Abstract
Long non‐coding RNAs (lncRNAs) play important roles in many cellular pathways, but their contribution to the defense of eukaryotic cells against pathogens remains poorly understood. A new study from Imamura et al in The EMBO Journal reports that Salmonella infection in human cells impacts nuclear RNA decay, which in turn drives the accumulation of otherwise unstable nuclear lncRNAs, some of which may have protective effects against this common bacterial pathogen. These unexpected findings demand more efforts to fully decrypt the molecular functions of lncRNAs in innate and adaptive immunity.
Subject Categories: Microbiology, Virology & Host Pathogen Interaction; RNA Biology
Infections are exquisitely complex organismic interactions ensuing from the dynamic interplay between a pathogen and its host. Infected host cells execute carefully orchestrated gene expression programs that involve pathways of both innate and adaptive immunity and integrate multiple different cues to mount and sustain an appropriate response. Traditionally, efforts to characterize these host gene expression changes have centered on the mRNA output, but more recently non‐coding RNAs (ncRNAs) have garnered considerable attention. This is well illustrated by studies of the non‐coding RNA arm of the host response to Salmonella enterica serovar Typhimurium (henceforth Salmonella), a well‐characterized invasive bacterium that causes a range of diseases in humans and livestock (Fig 1). For example, we now know of several members of the large family of ~22 nucleotides‐long microRNAs that respond to infection by Salmonella and contribute to its clearance (Rodriguez et al, 2007; Schulte et al, 2011; Maudet et al, 2014) (Fig 1).
Figure 1. The mammalian non‐coding RNA response to infections by the pathogenic bacterium Salmonella .
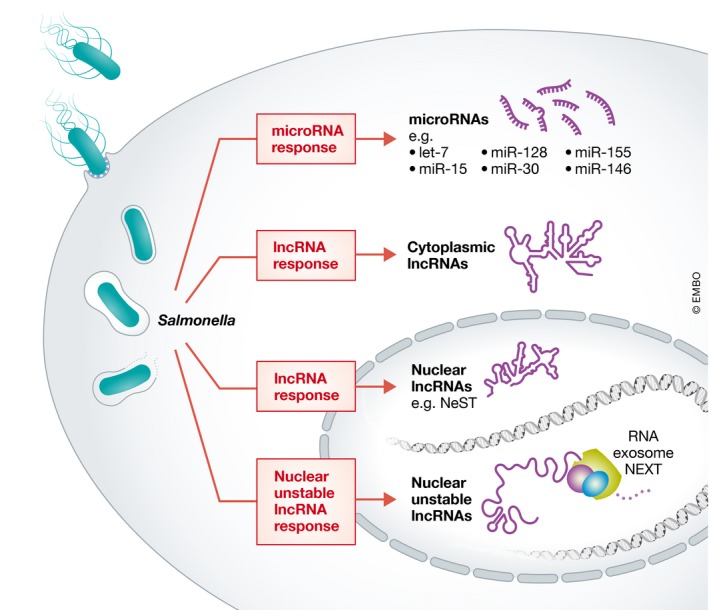
Host cells utilize microRNAs (e.g., let‐7, miR‐15, miR‐30, miR‐128, miR‐146, miR‐155) and lncRNAs (e.g., NeST) to respond to Salmonella infection. Imamura et al (2018) now report loss of nuclear RNA degradation factors and accumulation of unstable nuclear lncRNAs in response to intracellular Salmonella growth. These lncRNA candidates may contribute to host defense mechanisms that help clear the invading pathogen.
In addition to microRNAs, mammalian cells express many long non‐coding RNAs (lncRNAs), an increasing number of which are associated with dysregulation of immunity and host defense (Carpenter & Fitzgerald, 2018; Chen et al, 2017). This includes the lncRNA NeST, which promotes clearance of Salmonella through enhanced local IFN‐γ production in mice (Gomez et al, 2013) (Fig 1). Beyond individual examples, RNA‐seq profiling of various cell types infected with Salmonella has provided a treasure trove of lncRNAs that are regulated upon immune cell stimulation or pathogen invasion (Westermann et al, 2016; Avraham et al, 2015; Haber et al, 2017; Saliba et al, 2016), but their molecular functions remain uncharacterized. In this issue of The EMBO Journal, Imamura et al (2018), report the exciting observation that nuclear RNA degradation is dysregulated as Salmonella replicates inside host cells, prompting the accumulation of a distinct group of otherwise unstable lncRNAs, some of which are suggested to be important for antibacterial defense.
Unstable nuclear RNAs constitute a particularly enigmatic group of transcripts, which are often viewed as the mere result of pervasive transcription of the eukaryotic genome. Since the mammalian nuclear RNA exosome quickly degrades most of these RNAs (Schmid & Jensen, 2018), few accumulate to detectable levels. Therefore, it has been largely unclear how many of these transcripts are functional, and whether cells actively regulate the stability of specific nuclear RNAs in response to external or internal stimuli.
Profiling the transcriptome of HeLa cells at several time points after Salmonella invasion, the authors here observed 145 unstable nuclear ncRNAs that were significantly upregulated (Imamura et al, 2018). Of these, 26 were classified as enhancer RNAs (eRNAs) based on histone modifications of their genomic loci. The authors then selected two eRNAs and two other lncRNAs to understand what causes their infection‐induced accumulation. While all four of them required intracellular replication of Salmonella to accumulate, surprisingly, their transcription was found to be unchanged. Instead, they accumulated because their half‐lives increased upon Salmonella infection.
To explain this specific lncRNA stabilization, the authors broadly monitored infection‐induced changes in protein complexes that degrade unstable nuclear RNAs. Remarkably, protein levels of the nuclear exosome component RRP6 as well as of MTR4 (a component of the nuclear exosome targeting (NEXT) complex and the poly(A) exosome targeting (PAXT) complex; Schmid & Jensen, 2018) were diminished 18 h postinfection, just as the lncRNAs NEAT1_2 and eRNA07573 accumulated. Knocking down various RNA degradation factors in uninfected cells, the authors concluded that these candidate RNAs were predominantly targeted for degradation by the NEXT complex (Imamura et al, 2018).
The authors also obtained results to suggest that these nuclear lncRNAs are stabilized because they are required for effective host defense (Imamura et al, 2018). They show that NEAT1 knockout cells (lacking both the NEAT1_1 (3.7 kb) and NEAT1_2 (23 kb) isoforms) are more permissive to Salmonella infection and that there is a set of 126 genes, including those of several immune regulators, whose induction upon infection requires an intact NEAT1 locus. Thus, not only antiviral genes, as reported previously (Imamura et al, 2014), but also the antibacterial response seems to rely on the NEAT1_1 and NEAT1_2 lncRNAs. Similarly, deletion of the eRNA07573 locus renders HeLa cells more susceptible to Salmonella (Imamura et al, 2018). Whether this is because of a lost enhancer activity of this locus is currently unclear. Notably, eRNA07573 lies upstream of the genes encoding glucose transporters SLC2A3 and SLC2A14, and the authors show that both of these neighboring genes are transcriptionally induced upon Salmonella infection, in an eRNA07573 locus‐dependent manner. In a cis‐activation scenario, however, the putative enhancer function of the eRNA07573 locus would help to feed the enemy rather than to constrain it, since cytoplasmic glucose is a preferred carbon source of intracellular Salmonella (Eisenreich et al, 2010). Therefore, an alternative explanation for the observed phenotype may be found in those ~200 genes whose induction by Salmonella requires the eRNA07573 locus (Imamura et al, 2018).
Taken together, the authors present intriguing data suggesting that an infection‐induced loss of specific nuclear RNA degradation factors causes the accumulation of a subset of otherwise unstable nuclear lncRNAs, some of which may be involved in helping to clear the replicating pathogen. The extent to which the RNA products themselves regulate specific immune response pathways remains an open question that also highlights a grand challenge in the field: connecting lncRNA expression to lncRNA function. A mechanistic dissection of lncRNAs should include strategies that deplete the respective transcripts without perturbing genomic DNA elements, while monitoring infection phenotypes or the expression of distinct immune genes. One fast track to molecular functions and mechanisms of various infection‐induced lncRNAs would be a systematic dissection of biochemical interaction partners of endogenous lncRNAs in intact cells that undergo infection, for example, by RNA antisense purification in combination with next‐generation sequencing or quantitative mass spectrometry (Engreitz et al, 2013; McHugh et al, 2015).
The observations by Imamura et al (2018) prompt many additional questions as to host RNA metabolism being a target during infection. In the present case, it will be exciting to understand the chain of molecular events that leads to depletion of the RRP6 and MTR4 proteins. Does Salmonella secrete a specific effector protein that targets these components for proteolysis? Further downstream, how are the 145 affected unstable nuclear ncRNAs selected for targeting by the exosome/NEXT RNA degradation machineries? Is their stabilization a general phenomenon that Salmonella shares, at least to some extent, with other well‐characterized intracellular model bacteria such as Listeria or Shigella species? Addressing these questions from a viewpoint of lncRNA biology will certainly produce more unexpected insight into the many clever tricks that bacteria use to manipulate eukaryotic cells to their own benefit and into how host cells use their arsenal of thousands of non‐coding transcripts to mount the right response to their prokaryotic enemies.
The EMBO Journal (2018) 37: e99875
See also: https://doi.org/10.15252/embj.201797723 (July 2018)
Contributor Information
Mathias Munschauer, Email: mathias@broadinstitute.org.
Jörg Vogel, Email: joerg.vogel@uni-wuerzburg.de.
References
- Avraham R, Haseley N, Brown D, Penaranda C, Jijon HB, Trombetta JJ, Satija R, Shalek AK, Xavier RJ, Regev A, Hung DT (2015) Pathogen cell‐to‐cell variability drives heterogeneity in host immune responses. Cell 162: 1309–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, Fitzgerald KA (2018) Cytokines and long noncoding RNAs. Cold Spring Harbor Perspect Biol 10: a028589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Satpathy AT, Chang HY (2017) Gene regulation in the immune system by long noncoding RNAs. Nat Immunol 18: 962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Dandekar T, Heesemann J, Goebel W (2010) Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Micro 8: 401–412 [DOI] [PubMed] [Google Scholar]
- Engreitz JM, Pandya‐Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M (2013) The xist lncRNA exploits three‐dimensional genome architecture to spread across the X chromosome. Science 341: 1237973–1237973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JA, Wapinski OL, Yang YW, Bureau J‐F, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K (2013) The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon‐γ locus. Cell 152: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt‐Rosen O, Shi HN, Yilmaz O, Xavier RJ, et al (2017) A single‐cell survey of the small intestinal epithelium. Nature 551: 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, Kato A, Kawaguchi Y, Sato H, Yoneda M, Kai C, Yada T, Suzuki Y, Yamada T, Ozawa T, Kaneki K, Inoue T, Kobayashi M, Kodama T, Wada Y et al (2014) Long noncoding RNA NEAT1‐dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell 53: 393–406 [DOI] [PubMed] [Google Scholar]
- Imamura K, Takaya A, Ishida Y, Fukuoka Y, Taya T, Nakaki R, Kakeda M, Imamachi N, Sato A, Yamada T, Onoguchi‐Mizutani R, Akizuki G, Tanu T, Tao K, Miyao S, Suzuki Y, Nagahama M, Yamamoto T, Jensen TH, Akimitsu N (2018) Diminished nuclear RNA decay upon Salmonella infection upregulates antibacterial noncoding RNAs. EMBO J 37: e97723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudet C, Mano M, Sunkavalli U, Sharan M, Giacca M, Förstner KU, Eulalio A (2014) Functional high‐throughput screening identifies the miR‐15 microRNA family as cellular restriction factors for Salmonella infection. Nat Commun 5: 4718 [DOI] [PubMed] [Google Scholar]
- McHugh CA, Chen C‐K, Chow A, Surka CF, Tran C, McDonel P, Pandya‐Jones A, Blanco M, Burghard C, Moradian A, Sweredoski MJ, Shishkin AA, Su J, Lander ES, Hess S, Plath K, Guttman M (2015) The xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A (2007) Requirement of bic/microRNA‐155 for normal immune function. Science 316: 608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba A‐E, Li L, Westermann AJ, Appenzeller S, Stapels DAC, Schulte LN, Helaine S, Vogel J (2016) Single‐cell RNA‐seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat Microbiol 2: 16206 [DOI] [PubMed] [Google Scholar]
- Schmid M, Jensen TH (2018) Controlling nuclear RNA levels. Nat Rev Genet 91: 457 [DOI] [PubMed] [Google Scholar]
- Schulte LN, Eulalio A, Mollenkopf H‐J, Reinhardt R, Vogel J (2011) Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let‐7 family. EMBO J 30: 1977–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann AJ, Förstner KU, Amman F, Barquist L , Chao Y, Schulte LN, Müller L, Reinhardt R, Stadler PF, Vogel J (2016) Dual RNA‐seq unveils noncoding RNA functions in host‐pathogen interactions. Nature 529: 496–501 [DOI] [PubMed] [Google Scholar]


