Abstract
Electrical stimulation of tissues has many uses in pain management, antibacterial treatment, and wound healing. The electric field stimulates epidermal migration and increases fibroblast cell proliferation. Here we show the effects of electrical field (EF) stimulation of human dermal fibroblasts (HDF) on the expression of collagen, elastin, and collagenase (MMP1; matrix metalloproteinase 1). The effects of EF stimulation are evaluated in terms of changes in cell morphology and extracellular matrix (ECM) protein expression, defined as intracellular concentration of collagen, elastin, and MMP1. HDF are stimulated in a bioreactor using square wave voltage pulses for up to 24 h. The pulse voltage (0–10V), pulse bias (0, +), pulse time (10–1000 ms), and rest time (0.1–10 s) were varied. We show that expression of collagen, elastin, and MMP1 increases in response to applied EF. The intracellular concentration of ECM proteins more than doubles depending on stimulation conditions with a threshold of effective stimulation above 3V/cm. The short time voltage pulses used for EF stimulation are more effective, while the rest time between pulses has a small effect on intracellular concentration of collagen, MMP1 and elastin. The previously studied HDF stimulation with chemical factors (i.e. TNF-α, TGF-β) shows negative correlation between concentration of collagen and MMP1. Contrary to that observation, we show that EF stimulation causes increase in the intracellular concentration of both collagen and MMP1. We also demonstrate that the transdermal stimulation of HDF in subcutaneous tissue is possible, thus it might be utilized in the future to improve the wound healing and tissue regeneration process.
Keywords: Electrical stimulation, human dermal fibroblasts, ECM proteins, collagen synthesis
1. Introduction
When a wound occurs, the epithelial barrier is disrupted resulting in transepithelial potential [2]. In the process of natural wound healing, the epithelial cells migrate to the wound in response to chemical cues and electrical field (EF) generated by the wound. It has been shown that the cell migration in vitro can occur in response to externally applied EF. The direction of cell migration is the same as it would be after the wound formation in vivo. Thus the external electric field could facilitate the wound healing [2,3].
Electrical stimulation (ES) has been used as one of the treatments as it can accelerate wound healing, reduce infections, improve cellular immunity, and increase perfusion[1]. The direct current (DC), alternating current (AC), and high-voltage pulsed current (HVPC) has been employed in the past for tissue stimulation [1].
Dermal fibroblasts play a crucial role in several phases of the wound healing process [4]. Their major function is to synthesize and deposit large quantities of proteins (i.e. collagen I, II, fibronectin, elastin) that form extracellular matrix (ECM) needed to form a new tissue [4–6]. In addition, dermal fibroblasts are involved in tissue remodeling and cell migration that requires synthesis of matrix metalloproteinases i.e. MMP1 (matrix metalloproteinase 1) otherwise known as collagenase [7–9]. There are several signaling molecules that are known to regulate the synthesis of ECM proteins and MMPs [10]. If the regulatory process is unbalanced the wound does not heal properly. When the amount of deposited ECM is excessive, keloids and hypertrophic scars can form. On the other hand, when MMPs are upregulated, the wound cannot close properly, and the persistent wound developes, and tissue infection often follows [10].
The cytokines and growth factors form the largest group of signaling molecules involved in healing-related cellular activities [11]. Both cytokines and growth factors were extensively studied in biosynthesis regulation of ECM proteins and tissue remodeling [12–19]. The processes of ECM protein deposition and secretion of MMPs during cell migration and tissue remodeling, can be viewed as opposing; TNF-α downregulates synthesis of collagen I and at the same time upregulates synthesis of MMP1 [12,13]; in addition, TNF-α also downregulates expression of elastin [14]. In contrast, TGF-β upregulates synthesis of collagen in skin fibroblasts and at the same time inhibits the synthesis of MMP1 [18,19]. Recently Wang et al. showed that signal transduction between electro-stimulated skin fibroblasts and cellular response occurs by activation of the TGF-β1/ERK/NF-κB signaling pathway. Consequently, if the TGF-β1/ERK/NF-κB signaling pathway is the only activated signaling pathway responsible for signal transduction, one would expect a negative correlation between expression of collagen and MMP1 in electrically stimulated dermal fibroblasts, [20].
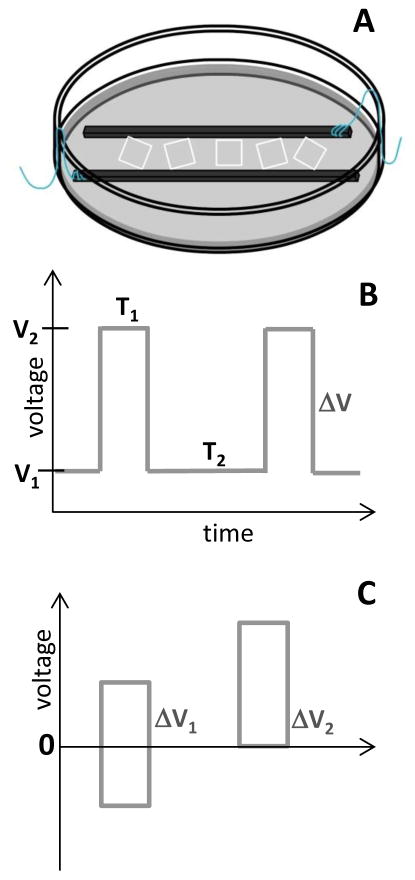
Bioreactors are often used for electrical cell stimulation. There are several designs of the bioreactors used for pulsed eclectic field cell stimulation [21–23]. One of the designs, described in detail by Tandon et al., was employed in our current work (Figure 1A) to perform EF stimulation of human dermal fibroblasts (HDF) [21]. In this design the two graphite electrodes are placed in the Petri dish 1 cm apart and cells are seeded between electrodes. In this configuration, the EF stimulation is applied to the monolayer of HDF cells that are positioned directly between the electrodes.
Figure 1.
Scheme of (A) bioreactor used for HDF stimulation; (B) voltage pulse scheme applied during stimulation; (C) no-bias (ΔV1) and bias (ΔV2) voltage pulse.
If one considers the application of the EF stimulation to wound healing, and tissue regeneration or remodeling, the EF has to penetrate the skin to be effective. The HDF that have to be stimulated are located in subcutaneous tissue between 0.3–0.9 mm below the skin surface [25]. Thus the simple transdermal stimulation of HDF may not be possible due to insulating properties of the skin. One of the previously proposed solutions was bending the skin in a U-shape fold and applying the EF across the fold [26].
Herein we report the effects of pulsed EF stimulation of HDF on ECM proteins synthesis. The reactor was operated by a potentiostat in the two electrode system configuration. The intracellular concentration of collagen I (procollagen), collagenase (MMP1) and elastin, was measured with Sircol Assay and ELISA, respectively, for different conditions of EF stimulation. The effect of EF stimulation on elongation of fibroblasts was quantified. In view of our results we have evaluated the previously proposed mechanisms of signal transduction during the EF stimulation. In addition, we have tested the EF depth penetration across the conductive spacer to establish plausibility of future transdermal stimulation of HDF for the wound healing and tissue regeneration applications.
2. Materials and methods
2.1 Cell Culture
The human dermal fibroblasts (PCS) cells were purchased from the American Type Culture Collection (ATCC) and cultured in a T-25 flask according to the specifications from ATCC. The PCS cells were cultured in Fibroblast Basal Medium (ATCC) supplemented with Fibroblast Growth Kit- Low Serum (ATCC) and 0.5% Penicillin Streptomycin L-Glutamine Mixture (Pen/Strep, Lonza). Once the cells reached an 80% or higher percentage confluence, they were counted (5.0 × 104 cells/cm2) and seeded on 1 cm2 polylysine slides (ThermoFisher Scientific) in 60 mm Petri dishes (Corning). Passages 3–10 were used for all experiments.
2.2 Electrical Stimulation of Cells
A reactor was assembled, as described by Tandon et al., using a petri dish (100 mm), two carbon rods, and platinum wires (Figure 1A) [21]. The platinum wires were attached to the carbon rods and glued 1 cm apart into a petri dish. Three polylysine slides with cells (90% or higher confluence) were transferred into the reactor. The reactor was transferred to a 37 °C incubator and attached to CHI 760B electrochemical workstation. The cells were stimulated with the pulsed electric field where voltage (0–10V), pulse time (10–1000 ms) and, rest time (0.1–10 s) was varied (Figure 1B) in the positive-bias or no-bias voltage mode (Figure 1C). The stimulation cycle was repeated for 0.5 – 24 hours stimulation time.
2.3 Measurements of EF
The field within the bioreactor was measured (multimeter, Klein Tools) by placing two Ag/AgCl electrodes in the reactor with a fixed distance from each other and between the carbon electrodes. Once the voltage was applied to the carbon electrodes, the voltage difference between the Ag/AgCl electrodes was measured independently by a multimeter during the pulse and rest time for several cycles.
To measure the depth of the EF penetration, the glass slide with deposited 1-mm gold electrodes 1.2 cm apart was covered with the spacer, Poly(2-hydroxyethyl methacrylate) (pHEMA) polymer (1% crosslinking, 0.89 ± 0.03 mm thicknesses), soaked in phosphate buffer pH = 7.6 (PBS). The two Ag/AgCl electrodes were placed on top of the spacer between the gold electrodes. Once the voltage was applied to the gold electrodes, the voltage difference between the Ag/AgCl electrodes was read independently from the stimulation voltage by a multimeter. To stimulate the cell in this configuration, polylysine slides with cells (90% or higher confluence) were positioned “upside down” on top of the p-HEMA soaked with cell media and EF stimulation was applied with gold electrodes.
2.4 Quantification of Protein Concentration
The HDF cell cultures on polylysine slides were transferred from the reactor into a 35 mm petri dish (Corning), trypsinized and after neutralization of trypsin, a cell scraper was used to ensure that all cells were removed from the slides and analyzed for survival with Trypan Blue exclusion assay. The cells were placed in a microcentrifuge vial then rinsed in 1 ml PBS three times. To determine the intracellular concentration of ECM proteins HDF cells were lysed using 0.5 ml RIPA buffer for 1 hour followed by centrifugation at 13,000 rpm for 10 min.
2.4.1 Collagen
The Sircol soluble collagen assay kit was employed, and protocol provided by Biocolor Ltd. was followed to determine the intracellular concentration of procollagen. Sircol assay is designed to detect acid-soluble and pepsin-soluble collagens, including newly synthesized collagen. It is a dye binding method where Sircol Dye reagent is binding to the [Gly-X-Y]n helical structure as found in collagens. The method is most specific to mammalian collagens type I–V.
1.0 ml of Sircol Dye Reagent was added to each tube with 100 μl of the sample. The tubes were capped and manually mixed at 5 minute intervals for 30 minutes. The tubes were then transferred to a microcentrifuge and unbound dye was removed by aspiration. 750 μl ice-cold Acid-Salt Wash Reagent was added and the tubes were transferred to a microcentrifuge to spin at 12,000 rpm for 10 minutes. The acid-salt wash reagent was removed by aspiration and 250 μl alkali reagent was added to each tube. The collagen bound dye was released into solution using a vortex mixture. 200 μl from each tube was then transferred to a 96-well plate and read at 555 nm using a plate reader (Thermo Electron Corporation). The quantification was determined in triplicates and compared to a control in which the cells were not electrically stimulated. The concentration of collagen produced was determined by comparing to standard curve and normalized by the number of cells that were lysed for the assay.
2.4.2 Collagenase
An MMP1 Human ELISA (enzyme-linked immunosorbent assay) Kit was utilized and protocol by Abcam was followed to quantify the intracellular concentration of collagenase (MMP1). 100 μl of the lysed electrically stimulated cells were added to 3 wells and 100 μl of the lysed control cells were added to another 3 wells of a 96-well plate. The samples were incubated at 4 °C overnight. The solutions were aspirated and each sample was washed 4 times with 1X Wash Solution. The samples were then incubated with 100 μl of 1X Biotinylated MMP1 detection antibody for 1 hour. The solutions were aspirated and cells washed. The samples were then incubated with 100 μl of 1X HRP-Streptavidin solution for 45 min. The solutions were aspirated and washed. The samples were then incubated with 100 μl of TMB One-Step Substrate Reagent for 30 min. After the addition of 50 μl of Stop solution, the absorbance of the samples were read at 450 nm and the concentration of collagenase was determined from a standard curve and normalized by the number of cells that were lysed for the assay. All experiments were performed in triplicates.
2.4.3 Elastin
A Human Elastin ELISA kit was utilized and the protocol by TSZ ELISA was followed to determine the intracellular concentration of elastin. 100 μl of the samples were added to each well of a 96 well plate and incubated at 37 °C for 30 min. The solutions were aspirated and washed 4 times with 1X Washing solution. 100 μl of 1X Enzyme Conjugate solution were added to each well and incubated at 37 °C for 30 min. The solutions were aspirated and washed.100 μl of TMB Substrate Solution was added to each well and incubated for 15 min. After the addition of 50 μl of Stop solution, the absorbance of the samples were read at 450 nm and the concentration of elastin was determined from standard curve and normalized by the number of cells that were lysed for the assay. All experiments were performed in triplicate.
2.5 Microscopy
The morphology of the HDF was studied using an inverted light microscope (Nikon Eclipse TE2000-U). For imaging HDF cells were fixed on the slides by 10–20 minutes incubation with 4% paraformaldehyde solution and washed. 1 mg/ml Hoechst dye solution was used to stain the nucleus of HDF. The images of HDF cells were taken immediately after the staining and the length and width of the nucleus of fibroblasts were measured using Image J (NIH, open source).
3. Results and Discussion
Human dermal fibroblasts were seeded on the 1cm2 polylysine coated glass slides and after reaching 80% to 90% confluence were placed in the bioreactor and stimulated by applying a voltage pulse (Figure 1). The voltage pulse (0–10V), pulse bias (0, +), pulse time (10–1000 ms) and, rest time (0.1–10 s) were varied. Because collagen is not easily soluble in media, it was detected as procollagen in the cell body before secretion to the media; after the EF stimulation the cells were lysed, and the content of the cells was analyzed to determine intracellular concentration of collagen, MMP1, and elastin. All the detected protein concentrations are reported normalized with respect to the number of cells present in the lysate.
The EF stimulation was performed with potentiostat in the two-electrode system configuration. First we report characterization and performance of the bioreactor, we then discuss effects of EF on cell morphology and protein expression. Lastly, we show the results of EF stimulation across hydrogel spacer.
3.1 Characterization of the bioreactor
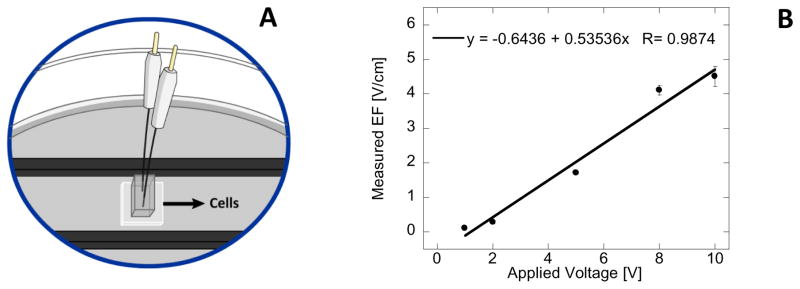
The bioreactor described by Tandon et al. has been used for pulsed EF stimulation of several different cell lines, however the performance details are not usually reported [21]. To measure the EF that is experienced by the HDF cells in the reactor constructed in our laboratory, two Ag/AgCl electrodes were placed and immobilized at the fixed distance from each other (3.31 mm) in the middle of the bioreactor (Figure 2A). The external voltage pulse was applied to the carbon electrodes, followed by the rest time, and the response of sensor Ag/AgCl electrodes was measured with a multimeter during the pulse and the rest time for multiple cycles. After the initial spike, the voltage stabilized and the data was collected. In Figure 2B the average voltage difference between the pulse voltage and rest voltage was calculated and plotted with respect to applied voltage. The data indicates that voltage experienced by the cells is never lower than 54% of applied voltage and that this percent of voltage drop is independent from the applied voltage. The voltage plateau, where data was collected, is observed for times longer than 400 – 600 ms, thus when EF stimulation utilizes short pulses (1Hz frequency with 10% duty cycle) the voltage experienced by HDF cells is likely larger.
Figure 2.
Scheme of EF sensing setup placed in the bioreactor containing two Ag/AgCl electrodes (A); Measured EF with respect of applied voltage within the reactor. Error bars represent standard deviation from 5 measurements (B).
3.2 Morphological changes of human dermal fibroblasts upon EF stimulation
All fibroblasts tend to migrate within the EF, however there are large differences in cell motility between different types of fibroblasts [27,28]. It was shown that EF applied to HDF has to be relatively large (4–30 V/cm) and/or applied for extended periods of time to effectively stimulate directional cell motility [29].
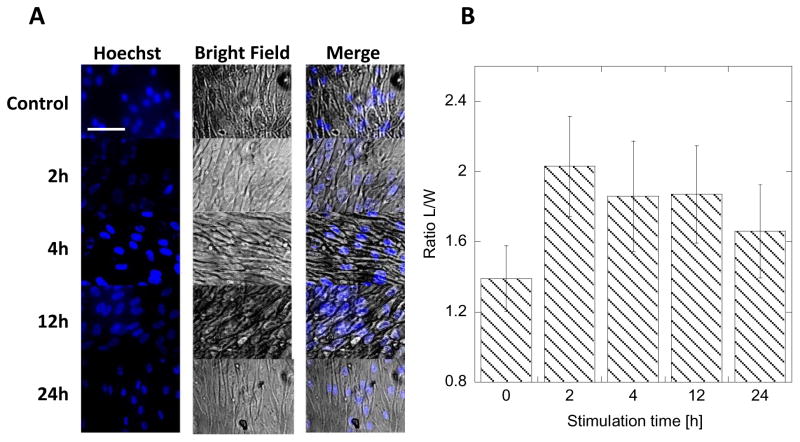
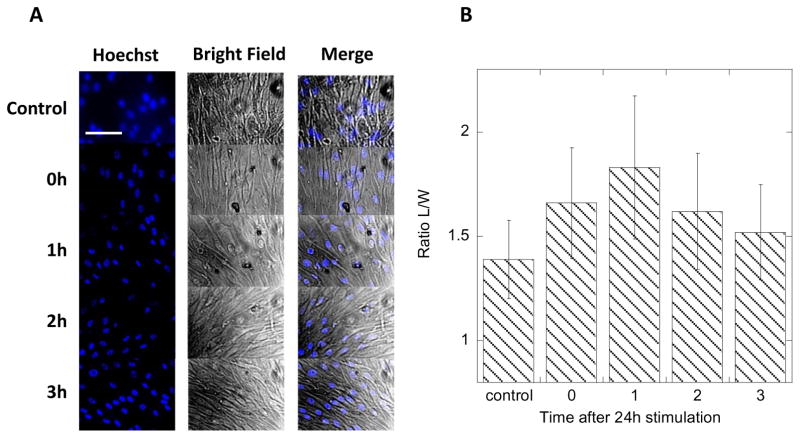
In order to initiate the migration process, cells need to elongate to extend lamellipodia towards the direction of the movement [29]. The elongation causes the shift in organelles. The initial changes in a cell’s shape preparing to migrate are difficult to track due to the irregular shape of fibroblasts and slow migration rate of dermal fibroblasts (<5 μm/h) [28,29]. Thus we hypothesized that observing the changes in the nucleus shape as an effect of EF stimulation will be much easier and faster to detect. To observe the nucleus, the cells were stained with Hoechst blue fluorescent dye that is selective for nucleus only, and light microscopy images of confluent cells stimulated by application of 8V, 1Hz frequency, and 10 % duty cycle over a period of 24h were recorded (Figure 3A). The ratio of nucleus length to width (L/W) was plotted in Figure 3B. It shows that after 2 hours the cells are already preferentially elongated towards the direction of the field. This elongation persists throughout the stimulation time (up to 24h recorded time). There is no statistically significant difference in average L/W in the course of EF stimulation, but a small decrease of average L/W is observed at longer times. We believe that this effect is related to the movement of the cell resulting in partial restoration of the original shape of the nucleus. We have also measured average L/W after the EF stimulation was terminated to determine if HDF are damaged during the EF application. In Figure 4 we show that the shape of the nucleus is restored after 3h of rest (no stimulation) post 24h stimulation (8V applied voltage, 1Hz, 10% duty cycle). This result suggests that the effects of EF stimulation at applied fields are reversible and well tolerated by HDF.
Figure 3.
Fluorescence and bright-field images of HDF stimulated with 8V biased, 1Hz, 10% duty cycle pulse for different periods of time. Bar represents 20 μm (A). The measured ratio of length-to-width (L/W) of HDF nucleus for different stimulation times in A. Error bars represent standard deviation from 20 measurements (B).
Figure 4.
Fluorescence and bright-field images of HDF after different time periods of stimulation with 8V biased, 1Hz, 10% duty cycle pulse for 24 h. Bar represents 20 μm (A). Plot of the ratio of length-to-width (L/W) of HDF nucleus for different times after stimulation in A. Error bars represent standard deviation from 20 measurements (B).
3.3 Collagen expression after EF stimulation
Once collagen is synthesized in the cell body, it is secreted to the media and deposited on the surface of the glass in a fibrillar form. This form of collagen is difficult to quantify. It is more accurate to detect collagen that is produced in the cell body in the form of procollagen before it is secreted to that media. Moreover, this scheme allows for early detection of protein expression changes.
HDF were stimulated in the reactor under varied conditions in pulsed mode. Most of the measurements were performed at 1Hz frequency. The cells were collected and lysed to detect differences in intracellular collagen concentration before collagen secretion to the media. To detect collagen concentration Sircol assay was performed on cell lysate. The assay was performed on the standard collagen solution provided by the manufacturer. All HDF cells were counted after EF stimulation, but before they were lysed, so all intracellular concentrations are reported normalized per single cell.
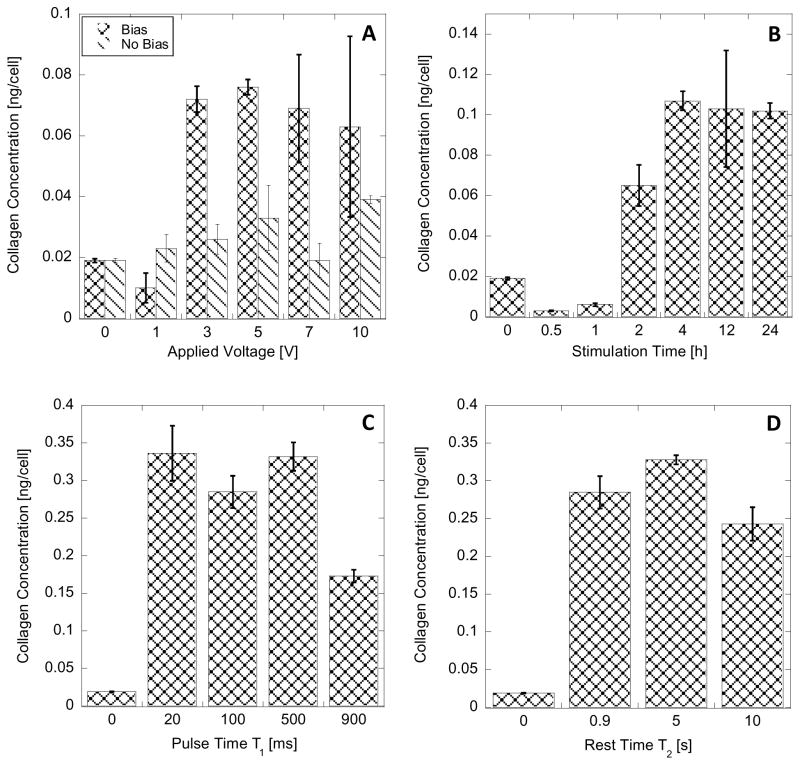
The collagen concentration was quantitatively measured by Sircol assay. The assay indicated that the average intracellular collagen concentration in control cells (not stimulated HDF), was 0.019 (± 0.001) ng/cell. This value agrees with the value measured by Bred et al in human lungs fibroblasts expressed as 3.72% of total protein content [27,30]. Since intracellular total protein content is 0.2g/mL and the average volume of HDF is 3.4pL, the expected literature value is about 0.025ng/cell [31].
The application of EF on HDF increases intracellular concentration of collagen in the cells (Figure 5). The concentration of collagen in HDF increases as the applied voltage pulse is increased as illustrated in Figure 5A. Positive-bias voltage had a larger effect on the fibroblasts than when no-bias voltage pulse was applied, as a higher intracellular concentration of collagen was detected, (all other parameters constant: 24h stimulation, 1Hz, 10% duty cycle). However, both the positive-bias and no-bias voltage pulse application show that the intracellular concentration of collagen within the cell increased as the voltage was increased. The significant changes in collagen concentration are detectable at applied voltage pulse above 3V (maximum tested pulse was 10V). This result correlates with the previous observations by Guo et. al. where in order to stimulate the motility of HDF, fields as high as 4V/cm were necessary [32]. This is in opposition to other types of fibroblasts, where fields as low as 0.1V/cm was able to initiate cell motility.
Figure 5.
Intracellular collagen concentration in HDF with varying experimental conditions: (A) varied applied voltage pulse for non-bias (ΔV1) and biased (ΔV2) pulsed; 24h, 1Hz, 10% duty cycle; (B) varied stimulation time; 8V biased voltage pulse,1Hz, 10% duty cycle; (C) varied pulse time (T1); 24 h, 8V biased voltage pulse,1Hz; (D) varied rest time (T2); 24h, 8V biased voltage pulse, 100 ms pulse time. Error bar represent standard deviation from 9 measurements.
There are no significant differences between measured intracellular concentrations of collagen in cells stimulated with positive-bias voltage pulse in range 3 to 10V (0.070 ± 0.005 ng/cell, Figure 5A). This behavior can indicate that the synthesized procollagen is secreted to the media and thus the intracellular concentration of collagen stays at constant level. Intracellular concentration of collagen in HDF cells stimulated by no-bias voltage pulses was on average 0.029 ± 0.008 ng/cell - about half of that recorded for cells stimulated by positive-bias voltage.
It was observed previously that while HDF will respond to EF above 4V/cm to initiate motility, there is a time delay in this response [32]. Here we tested how long after initiating the electrical stimulation the cells would respond in the procollagen synthesis. In Figure 5B we show that the increase of intracellular collagen concentration is observed no earlier than after 2 hours of stimulation (8V pulse, 1Hz, 10% duty cycle). In addition, the intracellular collagen concentration after EF stimulations longer than 4 h is significantly higher than control level and constant (0.104 ± 0.002 ng/cell).
The voltage pulse time (T1) and rest time between the pulses (T2) employed in EF generation was varied to investigate the conditions that result in changes of collagen expression in HDF. In Figure 5C, the short pulse times between 20–500 ms cause significant increase in the intracellular concentration of collagen in HDF stimulated at 1Hz frequency. Increasing the T1 to 900ms also caused a significant increase in the intracellular concentration of collagen (compared with control), but only about half of what was detected during short T1 pulses. This effect could be related to the fact that longer time pulses can generate more byproducts of the electrochemical reaction during the pulse application, and employing 1Hz frequency results in lack of sufficiently long recovery period. These conditions can chemically stress the HDF cells. We did not observe any effects related to cell membrane damage.
It seems that a rest time of T2 longer than 500 ms is sufficient for the cell to recover after each stimulation pulse (Figure 5D) and longer rest periods do not change the intracellular concentration of collagen in HDF.
3.4 Comparison of collagen, collagenase and elastin expression after EF stimulation
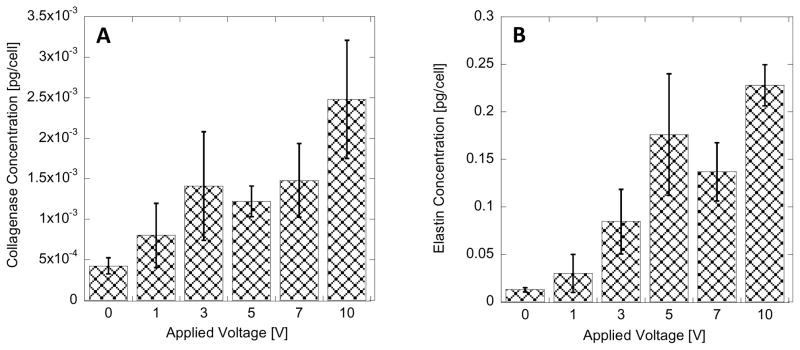
The EF stimulation conditions of HDF that resulted in the increased intracellular concentration of collagen were used to compare the intracellular concentrations of MMP1 (collagenase) and elastin. The quantification of intracellular concentration of collagenase and elastin was performed with ELISA. In Figure 6, the measured concentration of collagenase and elastin are plotted with respect to different voltage pulses in positive-bias configuration used to generate EF. Analogous to changes in intracellular collagen concentration (Figure 5A) the significant increase in collagenase and elastin intracellular concentration is observed when voltage pulse is larger than 3V. Increasing the voltage pulse above 3V does not cause an increase in protein concentration, but maintains the expression at 3–5 fold increased level.
Figure 6.
Intracellular collagenase (MMP1) (A) and elastin (B) concentration in HDF with varied applied voltage pulse for biased (ΔV2) pulsed; 24h, 1Hz, 10% duty cycle. Error bar represent standard deviation from 9 measurements.
On the other hand, the stimulation of fibroblasts by chemical factors, results in upregulation of collagen concentration and downregulation of collagenase concentration (TGF-β) or opposite when TNF-α is used. We believe that when the chemical signaling factors are used is cell stimulation, they are selective in regulating particular pathway of protein expression, while EF stimulation can activate several pathways simultaneously. Wang et al. showed that skin fibroblasts subjected to electrical stimulation activate the TGF-β1/ERK/NF-κB signaling pathway. However this pathway may be only one of the pathways that are activated during the EF stimulation [20]. The signal transduction between EF stimulation and cell response is not completely clear. One of the proposed mechanisms, the electrocoupling mechanism, postulated that regrouping of surface-bound integrins upon EF stimulation initiate calcium influx to the cytoplasm as the initial mechanism for (unknown) pathway activation [33,34]. We performed a set of experiments to initiate calcium influx to the cytoplasm without EF stimulation. We used ionomycin and thapsigargin to allow calcium influx from extracellular space or release it from ER, respectively. We did not observe any changes in intracellular concentration of collagen, collagenase or elastin. At this point, we are not able to identify the mechanism that translates the EF to changes in ECM proteins concentration. However, an unusual combination of ion channels types that are expressed on the surface of HDF, and the presence of “minimum” activation voltage and stimulation time, suggests that voltage gated potassium channels and calcium regulated potassium channels may be the transducers of the activation process [35].
3.5 EF depth penetration
The application of electrical stimulation in systems in vivo has been linked to wound healing, tissue regeneration and antimicrobial effects. The electrode geometry and localization has been debated because it can affect the EF field generated in the wound area. In the skin, the two main populations of HDF are located within the dermis at two depths, at about 300 μm and 700–900 μm [25]. Thus in order to apply the EF stimulation in wound healing or tissue regeneration, the EF has to be able to reach HDF.
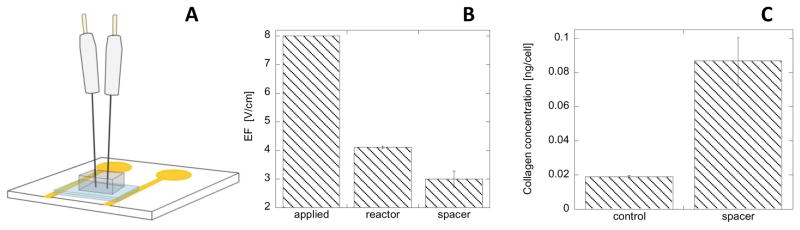
Here we have designed the simple in vitro system where HDF culture was separated from the stimulation electrodes by the 890 μm layer of hydrogel (poly-HEMA), Figure 7A. The EF that the cells experience in this configuration was measured with the same sensor as described in 3.1. In Figure 7B, we present the comparison of EF measured in the bioreactor, and with the spacer, in response to applied voltage pulse. Our data shows that the EF that HDF experience in the presence of the spacer is about 27% lower than in the reactor. The culture of HDF stimulated for two hours at 8V applied voltage across the hydrogel spacer (Figure 7C) behaves in a similar fashion as HDF cells in the reactor - an increase of intracellular concentration of collagen is observed. This is a very encouraging result that needs to be studied in more detail because it shows that it is feasible to use the two-electrode system positioned on one side of the spacer to stimulate HDF across the spacer. Thus transdermal stimulation with EF can be accomplished with the application of low voltage pulse. Naturally, the electrical resistance of epidermis is a substantial barrier for the EF penetration. However, one can envision that removal of the top layer of the epidermis (for example by microdermabrasion) could accomplish this goal and improve conductivity across the skin. Thus transdermal HDF stimulation could be possible.
Figure 7.
Scheme of EF sensing setup with a spacer between the sensing electrodes and voltage applied electrodes (see text) (A); Measured EF with respect of applied voltage within the reactor and with the spacer (B); Intracellular collagen concentration in HDF for biased (ΔV2) 8V voltage pulse; 2h, 1Hz, 10% duty cycle with a spacer compared to untreated HDF. Error bar represent standard deviation from 9 measurements.
4. Conclusions
In this work, we have shown the effects of electrical (EF) stimulation on ECM proteins i.e. collagen, MMP1, and elastin expression in HDF. The intracellular concentration of all tested ECM proteins increased with the EF generated by voltage pulses larger than 3V and stimulation time longer than 2h; with a larger concentration increase for biased than non-biased voltage pulse. Pulse time influenced the stimulation to the smaller extent, and rest time showed no significant effect on ECM protein concentration. Both voltage and time threshold for increased protein expression agree with the literature threshold needed for initiation of HDF motility. The positive correlation between collagen, MMP1, and elastin intracellular concentration was not observed in the past, when chemical factors were used for HDF stimulation. While we did not study in detail the EF stimulation mechanism of HDF, the previously proposed electrocoupling mechanism does not appear to be active in our experimental conditions. The proposed activation of the TGF-β1/ERK/NF-κB signaling pathway is a possible mechanism of signal transduction, but it seems to be not an exclusive activation pathway.
The testing of the bioreactor revealed that the generated field is not constant during the time of voltage pulse application, but it stabilizes and does not drop below 54% of applied voltage. In addition, the EF is able to penetrate the conductive spacer with a loss of about 27%. This data suggests that that transdermal stimulation of HDF in the subcutaneous tissue is possible, thus it could potentially be used in the future to improve the wound healing and tissue regeneration process.
Acknowledgments
We are grateful to Myungeun Oh for her help with graphics. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award number GM099594 and 5UL1GM118979-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabetic Foot & Ankle. 2013;4:22081–22090. doi: 10.3402/dfa.v4i0.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao M. Electrical fields in wound healing—An overriding signal that directs cell migration. Seminars in Cell & Developmental Biology. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang E, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chinese J Traumatology. 2010;13:55–61. [PubMed] [Google Scholar]
- 4.Gelse K, Poschl E, Aigner T. Collagens- structure, function, and biosynthesis. Adv Drug Delivery Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Farndale RW, Sixma JJ, Barnes MJ, Groot PGD. The role of collagen in thrombosis and hemostasis. J Thrombosis and Haemostasis. 2004;2:561–573. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 6.Almine JF, Wise SG, Weiss AS. Elastin signaling in wound repair. Birth Defects Research Part C: Embryo Today: Reviews. 2012;96:248–257. doi: 10.1002/bdrc.21016. [DOI] [PubMed] [Google Scholar]
- 7.Gill S, Parks W. Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caley MP, Martins VL, O’toole EA. Metalloproteinases and Wound Healing. Advances in Wound Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMPs Made Easy. Wounds Int. 2009;1:1–6. [Google Scholar]
- 10.Mast BA. Wound Healing: biochemical and clinical aspects. W.B. Saunders; Philadelphia: 1992. The Skin; pp. 344–355. [Google Scholar]
- 11.Mauviel A, Chen YQ, Kahari VM, Ledo I, Wu M, Rudnicka L, Uitto JJ. Evidence for transcriptional regulation in vitro and in vivo. J Biol Chem. 1993;268:6520–6524. [PubMed] [Google Scholar]
- 12.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Molec Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 13.Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. α2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res. 2005;33:3540–3549. doi: 10.1093/nar/gki275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahari V, Chen Y, Bashir M, Rosenbloom J, Uitto J. Transcriptional Regulation of the Elastin Gene by Insulin-like Growth Factor-I Involves Disruption of Sp1 Binding. J Biol Chem. 1992;267:26134–26141. [PubMed] [Google Scholar]
- 15.Reitamo S, Remitz A, Tamai K, Ledo I, Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. Biochem J. 1994;302:331–333. doi: 10.1172/JCI117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossert J, Terraz C, Dupont S. Regulation of type I collagen genes expression. Nephrology Dialysis Transplantation. 2000;15:66–68. doi: 10.1093/ndt/15.suppl_6.66. [DOI] [PubMed] [Google Scholar]
- 17.Varga J, Yufit T, Brown RR. Inhibition of collagenase and stromelysin gene expression by interferon-gamma in human dermal fibroblasts is mediated in part via induction of tryptophan degradation. J Clinic Investigation. 1995;96:475–481. doi: 10.1172/JCI118058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto T, Eckes B, Krieg T. Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Comm. 2001;281:200–205. doi: 10.1006/bbrc.2001.4321. [DOI] [PubMed] [Google Scholar]
- 19.Yuan W, Varga J. Transforming Growth Factor-β Repression of Matrix Metalloproteinase-1 in Dermal Fibroblasts Involves Smad 3. J Biol Chem. 2001;276:38502–38510. doi: 10.1074/jbc.M107081200. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Rouabhia M, Zhang Z. Pulsed electrical stimulation benefits wound healing by activating skin fibroblasts through the TGFβ1/ERK/NF-κB axis. Biochim Biophys Acta. 2016;1860:1551–1559. doi: 10.1016/j.bbagen.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Tandon N, Cannizzaro C, Chao P-HG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nature Protocols. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pires F, Ferreira Q, Rodrigues CAV, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta. 2015;1850:1158–1168. doi: 10.1016/j.bbagen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Rouabhia M, Park H, Meng S, Derbali H, Zhang Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE. 2013;8:e71660. doi: 10.1371/journal.pone.0071660. https://doi.org/10.1371/journal.pone.0071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao PHG, Roy R, Mauck RL, Liu W, Valhmu WB, Hung CT. Chondrocyte Translocation Response to Direct Current Electric Fields. J Biomech Eng. 2000;122:261–267. doi: 10.1115/1.429661. [DOI] [PubMed] [Google Scholar]
- 25.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 1999;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 26.Oliaei S, Manuel C, Karam B, Hussain SF, Hamamoto A, Protsenko DE, Wong BJF. In Vivo Electromechanical Reshaping of Ear Cartilage in a Rabbit Model A Minimally Invasive Approach for Otoplasty. JAMA Facial Plast Surg. 2013;15:34–38. doi: 10.1001/2013.jamafacial.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bred SD, Bradley KH, Hance AJ, Schafer MP, Berg RA, Crystal RG. Control of Collagen Production by Human Diploid Lung Fibroblasts. J Biol Chem. 1980;255:5250–5260. [PubMed] [Google Scholar]
- 28.Sillman AL, Quang DM, Farboud B, Fang KS, Nuccitelli R, Isseroff RR. Human dermal fibroblasts do not exhibit directional migration on collagen I in direct current electric fields of physiological strength. Exp Dermat. 2003;12:396–402. doi: 10.1034/j.1600-0625.2002.120406.x. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Koch D, Cardenas R, Ka J, Shih CK. Cell Motility and Local Viscoelasticity of Fibroblasts. Biophys J. 2005;89:4330–4342. doi: 10.1529/biophysj.104.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasmin H, Kabashima T, Rahman MS, Shibata T, Kai M. Amplified and selective assay of collagens by enzymatic and fluorescent reactions. Scientific Reports. 2014;4:4950. doi: 10.1038/srep04950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imaizumi T, Jean-Louis F, Dubertret M-L, Bailly C, Cicurel L, Petchot-Bacquk J-P, Dubertret L. Effect of human basic fibroblast growth factor on fibroblast proliferation, cell volume, collagen lattice contraction: in comparison with acidic type. J Dermatol Sci. 1996;11:134–141. doi: 10.1016/0923-1811(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 32.Guo A, Song B, Reid B, Gu JV, Forrester Y, Jahoda CAB, Zhao M. Effects of Physiological Electric Fields on Migration of Human Dermal Fibroblasts. J Invest Dermatol. 2010;130:2320–2327. doi: 10.1038/jid.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titushkin I, Cho M. Regulation of Cell Cytoskeleton and Membrane Mechanics by Electric Field: Role of Linker Proteins. Biophysical Journal. 2009;96:717–728. doi: 10.1016/j.bpj.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart FX. The Mechanical Transduction of Physiological Strength Electric Fields. Bioelectromagnetics. 2008;29:447–455. doi: 10.1002/bem.20411. [DOI] [PubMed] [Google Scholar]
- 35.Estacion M. Characterization of ion channels seen in subconfluent human dermal fibroblasts. J Physiol. 1991;436:579–601. doi: 10.1113/jphysiol.1991.sp018568. [DOI] [PMC free article] [PubMed] [Google Scholar]