Abstract
Objectives
We examined the impact of prenatal exposure to maternal antibiotics on risk of necrotizing enterocolitis (NEC), late onset sepsis (LOS), and death in preterm infants.
Study design
Secondary data analysis was conducted using an extant cohort of 580 infants born <32 weeks gestation and enrolled in three level III NICUs. Prenatal antibiotic exposure was defined as antibiotics received by the mother within 72 hours prior to delivery. Postnatal empiric antibiotic exposure was defined as antibiotic initiated within the first day of life without documented infection, categorized as low (<5 days) or high (>5 days) duration.
Results
Two-thirds of mothers received antibiotics within 72 hours prior to delivery, of whom 59.8% received more than one antibiotic. Ampicillin (37.6%) and azithromycin (26.4%) were the most common antibiotics given. NEC occurred in 7.5%, LOS in 11.1%, death in 9.6%, and the combined outcome of NEC, LOS, or death in 21.3% of study infants. In multiple logistic regression models adjusted for gestational age, postnatal empiric antibiotic exposure, and other factors, prenatal antibiotic exposure was associated with reduced risk of NEC (odds ratio [OR; CI=0.28; 0.14–0.56], P < .001), death ([OR; CI = 0.29; 0.14–0.60], p=0.001), but not LOS ([OR; CI=1.59; 0.84–2.99], p=0.15), though protection was significant for the combined outcome (OR=0.52, p<0.001). High postnatal empiric antibiotic exposure was associated with greater risk of death but not other outcomes in multiple regression models (OR=3.18, p=0.002).
Conclusions
Prenatal antibiotic exposure was associated with lower rates of NEC or death of preterm infants, and its impact on infant outcomes warrants further study.
Keywords: necrotizing enterocolitis, late onset sepsis, preterm infant, empiric antibiotics
Necrotizing enterocolitis (NEC) and late onset sepsis (LOS) cause significant morbidity and mortality among premature infants in neonatal intensive care units (NICUs). Among infants born <32 weeks gestational age (GA), 7% develop NEC, in whom case fatality is ~ 30%(1, 2). Late onset sepsis occurs in up to one-third of infants <32 weeks GA, with the most preterm infants experiencing the highest risk(3). High postnatal empiric antibiotic use is an epidemiologic correlate of both NEC and LOS(4). Cotten et al studied a national cohort of 5693 extremely low birth weight infants and found that infants receiving prolonged initial antibiotic therapy had an increased risk for developing NEC or death(5). Subsequently, Kuppala et al studied 365 premature infants in Cincinnati, Ohio, and reported that prolonged initial empiric antibiotics were associated with increased risk for NEC, LOS, or death(6). In a cohort of 328 premature infants in Saudi Arabia, Abdel Ghany et al also reported that each treatment day with empiric antibiotics was associated with an increased risk of death and the combined outcome of NEC or death(7). Greenwood et al examined the impact of high antibiotic use on the microbiome and found that infants receiving early antibiotics experienced a surge in Enterobacteriaceae as well as greater risk of NEC, sepsis or death(8).
However, infant antibiotic exposure may begin prenatally as a result of maternal exposure to antibiotic, and this prenatal exposure could potentially influence neonatal disease. Bizzarro et al noted an increase in ampicillin-resistant E.coli infections in ampicillin exposed mothers(9). Similarly, Didier et al found that maternal exposure led to an increase in amoxicillin resistant organisms(10). However, the effects of maternal antibiotic exposure prior to delivery on neonatal outcomes have not been well defined. With this concern in mind, we analyzed an extant cohort of preterm infants to test the hypothesis that infant antibiotic exposure prenatally is associated with increased incidence of NEC, LOS, or death.
Methods
We conducted a secondary analysis of an extant cohort of 580 infants <32 weeks gestational age. All infants in this study were part of a prospective, NIH-funded study of the preterm microbiome and risk of NEC, sepsis and death(11). Study infants were enrolled from two level III NICUs in Cincinnati, Ohio and one level III NICU in Birmingham, Alabama, from 2009 to 2012. Enrollment criteria included delivery <32 weeks gestation, being free of congenital anomalies, and survival free of NEC in the first week of life. One patient was excluded due to unknown maternal antibiotic status. Data collection followed the protocol of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) registry. Infants were enrolled immediately after delivery and were followed up until discharge, transfer, 120 days post-partum, or death. Maternal and infant data were abstracted from medical records. The Institutional Review Boards at the three participating hospitals approved the study. Maternal antibiotic exposure was defined as antibiotic treatment within 72 hours prior to delivery. Indications for maternal antibiotic exposure included Cesarean section, GBS prophylaxis, premature rupture of membranes, chorioamnionitis, and to prolong pregnancy (latency). Maternal antibiotic exposure did not include antibiotics given after the time of delivery, or antibiotic initiated by surgeons during (but not prior to) Cesarean-section delivery. Early empirical neonatal antibiotic exposure was defined as antibiotic treatment initiated within the first postnatal day without culture-identified infection. The duration of early antibiotic therapy was defined as the total number of continuous days of administration of antibiotics with sterile culture results. Empiric infant antibiotics used in this cohort were ampicillin and gentamicin, with empiric infant antibiotic exposure defined as either low (<5 days) or high (>5 days) (3, 5, 6, 8). NEC was defined using Bell stage II or III criteria(12). Spontaneous intestinal perforation (SIP) was excluded by including only cases of NEC that occurred after 7 days of life. LOS was defined as a positive blood, cerebrospinal fluid, urine or sterile site culture after the third postnatal day. If patients were diagnosed with NEC or LOS or died after day 7 of their NICU course, they were considered positive for the combined outcome, termed NSD.
Statistical analyses
Differences in clinical characteristics were tested utilizing the Fisher exact test for categorical variables and analysis of variance, and t-tests or Kruskal-Wallis for continuous variables, as appropriate. Maternal and neonatal baseline characteristics were compared across groups based on maternal antibiotic exposure alone and infant antibiotic exposure alone, and across the four combined maternal and infant antibiotic exposure groups. The individual outcomes of NEC, LOS, and death, and the occurrence of any of these outcomes were also compared across the groups as noted above. Multivariable logistic regression models were used to evaluate independent associations between maternal and infant antibiotic exposure and these outcomes. A set of clinical predictors of NEC, LOS and death were defined a priori and examined in the models. Gestational age, sex, race, mode of delivery, high human milk exposure, maternal chorioamnionitis, C-section delivery, premature rupture of membranes, maternal pre-eclampsia, prenatal steroids, parity, multiple birth, and hospital location were all included in the initial analysis. We defined high human milk exposure as feeding with human milk for more than 75% of the days from birth to 30 days of life or the onset of NEC or death, whichever occurred first.
Modeling was performed using backward elimination, with factors systematically eliminated in order of the highest (non-significant) p-values. Factors not included as potential confounders were postnatal infant health status measures that were considered to be on the same causal chain as (or potential biomarkers of) the study outcomes (eg, Apgar scores or respiratory status, which may be indicators of infant health). Factors that were eliminated from models did not appreciably affect the observed association between maternal or infant antibiotic use and study outcomes; the odds ratios of maternal antibiotic impact on study outcomes changed less than 4% after elimination of potential covariates (at most a single digit change at the second decimal point). Multiple logistic regression models reported here were standardized across outcomes by including a set of independent variables, including prenatal and postnatal antibiotic use and all factors with a p-value <0.10 in any of the outcome-specific final models.
Results
Clinical and demographic characteristics of study infants and their mothers are presented in Table I, compared by the number of maternal antibiotics used. Of the 580 infants included in the study, 362 (62.4%) were delivered to mothers who had received antibiotics in the 72 hours prior to delivery. Infant birthweight was similar across maternal antibiotic exposure groups, as was gestational age, infant sex and race. The median gestational age at the time of delivery in both maternal exposure groups was 28 weeks. Pre-eclampsia was more common among mothers without antibiotic exposure (46.3% and 24.5% respectively, p<0.001). Three hundred sixty-three infants (62.5%) were born via Cesarean delivery, which was more common among mothers who were not exposed to antibiotics (p<0.001). Antenatal corticosteroids were more regularly used in mothers who had antibiotic exposure (p<0.001). Clinical chorioamnionitis was higher among mothers who received antibiotics (p<0.001). Human milk feeding did not differ by maternal antibiotic exposure.
Table 1.
Clinical Characteristics of Study Infants and their Mothers by Maternal Antibiotic Exposure (N=580) 22
| Characteristic | 0 Antibiotics (218) | ≥1 Antibiotics (362) | P |
|---|---|---|---|
| Maternal Age, Years, Median (Range) | 28 (16–41) | 26 (14–44) | 0.011* |
| Primiparous, N (%) | 71 (32.5%) | 118 (32.5%) | 1.000 |
| Antenatal Steroids, N (%) | 181 (83.0%) | 348 (96.1%) | <0.001 |
| Premature Rupture of Membranes, N (%) | 54 (24.7%) | 206 (56.9%) | <0.001 |
| Clinical Chorioamnionitis, N (%) | 8 (3.7%) | 64 (17.6%) | <0.001 |
| Pre-eclampsia, N (%) | 101 (46.3%) | 89 (24.5%) | <0.001 |
| Cesarean section, N (%) | 167 (76.6%) | 196 (54.1%) | <0.001 |
| Gestational Age, Weeks, Median (Range) | 28 (23–32) | 28 (22–32) | 0.001* |
| Birth Weight, Grams, Median (Range) | 1050 (400–2590) | 1062 (360–2485) | 0.73* |
| Male, N (%) | 117 (53.6%) | 178 (49.1%) | 0.39 |
| Black, N (%) | 66 (30.2%) | 143 (39.5%) | 0.03 |
| ≥5 Days Empiric Infant Antibiotics, N (%) | 35 (16.0%) | 100 (27.6%) | 0.002 |
| High (>75%) Human Milk Exposure | 118 (54.1%) | 213 (58.8%) | 0.23 |
| NEC | 25 (11.4%) | 19 (5.2%) | 0.009 |
| Late Onset Sepsis (LOS) | 16 (7.3%) | 49 (13.5%) | 0.02 |
| Death | 26 (11.9%) | 30 (8.2%) | 0.19 |
| NEC/LOS/Death | 50 (22.9%) | 74 (20.4%) | 0.53 |
P values for maternal age, gestational age and birth weight calculated with Kruskal Wallis test
Overall 135 (23.2%) study infants received five or more days of postnatal empiric antibiotic. Infants who were born with prenatal antibiotic exposure were more likely to experience high postnatal empiric antibiotic exposure than infants without prenatal antibiotic exposure (p=0.002): Among the 362 infants exposed to prenatal antibiotic, 100 (27.5%) had high postnatal antibiotic exposure, whereas among the 218 infants with no prenatal antibiotic exposure, 35 (16.0%) had high postnatal antibiotic exposure.
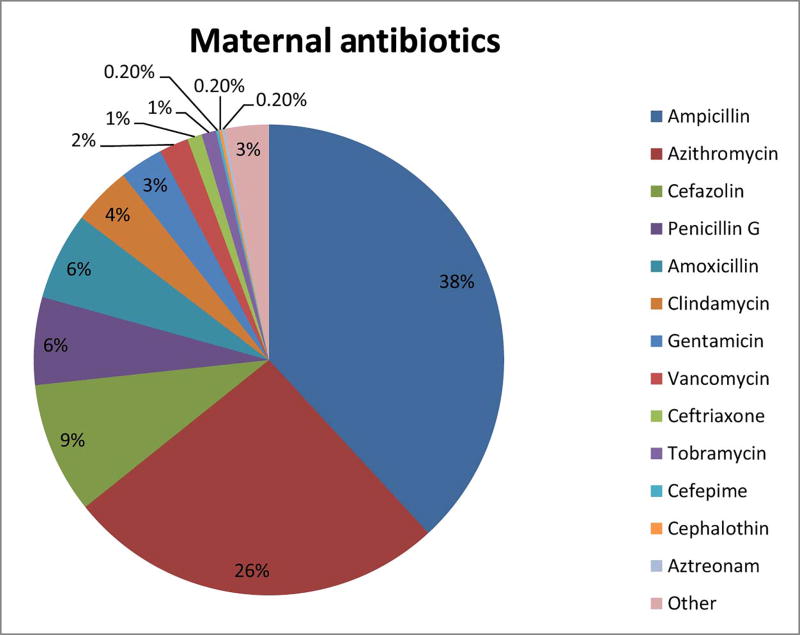
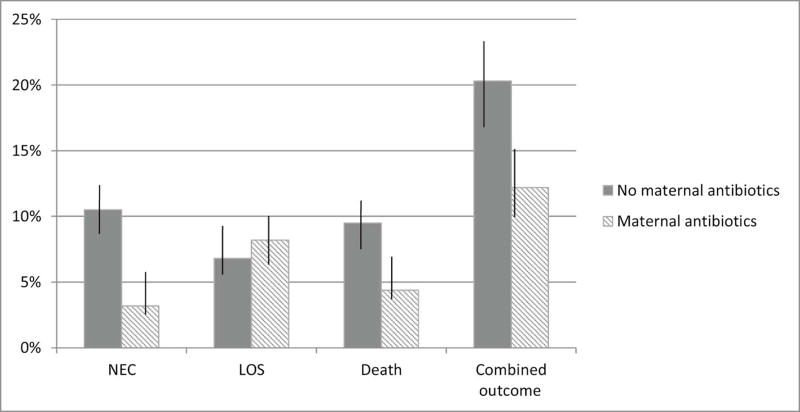
Of the 580 study infants, there were 106 twin deliveries and 10 triplet deliveries. There was no difference in prenatal antibiotic exposure between multiple and singleton births. Nevertheless, analysis of maternal antibiotic use was based on the 309 individual mothers who were provided antibiotics prenatally (rather than the 362 study infants who also received antibiotics via their mothers prenatally). In the 72 hours prior to delivery, a total of 534 antibiotic courses were given, which could be grouped into 55 distinct combinations of the 14 different antibiotics that were utilized. Sixty percent of mothers who received antibiotics had more than one type. (Figure 1, Tables 2–4; available at www.jpeds.com) Of the 580 infants studied, 44 (7.5%) developed NEC, 65 (11.1%) developed LOS, 56 (9.6%) died before discharge from the NICU, and 124 (21%) experienced at least one of the (combined outcome) of NEC, LOS, or death (Table 1). Death was attributable to NEC in nineteen cases (34%) and to LOS in 12 cases (21%). Bacterial organisms causing LOS were coagulase-negative Staphylococcus (48%), Klebsiella (10%), E. coli (8%), Enterococcus (6%), Group B Streptococcus (5%), methicillin-resistant Staphylococcus aureus (5%), and Staphylococcus aureus (4%), Streptococcus viridans (2%), and 1% each for Pseudomonas, Enterobacter, and Bacillus species. Fungal species accounted for 1% of LOS cases. Neither probiotic therapy nor fluconazole prophylaxis were used routinely in the study NICUs when this cohort was accrued. To adjust for potential confounding, the rates of disease were corrected for gestational age utilizing infants who did not receive prenatal or high empiric postnatal antibiotics as the comparison group. NEC was diagnosed less commonly among infants with prenatal exposure to antibiotics (3.2%) compared with infants with no prenatal antibiotic exposure (10.5%, p<0.001, Figure 2). Death rates followed a pattern similar to that of NEC (p=0.002). LOS was not significantly different in infants with prenatal exposure (8.2%) compared with infants with no prenatal antibiotic exposure (6.8%, p=0.159). Moreover, the combined outcome of NEC, LOS or death was more common in infants without prenatal antibiotic exposure compared with infants lacking prenatal antibiotic exposure (p=0.012) (Figure 2).
Figure 1; online only.
Percentage of individual antibiotic use out of 534 total maternal antibiotics used in 309 mothers prior to delivery
Table 2; online only.
Type of maternal antibiotic use by medical indication
| Maternal Antibiotic by Indication | ||
|---|---|---|
| Maternal Condition (N) | Antibiotic | N (%) |
| Cesarean Prophylaxis Only (27) | Cefazolin | 27 (100%) |
| GBS Prophylaxis Only (78) | Ampicillin | 46 (59%) |
| Penicillin G | 23 (29%) | |
| Vancomycin | 3 (4%) | |
| Clindamycin | 6 (8%) | |
| Premature Rupture of Membranes Only (87) | Ampicillin + Azithromycin | 87 (100%) |
Table 4; online only.
Breakdown of neonatal disease by combination of maternal antibiotic exposure
| Antibiotic Combination |
Patients With Antibiotic Combination |
Patients With Sepsis |
Patients With NEC |
Patients With Death |
|---|---|---|---|---|
| None | 183 | 18 | 15 | 22 |
| 1, 0, 0, 0 | 46 | 7 | 3 | 1 |
| 1, 10, 0, 0 | 6 | 0 | 0 | 1 |
| 1, 10, 21, 0 | 1 | 0 | 0 | 0 |
| 1, 10, 21, 68 | 1 | 0 | 0 | 0 |
| 1, 10, 26, 0 | 1 | 0 | 0 | 0 |
| 1, 10, 32, 68 | 1 | 0 | 0 | 0 |
| 1, 10, 34, 0 | 1 | 0 | 0 | 0 |
| 1, 10, 49, 68 | 1 | 0 | 0 | 0 |
| 1, 10, 61, 0 | 1 | 0 | 0 | 0 |
| 1, 10, 68, 0 | 11 | 0 | 0 | 0 |
| 1, 21, 0, 0 | 4 | 3 | 0 | 1 |
| 1, 21, 43, 68 | 1 | 0 | 1 | 0 |
| 1, 21, 68, 0 | 3 | 0 | 2 | 1 |
| 1, 26, 0, 0 | 2 | 0 | 0 | 0 |
| 1, 26, 68, 0 | 1 | 0 | 0 | 0 |
| 1, 32, 0, 0 | 6 | 0 | 0 | 0 |
| 1, 32, 49, 0 | 1 | 0 | 0 | 0 |
| 1, 32, 49, 68 | 1 | 0 | 0 | 0 |
| 1, 32, 68, 0 | 1 | 0 | 0 | 0 |
| 1, 32, 99, 0 | 1 | 0 | 0 | 0 |
| 1, 34, 0, 0 | 1 | 0 | 0 | 0 |
| 1, 34, 71, 0 | 1 | 0 | 0 | 0 |
| 1, 4, 32, 68 | 1 | 0 | 0 | 0 |
| 1, 4, 68, 0 | 7 | 0 | 0 | 1 |
| 1, 49, 0, 0 | 1 | 0 | 0 | 0 |
| 1, 49, 68, 0 | 1 | 0 | 0 | 0 |
| 1, 63, 0, 0 | 2 | 0 | 0 | 0 |
| 1, 68, 0, 0 | 87 | 9 | 4 | 12 |
| 1, 68, 71, 0 | 1 | 0 | 0 | 0 |
| 1, 68, 71, 99 | 1 | 0 | 0 | 0 |
| 1, 68, 99, 0 | 2 | 0 | 0 | 0 |
| 1, 71, 0, 0 | 3 | 0 | 0 | 0 |
| 1, 99, 0, 0 | 2 | 2 | 0 | 1 |
| 10, 0, 0, 0 | 3 | 1 | 2 | 1 |
| 10, 49, 0, 0 | 1 | 0 | 1 | 0 |
| 10, 71, 0, 0 | 1 | 0 | 1 | 0 |
| 10, 98, 0, 0 | 1 | 0 | 0 | 0 |
| 20, 21, 0, 0 | 1 | 0 | 0 | 0 |
| 21, 0, 0, 0 | 27 | 0 | 0 | 1 |
| 21, 32, 0, 0 | 1 | 0 | 0 | 0 |
| 21, 44, 0, 0 | 1 | 0 | 0 | 0 |
| 21, 44, 68, 0 | 2 | 0 | 0 | 0 |
| 21, 49, 0, 0 | 2 | 0 | 0 | 0 |
| 21, 49, 68, 0 | 2 | 0 | 0 | 0 |
| 21, 68, 0, 0 | 1 | 0 | 0 | 0 |
| 21, 71, 0, 0 | 1 | 0 | 0 | 0 |
| 26, 0, 0, 0 | 1 | 0 | 0 | 0 |
| 26, 49, 68, 99 | 1 | 0 | 0 | 0 |
| 32, 0, 0, 0 | 1 | 0 | 0 | 0 |
| 32, 44, 0, 0 | 1 | 0 | 0 | 0 |
| 32, 49, 0, 0 | 1 | 0 | 0 | 1 |
| 32, 49, 99, 0 | 1 | 0 | 0 | 1 |
| 4, 0, 0, 0 | 23 | 0 | 0 | 1 |
| 4, 21, 0, 0 | 1 | 0 | 0 | 0 |
| 4, 64, 0, 0 | 1 | 0 | 0 | 0 |
| 4, 68, 0, 0 | 2 | 0 | 0 | 0 |
| 44, 0, 0, 0 | 3 | 0 | 0 | 0 |
| 44, 68, 0, 0 | 2 | 0 | 0 | 0 |
| 49, 0, 0, 0 | 6 | 0 | 0 | 1 |
| 49, 68, 0, 0 | 3 | 0 | 0 | 0 |
| 49, 68, 71, 0 | 1 | 0 | 0 | 0 |
| 49, 68, 99, 99 | 1 | 0 | 0 | 0 |
| 68, 0, 0, 0 | 6 | 5 | 0 | 0 |
| 70, 0, 0, 0 | 1 | 1 | 0 | 0 |
| 71, 0, 0, 0 | 1 | 1 | 0 | 1 |
| 99, 0, 0, 0 | 6 | 4 | 2 | 0 |
| 99, 99, 0, 0 | 2 | 2 | 2 | 0 |
Figure 2.
Bar graph depicting gestional age-adjusted rates of NEC, LOS and the combined outcome NSD based on maternal antibiotic exposure groups. Vertical lines represent the 95% confidence intervals. Adjustment for gestational age was calculated using gestational age-specific rates and a standardized age distribution between groups. No maternal antibiotic exposure included n=218 infants; maternal antibiotic exposure included n=362 infants.
Because maternal indication for use of antibiotics could be a critical factor, we evaluated neonatal outcomes in relation to the maternal circumstances of use. Among all 580 study infants, 33 (5.6%) were born of mothers given antibiotic for prophylaxis of Cesarean delivery only; 83 (14.3%) were born of mothers given antibiotic for Group B Streptococcus prophylaxis only; 107 (18.4%) were born of mothers given antibiotic for premature rupture of membranes only; and the remaining 139 (23.9%) infants’ mothers were given antibiotics for a combination of indications. Comparing outcomes based on these maternal indication groups (Table 5), NEC occurred most frequently in infants without maternal antibiotic exposure (11.4%), and least frequently in infants whose mothers received antibiotics for GBS prophylaxis only (3.6%), but NEC rates were low across all prenatally-antibiotic exposed infants regardless of maternal indication. Similar to NEC, death occurred most frequently in infants without maternal antibiotic exposure (11.9%), and was lower among all prenatally-exposed infants regardless of maternal indication. In contrast, LOS occurred least frequently in infants without maternal antibiotic exposure (7.3%), with LOS rates higher regardless of maternal indication for antibiotics.
Table 5.
Neonatal Disease by Maternal Antibiotic Indication
| No Antibiotics (n=218) |
CS Prophylaxis Only (n=33) |
GBS Prophylaxis Only (n=83) |
Premature Rupture of Membranes Only (n=107) |
All other indications for antibiotics (n=139) |
|
|---|---|---|---|---|---|
|
| |||||
| NEC | 25 (11.4%) | 2 (6.1%) | 3 (3.6%) | 7 (6.5%) | 7 (5.0%) |
| OR = 1.00 | OR = 0.49 | OR = 0.29 | OR = 0.54 | OR = 0.41 | |
| Referent | (0.11–2.21) | (0.08–0.99) | (0.23–1.29) | (0.17–0.97) | |
| P = 0.36 | P = 0.05 | P = 0.17 | P = 0.04 | ||
|
| |||||
| LOS | 16 (7.3%) | 6 (15.1%) | 12 (14.5%) | 12 (11.2%) | 19 (13.6%) |
| OR = 1.00 | OR = 2.25 | OR = 2.13 | OR = 1.59 | OR = 2.10 | |
| Referent | (0.77–6.63) | (0.96–4.73) | (0.73–3.50) | (1.05–4.21) | |
| P = 0.14 | P = 0.06 | P = 0.25 | P =0.04 | ||
|
| |||||
| Death | 26 (11.9%) | 1 (3.0%) | 7 (8.4%) | 7 (6.5%) | 15 (10.7%) |
| OR = 1.00 | OR = 0.23 | OR = 0.68 | OR = 0.52 | OR = 0.88 | |
| Referent | (0.03–1.76) | (0.28–1.63) | (0.22–1.23) | (0.45–1.74) | |
| P = 0.16 | P = 0.39 | P = 0.14 | P =0.73 | ||
|
| |||||
| NSD | 50 (22.9%) | 8 (24.2%) | 19 (22.9%) | 19 (17.8%) | 28 (20.1%) |
| OR = 1.00 | OR = 1.07 | OR = 0.99 | OR = 0.73 | OR = 0.84 | |
| Referent | (0.45–2.53) | (0.55–1.82) | (0.40–1.31) | (0.49–1.41) | |
| P = 0.87 | P = 0.99 | P =0.29 | P =0.51 | ||
Parentheses indicate the 95% confidence interval
Multiple logistic regression modeling was performed to adjust for potential confounding factors (Table 6). Maternal antibiotic use (y/n) and high infant antibiotic use (y/n) were included in all models regardless of significance. Repeat data analysis utilizing only patients from singleton births was compared with the analysis of the entire study population, and the results were not affected by the inclusion of the entire cohort (Table 7; available at www.jpeds.com).
Table 6.
Logistic Regression Model
| Variables | NEC | LOS | Death | NSD |
|---|---|---|---|---|
|
| ||||
| Maternal Antibiotics (Y/N) | OR 0.28 | OR 1.59 | OR 0.29 | OR 0.52 |
| (0.14–0.56) | (0.84–2.99) | (0.14–0.60) | (0.32–0.85) | |
| P < 0.001 | P = 0.15 | P = 0.001 | P = 0.01 | |
|
| ||||
| High Infant Antibiotics (Y/N) | OR 1.11 | OR 0.99 | OR 3.18 | OR 1.45 |
| (0.52–2.39) | (0.53–1.88) | (1.55–6.54) | (0.85–2.45) | |
| P = 0.79 | P = 0.99 | P = 0.002 | P = 0.17 | |
|
| ||||
| Gestational Age (Weeks) | OR 0.71 | OR 0.66 | OR 0.55 | OR 0.60 |
| (0.61–0.83) | (0.58–0.75) | (0.46–0.65) | (0.53–0.67) | |
| P <0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
|
| ||||
| Primiparous (Y/N) | OR 1.58 | OR 0.62 | OR 1.61 | OR 1.17 |
| (0.79–3.14) | (0.32–1.21) | (0.76–3.42) | (0.69–2.01) | |
| P = 0.19 | P = 0.16 | P = 0.22 | P = 0.56 | |
|
| ||||
| >75% Maternal Milk (Y/N) | OR 1.47 | OR 0.46 | OR 0.24 | OR 0.42 |
| (0.73–2.96) | 0.27–0.82) | (0.12–0.50) | (0.26–0.67) | |
| P = 0.28 | P = 0.008 | P < 0.001 | P < 0.001 | |
|
| ||||
| Multiple birth (Y/N) | OR 0.21 | OR 0.89 | OR 0.76 | OR 0.68 |
| (0.07–0.63) | (0.47–1.70) | (0.33–1.75) | (0.39–1.19) | |
| P = 0.005 | P = 0.73 | 0.52 | P = 0.18 | |
Controlled for prenatal and postnatal antibiotic exposure, gestational age, sex, race, mode of delivery, high human milk exposure, maternal chorioamnionitis, C-section delivery, premature rupture of membranes, maternal pre-eclampsia, prenatal steroids, parity, multiple birth, and hospital location.
Table 7; online only.
Logistic Regression Model including only singleton births
| Confounder | NEC | LOS | Death | NSD |
|---|---|---|---|---|
|
| ||||
| Maternal Antibiotics (Y/N) | OR 0.35 | OR 1.32 | OR 0.20 | OR 0.36 |
| (0.17–0.70) | (0.63–2.78) | (0.09–0.46) | (0.20–0.66) | |
| P =0.003 | P = 0.465 | P < 0.001 | P = 0.001 | |
|
| ||||
| High Infant Antibiotics (Y/N) | OR 1.17 | OR 1.32 | OR 2.36 | OR 1.61 |
| (0.53–2.58) | (0.63–2.76) | (1.03–5.43) | (0.87–2.99) | |
| P = 0.702 | P = 0.463 | P = 0.043 | P = 0.130 | |
|
| ||||
| Gestational Age (Weeks) | OR 0.72 | OR 0.69 | OR 0.53 | OR 0.61 |
| (0.62–0.85) | (0.59–0.81) | (0.43–0.66) | (0.53–0.69) | |
| P <0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
|
| ||||
| Primiparous (Y/N) | OR 1.29 | OR 0.60 | OR 1.58 | OR 1.03 |
| (0.65–2.58) | (0.30–1.21) | (0.71–3.50) | (0.59–1.81) | |
| P = 0.470 | P = 0.165 | P = 0.261 | P = 0.914 | |
|
| ||||
| >75% Maternal Milk (Y/N) | OR 1.61 | OR 0.49 | OR 0.29 | OR 0.50 |
| (0.77–3.34) | 0.25–0.96) | (0.13–0.65) | (0.28–0.87) | |
| P = 0.205 | P = 0.037 | P = 0.003 | P = 0.015 | |
Controlled for prenatal and postnatal antibiotic exposure, gestational age, sex, race, mode of delivery, high human milk exposure, maternal chorioamnionitis, C-section delivery, premature rupture of membranes, maternal pre-eclampsia, prenatal steroids, parity, and hospital location.
Overall, we found maternal antibiotics to be associated with improved outcomes. In these multiple logistic regression models, high postnatal empiric infant antibiotic exposure was not significantly associated with risk for NEC or LOS. But, consistent with previous studies (5, 6) receipt of high empiric infant antibiotics was associated with a significantly increased risk of death (OR=3.18, p=0.002). The combined outcome of NEC, LOS or death was not significantly different in infants with high empiric antibiotic exposure (OR=1.44, p=0.17). We then examined the combination of maternal antibiotic exposure and high empiric infant antibiotics. In infants who later received low empiric antibiotics, maternal antibiotic exposure remained protective for NEC (OR=0.24, p=0.001). Maternal antibiotics in combination with high infant antibiotics were not significantly associated with a reduction in NEC (OR=0.36, p=0.09). Maternal antibiotic exposure was not a risk factor for the development of LOS in the high or low infant antibiotic groups (OR=1.06, p=0.9 and OR=2.13, p=0.07, respectively). Similar to NEC, receipt of maternal antibiotics was protective for death in the high (OR=0.33, p=0.048), and for death and NSD in low (OR=0.19, p=0.003 and OR=0.53, p=0.04) infant postnatal antibiotic receipt groups. Maternal antibiotics in combination with high infant postnatal antibiotics were not statistically different for NSD (OR=0.48, p=0.12)
Discussion
Contrary to our original hypothesis, we found that prenatal antibiotic exposure within 72 hours prior to delivery was significantly associated with three-fold protection against NEC and similar protection against death, based on multiple logistic regression models that controlled for multiple confounding factors. However, in contrast, prenatal antibiotic exposure was associated with a 1.6-fold greater risk for LOS in the same infants. Although the association with LOS was not statistically significant, the potentially disparate effect of maternal antibiotics on these outcomes is intriguing. Nevertheless, prenatal antibiotic use remained significantly associated with protection against the combined metric of NEC, LOS, or death.
Antibiotic prophylaxis prior to delivery has become commonplace as therapy for a number of potential diseases. Some estimates report that over 40% of all pregnant women receive antibiotics prior to delivery for either prophylaxis for GBS or Cesarean delivery(13, 14). When other indications for antibiotics are taken into account such as prevention of premature birth and chorioamnionitis, the percentage of mothers receiving antibiotics is even higher. In a 2015 study from Canada, the authors noted that 39% of 449 mothers undergoing term delivery were given intrapartum antibiotics, indirectly exposing their infants to antibiotics(15). In our study, maternal indications for antibiotic therapy included, but were not limited to GBS prophylaxis, Cesarean delivery, and premature rupture of membranes. In our study, 62.3% of mothers received antibiotics prior to delivery, most commonly ampicillin (37.6%) and azithromycin (26.4%). The prevalence of maternal antibiotic use (62.3%) found in this study may be higher than previously reported (40%) due to the different study populations (premature vs term infants).
Our finding regarding prenatal antibiotic exposure and protection against NEC is in stark contrast to the findings reported by Weintraub et al(16). In their retrospective case-control study, they noted that in 97 matched pairs of preterm infants, NEC was more than twice as likely to occur among infants with prenatal exposure to ampicillin(16). A Cochrane review of prenatal antibiotics given to mothers for premature rupture of membranes, noted that among the various antibiotics given, amoxicillin-clavulanate was the only antibiotic that increased neonatal risk for NEC(17). Our findings are also contrary to our expectation due to increased risk of NEC reported in previous studies associated with postnatal empiric antibiotic use(5–7, 18, 19). Speculation that prenatal antibiotics would have a similar association with neonatal disease as postnatal antibiotics appears to be incorrect.
The trend that we observed towards increased risk for LOS associated with prenatal antibiotic exposure was not statistically significant. Nevertheless, we consider the findings of concern given previous reports. Lin et al noted that in one hospital, after the initiation of a standardized treatment protocol for intrapartum antibiotic prophylaxis for GBS, the utilization of maternal antibiotics increased from 40% to 90% over 4 years in GBS-positive mothers, during which time GBS-associated early onset sepsis in neonates decreased (45% to 20%) and E. coli-associated early onset sepsis increased proportionally (41% to 70%)(20). Similarly, in a study of over 5400 very low birth weight infants from the NICHD in 2002, researchers reported a change in the pathogens causing early onset sepsis from predominantly gram-positive to predominantly gram-negative organisms, which correlates with the increase in maternal antibiotic prophylaxis during labor and delivery(21). A retrospective review of early and late onset sepsis and ampicillin resistance demonstrated that increasing use of intrapartum antibiotics was an independent risk factor for ampicillin-resistant E. coli-associated early onset sepsis and an increase in E. coli-associated late onset sepsis(9). Similarly, Didier et al found that maternal antibiotic exposure was significantly associated with the risk of amoxicillin-resistant E. coli infections(10). Coagulase-negative Staphylococcus was the causal organism in nearly half of LOS cases. Although this organism is generally considered to have low pathogenic potential, this is not always the case for extremely preterm infants. Moreover, in this study two deaths attributable to LOS were caused by coagulase-negative Staphylococcus.
Use of antibiotics would be expected to alter the maternal microbiome, thereby changing the infant’s initial exposure is to the microbial environment, which could potentially lead to an abnormal succession of microbial colonization in the infant. Alterations in early microbial transfer and succession could have significant downstream effects, as the maternal microbiome has been shown to promote development of neonatal immunity(22). For example, Deshmukh et al showed neonatal mice from dams exposed to antibiotics had a decreased number of intestinal microbes, altered structure of the microbiota and colonization, decreased bone marrow neutrophils and granulocyte/macrophage progenitor cells, reduced interleukin (IL) 17-producing cells in intestine and consequent production of granulocyte colony stimulating factor (G-CSF)(23). Relative granulocytopenia contributed to increased susceptibility of antibiotic-exposed neonatal mice to Escherichia coli K1 and Klebsiella pneumoniae sepsis.
Transplacental passage of maternal antibiotics also may directly alter the neonatal microbiome, reducing microbial diversity and creating a preponderance of pathogenic bacteria. It is well established that maternal antibiotics cross the placenta to reach the fetus(24). Mazzolo et al demonstrated that infants whose mothers received intrapartum antibiotic prophylaxis against GBS had an abundance of Enterobacteriaceae compared with control infants(25). As previous studies have shown, dysbiosis of the neonatal microbiome has been increasingly associated with development of disease. We have previously reported that early dysbiosis strongly predicts NEC(11). Stool samples from patients with NEC had lower bacterial diversity, lacked Proprionibacterium, and were dominated by either Firmicutes or Proteobacteria, compared to controls(11). Based on a meta-analysis of cohort studies in diverse locations, Pammi et al reported a greater relative abundance of Proteobacteria and decreased Firmicutes and Bacteroidetes in patients with NEC(26). However, NEC and LOS are multifactorial diseases, and risk factors should not be considered in a vacuum. Different microbes or forms of dysbiosis may distinguish the etiologies of NEC(11, 27) and LOS(28), which may account for the opposite effects of prenatal antibiotics on NEC and LOS seen in our study.
Strengths of our study were that it includes a relatively large number of participants and our cohort was representative of the neonatal population at high risk referral centers in two large US catchment areas. Because we previously reported temporal, regional, and inter-institutional difference between the neonatal microbiome in this cohort(29), our findings may not be completely generalizable to other centers for delivery of high-risk infants. The results of our study are compelling enough to warrant further research.
The main limitation to our study was that relevant maternal samples were not collected, thus simultaneous study of maternal and infant microbiomes was not possible. As noted above, our results might not be generalizable due to temporal, geographic, and population differences in the microbial milieu. Also, there were too many combinations of maternal antibiotic exposure to separate results by the antibiotic given, so antibiotic indication was used as a substitute. Due to data availability and accessibility limitations, we did not include mothers in the exposure group if their only antibiotic exposure was at the time of Cesarean delivery. Although this was a potential limitation, we completed an analysis of our data testing as if every mother who had a Cesarean delivery was exposed to antibiotics, and noted that this did not significantly change our findings. Moreover, it was felt that if antibiotics were given only at the time of Cesarean delivery, they would not have enough time to significantly affect the neonate. Another limitation is that as a retrospective review, the study is at risk for information bias. Furthermore, maternal antibiotics could suppress bacterial growth in cultures and cause false-negative culture results and limit identification of LOS cases.
Understanding the interactions between risk factors for NEC and LOS may help clinicians provide better care for patients in the future. For instance, if resistance patterns in the neonatal microbiome are identified based on maternal antibiotic exposure, knowledge of maternal antibiotic exposure could assist clinicians in choosing more appropriate antibiotics as empiric therapy. Maternal antibiotic exposure prior to delivery occurs in a large portion of mothers; nevertheless, there is a paucity of information about the potential effects this exposure might have on the health of the infant. Our study indicates that in our cohort maternal antibiotic administration was associated with protective effects, reducing NEC, death and the combined outcome of NEC, LOS and/or death. On the contrary, we found maternal antibiotic use was associated with a potentially deleterious effect in that the LOS rate was higher among exposed infants. Research incorporating the concomitant study of maternal and infant microbiota will be needed to understand these disparate outcomes.
Table 3; online only.
List of antimicrobial agents given to mothers prior to delivery
| Numerical Identifier |
Antibiotic |
|---|---|
| 1 | Ampicillin |
| 4 | Penicillin G |
| 10 | Amoxicillin |
| 20 | Cephalothin |
| 21 | Cefazolin |
| 26 | Ceftriaxone |
| 70 | Cefepime |
| 32 | Gentamicin |
| 34 | Tobramycin |
| 43 | Flucytosine |
| 44 | Vancomycin |
| 49 | Clindamycin |
| 61 | Fluconazole |
| 64 | Aztreonam |
| 68 | Azithromycin |
| 71 | Acyclovir |
| 98 | Micafungin |
| 99 | Other |
Acknowledgments
We thank Cathy Grisby, Barbara Alexander, Lenora Jackson, Kristen Kirker, and Greg Muthig for clinical data collection and data entry, and Donna Wuest for assistance with manuscript preparation. We also thank the members of the NICU staff at the University of Alabama Birmingham Medical Center, Good Samaritan Hospital, Cincinnati, University Hospital, Cincinnati, and Cincinnati Children’s Medical Center, for their contributions to this study.
Funded by the Eunice Kennedy Shriver National Institute of Health and Human Development Neonatal Research Network (HD27853) and The National Institute of Health and Human Development (HD059140 and HD13021).
Abbreviations
- NEC
Necrotizing enterocolitis
- LOS
Late onset sepsis
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):e298–304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314(10):1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–6. doi: 10.5144/0256-4947.2012.521. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23–9. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121(4):689–96. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 10.Didier C, Streicher MP, Chognot D, Campagni R, Schnebelen A, Messer J, et al. Late-onset neonatal infections: incidences and pathogens in the era of antenatal antibiotics. Eur J Pediatr. 2012;171(4):681–7. doi: 10.1007/s00431-011-1639-7. [DOI] [PubMed] [Google Scholar]
- 11.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014;11(8):7993–8009. doi: 10.3390/ijerph110807993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledger WJ, Blaser MJ. Are we using too many antibiotics during pregnancy? BJOG. 2013;120(12):1450–2. doi: 10.1111/1471-0528.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persaud RR, Azad MB, Chari RS, Sears MR, Becker AB, Kozyrskyj AL, et al. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: a population-based study. J Matern Fetal Neonatal Med. 2015;28(10):1190–5. doi: 10.3109/14767058.2014.947578. 19. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub AS, Ferrara L, Deluca L, Moshier E, Green RS, Oakman E, et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. J Perinatol. 2012;32(9):705–9. doi: 10.1038/jp.2011.180. [DOI] [PubMed] [Google Scholar]
- 17.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2013;(12):CD001058. doi: 10.1002/14651858.CD001058.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afjeh SA, Sabzehei MK, Fahimzad SA, Shiva F, Shamshiri AR, Esmaili F. Antibiotic Therapy for Very Low Birth Weigh Newborns in NICU. Iran J Pediatr. 2016;26(2):e2612. doi: 10.5812/ijp.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman MA, Konnikova L, Gerber JS. Impact of Antibiotics on Necrotizing Enterocolitis and Antibiotic-Associated Diarrhea. Gastroenterol Clin North Am. 2017;46(1):61–76. doi: 10.1016/j.gtc.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CY, Hsu CH, Huang FY, Chang JH, Hung HY, Kao HA, et al. The changing face of earlyonset neonatal sepsis after the implementation of a maternal group B Streptococcus screening and intrapartum prophylaxis policy--a study in one medical center. Pediatr Neonatol. 2011;52(2):78–84. doi: 10.1016/j.pedneo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 22.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 23.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20(5):524–30. doi: 10.1038/nm.3542. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacifici GM. Placental transfer of antibiotics administered to the mother: a review. Int J Clin Pharmacol Ther. 2006;44(2):57–63. doi: 10.5414/cpp44057. [DOI] [PubMed] [Google Scholar]
- 25.Mazzola G, Murphy K, Ross RP, Di Gioia D, Biavati B, Corvaglia LT, et al. Early Gut Microbiota Perturbations Following Intrapartum Antibiotic Prophylaxis to Prevent Group B Streptococcal Disease. PLoS One. 2016;11(6):e0157527. doi: 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner BB, Tarr PI. Necrotizing enterocolitis and preterm infant gut bacteria. Semin Fetal Neonatal Med. 2016;21(6):394–9. doi: 10.1016/j.siny.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carl MA, Ndao IM, Springman AC, Manning SD, Johnson JR, Johnston BD, et al. Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin Infect Dis. 2014;58(9):1211–8. doi: 10.1093/cid/ciu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taft DH, Ambalavanan N, Schibler KR, Yu Z, Newburg DS, Ward DV, et al. Intestinal microbiota of preterm infants differ over time and between hospitals. Microbiome. 2014;2:36. doi: 10.1186/2049-2618-2-36. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]