Summary
The paper discusses the means in which exercise hormonal data are transformed and expressed as a way to deal with the inherent variability in endocrine measurements. Specifically, the aim of the paper is to present an alterative transformation adjustment method for expressing the exercise responses of hormones, especially those which exhibit a diurnal behaviour in their circulating concentrations. The suggested alterative adjustment method attempts to account for the influence of the “range effect” on diurnal hormonal data and the subsequent effects it may have on statistical, and perhaps physiological, outcomes and data interpretation.
Keywords: Endocrinology, Hormones, Exercise, Stress, Data management
Introduction
An acute session of exercise provokes significant changes in the circulating levels of many hormones and, typically, the magnitudes of such responses are modulated primarily by the intensity and/or duration of the exercise session (i.e., “exercise dosage”) [3]. In the majority of exercise-related research studies, hormonal levels are reported as either absolute values (i.e., raw scores) or with respect to some value obtained before exercise (e.g., such as percent change scores, i.e. post-exercise vs. pre-exercise values), or as a delta scores (post-exercise -pre-exercise, etc.). Such transformation methods are a common approach and are used in an attempt to deal with the inherent variability found in many human-based endocrinological measurements [1,6,9,11].
Several hormones in humans display large diurnal hormonal concentration shifts in the blood during the course of a 24-hour period [7]. For example, in a normal healthy person (male or female) it would not be unusual for circulating basal cortisol levels to range from 5 to 25 (± 5) μg/dL during the course of a single day. The concentration of cortisol varies because of the endogenous rhythm of hypothalamic secretion (i.e. corticotropin releasing factor), which is a key controller of cortisol release [7,11]. This type of rhythmical oscillations in the circulating blood values is common in hormones associated with hypothalamic control [11]. In response to a stressful stimulus, the cortisol concentration will readily increase to a level of 30–35 μg/dL [8,9]. It is important to recognise, however, that blood cortisol concentrations of 60, 70 or 80 μg/dL or greater would not occur, or be expected regardless of the stimulus, unless an individual had a serious medical condition such as an adrenal gland abnormality, e.g., Cushing’s Syndrome [8]. In other words, hormonal responses are not infinitely continuous and there is a limit to the normal responses of a hormone at rest or in response to a stimulus within healthy individuals. Research suggests this type of limitation in both the normal basal range of responses due to rhythmical oscillations or in response to a stimulus is found for the blood values of certain circulating hormones (e.g., growth hormone, prolactin, testosterone) [1,6,8,9,11].
These occurrences (limitation in response) have the ability to impact upon exercise research looking at endocrinological measurements. To illustrate this point, for example, some research evidence indicates if exercise perturbations of an equal stimulus (dosage) are given to an individual at two distinctly different time points within the normal basal hormonal diurnal pattern, the magnitude of the hormonal response measured in the blood to the exercise can vary. This suggests that the variability of the measured response may be affected [4,5]. Accordingly, these findings would imply that for some hormones the magnitude of a response to exercise is influenced not only by the exercise intensity and duration, but also by the time of day when the exercise is conducted and by the basal hormonal level of the diurnal response at that time. Specifically, observations from our laboratory as well as those of others have led our research group to surmise that hormonal responses to exercise can be reduced when basal hormonal levels are elevated (near ‘acrophase’), and enhanced when the basal levels are reduced/lower (near ‘nadir’) [5, 9,10].
Figure 1 is an example illustrating this point in the case of a hypothetical hormone referred to here as ‘H’. In the figure, two equal exercise dosage perturbations are given at different time points during the day (A - high diurnal basal circulating level; B - low diurnal basal circulating level). In situation A, the exercise produces a 5-unit peak response change in ‘H’ from the pre-exercise level. This peak response nearly reaches the upper limit of the normal range of values for this hypothetical hormone. In situation B the exercise produces a larger (10 units) peak response change in ‘H’ from the pre-exercise level, but this peak response is still more than 15 units from the upper limit of possible responses. In other words, in situation B there is a much greater potential increase for peak responses in the normal physiologic range in this situation. Conversely, the potential variability of the response in situation A is suppressed due to the normal physiological upper limit for this hormone being reached. In statistical terminology, this is the consequence of the “range effect”. This term is statistically defined as a situation where values of a variable have an upper or lower limit of response, and this limit is encountered during the course of an observation [12].
Fig. 1.
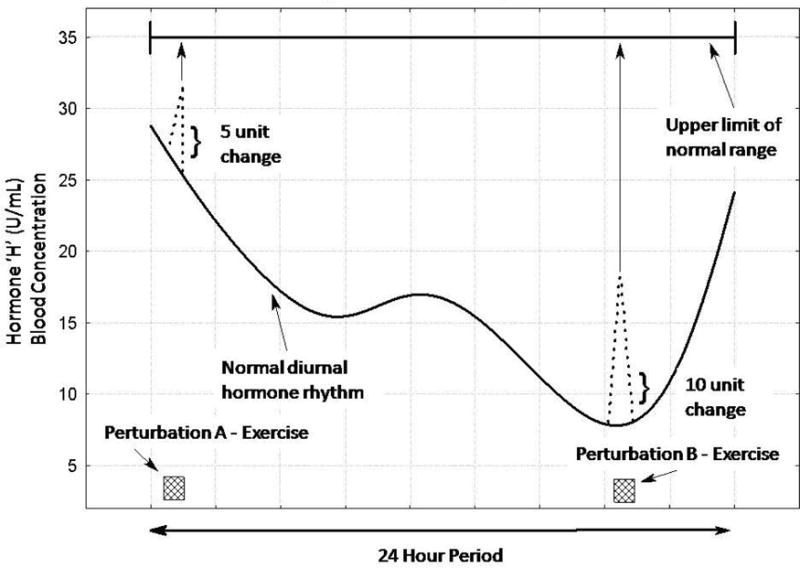
An example of the influence of diurnal rhythms and exercise on a hypothetical hormone (named ‘H’). Equal exercise dosage perturbations (exercise bouts A and B) are given at different time points during the day. The changes in the normal hormonal levels due to the exercise are depicted as the dotted lines. The arrows from the peak exercise response of each perturbation to the upper limit of the normal hormonal range (top of figure) represent the potential response levels, and illustrate that in perturbation A there is a compression of the possible data variability (i.e., ceiling effect) compared to perturbation B.
Within the area of exercise endocrinology the most common influence of exercise per se on hormonal values is to increase circulating levels [7]. Few hormones actually decrease with exercise of increasing duration and/or intensity (insulin being one notable exception) [7]. Therefore, the most likely impact of the range effect on hormonal responses would be with regards to the upper limit of response; this is called the “ceiling effect” on high-end responses of the “range effect”. With regard to low end responses, the impact of the range effect is referred to as the “basement effect”.
Range Effect
Range effects influence data in two distinct ways. First, by limiting the values of the highest (or lowest) data points, the range effect decreases the difference between treatments means (in comparative research designs). Consequently, the apparent effects of independent variables are potentially lessened. This in turn, in most situations, leads to an increased risk of a Type II statistical error (i.e. declaring that treatment means are not significantly different when in fact they potentially could be). Secondly, with the range effect the variability of scores within the affected treatment is reduced. Because most inferential statistical analysis rely upon estimating variance due to random causes from the variability of scores within a treatment, the statistical validity of certain analyses can be compromised by this reduced variability - leading to ambiguous results and potentially a Type I statistical error (i.e. declaring that treatment means are significantly different when in fact they might not be) [12].
As a means of dealing with this possible bias within the exercise responses of hormones exhibiting diurnal rhythms, and thus probably being influenced by the “range effect”, it may be necessary to adjust the data values differently from the traditional transformation methods noted earlier. Over the last several decades our laboratory, in dialogue with scientists from other countries, have examined different ways of adjusting and transforming hormonal data in order to get a clearer understanding of the physiological meaning of our research outcomes. These dialogues came about due to observations of greater than expected variability in the physiologic hormonal responses and their effects occurring across exercise trials, even when strict scientific controls were enforced in conducting experiments. These ongoing dialogues involved a “trial and error” pragmatic process which has ultimately lead to the suggestion of the following alternative adjustment method as a possible means for examining diurnal hormones responses in exercise studies. The data for the hypothetical hormone ‘H’ data in Figure 1 is used for illustrative purposes to explain the adjustment.
Alternative (Transformation) Adjustment
Observed physiological range of hormone ‘H’ equals 0 to 35 units (U/mL), i.e., high ‘H’ response = 35 U/mL and low ‘H’ response = 0 U/mL;
Range max difference, i.e., (High response - Low response) = (35 U/mL − 0 U/mL) = 35 U/mL
Perturbation A
Pre-concentration = 27 U/mL; Post-concentration = 32 U/mL; Change from post- to pre-concentration = 5 U/mL
Adjustment = ((Post-concentration - Pre-concentration)/([Range max - Pre-concentration]) × 100) = ((32 U/mL − 27 U/mL)/([35 U/mL − 27 U/mL]) × 100) = 62.5 %
Perturbation B
Pre-concentration = 8 U/mL; Post-concentration = 18 U/mL; Change from post- to pre-concentration = 10 U/mL
Adjustment = ((Post-concentration - Pre-concentration)/([Range max - Pre-concentration]) × 100) = ((18 U/mL − 8 U/mL)/([35 U/mL − 8 U/mL]) × 100) = 37.0 %
Table 1 presents a comparison of several traditional means of adjusting and transforming hormonal values as well as the suggested alternative adjustment method which attempts to account for the influence of the range effect. Review of the comparative results in the table reveals that the interpretation of the size of a hormonal change that occurred in exercise perturbation A versus B is dramatically different. Most certainly, each method depicts an increase occurring in the representative hormonal values. However, the alternative adjustment method demonstrates that the observed relative change in perturbation A is actually much larger than in B representing, in fact, a reversal of the approaches 1, 2 and 3.
Table 1.
Comparison of several common hormonal adjustment-transformation methods
| Adjustment Methods | Perturbation A | Perturbation B |
|---|---|---|
| 1. Delta value = (Post - Pre) | 5 U/mL | 10 U/mL |
| 2. Percentage = (Post/Pre) × 100 | 118.5 % | 225.0% |
| 3. Delta percentage= ((Post - Pre)/Pre) × 100 | 18.5% | 125.0% |
| 4. Alternative adjustment | 62.5% | 37.0% |
The suggested alternative adjustment method for the transformation of hormonal data presented here is most certainly not appropriate for all endocrine measurements. For example, if there is no strong diurnal shift in the basal levels of a hormone across the course of day, then it would not be necessary to apply the transformation (see [7] for a discussion of hormones with diurnal shifts; e.g. testosterone, growth hormone, IGF-1, etc.). Also, the upper range for responses necessary to calculate the “range max difference” value may not be known or have been determined for some hormones, invalidating use of the proposed alternative adjustment method (N.B., a thorough literature review of exercise endocrinology research can reveal range max difference values for many hormones, or the laboratories may choose to determine their own values through empirical means). Many hormones are released in a pulsatile manner, which can complicate examination of exercise responses [7]. Provided a blood sampling protocol is frequent enough to account for pulses within a specific hormone, the alternative adjustment could be useful in examining data, but if infrequent sampling is employed the adjustment may not be applicable. Furthermore, the alternative adjustment would also need to be limited in its application to hormonal responses from only normal healthy individuals; if not, the upper range for response could be extremely variable and the range max value necessary for the calculations not standardised, thus compromising validity of the calculation of the adjustment.
Regardless of how exercise hormonal data are adjusted or expressed, it is important to recognise that the ultimate physiological effect of any observed hormonal change is governed by several factors, which are fundamental to interpretation of the response-effect relationship. These factors that govern the magnitude of the response-effect in hormonal changes include: (1) the concentration of the hormone that is available to bind to receptors, (2) the number of competent target cells that express functional receptors, and (3) the sensitivity of the target cells to hormonal stimulation. This latter factor is, in turn, dependent upon: (a) the number of functional target tissue receptors that are expressed, (b) the affinity of the receptor for the hormone, (c) the status of post-receptor amplification mechanisms, and (d) the status and abundance of effector molecules such as enzymes, ion channels, or contractile proteins at the target tissue [2,10]. It is important to realise the suggested alternative adjustment method only allows the researcher to examine the magnitude of the concentration change but reveals no insight into the other critical factors influencing the hormonal response effectiveness.
Concluding Remarks
This paper suggests a different means by which hormonal responses to exercise could be expressed using an alternative adjustment (transformation) method accounting for basal diurnal rhythmical fluctuations and the potential range of responses for a hormone. The suggested adjustment method should be viewed as provisional (“a work in development”) and one that will need refinement over time. Nevertheless, it is presented as a means to stimulate exercise scientists to “think outside the box” concerning their hormonal data. That is, the intent of the suggested adjustment method is to provide an alternative means for exercise scientists to examine and view certain hormonal data. This alternative means may provide for a different view of findings and spark new thoughts on the effects exercise, or any similar stimulus, have on the endocrine system in humans. Such different perspectives, it is hoped, might lead to novel insights into the human response to physical stress and the adaptive process to such stress exposure.
References
- 1.Dantas EHM, Pires T, Castro JC, Bastos FAC, Santos CAS, Caetano LF. Serum levels of IGF-1 in elderly women engaged in various motor activities. Phys Educ Sport. 2008;52:81–83. doi: 10.2478/v10030-008-0017-3. [DOI] [Google Scholar]
- 2.Goodman HM. Endocrinology concepts for medical students. Adv Physiol Educ. 2001;25:213–224. doi: 10.1152/advances.2001.25.4.213. [DOI] [PubMed] [Google Scholar]
- 3.Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocr Metab. 2006;1:783–792. doi: 10.1586/17446651.1.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackney AC, Premo MC, McMurray RG. Influence of aerobic versus anaerobic exercise on the relationship between reproductive hormones in men. J Sports Sci. 1995;13:305–311. doi: 10.1080/02640419508732244. [DOI] [PubMed] [Google Scholar]
- 5.Hackney AC, Viru A. Twenty-four hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin Physiol. 1999;19:178–182. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 6.Hübner-Woźniak E, Ochocki P. Effects of training on resting plasma levels of homocysteine and C-reactive protein in competitive male and female wrestlers. Biomed Hum Kinet. 2009;1:42–46. doi: 10.2478/v10101-009-0011-0. [DOI] [Google Scholar]
- 7.McMurray RG, Hackney AC. Endocrine responses to exercise and training. In: Garrett W, Kirkendall DT, editors. Exercise and Sport Science. Lippincott, Williams & Wilkins; Philadelphia: 2000. pp. 135–161. [Google Scholar]
- 8.Tietz NW. Clinical Guide to Laboratory Tests. Saunders Publishing; Philadelphia: 1990. pp. 78–130. [Google Scholar]
- 9.Viru A, Viru M. Biochemical Monitoring of Sport Training. Human Kinetics Publishing; Champaign IL: 2001. pp. 170–192. [Google Scholar]
- 10.Viru A. Adaptive regulation of hormone interaction with receptors. Exp Clin Endocr. 1991;97:13–28. doi: 10.1055/s-0029-1211033. [DOI] [PubMed] [Google Scholar]
- 11.Widmaier EP. Metabolic feedback in mammalian endocrine systems. Horm Metab Res. 1992;24:147–153. doi: 10.1055/s-2007-1003282. [DOI] [PubMed] [Google Scholar]
- 12.Winer BJ. Statistical Principles in Experimental Design. McGraw-Hill; New York, NY: 1971. pp. 110–210. [Google Scholar]


