4% trypan blue exclusion staining (see Note 8).
The cells are washed three times with BSA–EDTA–PBS buffer (ice-cold PBS containing 0.5% BSA and 2 mM EDTA) (see Note 9) for 5 min each time and are suspended in 80 μl of the BSA–EDTA–PBS buffer.
A total of 20 μl of goat anti-rabbit IgG magnetic microbeads is added to the cells and the cell mixture is incubated at 4°C with rotation at a 50 cycles/min for less than 20 min (see Note 10).
After incubation, 400 μl of BSA–EDTA–PBS buffer are added to the cells to get a final volume of 500 μl.
Around 5 μl of cell suspension is used to perform a viability test with 0.4% trypan blue exclusion staining.
The cells are loaded on the column that is equilibrated in the BSA–EDTA–PBS buffer.
The MACS preseparation filters (30 μm) are mounted on the column to filter out any aggregates before loading.
A total of 0.5 ml of cells is loaded on the column while the column is attached on the magnet.
The unlabelled cell fraction (i.e., GPR125- or GFRA1-negative cells) is first collected.
The column is washed three times with PBS–BSA–EDTA buffer (0.5 ml each time) and the unlabelled cell fraction (i.e., GPR125 or GFRA1-negative cells) is collected.
The column is removed from the magnet and GPR125- or GFRA1-positive spermatogonia are collected by adding 1 ml of BSA–EDTA–PBS buffer and pressing very gently with the plunger without making bubbles (see Note 11).
A second MACS is performed pursuant to the methods described as above so as to get a better purity of human GPR125- or GFRA1-positive spermatogonia.
3.5. Immunocytochemistry of Isolated Human GPR125- and GFRA1-Positive Spermatogonia
Human GPR125- or GFRA1-positive spermatogonia and GPR125- or GFRA1-negative male germ cells are washed with PBS and centrifuged at 112 g for 5 min and the cell pellet is suspended in PBS.
The cells are cytospun onto the microscope slides at 112 g for 5 min using a Cyto-Tek unit, and they are fixed with 4% paraformaldehyde for 30 min.
The cells are washed twice with PBS for 3 min at room temperature and blocked with 200 μl of normal 10% goat serum for 30 min at room temperature.
Primary antibody to GPR125, GFRA1, THY1, UCHL1, or ITGA6, or normal rabbit IgG, or normal mouse IgG, is diluted in 100 μl of PBS (dilution at 1:100–1:500); the diluted primary antibody is then added to the cells, and incubated for 1 h at 34°C or overnight at 4°C.
Replacement of the primary antibody with PBS serves as negative controls.
After incubation, the cells are washed with 200 μl of PBS.
After three washes in PBS, the cells are incubated with the secondary antibody, including Fluorescein (FITC)-conjugated goat anti-rabbit IgG, or Rhodamine-conjugated goat anti-rabbit or anti-mouse IgG.
DAPI is used to stain the nuclei and the cells are observed for epifluorescence using an Olympus Fluoview 500 Laser Scanning Microscope.
Footnotes
It is important to note that as soon as the blood supply to the testes is interrupted by “clamping” of the aorta, the Viaspan™ solution enter the testes.
It is essential to use glass pipettes to transfer the seminiferous tubules or male germ cells and Sertoli cells. Plastic pipettes are not suitable because the tubules or cells attach to the walls of plastic pipettes and significantly reduce the yield of male germ cells.
The time for the first digestion may vary and is dependent on the age of donors. Usually, 10 min are required for the first digestion, but it may take more time.
After the first digestion, extensive washes of the tubules (at least four times) with DMEM/F12 are required to remove the Leydig cells and other interstitial cells.
The time for the second digestion of the tubules to obtain single cells varies based on the age of the donors. Usually, 20 or 30 min are required, but it may take more time.
Tissue culture dishes are precoated with 0.1% gelatin, which facilitates Sertoli cell attachment to the dish within 3 h.
It is essential to adjust cell number to no more than ten million cells per ml of DMEM with 10% NU serum and DNase I.
Cell viability prior to MACS should be more than 95%. A higher percentage of dead cells can cause nonspecific binding to the separator column.
The BSA–EDTA–PBS buffer should be prefiltered and freshly prepared.
The cells cannot remain on ice with the microbeads for more than 20 min, since they are very sensitive at this stage and die if they are incubated for longer periods of time.
The cells can pass through the column without using the plunger. Only in the end, the plunger is placed on the top of the column and pressed gently and slowly for about 0.5 cm of the column until most of the liquid is out of the column. Bubbles in the column can kill the cells.
References
- 1.Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, Oshimura M, Ishino F, Ogura A, Shinohara T. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development. 2005;132:4155–4163. doi: 10.1242/dev.02004. [DOI] [PubMed] [Google Scholar]
- 5.He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 7.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 8.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, Valenzuela DM, Hobbs RM, Pandolfi PP, Rafii S. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ikawa M, Nakamura T, Ogura A, Shinohara T. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–687. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 11.Ko K, Tapia N, Wu G, Kim JB, Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, Hausdorfer K, Sebastiano V, Stehling M, Fleischmann BK, Brustle O, Zenke M, Scholer HR. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Golestaneh N, Kokkinaki M, Pant D, Jiang J, Destefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, Van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, De Reijke TM, De La Rosette JJ, Knegt AC, Belmonte JC, Van Der Veen F, De Rooij DG, Repping S, Van Pelt AM. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- 15.Payne CJ, Braun RE. Human adult testis-derived pluripotent stem cells: revealing plasticity from the germline. Cell Stem Cell. 2008;3:471–472. doi: 10.1016/j.stem.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Geijsen N, Hochedlinger K. gPS navigates germ cells to pluripotency. Cell Stem Cell. 2009;5:3–4. doi: 10.1016/j.stem.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biology of Reproduction. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, Lindahl M, Hyvonen ME, Parvinen M, De Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Jiang J, Hofmann MC, Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod. 2007;77:723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–1540. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 27.Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- 28.Clermont Y. Renewal of spermatogonia in man. Am J Anat. 1966;118:509–524. doi: 10.1002/aja.1001180211. [DOI] [PubMed] [Google Scholar]
- 29.Clermont Y. Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril. 1966;17:705–721. [PubMed] [Google Scholar]
- 30.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 31.Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- 32.Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M. Age affects gene expression in mouse spermatogonial stem/progenitor cells. Reproduction. 2010;139:1011–1120. doi: 10.1530/REP-09-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]