Abstract
The combination of neurologic disease and ichthyosis defines a heterogeneous group of rare inherited disorders that present in infancy through early adulthood. Although affected patients share the cutaneous feature of ichthyosis, there is variability in the nature and severity of neurologic disease. Impaired cognition, spasticity, sensorineural deafness, visual impairment, and/or seizures are the primary neurologic findings. Most of these disorders are caused by genetic defects in lipid metabolism, glycoprotein synthesis, or intracellular vesicle trafficking. The clinical features of some of the neuro-ichthyoses are distinct enough to allow their clinical recognition, but confirmatory biochemical or genetic tests are necessary for accurate diagnosis. Treatment of the ichthyosis is largely symptomatic, and except for Refsum’s disease, there are no effective pathogenesis-based therapies for the neurologic disease.
Keywords: ichthyosis, lipids, mutation, skin, myelin
Neurocutaneous diseases comprise a large and heterogeneous group of disorders that are predominantly genetic in nature. Among these disorders, the distinctive coexistence of neurologic symptoms and ichthyosis is seen in a subgroup of genetic diseases referred to as the neuro-ichthyoses. These diseases may appear superficially similar at a clinical level and can seem bewildering for the clinician who first encounters a patient. Upon closer inspection, however, many neuro-ichthyoses have distinguishing historical and clinical features that allow them to be recognized and reliably diagnosed using biochemical or DNA tests. The number of neuro-ichthyotic syndromes with identified genetic etiologies has increased over the past decade, and knowledge about their molecular etiologies has expanded accordingly. Although effective treatments for most of these disorders remain an elusive challenge, insights into therapeutic approaches are starting to emerge.
The major neuro-ichthyotic syndromes will be reviewed with an emphasis on their clinical recognition and a practical diagnostic approach provided that can be used by the neurologist and other subspecialists.
Clinical Recognition of Ichthyosis
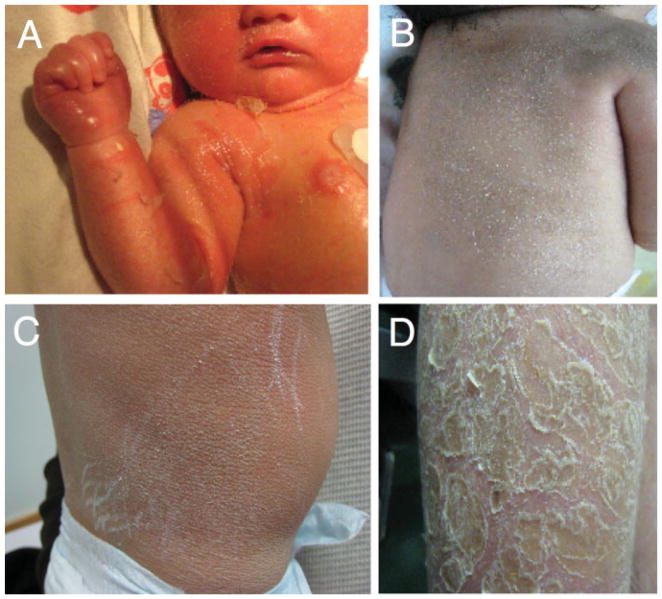
Neurologists, and many other physicians, may not recognize the presence of ichthyosis in patients, in part due to the rarity of the symptom, the failure to focus on the skin in patients who have severe neurologic disease, the wide variation in its severity—even in the same patient—and the general tendency to simply ascribe it to “dry skin.” In fact, mildly affected patients may partially mask the cutaneous symptoms by the liberal use of moisturizing lotions. It is uncommon, however, to miss ichthyosis in patients who have more severe forms, especially those that present at birth. Some newborn infants are covered by a parchment-like “collodion membrane,” which resolves over the first weeks of life, leaving behind a dry, scaly appearance. To assist in its recognition, variation in the appearance of ichthyosis is illustrated in Fig. 1.
Figure 1.
Variation in the appearance of ichthyosis. (A) Newborn infant with healing collodion membrane. Note the tight, shiny skin on the hand and the areas of desquamation with underlying erythema. The skin later developed hyperkeratosis with scaling. (B) Fine scales on the back and shoulders of a 10-month-old infant with developmental delay. (C) Thick hyperkeratotic skin with a lichenified appearance on the trunk of a 2-year-old infant with Sjögren-Larsson’s syndrome. Note excoriations due to pruritus. (D) Large lamellar-like scales on the lower leg of a patient with Sjögren-Larsson’s syndrome.
Neuro-Ichthyotic Syndromes of Known Etiology
The neuro-ichthyotic diseases are clinically and genetically heterogeneous. They include 16 distinct disorders with a known genetic etiology (Table 1). Many of them affect lipid metabolism, glycoprotein synthesis, or intracellular vesicle trafficking. The existence of biochemical or genetic markers has permitted their reliable diagnosis and provided insight into their clinical variation and pathogenic mechanisms.
Table 1.
Clinical Features of Neuro-Ichthyotic Disorders with a Known Genetic Etiology*
| Disorder | Gene Defect | Impaired Cognition | Dysmorphic Facies | Peripheral Neuropathy | Deafness | Eye Abnormality | Seizures | Abnormal Hair | Brain MRI Abnormalities | Associated Clinical Features |
|---|---|---|---|---|---|---|---|---|---|---|
| Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome | VPS33B | ± | ± | + | − | − | − | − | ± Brain dysgenesis | Peripheral denervation of muscles, 50% have ichthyosis, early lethality |
| CEDNIK syndrome (Cerebral dysgenesis, neuropathy, ichthyosis and palmoplantar keratoderma syndrome) | SNAP29 | + | + | + | + | ± Visual impairment, OptN, macular atrophy | − | ± Brittle course hair, ± scarring alopecia | Brain dysgenesis, ± hypoplastic corpus callosum | Failure to thrive, early death |
| Chondrodysplasia punctata, X-linked recessive | ARSE | ± | + | − | + | ± Cat | − | − | NE | Ichthyosis <20%, punctate epiphyseal calcifications, hypoplastic nasal cartilage and distal phalanges, short stature |
| Dolichol kinase deficiency | DOLK | ? | − | − | − | ± Nyst | + | Alopecia ± | NE | Hypotonia, spastic tetraplegia, microcephaly. ± DCM, early death. |
| ELOVL4 deficiency | ELOVL4 | + | ± | − | − | ± Pp | + | − | Brain atrophy, deficient myelin | Spastic quadriparesis, inguinal hernias, abnormal VEP |
| Gaucher’s disease type 2 | GBA | + | ± | − | − | − | + | − | NE | Congenital ichthyosis (±), hepatosplenomegaly, neuroregression |
| Keratitis-ichthyosis-deafness (KID) syndrome | GJB2 | − | − | − | + | + Ker | − | Alopecia | NE | Increased risk for squamous cell carcinoma, hyperkeratotic plaques |
| MEDNIK syndrome (Mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma syndrome) | AP1S1 | + | + | + | + | ± Cat | − | − | NE | Early lethality, elevated very long chain fatty acids |
| Multiple sulfatase deficiency | SUMF1 | + | + | + | − | + CC | + | − | WMD | Hepatosplenomegaly, gait disturbance, ataxia, neuroregression, course facies, ± ichthyosis |
| Neutral lipid storage disease with ichthyosis (Chanarin-Dorfman’s syndrome) | CGI-58 (ABHD5) | ± | − | ± | + | + Cat | − | − | NE | Vacuolated leukocytes; myopathy; hepatomegaly, lipid droplets in skin |
| Refsum’s disease | PHYH, PEX7 | − | − | + | + | + RP | − | − | NE | Late-onset, ataxia, ± ichthyosis, cardiac conduction defects, lipid droplets in skin |
| Rhizomelic chondrodysplasia punctata | PEX7, AGPS, GNPAT | + | + | ± | ± | + Cat | ± | − | NE | Ichthyosis 25%, short proximal limbs, failure to thrive, punctate epiphyseal calcifications, ± ichthyosis |
| Steroid sulfatase contiguous gene deletion | STS and contiguous genes | ± | − | − | − | + RP | + | − | NE | Hypogonadism; short stature |
| Sjögren-Larsson’s syndrome | ALDH3A2 | + | − | − | − | +Dots, Pp | ± (30%) | − | WMD, lipid peaks on MRS | Spastic diplegia > tetraplegia, pruritus |
| Steroid 5α-reductase type-3 deficiency | SRD5A3 | + | + | − | − | + Nyst, Colob, OptN | − | − | Cereb Hypo | Elevated serum transaminases, ± congenital cardiac defects ASD, TGA, pulmonary valve |
| Trichothiodystrophy with Ichthyosis (Tay’s syndrome) | XPD, XPB, P8/TTDA or TTDN1 | + | − | − | − | +Pp | − | + | WMD, Cereb Hypo | Sparse brittle hair (trichoschisis), ± photosensitivity |
ASD, atrial septal defect; Cat, cataracts; CC, corneal cloudiness; Cereb Hypo, cerebellar and/or vermis hypoplasia or atrophy; Colob, coloboma of iris or retina; DCM, dilated cardiomyopathy; Dots, perifoveal glistening white dots; HydroC, hydrocephalus; Ker, keratitis; MRS, magnetic resonance spectroscopy; NE, not established; Nyst, nystagmus; OptN, optic nerve hypoplasia or atrophy; Pp, photophobia; RP, retinitis pigmentosa; TGA, transposition of the great arteries; VEP, visual-evoked potentials; WMD, white matter disease.
+, indicates a clinical feature is usually present; -, indicates its absence; ±, indicates it has a variable incidence.
Sjögren-Larsson’s Syndrome
Sjögren-Larsson’s syndrome (SLS) is the most widely recognized form of neuro-ichthyosis. The disease is characterized by the presence of ichthyosis, spastic diplegia or tetraplegia, cognitive delay, seizures, and a maculopathy with glistening white dots.1 The cutaneous abnormalities are usually present at birth, at which time the skin appears erythematous and hyperkeratotic. About 15% of newborn infants have a collodion membrane. The cutaneous disease in SLS has a characteristic pruritic nature, and the itchiness is an agonizing feature for some patients. Neurologic symptoms are rarely present in the first few months of life, but appear later in the first year with delay in achieving motor milestones due to spastic diplegia or tetraplegia. Intellectual impairment varies from mild to profound, and rare patients are cognitively normal. Most patients have pseudobulbar dysarthria and exhibit speech delay beyond that expected from their cognitive impairment. Ophthalmologic examination shows perifoveal glistening white dots in ~85% of patients, but these retinal abnormalities may only appear after infancy. Myopia and photophobia are common. Seizures occur in 30% of patients. Brain MRI may be normal in the first few years of life, but older children and adults typically show white matter disease involving the centrum semiovale, corpus callosum, periventricular regions, and parietal and frontal lobes.2 Magnetic resonance spectroscopy (MRS) characteristically reveals unusual lipid peaks at 1.3 ppm and 0.9 ppm in the affected white matter. Peripheral nerve function is unaffected. The neurologic disease is usually static and patients routinely survive into adulthood.
Sjögren-Larsson’s syndrome is caused by mutations in the ALDH3A2 gene that codes for fatty aldehyde dehydrogenase.3 Fatty aldehyde dehydrogenase is necessary for the oxidation of long-chain aldehydes and alcohols to fatty acids. Deficiency of this enzyme leads to accumulation of these lipids in skin and brain, which is thought to be directly responsible for the symptoms.1 Fatty aldehydes and alcohols intercalate into membranes and may disrupt myelin by physically altering its membrane structure or forming covalent Schiff-base adducts with myelin lipids and proteins. In the skin, stratum corneum membranes are defective, which results in a leaky water barrier and reactive hyperkeratosis.4
The diagnosis of SLS requires measurement of fatty aldehyde dehydrogenase in cultured skin fibroblasts or demonstration of mutations in ALDH3A2, which are detected in > 95% of patients.
The ichthyosis in SLS is improved with topical moisturizing lotions, keratolytic agents, and systemic retinoids, but therapy of the neurologic disease is limited. Botox injections are a temporary measure for the spasticity in most patients, who ultimately require surgical procedures.
Neutral Lipid Storage Disease With Ichthyosis
Neutral lipid storage disease with ichthyosis (NLSDI) (also known as Chanarin-Dorfman’s syndrome) is characterized by congenital ichthyosis, mental retardation, neurosensory deafness, ataxia, hepatomegaly, myopathy, and cardiomyopathy.5 There is considerable clinical variation and some patients have normal intelligence or lack liver disease. The ichthyosis may have a pruritic nature. Myopathy is associated with elevated serum creatine kinase and abnormal electromyography (EMG) studies. Progressive neurodegeneration is not typical.
Neutral lipid storage disease with ichthyosis is caused by mutations in the CGI-58 gene (also known asABHD5), which codes for a protein that stimulates triglyceride hydrolysis.6 A convenient diagnostic finding is the presence of cytoplasmic lipid droplets in tissues. Examination of a peripheral blood smear reveals vacuolated neutrophils and eosinophils (Jordan’s anomaly) due to triglyceride storage, although serum triglycerides are normal. The ichthyosis is likely caused by impaired hydrolysis of fatty acids from triglyceride stores, which are normally used for synthesis of ω-hydroxy-acylceramide, a key lipid component of the stratum corneum membranes required for the epidermal water barrier.7 The mechanism for neurologic disease has not been elucidated. Mutation analysis of the CGI-58 gene is required to confirm the diagnosis. The skin can be treated with topical moisturizing lotions, but no specific therapy is available for the neurologic symptoms.
Refsum’s Disease
Refsum’s disease, also termed hereditary sensory motor neuropathy type IV, is an autosomal recessive disorder caused by mutations in the PHYH gene that encodes phytanoyl-CoA hydroxylase. This enzyme is necessary for peroxisomal fatty acid α-oxidation of phytanic acid, a methyl branched-chain fatty acid that is derived from the diet.8 Due to defective α-oxidation, patients with Refsum’s disease slowly accumulate phytanic acid in tissues, resulting in clinical symptoms.9 The age of symptom onset is quite variable, but typically begins insidiously in late childhood with loss of night vision due to retinitis pigmentosa, and anosmia, which is an almost universal finding. Deafness, ataxia, ichthyosis, and polyneuropathy slowly develop over the next 10 to 15 years. Cognition is not affected. Cardiac arrhythmias are frequently seen and can cause premature death. Sudden death can rarely occur as a result of acute release of phytanic acid from the liver following stress-induced catecholamine release or infection.
The diagnosis of Refsum’s disease is made by demonstrating elevated plasma phytanic acid concentrations >200 μmol/L (normal <3). Mutation analysis of two genes will identify >95% of patients with Refsum’s disease. More than 90% of patients have genetic mutations in PHYH, and the remaining patients have mutations in PEX7, which is necessary for import of phytanoyl-CoA hydroxylase into peroxisomes.
Treatment of Refsum’s disease is initiated by removal of phytanic acid through plasmapheresis or lipid apheresis. Dietary restriction of phytanic acid through elimination of dairy products and certain vegetables subsequently reduces phytanic acid levels by 50 to 70% and typically resolves the ichthyosis, sensory neuropathy, and ataxia. Retinitis, anosmia, and deafness, however, may not improve with dietary therapy. Long-term dietary therapy is effective in limiting clinical progression of symptoms. Avoidance of rapid weight loss or fasting is recommended as this can precipitate the acute release of phytanic acid from lipid stores. The intestinal lipase inhibitor, Orlistat has shown some efficacy in reducing phytanic acid absorption with less-strict dietary therapy.10
Gaucher’s Disease Type 2
Some infants with Gaucher’s disease type 2 are born with a collodion membrane and exhibit ichthyosis, contractures, and dysmorphic facial features.11 They develop opisthotonic posturing, seizures, hypertonicity, hepatosplenomegaly, and strabismus. The neurologic disease progresses over the first few months with dysphagia, cachexia, apnea, and a fatal outcome in the first year of life.
Gaucher’s disease type 2 is caused by mutations in the GBA gene for glucocerebrosidase.12 Deficient activity of this lysosomal enzyme results in accumulation of glucosylceramide in brain and most other tissues. The neurologic disease may be caused by accumulation of glucosylsphingosine. In the skin, glucocerebrosidase normally hydrolyzes glucosylceramide to produce ceramide, which is a major lipid in the membranes of the stratum corneum. With reduced ceramides, the membranes are structurally abnormal and the epidermal water barrier is impaired, resulting in ichthyosis. The diagnosis is made by testing leukocytes for enzyme activity or detecting mutations in GBA. There is no effective therapy for this form of Gaucher’s disease.
ELOVL4 Deficiency
Very long-chain fatty acids (VLCFA) >22 carbons long are essential components of certain lipids, such as acylceramide in skin and sphingolipids in brain. Very long-chain fatty acids are synthesized through elongation of shorter fatty acids, which is catalyzed by microsomal elongating enzymes that sequentially add 2-carbons to the growing fatty acid chain. Seven elongating enzymes (designated ELOVL1–7) are known in man. Patients who carry recessive mutations in the gene for ELOVL4 have recently been reported to have congenital ichthyosis, intellectual disability, seizures, and spastic quadriparesis.13 The ichthyosis appears erythematous and patchy. Brain magnetic resonance imaging (MRI) in one patient showed brain atrophy and delayed myelination. Seizures are recalcitrant to anticonvulsant therapy and survival appears limited. The pathogenic mechanism for neurologic and cutaneous symptoms presumably arises from deficient VLCFA-containing lipids. An ELOVL4−/− knockout mouse dies within hours of birth due to profound deficiency of VLCFA-containing acylceramide in skin that results in a defective epidermal water barrier.14 The diagnosis of ELOVL4 deficiency in man is made by mutation analysis of the ELOVL4 gene. Interestingly, certain dominant mutations in ELOVL4 cause a form of juvenile macular dystrophy (Stargardt’s disease type 3) without the neuro-ichthyotic symptoms. No effective therapy is known.
Multiple Sulfatase Deficiency
Multiple sulfatase deficiency (MSD) is a rare, autosomal recessive inborn error of metabolism characterized by deficient activity of sulfatase enzymes due to mutations in the SUMF1 gene encoding formylglycine-generating enzyme.15 This enzyme is responsible for the posttranslational activation of sulfatase enzymes via conversion of a crucial cysteine residue to a formylglycine residue.16 Without posttranslational activation, sulfatase enzymes are nonfunctional and various sulfated molecules accumulate in tissues.
The clinical features appear as an amalgam of single sulfatase enzyme deficiencies. Similar to metachromatic leukodystrophy, multiple sulfatase deficiency patients exhibit neurodegenerative disease in early childhood due to central nervous system (CNS) and peripheral demyelination and loss of sensory and motor functions. They also develop mental retardation, hepatosplenomegaly, coarse facies, and corneal clouding as seen in patients with mucopolysaccharidoses. Ichthyosis and skeletal changes reffect enzyme deficiencies of steroid sulfatase (X-linked ichthyosis) and arylsulfatase E (chondrodysplasia punctata), respectively. The unique combination of neurodegeneration, coarse facial features, hepatosplenomegaly, and ichthyosis is not seen in other neuro-ichthyotic disorders. However, the sequential appearance of these clinical signs often delays the diagnosis of MSD.
Brain MRI shows hyperintense T-2 signals in posterior periventricular and subcortical white matter. X-ray findings of chondrodysplasia punctata may be seen. Urine glycosaminoglycans and sulfatides are often elevated. The molecular diagnosis is confirmed by measuring sulfatase enzyme activities in leukocytes and sequencing the SUMF1 gene. There appears to be a correlation between SUMF1 genotype and clinical severity.17
Steroid Sulfatase Contiguous Gene Deletion
Contiguous gene deletions of the short arm of the X chromosome (Xp22.3) involving steroid sulfatase (STS) and neighboring genes can present with ichthyosis and neurologic symptoms.18,19
Small deletions in STS are a frequent cause of isolated steroid sulfatase deficiency (X-linked ichthyosis), a common disorder that affects 1 in 1500 males and presents with ichthyosis in the first weeks of life. This enzyme normally metabolizes cholesterol sulfate, which accumulates in the skin of patients and causes the ichthyosis. Larger contiguous gene deletions of Xp22.3 can involve several notable genes for mental retardation, short stature, chondrodysplasia punctata, and Kallman’s syndrome (hypothalamic hypogonadism and anosmia).18,19 Depending on the size of the microdeletion of Xp22.3, patients can present with ichthyosis and any combination of gene-related symptoms. Xp22.3 deletions can be detected by microarray comparative genomic hybridization or fluorescent in situ hybridization (FISH) using a STS probe.
Glycoprotein Synthesis Disorders: Dolichol Kinase and Steroid 5α-Reductase Type-3 Deficiency
Two inherited disorders of glycoprotein synthesis with prominent neuroichthyotic symptoms have recently been recognized. Both disorders are caused by mutations in genes that are necessary for the synthesis of dolichol-phosphate, which is a phosphorylated very long-chain isoprenoid lipid alcohol that functions as the N-glycan acceptor for glycoprotein biosynthesis.
DOLK (or DK1) codes for dolichol kinase, an enzyme that catalyzes the final step in synthesis of dolichol-phosphate. Mutations in DOLK have been described in four patients, who exhibited ichthyosis, hypotonia, seizures, tetraplegia, microcephaly, nystagmus, and alopecia.20 Some patients had dilated cardiomyopathy. All died in the first year of life.
Patients with deficiency of steroid 5α-reductase type-3, which catalyzes the penultimate step in dolichol-phosphate synthesis, have clinical features of early visual impairment with various eye malformation (hypoplasia or colobomas of the retina and iris, congenital cataracts, and optic atrophy), nystagmus, cerebellar hypoplasia, ataxia, hypotonia, mental retardation, and ichthyosis or dry skin.21 Some had heart malformations. Mutations in the SRD5A3 gene are causative.
The pathogenic mechanisms in both disorders are not clear, but undoubtedly involve deficient synthesis of key glycoproteins in the skin, brain, and other organs. The diagnosis can be supported by detecting abnormally glycosylated serum transferrin, but requires confirmatory mutation analysis of the DOLK or SRD5A3 genes. There is no effective therapy for either disease.
Keratitis-Ichthyosis-Deafness Syndrome
Keratitis, ichthyosis, and deafness (KID) syndrome is a rare sporadic or autosomal dominant congenital disorder that is caused by mutations in the GJB2 gene encoding the gap junction protein connexin-26.22,23 Gap junctions are composed of hexameric connexin proteins that form channels to allow exchange of ions, metabolites, and small proteins between the cytoplasm of adjacent cells. Mutated connexin-26 results in functional defects in gap junctions, which allow increased calcium influx and cell necrosis.24 Connexin-26 mutations are also a common cause of genetic nonsyndromic deafness.
KID patients develop a vascularizing keratitis that leads to decline in visual acuity and possibly blindness.24 Sensorineural deafness is the primary neurologic manifestation. Cutaneous findings include ichthyosiform erythroderma, palmoplantar keratoderma, erythematous verrucous lesions, and an aged facial appearance. Scarring alopecia and onycho-dystrophy are frequently present. Patients are at increased risk for developing squamous cell carcinomas and inflammatory nodules.23 The diagnosis is based on dermatologic, ophthalmologic, and hearing evaluations, and confirmed with molecular analysis of GJB2. No specific therapy exists for the disease. A significant number of familial cases have been reported and the possibility of germinal mosaicism should be considered during genetic counseling.23
Disorders of Vesicle Trafficking: CEDNIK Syndrome, MEDNIK Syndrome, and ARC Syndrome
CEDNIK Syndrome
CEDNIK syndrome is a rare, autosomal recessive disease named for its defining features of cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma.25,26 The clinical symptoms of the patients progress with age. During the first 4 months, patients exhibit roving eye movements, poor head and trunk control, microcephaly, and failure to thrive. Facial dysmorphisms are apparent, including elongated faces, antimongolian eye slant, mild hypertelorism, and a flat broad nasal root. Later in the first year of life, ichthyosis and palmoplantar keratoderma appear. Psychomotor retardation becomes apparent between 8 and 15 months. MRI of the brain displays corpus callosum abnormalities and cortical dysplasia with pachygyria and polymicrogyria. Loss of deep tendon reflexes is associated with low amplitude responses on peripheral nerve conduction studies. Muscle biopsies show neurogenic atrophy. Ophthalmologic examination reveals hypoplastic optic disks and macular atrophy. Many patients do not survive beyond childhood.
CEDNIK syndrome is caused by mutations in SNAP29 that leads to decreased soluble n-ethylmaleimide sensitive factor receptor (SNARE) protein SNAP29 that mediates endocytic vesicle trafficking between organelles and plasma membrane.27 The decrease in SNAP29 leads to abnormal endocytic vesicle maturation, fusion, and secretion in tissues.25 Lamellar body vesicles in the skin do not mature and fuse properly with the plasma membrane to release their cargo membrane contents between the stratum granulosum and stratum corneum, which results in diminished stratum corneum membranes and a leaky epidermal water barrier. SNAP29 deficiency also results in defective endocytic recycling of β1-integrin required for cell migration, which may underlie the neuronal migration defect in CEDNIK syndrome.27 The diagnosis requires DNA analysis of SNAP29. No specific therapy exists.
MEDNIK Syndrome
MEDNIK syndrome is an autosomal recessive disease characterized by mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma.28 The patient’s facial features can appear mongoloid with a high forehead. Additional cutaneous symptoms include erythrodermia and erythrokeratodermia variabilis. Neurologic deficits include psychomotor retardation, hypotonia, peripheral neuropathy, and sensorineural deafness. Gastrointestinal symptoms such as congenital diarrhea may be present. Patients have elevated levels of very long-chain fatty acids, suggesting peroxisomal dysfunction.
The disease was first described in four French-Canadian families who segregated a founder mutation in AP1S1, which encodes a subunit of adapter protein AP-1 protein complex that participates in endocytic vesicle assembly, protein cargo sorting, and vesicular trafficking. Like CEDNIK syndrome, the mechanisms for ichthyosis and impaired development of various neural networks may be related to abnormal endocytic vesicle trafficking. The diagnosis of MEDNIK syndrome requires DNA analysis of AP1S1. There is no specific therapy.
ARC Syndrome
Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome is a rare, autosomal recessive multisystem disorder caused by mutations in the VPS33B gene, which codes for a protein that interacts with SNARE proteins and functions in intracellular protein and vesicle trafficking.29 One-half of ARC patients have associated ichthyosis.30 The arthrogryposis is thought to be secondary to in utero muscle atrophy from peripheral denervation. Autopsy results have demonstrated alterations in the motor neurons of the anterior horn of the dorsal spinal cord.31 Neurologic symptoms include hypotonia and global developmental delay. MRI of the brain may reveal agyria and loss of sulci consistent with defective neuronal migration. Renal tubular dysfunction ranges from isolated renal tubular acidosis to a more severe renal Fanconi syndrome with glucosuria, proteinuria, aminoaciduria, phosphaturia, and bicarbonate wasting. A kidney ultrasound typically shows nephrocalcinosis or small dysplastic kidneys. Cholestasis with elevated transaminases and hyperbilirubinemia completes the triad of ARC syndrome. Liver biopsies show a wide spectrum of changes including paucity of bile ducts, lipofusion deposition, bile plugs, bile duct proliferation, giant cell hepatitis, and portal tract fibrosis. Associated dysmorphic features include prominent occiput, posteriorly angulated and low set ears, flattened nasal bridge, upslanting palpebral fissures, simian crease, high arched palate, beaked nose, small anterior fontanel, lax skin, low implantation of the thumb, and cryptorchidism. Frequent complications of the disease include hypernatremic dehydration and polyuria. Most patients with this disorder die before 1 year of age from sepsis, severe dehydration, or acidosis.31 The diagnosis is confirmed by DNA analysis.
Trichothiodystrophy (TAY SYNDROME)
Trichothiodystrophy (TTD) is a rare, autosomal recessive neuroectodermal disease that features brittle hair and a variety of skin and systemic abnormalities.32 Patients have mutations in one of four genes (XPD, XPB, P8/TTDA, TTDN1) resulting in abnormal production of a multiprotein complex that functions as transcription factor-IIH (TFIIH) and is involved in DNA excision repair.33 TFIIH functions in both basal and activated transcription of many genes expressed in skin and brain. The nature and severity of symptoms in TTD correlate with the amount of dysfunctional TFIIH and reduced gene transcription.
Affected infants may have low birth weight and a collodion membrane at birth.32 Neurologic abnormalities include developmental delay, microcephaly, and ataxia. Neuroimaging reveals dysmyelination, cerebellar atrophy, and dilated ventricles. Brittle, sulfur-deficient hair with a “tiger-tail” banding under polarized light microscopy is the defining clinical sign. Additional findings include ichthyosis (65%), short stature, photosensitivity, and increased incidence of infections. Clinical features of TTD, such as age of clinical symptom and mortality, vary greatly. Mortality rate is 20-fold higher than the general population. Infection, especially sepsis, is the most common cause of death.
TDD is usually suspected from the constellation of clinical features, including the characteristic hair abnormality. DNA analysis will show mutations in XPD, XPB, P8/TTDA or TTDN1. Treatment of TTD is supportive and multidisciplinary. Prevention of infection is an important component of effective management.
Disorders with Low Incidence of Ichthyosis and Neurologic Disease
Some disorders are associated with a low (<25%) incidence of ichthyosis and variable neurologic symptoms. These include rhizomelic chondrodysplasia punctata and X-linked chondrodysplasia punctata (Table 1).
Neuro-Ichthyotic Syndromes of Unknown Etiology
Several neuroichthyotic syndromes for which no etiology is known have been reported (see Online Mendelian Inheritance in Man: http://www.ncbi.nlm.nih.gov/omim). Many of these disorders are less well characterized and some have been reported only in a single pedigree.
Diagnostic Approach to the Neuro-Ichthyoses
The diagnosis of a neuro-ichthyotic disorder is typically delayed until the neurologic and cutaneous symptoms appear together. Most patients present with isolated skin manifestations at birth or later in infancy, but in the absence of other systemic findings, a consulting dermatologist erroneously concludes that the patient has a form of pure ichthyosis. Similarly, in some disorders, neurologic symptoms may precede the onset of the skin disease. The recognition that both organ systems are affected is usually the key to diagnosing these diseases.
The diagnostic approach for patients with neuro-ichthyosis is outlined in Table 2. The physician should begin by collecting as much clinical information as possible using Table 1 as a guide. A clinical geneticist should be consulted to evaluate family history and provide expertise in dysmorphology. Consultations by ophthalmologists and audiologists are mandatory to determine whether ocular abnormalities or deafness exist. Additional clinical and laboratory tests are particularly useful to define the extent of neurologic disease and narrow down the differential diagnosis. Brain MRI and MRS should be done on all patients to reveal congenital dysplasia, white matter disease, or abnormal lipid peaks. For appropriate patients, cardiovascular evaluation with echocardiogram and electrocardiogram is indicated. A skin biopsy for routine histologic analysis with light microscopy is of limited value for diagnosing most of the ichthyoses because these diseases typically exhibit nonspecific findings of hyperkeratosis. However, a search for lipid inclusions in the skin is worthwhile, and electron microscopy may reveal characteristic structural abnormalities in some disorders.34 Routine laboratory testing should include serum transaminases, creatine kinase, and examination of a blood smear to search for vacuolated leukocytes, which is a diagnostic finding for NLSDI. Several more-specialized biochemical tests, including glycosylated transferrin, lysosomal enzymes, and peroxisomal markers, should help pin down the diagnosis or justify more targeted genetic testing. Ultimately, mutation analysis is necessary for the diagnosis of many of these diseases.
Table 2.
Diagnostic Approach to Evaluating a Patient with Neuro-Ichthyosis
| 1. Clinical Evaluations | 2. Laboratory Investigations | 3. Confirmatory Testing |
|---|---|---|
| Genetic evaluation Ophthalmologic exam Audiology evaluation with BAER Brain MRI with MRS EMG/NCV Bone X-rays, as appropriate echocardiogram/ECG, as appropriate |
Blood smear for vacuolated leukocytes CK, transaminases Enzyme measurements (lysosomal sulfatases, glucocerebrosidase, fatty aldehyde dehydrogenase) Urine glycosaminoglycans and sulfatides Glycosylated transferrin Skin biopsy for lipid inclusions, histology, and EM Phytanic acid, erythrocyte plasmalogens, very long-chain fatty acids Hair microscopic examination |
DNA mutation analysis Comparative genomic hybridization (microarray) |
BAER, brainstem auditory-evoked responses; EM, electron microscopy; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; EMG, electromyography; NCV, nerve conduction velocity; ECG, electrocardiogram; CK, creatine kinase.
Despite a thorough clinical and laboratory workup, a significant proportion of neuro-ichthyotic patients remain undiagnosed at this time. In the absence of a positive family history, it is possible that a patient may have two distinct diseases affecting the skin and nervous system, especially if consanguinity exists. With thorough clinical characterization and the application of next-generation DNA sequencing to these patients, however, we can expect new neuro-ichthyotic diseases to be discovered at an increasing pace.
Acknowledgments
This work was supported in part by grant R01 AR044552 from the National Institute of Arthritis, and Musculoskeletal and Skin Diseases.
References
- 1.Rizzo WB. Sjögren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency. Mol Genet Metab. 2007;90(1):1–9. doi: 10.1016/j.ymgme.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willemsen MA, Van Der Graaf M, Van Der Knaap MS, et al. MR imaging and proton MR spectroscopic studies in Sjögren-Larsson syndrome: characterization of the leukoencephalopathy. AJNR Am J Neuroradiol. 2004;25(4):649–657. [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo WB, Carney G. Sjögren-Larsson syndrome: diversity of mutations and polymorphisms in the fatty aldehyde dehydrogenase gene (ALDH3A2) Hum Mutat. 2005;26(1):1–10. doi: 10.1002/humu.20181. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo WB, S’Aulis D, Jennings MA, Crumrine DA, Williams ML, Elias PM. Ichthyosis in Sjögren-Larsson syndrome reflects defective barrier function due to abnormal lamellar body structure and secretion. Arch Dermatol Res. 2010;302(6):443–451. doi: 10.1007/s00403-009-1022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demerjian M, Crumrine DA, Milstone LM, Williams ML, Elias PM. Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin-Dorfman syndrome) J Invest Dermatol. 2006;126(9):2032–2038. doi: 10.1038/sj.jid.5700332. [DOI] [PubMed] [Google Scholar]
- 6.Lefèvre C, Jobard F, Caux F, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin - Dorfman syndrome. Am J Hum Genet. 2001;69(5):1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida Y, Cho Y, Moradian S, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of Dorfman-Chanarin syndrome. J Invest Dermatol. 2010;130(10):2497–2499. doi: 10.1038/jid.2010.145. [DOI] [PubMed] [Google Scholar]
- 8.van den Brink DM, Wanders RJ. Phytanic acid: production from phytol, its breakdown and role in human disease. Cell Mol Life Sci. 2006;63(15):1752–1765. doi: 10.1007/s00018-005-5463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanders RJ, Jakobs C, Skjeldal OH. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. Refsum disease; pp. 3303–3321. [Google Scholar]
- 10.Perera NJ, Lewis B, Tran H, Fietz M, Sullivan DR. Refsum’s disease-use of the intestinal lipase inhibitor, Orlistat, as a novel therapeutic approach to a complex disorder. J Obes. 2011;2011 doi: 10.1155/3011/482021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone DL, Carey WF, Christodoulou J, et al. Type 2 Gaucher disease: the collodion baby phenotype revisited. Arch Dis Child Fetal Neonatal Ed. 2000;82(2):F163–F166. doi: 10.1136/fn.82.2.F163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15(2):181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Aldahmesh MA, Mohamed JY, Alkuraya HS, et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89(6):745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasireddy V, Uchida Y, Salem N, Jr, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique ω-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16(5):471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosma MP, Pepe S, Annunziata I, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113(4):445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 16.Dierks T, Dickmanns A, Preusser-Kunze A, et al. Molecular basis for multiple sulfatase deficiency and mechanism for formylglycine generation of the human formylglycine-generating enzyme. Cell. 2005;121(4):541–552. doi: 10.1016/j.cell.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Schlotawa L, Ennemann EC, Radhakrishnan K, et al. SUMF1 mutations affecting stability and activity of formylglycine generating enzyme predict clinical outcome in multiple sulfatase deficiency. Eur J Hum Genet. 2011;19(3):253–261. doi: 10.1038/ejhg.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curry CJ, Magenis RE, Brown M, et al. Inherited chondrodysplasia punctata due to a deletion of the terminal short arm of an X chromosome. N Engl J Med. 1984;311(16):1010–1015. doi: 10.1056/NEJM198410183111603. [DOI] [PubMed] [Google Scholar]
- 19.Bick DP, Schorderet DF, Price PA, et al. Prenatal diagnosis and investigation of a fetus with chondrodysplasia punctata, ichthyosis, and Kallmann syndrome due to an Xp deletion. Prenat Diagn. 1992;12(1):19–29. doi: 10.1002/pd.1970120104. [DOI] [PubMed] [Google Scholar]
- 20.Kranz C, Jungeblut C, Denecke J, et al. A defect in dolichol phosphate biosynthesis causes a new inherited disorder with death in early infancy. Am J Hum Genet. 2007;80(3):433–440. doi: 10.1086/512130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morava E, Wevers RA, Cantagrel V, et al. A novel cerebello-ocular syndrome with abnormal glycosylation due to abnormalities in dolichol metabolism. Brain. 2010;133(11):3210–3220. doi: 10.1093/brain/awq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard G, Rouan F, Willoughby CE, et al. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70(5):1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazereeuw-Hautier J, Bitoun E, Chevrant-Breton J, et al. Keratitis-ichthyosis-deafness syndrome: disease expression and spectrum of connexin 26 (GJB2) mutations in 14 patients. Br J Dermatol. 2007;156(5):1015–1019. doi: 10.1111/j.1365-2133.2007.07806.x. [DOI] [PubMed] [Google Scholar]
- 24.Terrinoni A, Codispoti A, Serra V, et al. Connexin 26 (GJB2) mutations, causing KID syndrome, are associated with cell death due to calcium gating deregulation. Biochem Biophys Res Commun. 2010;394(4):909–914. doi: 10.1016/j.bbrc.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 25.Sprecher E, Ishida-Yamamoto A, Mizrahi-Koren M, et al. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am J Hum Genet. 2005;77(2):242–251. doi: 10.1086/432556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs-Telem D, Stewart H, Rapaport D, et al. CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br J Dermatol. 2011;164(3):610–616. doi: 10.1111/j.1365-2133.2010.10133.x. [DOI] [PubMed] [Google Scholar]
- 27.Rapaport D, Lugassy Y, Sprecher E, Horowitz M. Loss of SNAP29 impairs endocytic recycling and cell motility. PLoS ONE. 2010;5(3):e9759. doi: 10.1371/journal.pone.0009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montpetit A, Côté S, Brustein E, et al. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 2008;4(12):e1000296. doi: 10.1371/journal.pgen.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gissen P, Johnson CA, Morgan NV, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36(4):400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- 30.Choi HJ, Lee MW, Choi JH, Moon KC, Koh JK. Ichthyosis associated with ARC syndrome: ARC syndrome is one of the differential diagnoses of ichthyosis. Pediatr Dermatol. 2005;22(6):539–542. doi: 10.1111/j.1525-1470.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Sa’da O, Barbar M, Al-Harbi N, Taha D. Arthrogryposis, renal tubular acidosis and cholestasis (ARC) syndrome: two new cases and review. Clin Dysmorphol. 2005;14(4):191–196. [PubMed] [Google Scholar]
- 32.Faghri S, Tamura D, Kraemer KH, Digiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45(10):609–621. doi: 10.1136/jmg.2008.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanini M, Botta E, Lanzafame M, Orioli D. Trichothiodystrophy: from basic mechanisms to clinical implications. DNA Repair (Amst) 2010;9(1):2–10. doi: 10.1016/j.dnarep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Elias PM, William ML, Crumrine D, Schmuth M. Current Problems in Dermatology. Vol. 39. Basel: Karger; 2010. Ichthyosis: clinical, biochemical, pathogenic and diagnostic assessment. [Google Scholar]