Abstract
Cardio-oncology is an emerging discipline focused predominantly on the detection and management of cancer treatment-induced cardiac dysfunction (cardiotoxicity), which predisposes to development of overt heart failure or coronary artery disease. The direct adverse consequences, as well as those secondary to anticancer therapeutics, extend beyond the heart, however, to impact the entire cardiovascular-skeletal muscle axis (i.e., whole-organism cardiovascular toxicity). The global nature of impairment creates a strong rationale for treatment strategies that augment or preserve global cardiovascular reserve capacity. In non-cancer clinical populations, exercise training is an established therapy to improve cardiovascular reserve capacity, leading to concomitant reductions in cardiovascular morbidity and its attendant symptoms. Here we overview the tolerability and efficacy of exercise on cardiovascular toxicity in adult patients with cancer. We also propose a conceptual research framework to facilitate personalized risk assessment and development of targeted exercise prescriptions to optimally prevent and/or manage cardiovascular toxicity following a cancer diagnosis.
Keywords: cardiorespiratory fitness, cardiotoxicity, heart failure, physical activity, cancer survivorship
INTRODUCTION
The adverse physiological consequences of anticancer therapy have been recognized for over half a century.1 Exposure to the first combination chemotherapy regimens (e.g., ‘VAMP’ vincristine, amethopterin, 6-mercaptopurine, and prednisone) in the 1950s among patients with childhood acute leukemia were associated with severe nausea, extreme fatigue, hepatotoxicity, and thrombocytopenia.1,2 The cardiac-centric adverse consequences were first described in 1968 with anthracycline-containing regimens causing arrhythmias, dose-dependent overt heart failure (HF), and/or sudden cardiac-related death in adult leukemia patients.3 This seminal report ignited a flurry of investigations to identify risk factors (e.g., age,4 anthracycline dose5), and invasive (i.e., endomyocardial biopsy6) and non-invasive [(e.g., left ventricular ejection fraction (LVEF)]7 methods to detect cancer therapy-related cardiotoxicity.
The first cancer-specific cardiac monitoring guidelines for cancer patients were not published for another two decades8 essentially launching the sub-discipline now known as cardio-oncology. Widespread recognition of this field did not occur, however, until evidence that anti-human epidermal growth factor receptor 2 (HER2)-directed agents (e.g., Herceptin) in combination with anthracycline increased LV dysfunction and HF in women with advanced and primary HER2 positive breast cancer.9 Major cardiology and oncology agencies now recommend assessment of resting ECG (to monitor for prolonged QT) and/or resting LVEF prior to and during exposure to known cardiotoxic agents in high-risk patients [e.g., anthracycline dose >200 mg/m2; HER2 therapy or history of cardiovascular disease (CVD)].10
Despite the cardiac-centricity of current guidelines, therapy-induced direct as well as indirect (e.g., deconditioning, unfavorable changes in body composition) consequences extend across the entire cardiovascular-skeletal muscle axis (i.e., whole-organism cardiovascular toxicity). Cardiorespiratory fitness (CRF), an integrative assessment of global cardiovascular function,11 declines between 5% to 26% during exposure to various systemic combinational regimens12,13 and may not recover following treatment cessation.14,15 Such impairments may predispose to excess non-cancer competing morbidity and its attendant symptom burden (e.g., poor quality of life, fatigue).16 The global nature of cardiovascular toxicity portends the requirement for multifactorial treatment strategies with the capacity to augment and/or preserve whole-organismal cardiovascular function. Structured exercise therapy (hereto referred to as exercise) is a central component of comprehensive rehabilitation among a wide number of cardiac and pulmonary conditions.17 In contrast, neither cancer nor treatment with known cardiotoxic regimens are qualifying conditions for exercise rehabilitation in North America and, as such, exercise is not currently considered a standard aspect of cancer management.18 Nevertheless, a growing body of work is emerging investigating the efficacy of exercise in cancer – a field known as “exercise oncology”.19
Here we overview the tolerability and efficacy of exercise on cardiovascular toxicity outcomes in adult patients with cancer. We also propose a conceptual research framework to facilitate personalized risk assessment and development of targeted exercise prescriptions to optimally prevent and/or manage cardiovascular toxicity in cancer.
Current Evidence
We conducted a comprehensive review of definitive (phase 3) clinical trials, observational cohorts, and smaller randomized controlled trials (RCT) evaluating the association between exercise and subclinical (e.g., CRF, CVD risk factors) or overt (e.g., HF, CVD-related mortality) cardiovascular outcomes either during or after primary definitive therapy.
During Therapy
Phase 3 trials during definitive therapy (e.g., chemotherapy, radiation) are not yet available. One observational cohort study from Palomo and colleagues20 found that compared <2.5 metabolic equivalent hours per week (MET-hrs.wk−1), ~18 MET-hrs.wk−1 was associated with an adjusted 47% (95% CI: 0.43 to 0.80) and 31% (95% CI: 0.46 to 1.04) lower risk of any CVD event and coronary artery disease death, respectively in 4015 patients with primary breast cancer after median follow-up of 12.7 years.20
There is a paucity of RCT data investigating the efficacy of exercise on subclinical (e.g., CRF, CVD risk factors) cardiovascular outcomes in this setting. Nevertheless, at least 11 trials have examined the efficacy of various exercise prescriptions on CRF (Table 1). In the first pioneering study, MacVicar et al.21 assessed the efficacy of 10 weeks of supervised standard prescription (i.e., a prescription that maintains a fixed intensity, frequency, and duration throughout the intervention after an initial lead-in period) interval training (3d/wk, intensity between 60%-85% heart rate reserve), supervised stretching, or usual care on peak oxygen consumption (VO2peak) in 45 patients with primary breast cancer initiating various chemotherapy regimens. In comparison to non-exercise groups, aerobic training led to a mean VO2peak improvement of 40%.21 The next trial was not published until over a decade later with Segal et al.22 comparing the efficacy of a 26 week home-based or supervised standard aerobic prescription (3 to 5d/wk, session duration not reported, at 50%-60% of estimated VO2peak) to usual care in primary breast cancer patients initiating various therapy regimens (66% received anthracycline-based regimens). No significant changes in CRF were observed in any group.22 Additional trials have investigated the effects of exercise on CRF in breast cancer receiving contemporary adjuvant chemotherapy.23-25 For instance, van Waart et al.24 randomized 230 primary breast cancer patients to a standard home-based low intensity (5d/wk, 30 min/session at 12 to 14 on Borg scale), the combination of aerobic and resistance training (5d/wk; 2 supervised combined exercise, 3 home-based aerobic, 30 min aerobic at 12 to 16 RPE and 20 min resistance), or usual care control. From baseline to post-intervention (12 weeks), CRF declined ~17% in usual care, a decline significantly attenuated in the home-based group only (although a decline of ~9% was still observed).24 In a randomized pilot trial, our group investigated the efficacy of non-linear aerobic training prescription (i.e., intensity and duration of the exercise stimulus continually altered across the entire study period) in 20 locally-advanced breast cancer patients initiating neoadjuvant anthracycline-cyclophosphamide (AC) chemotherapy.25 After 12 weeks, VO2peak decreased by 1.5 ± 2.2 ml.kg.-1min−1 (-9%) in AC alone group and increased by 2.6 ± 3.5 ml.kg.-1min−1 (+13%) in the AC plus aerobic training group.25 Finally, in a study evaluating non-linear aerobic exercise (3d/wk, 15-45 min/session at 55%-80% VO2peak) or attention control (stretching) in 65 women with metastatic (stage IV) breast cancer (57% receiving chemotherapy; >40% ≥ 2 lines of prior therapy), on the basis of predefined criteria (i.e., attendance >70%), supervised aerobic exercise at the dose and schedule tested was safe (no serious adverse events) but not tolerated in all patients (mean attendance of 63%); exercise was associated with significant improvements in VO2peak among patients with exercise tolerability (J.Scott, PhD; unpublished data; 2018).
Table 1.
Key randomized controlled trials during cancer therapy.
| Study | N | Cohort/Setting | CVD status | Modality | Length | Fq | Duration (range) | Intensity (range) | LTF | Safety | Attendance | Adherence | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MacVicar et al. (1989)21 | 45 | Breast cancer patients undergoing adjuvant chemotherapy randomized to AT, stretching, or UC | NR | CE | 10 wks | 3 | NR | 60 – 85% HRR |
27% | NR | NR | NR |
Measured VO2peak. AT: ↑40% Stretching and UC: no change; significant between group difference |
| Segal et al. (2001)22 | 123 | Breast cancer patients undergoing adjuvant chemotherapy randomized to supervised AT, self-directed AT, or UC | NR | TM | 26 wks | 3-5 | NR | 50 – 60% VO2peak | 27% | NR | NR | NR |
Estimated VO2peak: Self: ↑3.5% Supervised: ↑2.4%, UC: 0% non-significant between group difference |
| Courneya et al. (2007)23 | 242 | Breast cancer patients undergoing adjuvant chemotherapy randomized to supervised RT, AT, or UC | NR |
AT CE, ET, TM RT 2 sets of 8-12 reps of 9 exercises |
17 wks | 3 | 15 – 45 mins |
AT 60 – 80% VO2peak RT 60 – 70% estimated 1 RM |
9% | 2 AE | AT: 72% RT: 68% |
AT: 93%, RT: 96% |
Measured VO2peak: AT: ↑0.2% in AT; RT:↓5% UC: ↓6% significant difference between AT and UC and RT |
| Courneya et al. (2009)27 | 122 | Lymphoma patients undergoing therapy or immediately after therapy randomized to supervised AT or UC | HTN (29%) HPL (30%) |
CE | 12 wks | 3 | 15 – 45 mins | 60 – 100% VO2peak | 11% | 3 AE | 78% | 95% |
Measured VO2peak: AT: ↑17% UC: ↓2% significant between group difference |
| Segal et al. (2009)26 | 121 | Prostate cancer patients initiating radiotherapy with or without ADT randomized to supervised AT, RT, or UC | NR |
AT CE, ET, TM RT 2 sets of 8-12 reps of 10 exercises |
24 wks | 3 | 15 - 45 mins |
AT 50 – 75% VO2peak RT 60 – 70% estimated 1 RM |
7% |
3 AE | AT: 83% RT: 88% |
NR |
Measured VO2peak: RT: ↑0.5% AT: ↑0.1% UC: ↓5% significant difference between RT and UC |
| Courneya et al. (2013)33 | 301 | Breast cancer patients initiating adjuvant chemotherapy randomized to standard AT, high dose AT, or CT | Obese (23%) |
Standard and high dose AT CE, ET, TM, row RT 2 sets of 8-12 reps of 9 exercises |
16 wks | 3 |
Standard AT 15 – 30 min High dose AT 15 – 60 min CT 15 – 60 min |
Standard and high dose AT CE, ET, TM, row RT 60 – 70% estimated 1 RM |
7% | 3 AE | Standard AT: 88% High AT: 82% CT: 78% |
NR |
Measured VO2peak: Standard:↓12% High: ↓9% CT:↓13% significant difference between high AT and CT |
| Jones et al. (2014)25 | 20 | Breast cancer patients undergoing neoadjuvant chemotherapy randomized to supervised AT or UC | NR | CE | 12 wks | 3 | 20 – 45 mins | 55 – 100% VO2peak | 5% | 4 AE | 82% | 66% |
Measured VO2peak: AT: ↑13% UC: ↓9% significant between group difference FMD: AT:↑0.7% UC: ↑0.5% non-significant between group difference |
| Van Waart et al. (2015)24 | 230 | Breast or colon cancer patients initiating adjuvant chemotherapy randomized to home AT, supervised CT, or UC | NR |
Home NR Supervised NR |
NR | 5 |
Home 30 mins Supervised AT: 30 mins; RT: 20min |
Home 12-14 Borg Supervised 50-80% maximal workload |
11% | NR | 71% | NR |
Estimated exercise capacity: Home: ↓9% Supervised: ↓14% UC: ↓18% significant difference between home and UC and supervised |
| Scott et al. (2017)68 | 65 | Breast patients with metastatic disease (57% receiving chemotherapy) randomized to AT or stretching (attention control) | Co-morbidities: 34% |
TM | 12 wks | 3 | 20 – 45 mins | 55 – 100% VO2peak | 3% | 0 AE | 63% | RDI: 61% |
Measured VO2peak: unchanged in AT and stretching |
All interventions were described according to the classic components of exercise prescription: (1) type (modality), (2) program length (total number of training weeks), (3) frequency (mean number of exercise sessions/week), (4) duration (duration spent on 1 session of exercise), and (5) intensity (percentage of a predetermined physiological parameter such as maximum heart rate obtained from baseline cardiopulmonary exercise test). All outcomes were described according to LTF (number of patients that dropped out divided by total number of patients), safety (number of AE), attendance (the number of exercise sessions attended divided by the total number of planned sessions), adherence (number of exercise sessions that were completed at the planned duration and intensity divided by the number of planned sessions attended), and efficacy (change in outcome).
Abbreviations: Fq; frequency; RCT, randomized controlled trial; AT, aerobic training; UC, usual care; RT, resistance training; CT, combined aerobic and resistance training; CE, cycle ergometer; ET, elliptical trainer; TM, treadmill; RM, repetition maximum; HRR, heart rate reserve; HR, heart rate; ADT, androgen deprivation therapy; NR, not reported; LTF, loss to follow up rate; AE, adverse event; RDI, relative dose intensity (ratio of total completed to total planned cumulative dose).
Beyond breast cancer, Segal and colleagues26 compared the efficacy of 3d/wk of resistance (60%-70% 1 repetition maximum) or aerobic training (15-45 min/session, 50-75% VO2peak) following a standard prescription approach versus usual care in 121 patients initiating radiotherapy with or without receiving androgen deprivation therapy (ADT) for early or locally advanced prostate cancer. After 24 weeks, both exercise groups abrogated the ~5% significant decline observed in the usual care group. Courneya et al.27 evaluated the efficacy of non-linear aerobic exercise (3d/wk, 15-45 min/session at 60%-100% VO2peak) in 122 Hodgkin’s or non-Hodgkin’s lymphoma patients during (n=54, 44%) or after chemotherapy or radiation therapy (n=68, 56%). After 12 weeks, mean VO2peak increased 4.6 ml.kg.-1min−1 (~17%) compared with a mean decrease of 0.6 ml.kg.-1min−1 (~2%) in usual care. There was no interaction between treatment status and VO2peak response to aerobic training (p = 0.40).
Only one RCT, to our knowledge, has investigated the effects of exercise on cardiovascular outcomes other than CRF. Jones et al.25 (described above) found that increases in VO2peak occurred in conjunction with improvements in vascular endothelial function (as measured by flow mediated dilatation of brachial artery), with no changes in hemoglobin or resting LVEF. Only one study has targeted recruitment of patients with a pre-existing CVD risk factor. Courneya and colleagues28 randomized 55 patients with various solid tumors (47% metastatic disease; 60% breast cancer patients, 93% receiving chemotherapy) and clinically-defined anemia (hemoglobin 80-110 g/l), to either 12 weeks of an erythropoiesis-stimulating agent (ESA) alone or the combination of ESA plus non-linear aerobic training (3d/wk, 20-45 mins/session at 60%-100% VO2peak). Combination treatment significantly increased VO2peak (+3.5 ml.kg.−1min−1; 22%) compared to no change in ESA alone (+0.6 ml.kg.-1min−1; 4%). Anemia is associated with LV dilation,29 and was an independent risk factor for CVD in 14,410 subjects without CVD.30
In summary, short-term (12 to 26 weeks) anticancer therapy causes marked and significant impairments in CRF (up to 26%), compared with a typical 10% decline every decade in normal aging,31 indicating an “accelerated cardiovascular aging” phenotype.12 Although the molecular mechanisms are incompletely understood, recent elegant work demonstrated that anthracycline-containing chemotherapy increased expression of the cellular senescence marker p16INK4a by almost one log2 order of magnitude immediately following chemotherapy and remained elevated for up to 12 months after treatment in patients with primary breast cancer;32 the magnitude of increase corresponded to 14.7 years of chronological aging.32
In this context, whether exercise completely abrogates the cancer treatment-induced decline in CRF remains unclear. The available mixed findings may be attributable to the differences in adjuvant therapy regimens (e.g., taxane,33 nontaxane-based,34 ADT26), baseline profile of patients (e.g., inclusion28 or exclusion24,26 of patients with pre-existing CVD risk factors), prescription approach (i.e. standard33 vs. non-linear34), or intensity (i.e. moderate intensity only33 vs. prescriptions incorporating higher-intensity exercise28,34). In addition, despite the general consensus that exercise is safe and tolerable for cancer patients,35,36 few studies systematically monitored and/or reported these end points.
After Therapy
Data from phase 3 trials are not currently available. In observational evidence, after median follow-up of 10 years, compared with <3 MET-hrs.wk−1, ≥ 3 MET-hrs.wk−1 was associated with a 19% (p=0.026), 39% (p=0.026), and 11% (p=0.17) reduction in all-cause, recurrence/progression, and health-related deaths, respectively in 15,450 adult survivors of childhood cancer (J.Scott, PhD; unpublished data; 2018). In addition, increase in exercise exposure (+7.9 ± 4.4 MET-h/wk) over an 8-year period was associated with a 40% reduction in all-cause mortality rate compared to maintenance of low exercise exposure (RR=0.60; 95% CI, 0.44 to 0.82, p=0.01). In related work, increasing exercise exposure was associated with a strong, graded reduction in the risk of CVD events and CVD mortality in 2973 women with primary breast cancer;37 adherence to exercise guidelines was associated with a 23% reduction in CVD events.37
Similar to during therapy, few studies have investigated the effects of exercise on subclinical cardiovascular outcomes in this setting with most data available on CRF (Table 2). For instance, Courneya et al.38 demonstrated that 15 weeks of supervised cycle ergometry following a standard prescription led to a 2.7 ml.kg.-1min−1 (15%) VO2peak increase compared to usual care in 53 postmenopausal patients with primary breast cancer. In contrast, Rogers et al.39 reported no differences in VO2peak following 12 weeks of standard aerobic exercise (4 weeks supervised, 8 weeks unsupervised, 3-5d/wk, 15-50 min/session at 40% - 59% heart rate reserve) compared with usual care in 222 primary breast cancer survivors. Beyond breast cancer, Pinto et al.40 randomized 46 colorectal patients to home-based standard aerobic exercise (2-5d/wk, 10-30 min/session at 65-75% estimated maximum heart rate) or usual care. After 12 weeks, estimated VO2peak increased by 4.7 ml.kg.-1min−1 (20%) in the aerobic training group with no change in the usual care group.
Table 2.
Key randomized controlled trials after cancer therapy.
| Study | N | Cohort/Setting | CVD status | Modality | Length | Fq | Duration (range) | Intensity (range) | LTF | Safety | Attendance | Adherence | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Courneya et al. (2003)38 | 53 | Breast cancer patients ~14 mo post therapy randomized to supervised AT or UC | NR | CE | 15 wks | 3 | 15 – 35 mins | 70 – 75% Power output at ventilation threshold |
6% | 0 AE | 98% | NR |
Measured VO2peak: AT: ↑15% UC: no change significant between group difference |
||
| Thorsen et al. (2005)45 | 139 | Breast, gynecologic, lymphoma, testicular cancer patients ~ 30 d post therapy randomized to unsupervised AT or UC | NR | TM, CE, Skiing | 15 wks | 2 | NR | 13 -15 Borg scale | 20% | NR | NR | NR |
Estimated VO2peak: AT and UC: no change |
||
| Pinto et al, 201340 | 46 | Colorectal cancer patients ~ 3 years post diagnosis randomized to AT or UC | NR | Walk | 48 weeks | 2-5 | 10 – 30 mins | 60-75% estimated VO2peak | 9% | NR | NR | NR |
Estimated VO2peak: CT:↑32% UC:↑15% significant between group difference |
||
| Jones et al. (2014)64 | 90 | Cancer patients with HF post therapy randomized to 3 mo supervised + 4 to 12 mo unsupervised AT or UC | HTN (94%) Diabetes (38%) HF (100%) |
CE/TM | 52 wks | 4 | 20 – 45 mins | 60 – 70% HRR | 14% |
AT 2 (4%) AE post- exercise; 21 (45%) total AE UC 1 (2%) AE post-exercise; 10 (23%) total AE |
53% | NR |
Measured VO2peak: AT:↑4% UC:↑6% non-significant between group difference |
||
| Jones et al. (2014)42 | 50 | Prostate cancer patients ~ 75d post-therapy randomized to supervised AT or UC | HTN (54%) HPL (60%) Diabetes (16%) CVD (8%) Low CRF (100%) |
TM | 24 wks | 5 | 30 - 45 mins | 55 – 100% speed at VO2peak | 8% | 0 AE | 83% | 79% |
Measured VO2peak: AT:↑9% UC: ↑1% significant between group difference FMD: AT:↑1.7% UC: ↑0.27% significant between group difference |
||
| Rogers et al. (2015)39 | 222 | Breast cancer patients ~54 mo post therapy randomized to supervised + unsupervised AT or UC | HTN (11%) |
AT CE, ET, TM |
12 wks | 3 - 5 | 15 - 50 mins |
AT 40 – 59% HRR |
2% | 17 AE | 98% | NR |
Estimated VO2peak: AT: ↑12% UC: ↑10 non-significant between group difference |
||
| Adams et al. (2017)41 | 63 | Testicular cancer patients ~8 years post-therapy randomized to supervised AT or UC | Obese (21%) Pre HTN (19%) Metabolic syndrome (19%) Mild carotid plaque (57%) Moderate to severe carotid plaque (24%) |
TM | 12 wks | 3 | 35 min (4×4 min interval) | 75-95% VO2peak | 3% | 0 AE | 99% | 98% |
Measured VO2peak: AT: ↑ 11% UC: no change significant between group difference Carotid intima-media thickness AT: ↑7% UC: no change significant between group difference; Carotid distensibility: AT: ↑16% UC: no change significant between group difference Framingham risk score: AT: ↑0.5% UC: no change significant between group difference |
||
All interventions were described according to the classic components of exercise prescription: (1) type (modality), (2) program length (total number of training weeks), (3) frequency (mean number of exercise sessions/week), (4) duration (duration spent on 1 session of exercise), and (5) intensity (percentage of a predetermined physiological parameter such as maximum heart rate obtained from baseline cardiopulmonary exercise test). All outcomes were described according to LTF (number of patients that dropped out divided by total number of patients), safety (number of serious AE), attendance (the number of exercise sessions attended divided by the total number of planned sessions), adherence (number of exercise sessions that were completed at the planned duration and intensity divided by the number of planned sessions attended), and efficacy (change in outcome).
Abbreviations: Fq; frequency; RCT, randomized controlled trial; AT, aerobic training; UC, usual care; RT, resistance training; CT, combined aerobic and resistance training; CE, cycle ergometer; ET, elliptical trainer; TM, treadmill; RM, repetition maximum; HRR, heart rate reserve; HR, heart rate; ADT, androgen deprivation therapy; NR, not reported; LTF, loss to follow up rate; AE, adverse event; FMD, flow mediated dilatation.
Few studies have assessed effects of exercise on cardiovascular end points beyond CRF. Adams and colleagues41 reported that compared to usual care, 12 weeks of high-intensity interval aerobic training (3d/wk, 35 min/session at 75%-95% VO2peak) improved VO2peak, (adjusted mean group difference: 3.7 ml.kg.-1min−1) as well as vascular function (adjusted mean group differences of −0.6 mm, 1.54 10−3/kPa, and −2.02 m/s for carotid intima-media thickness, carotid distensibility, arterial stiffness, respectively), and Framingham risk score (adjusted mean group difference: −0.6%) in 63 patients with testicular cancer. Two RCTs specifically recruited patients at high-risk of or with overt CVD. In a RCT by Jones et al.,42 a protocol-defined eligibility criteria was a VO2peak below age-sex-matched normative sedentary norms in men with prostate cancer. Non-linear aerobic training [5d/wk (x3 supervised, x2 home-based); 30-60 min/session at 55%-100% of measured VO2peak for 24 weeks] increased VO2peak which occurred in conjunction with improvements in endothelial function but no changes in other CVD markers (e.g., lipid profile, blood pressure, body composition).42 The same investigators conducted an unplanned, ancillary retrospective analysis of 90 patients enrolled in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial43 with a prior history of cancer.44 Intention-to-treat analyses indicated no differences in primary end point (all-cause mortality or hospitalization) after a median follow-up of 35 months (HR: 1.11; 95% CI: 0.69-1.77).44 For secondary endpoints, the incidence of cardiovascular mortality or cardiovascular hospitalization was significantly higher in the exercise group compared with usual care (67% vs. 41%; HR: 1.94; 95% CI: 1.12 to 3.16), while no significant differences in VO2peak were observed in either group.44
In sum, there is reasonable evidence to support the conclusion that exercise improves CRF after the completion of cancer therapy, although several studies found no effects of exercise.39,44,45 The discrepant findings may be due to methodological differences or possibly differences in the long-term, persistent effects of certain anticancer therapies. There is insufficient evidence to conclude that exercise improves other markers of cardiovascular health in this setting.
Summary of Current Evidence
As reviewed here, the efficacy and mechanisms of exercise to prevent and/or mitigate cardiovascular toxicity following a cancer diagnosis is limited. Moreover, beyond observational studies, investigation of exercise is primarily limited to CRF, an end point of significant clinical importance since poor CRF is associated with a higher prevalence of acute and chronic treatment-related toxicities (e.g., CVD),15,46-49 higher symptom burden (e.g., fatigue),50-52 and increased risk of all-cause and cancer-specific mortality in patients with cancer.14,53,54 Nevertheless, CRF is not a traditional CVD risk factor nor is it a component of ideal cardiovascular health score.55 Against this background, current and exploratory exercise-oncology paradigms are presented in the following section.
Personalized Exercise-Oncology Research
Current Paradigm
Precision or high-definition medicine – understanding the mechanisms or predictors of disease risk or treatment response in an individual patient or phenogroup of patients to guide tailored treatment strategies – is becoming the paradigm in clinical investigation.56 In stark contrast, investigation of exercise as a therapeutic strategy in chronic disease has not yet adopted such an approach. Instead, the traditional approach has been to test the efficacy of standard prescriptions that closely adhere to the national exercise guidelines (i.e., aerobic alone, resistance alone or the combination 3 – 5d/wk, 20 – 60 mins/session at 55% to 75% of age-predicted or measured heart rate maximum or reserve or 12 to 24 weeks). Although this approach has an aspect of personalization (exercise dosing intensity is targeted to each individual patient on the basis of measured or predicted heart rate), the dose [i.e., modality, frequency (per week), duration (per session), intensity (per session), and length of treatment exposure] as well as the scheduling (linear prescription)] is similar both within and across major disease conditions. Thus, the current paradigm operates under the overarching assumption that all patients respond equally to a standard exercise dose (“one size, fits all’).
Clearly, despite this, it could be argued that relatively homogeneous exercise prescriptions have consistently been shown to improve a diverse range of end points largely irrespective of disease condition and setting, therefore questioning the rationale to investigate targeted approaches. However, the vast majority of observational and RCT studies as well as related meta-analyses/systematic reviews36,57 focus on the overall treatment effect for the entire study sample. Accordingly, presentation of the mean result masks the variability in responses (i.e., those with lesser or greater benefit than the overall population) that could be observed within a heterogeneous population.58 Emerging data from several ancillary analyses indicates there is considerable heterogeneity in exercise response even for changes in CRF. For instance, in the HF-ACTION trial, despite a mean increase in VO2peak of 0.6 ml.kg.-1min−1 (4%) following 12 weeks of aerobic training, change in VO2peak ranged from −12 ml.kg.-1min−1 (−83%) to +14 ml.kg.-1min−1 (+97%).59 Moreover, only ~50% of patients randomized to exercise experienced a VO2peak ≥1.0 ml.kg.-1min−1 – a change considered clinically important.60 In the oncology setting, we found that the mean change in VO2peak following 24 weeks of aerobic training in prostate cancer patients was ~9%; patient-level data, however, revealed the delta in VO2peak ranged from −18% to +32%.42 A similar response variability has been observed in other cardiovascular end points. Leon et al.61 reported a significant 4% group mean increase in HDL cholesterol; however, the individual patient change ranged from −24% to +66% in 675 sedentary subjects following 20 weeks of standard aerobic training (3d/wk, 30-50 min at 55-75% VO2peak). A threshold effect (i.e., prescribed exercise dosing intensity is insufficient to confer meaningful cardiovascular adaptation) has been proposed to explain ‘low responders’ to exercise.62,63 Ross and colleagues62 examined this hypothesis in an ancillary analysis of standard aerobic training in sedentary obese adults. Results indicated that either higher exercise intensity or volume decreased the number of ‘low responders,’ as defined by improvements in CRF. Nevertheless, increasing exercise intensity or volume is unlikely to be an all-encompassing solution to improve exercise response variability, and may even be contraindicated in certain clinical populations.64
In addition to heterogeneity in a specific end point (e.g., CRF), there also appears to be heterogeneity across study end points within a specific study cohort. For instance, Kraus et al.65 found that improvements in CRF were similar for high-duration–high-intensity (approximately 20 miles/wk at 65% to 80% of VO2peak) as well as low-duration-high-intensity (approximately 12 miles/wk at 65% to 80% of VO2peak) training, yet improvements in lipoprotein profile were superior with high-duration-high-intensity exercise among 84 overweight men and women with mild-to-moderate dyslipidemia. Similarly, Ross and colleagues66 found that a standard aerobic training prescription was associated with substantial reductions in abdominal obesity whereas improvements in CRF and 2-hr glucose levels were confined to high-dose exercise among 300 abdominally obese adults. These data indicate that same exercise prescription confers differential effects across different end points, supporting the notion that exercise should be designed to target the primary end point of interest. This is consistent with the principle of specificity – the selected exercise stress must be specific and targeted to the primary underlying system(s) or pathway(s) known or postulated to underpin the primary end point of interest.67
Finally, in addition to exercise response heterogeneity in efficacy end points, it is also important to consider variability in exercise safety and tolerability. Even within a seemingly homogenous cohort (e.g., primary breast cancer), there may be considerable variability in treatment (e.g., radiation, chemotherapy), CVD risk factors (e.g., age, hypertension, dyslipidemia), and baseline physiological status (e.g., below or comparable to age-sex-matched VO2peak).65 Dependent on patient’s baseline status (and therefore inherent capacity to respond to external physiological stress in the form of exercise), a standard exercise prescription dose may be insufficient (under training), sufficient (physiologic adaptation), or excessive (over training). Such a consideration may be especially important in cancer patients given the potential of anticancer therapies to alter the exercise – adaptation relationship.67 As outlined above, supervised aerobic exercise was not tolerated in all patients with metastatic breast cancer,68 while the incidence of cardiovascular mortality or cardiovascular hospitalization was significantly higher in HF patients with a history of cancer randomized to aerobic exercise compared to control.64 These findings provide initial evidence to suggest that a subgroup of patients with cancer could be too ill to adhere to a prescribed exercise dose or may even experience an adverse exercise response.
Collectively, the above examples create a strong rationale for the development and testing of alternative tailored approaches to optimize efficacy, tolerability, and safety of exercise in the oncology setting (Figure 1).
Figure 1.
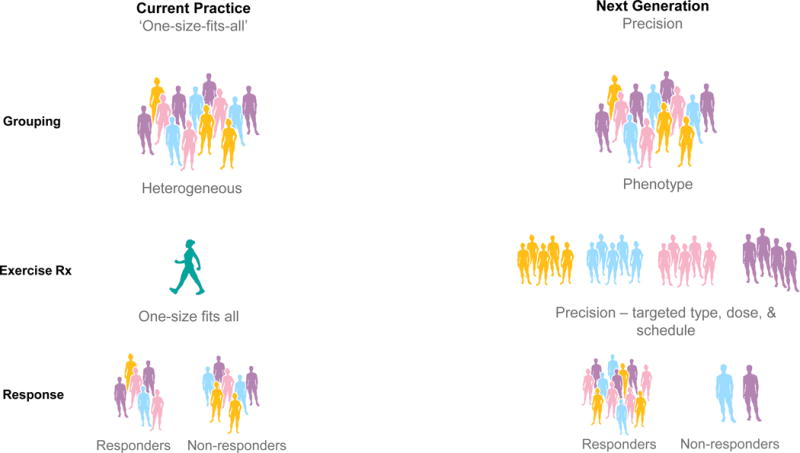
Current and next generation practice in exercise oncology.
Current practice (left column) stratifies patients based on tumor type, provides a generic exercise prescription (typically based on predicted maximum heart rate), resulting in a heterogeneous response. Next generation practice (right column) stratifies patients based on multiple factors, provides a targeted exercise prescription based on phenogroup, resulting in optimized efficacy, safety, and tolerability of exercise therapy. CPET, cardiorespiratory exercise test, CRF, cardiorespiratory fitness, Rx, prescription.
Exploratory Paradigms
The development of targeted exercise prescriptions requires an initial evaluation (i.e., phenotyping) of clinical and/or medical parameters that permits stratification of patients with a common but heterogeneous condition into homogeneous subgroups (i.e., phenogroups).56 In this context, below we overview three potential screening/evaluation (phenogrouping) approaches that could be applied to research investigations designed to assess the efficacy of exercise on cardiovascular toxicity in the oncology setting (Figure 2). These screening approaches range from methods that leverage existing risk stratification models to increasingly multifaceted approaches that incorporate more detailed physiological and potentially biological phenotyping. We further speculate on how these approaches might facilitate the design of phenogroup-targeted exercise prescriptions, and illustrate how more detailed phenotyping may permit the design of even more targeted/personalized prescriptions. Given the preliminary nature of this paradigm and approach, we explore the application of these tenets to investigation of exercise on CRF. We selected CRF since this the majority of exercise-oncology research to date has used this outcome, and, CRF is a strong predictor of late-occurring CVD.11
Figure 2.
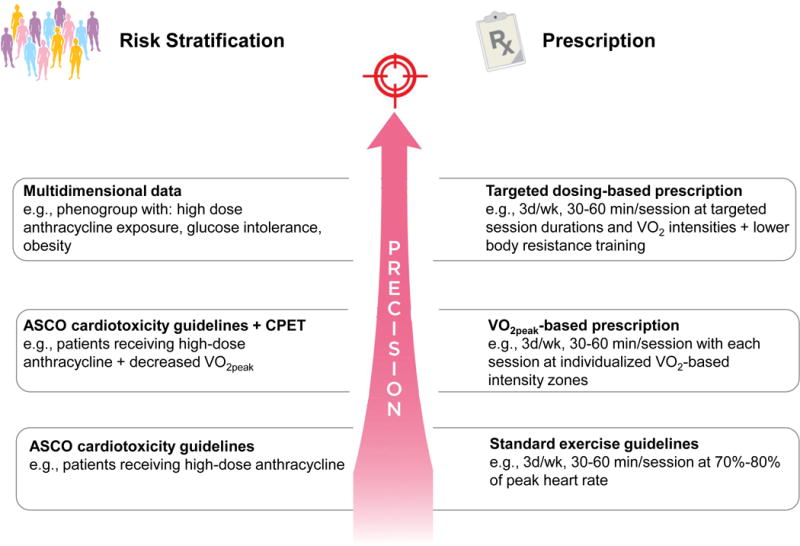
Screening/exercise prescription approaches in oncology.
Three example screening/exercise prescription approaches that could be applied to research investigations designed to assess the efficacy of exercise on cardiovascular toxicity in the oncology setting: (1) guideline based approach (bottom row) applies ASCO cardiotoxicity guidelines and standard exercise guidelines; (2) ASCO guidelines and VO2peak-based approach (middle row) applies the addition CPET for risk stratification and exercise prescription design; (3) multidimensional data approach (top row) applies advanced analytics for both risk stratification and targeted exercise prescription design. ASCO, American Society of Clinical Oncology; CPET, cardiorespiratory exercise test.
Approach 1: Model/Guideline-Based Risk Stratification
Screening
The extent of patient evaluation and pre-exercise screening in the majority of exercise-oncology studies is physician/oncologist clearance, whereas stratification is typically confined to type of diagnosis (e.g., breast, prostate) and/or setting (e.g., during, after therapy), rather than cardiovascular toxicity risk profile. Although decline in CRF and impaired CRF14,16 appear to be cardinal features following a cancer diagnosis, predictors of individual risk of CRF decline are not known.69 Therefore, a logical and practical first step in studies designed to prevent and/or mitigate cardiovascular toxicity is selection of patients on the basis of late cardiovascular toxicity risk. Several models accurately predict individual patient risk of late-occurring CVD following a cancer diagnosis and assist in the stratification of patients into low, moderate, and high-risk groups on the basis of widely available clinical inform such as sex and radiation/chemotherapy exposures.70 For instance, the American Society of Clinical Oncology (ASCO) guidelines10 has identified patient subgroups considered “high” risk of LV dysfunction or HF (Table 3).10 Unfortunately, available risk stratification models provide limited information on which to design targeted exercise prescriptions, but do identify those at highest risk of future events for prophylactic intervention.
Table 3.
American Society of Clinical Oncology (ASCO) guidelines on patients at high cardiac dysfunction risk.10
| Risk | Example |
|---|---|
| High-dose anthracycline | ≥ 250 mg/m2 doxorubicin |
| High-dose radiotherapy | ≥ 30 Gy radiation with the heart in the treatment field |
| Low-dose anthracycline + low dose radiotherapy | < 250 mg/m2 doxorubicin in combination with < 30 Gy radiation with the heart in the treatment field |
| Low-dose anthracycline + ≥ 2 CVD risk factors | < 250 mg/m2 doxorubicin in combination with smoking, hypertension, diabetes, dyslipidemia, and/or obesity |
| Low-dose anthracycline + older age | < 250 mg/m2 doxorubicin in combination with ≥ 60 years at cancer treatment |
| Low-dose anthracycline + comprised cardiac function | < 250 mg/m2 doxorubicin in combination with history of myocardial infarction, moderate valvular disease, LVEF between 50% and 55% |
| Trastuzumab + ≥ 2 CVD risk factors | Trastuzumab in combination with smoking, hypertension, diabetes, dyslipidemia, and/or obesity |
| Trastuzumab + older age | Trastuzumab in combination with ≥ 60 years at cancer treatment |
| Trastuzumab + comprised cardiac function | Trastuzumab in combination with history of myocardial infarction, ≥ moderate valvular disease, LVEF between 50% and 55% |
| Low-dose anthracycline followed by trastuzumab | Sequential therapy of < 250 mg/m2 doxorubicin and trastuzumab |
Abbreviations: CVD, cardiovascular disease; Gy; Gray; LVEF, left ventricular ejection fraction.
Targeted exercise prescription
The optimal exercise dose to prevent CVD events and mortality is not known; however, exercise recommendations from the Centers for Disease Control and Prevention,71 and the American Heart Association72 (i.e., 3d.wk, 30-60 min/session at 70%-80% of peak heart rate determined from a symptom-limited exercise stress test) affirm the primary role of exercise in preventing chronic disease. The clinical applicability of generic exercise guidelines (which are identical to current national and international exercise guidelines for cancer patients73) is high; however, as outlined above, precision for the individual patient is low.
Approach 2: Model/Guideline-Based Risk Stratification plus Exercise Stress Testing
Screening
Incorporation of data from exercise stress testing (in conjunction with available risk models overviewed in approach 1) may further facilitate risk stratification. Exercise stress tests can identify contraindications (e.g., hypertension, ischemia) and exertional symptoms, provide an objective determination of CRF, and guide targeted interventions.72 International guidelines on the proper conduct of a range of submaximal and maximal exercise stress testing are available.74 For instance, with minimal instrumentation and trained personnel requirements, functional testing (e.g., 6 min walk test) and submaximal exercise testing (e.g., submaximal work rates) are viable options in markedly deconditioned patients and can be performed with minimal risk to patients.11 Maximal stress testing with ECG monitoring can also be used to estimate CRF,11 and measured peak heart rate can be used to facilitate prescription design.11 Finally, cardiopulmonary exercise tests (CPET) with measurement of ventilatory gas exchange may be the preferable method since it can provide: (1) an objective assessment of submaximal and peak VO2, and (2) delineation of the pathophysiological mechanisms underlying exercise limitations.74
VO2peak is determined by a series of steps that transports oxygen from the environment to the skeletal muscle mitochondria, also known as the ‘oxygen pathway’.69 As a result, additional patient stratification could occur through assessment of any defective step(s) along the oxygen pathway via: (1) CPET with gas exchange to determine VO2peak, (2) cardiac output assessed noninvasively (e.g., echocardiography)75 or invasively (e.g., intracardiac hemodynamic data from a pulmonary artery),76 (3) blood hemoglobin concentration, and (4) arterial-venous oxygen content difference (A-VO2 Diff) assessed non-invasively (e.g., calculated from the Fick equation),74 or invasively (e.g., arterial blood gas data from a radial catheter).77 To date, few studies have directly investigated the mechanisms of reduced CRF in cancer patients.69 Nevertheless, important insights can be gleaned from other clinical settings that have assessed determinants of poor CRF.77 For example, Houstis and colleagues77 quantified oxygen pathway deficiencies with invasive monitoring among 134 HF patients and found that two of the steps, cardiac output and skeletal muscle oxygen diffusion, were impaired in a subgroup of patients by an average of 27±3% and 36±2%, respectively. Thus identification of a subgroup of patients with shared defects could be used not only to stratify patients, but, as outlined below, to tailor therapy.
Targeted exercise prescriptions
Functional and submaximal exercise testing heart rate and blood pressure responses can be used to estimate peak values and prescribe different exercise intensities for each patient that are independent of disease severity or baseline fitness.78 Exercise prescriptions that are based on estimated baseline physiological endpoints have high clinical applicability, but increase the susceptibility for under-dosing and/or over-dosing of exercise therapy. For instance, use of age-predicted maximum heart rate may result in overtraining in primary breast cancer patients treated with polychemotherapy due to the resulting autonomic dysfunction and decreased heart rate reserve.79 Therefore, utilization of CPET-based metabolic or ventilatory responses to generate three to five unique exercise ‘intensity zones’ increases personalization and allows for specificity of exercise prescriptions.42,67 For example, high-intensity, interval exercise sessions (e.g., 6 × 2 min above ventilatory threshold) activate mitochondrial biogenesis within skeletal muscle,80 and may be more effective for augmenting CRF. Finally, exercise prescriptions based on oxygen pathway defects could be implemented. In the case of CRF where the primary limitation is identified as a peripheral limitation (e.g., decreased A-VO2 Diff due to decreased capillary density or impaired oxygen utilization by the exercising skeletal muscles), as could occur in sarcopenia or cachexia, whole body exercise may not be the most effective mode of exercise to increase VO2peak. Among cachexic HF patients, Esposito et al.81 demonstrated that 2 months of one leg knee extensor exercise resulted in a significant increase in VO2peak due to improvements in A-VO2 Diff.
Approach 3: Multidimensional Data-Based Risk Stratification
Screening
Addressing the heterogeneity of cancer patients on the basis of multiple medical (e.g., cancer therapy, comorbidities, performance status) and physiological characteristics (e.g., lipids, glucose, CPET variables, cardiac function) could enable novel subgroup risk classifications.58 Such integration of multidimensional data using machine learning, an approach that integrates statistical relationships and computer algorithms,82 has been applied to combine complex datasets to cluster HF patients into distinct, mutually exclusive groups.83,84 For example, using unsupervised learning (i.e., identifying subgroups without a predicted outcome) in an ancillary analysis of the HF-ACTION trial, Ahmad and colleagues84 identified four novel subgroups that varied in baseline clinical characteristics (e.g., age, sex, race, symptoms, comorbidities, biomarkers), response to aerobic exercise, and incidence of CV death and/or CV hospitalization. Similarly, based on 67 candidate variables (e.g., echocardiography, electrocardiogram-based data points) Shah and colleagues83 identified three mutually exclusive subgroups in a cohort of 397 HF patients. Whether machine learning could be applied to identify subgroups of cancer patients at high risk of cardiovascular toxicity is unknown, but based on previous studies in oncology and cardiology it appears to be a promising avenue for future work.
Targeted exercise prescriptions
Classifying patients a priori into distinct subgroups based on multi-dimensional data to guide the design of exercise prescriptions undoubtedly represents a significant challenge.85 However, a landmark framework by Zeevi and colleagues86 provided ‘proof-of-concept’ that applying deep clinical phenotyping in conjunction with machine-based learning enabled the design of an effective personalized nutrition intervention. To exemplify the potential application of multidimensional data and machine learning to guide exercise prescription design, the following steps could be applied in a cohort of breast cancer patients that had completed a 12 week exercise program with CRF as the primary end point (Figure 3). First, patients would be extensively characterized with multidimensional data (e.g., treatment, medical, physiological). Second, unsupervised learning would be applied to develop a parsimonious number of subgroups (with internal validation). Third, an independent validation analysis in a separate cohort using the same multidimensional data would be performed, and finally, supervised learning within each subgroup would be applied to ascertain predictors of CRF. Once subgroup-specific predictors of CRF were identified the next logical step would be to design and test personalized exercise prescriptions that are specifically targeted to CRF predictors in a de novo breast cancer cohort. This approach is consistent with the Precision Medicine Initiative to assess individual variability and personalize prevention and treatment strategies;56 nevertheless, has not yet been tested in any exercise setting.
Figure 3.
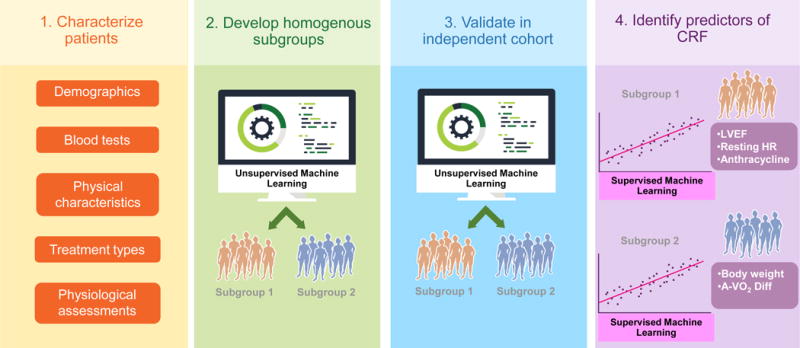
Phenogrouping model.
Phenogrouping model with four steps: (1) characterize patients using multidimensional data (e.g., demographics, blood tests, physical characteristics, treatments types, physiological assessments); (2) develop and internally validate a parsimonious number of homogenous subgroups using machine learning; (3) validate model in an external cohort; (4) identify predictors of primary end point (e.g., CRF) within each subgroup. CRF, cardiorespiratory fitness, A-VO2 Diff, arterial-venous oxygen content difference, LVEF, left ventricular ejection fraction; HR, heart rate.
Challenges and Future Directions of Personalized Exercise Therapy
Many critical questions pertaining to the implementation of precision medicine have been outlined previously;56,85 here we briefly discuss major barriers germane to personalized exercise therapy to optimize CRF. Addressing these, and other challenges is not only scientifically intriguing, but also critical to inform policy, evidence-based guidelines, and daily clinical care.
Screening
A fundamental step is to discern what factors should be included in risk screening. Unlike identification of therapy-related factors predictive of LV dysfunction or HF,10 factors predictive of: (1) CRF decline, and (2) CRF response to exercise in cancer patients are unknown. Accordingly, characterization of deficits in the oxygen pathway is arguably the first knowledge gap that needs to be addressed. Investigation of whether incorporation of additional biomarkers (e.g., troponins87) or ‘omics’ (e.g., genomics88) improves risk stratification is needed. For example, results from a genome-wide association study in childhood cancer survivors suggests there is a modifying effect of a polymorphism of CELF4 on anthracycline dose-dependent HF risk;89 whether this polymorphism is associated with CRF decline is unknown. Similarly, approximately 200 genetic variants have been associated with physical performance to date;88 however, even in genome wide association studies the physiologic and clinical significance of genetic predictors is low.90 Finally, risk stratification will become increasingly complex with multidimensional screening factors and there will be a need for advanced analytic approaches to aid in the development of parsimonious subgroups. Nevertheless, advanced analytic solutions are emerging in other areas of medicine91 which could be applied to exercise-oncology research.
Targeted exercise prescriptions
The approach of personalizing interventions by first categorizing patients into more homogeneous subgroups to then deliver targeted therapy has been in practice in oncology for over 40 years.92 Application of this model to exercise-oncology will clearly represent a paradigm shift for both patients and researchers and require a novel framework. Phase I/II trials that evaluate exercise safety and tolerability may be a prerequisite for initiating clinical exercise trials, while rigorous clinical trials conducted in a highly structured, clinical-based setting with all sessions monitored and supervised by certified exercise professionals may be necessary to demonstrate efficacy of a novel exercise training paradigm. In theory, different training modalities as well as different doses and lengths of training programs will be indicated. Such ‘personalized dosing’ has been successfully tested in several clinical research settings – in a pilot RCT, Zarrinpar and colleagues93 used multidimensional patient phenotyping and machine learning to develop personalized dosing of tacrolimus to prevent under- and overdosing among liver transplant patients. These research approaches that integrate multiple fields (e.g., exercise, oncology, cardiology, computational medicine) will be essential in order to test and implement targeted exercise prescriptions.
Implementation
Transition from research settings to widespread clinical applicability represents a significant challenge. Delivery of personalized exercise therapy could consist of several different supervised, unsupervised, or hybrid clinic-/community-/home-based models. In certain settings application of a cardiac rehabilitation model to the oncology setting could allow patients access to structured exercise interventions across the cancer continuum. For instance, given the emergent data on the prognostic importance of pre-surgical CRF on post-surgical outcomes,48,49,54 the introduction of exercise therapy to improve CRF in the interval between diagnosis and cancer interventions may have considerable clinical benefit.94 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) guidelines outline a continuum of services ranging from inpatient programs (during hospitalization), transitional programs (during post-acute care), outpatient programs (6 weeks following hospital discharge and continuing for up to 12 weeks), and long-term maintenance programs (following completion of outpatient programs).95 An alternative, community-based model is the LIVESTRONG at the YMCA exercise program.96 This 12-week, supervised group-based program for patients with a history of cancer is currently offered in approximately 20% of YMCA branches across the US.96 Finally, widespread implementation of home-based tele-exercise programs with monitoring97 could ensure safety and efficacy of exercise programs with lower patient burden.
Conclusions
Cardiovascular toxicity is a devastating adverse consequence of cancer therapy for numerous patients, especially those living 5 years beyond initial diagnosis.98 In certain cancer populations with primary disease, CVD mortality is not only more common (2-fold to 4-fold higher), but also occur at an earlier age than in the general population.98 With ~16.7 million adults living with a history of cancer in the United States, a figure expected to reach ~26 million by 2040,99 cardiovascular medicine specialists can expect or are already managing a large proportion of cancer patients with or at high-risk of cardiovascular toxicity. Although the current evidence base is limited, the demonstrated benefit and centricity of exercise in other clinical populations suggest that it may also become a key feature of future programs in the oncology setting. In the design of such programs, the adoption and implementation of a targeted/precision medicine approach could be critical to optimize the efficacy, safety, and tolerability of exercise for patients with a history of cancer.
Acknowledgments
The authors thank Wenjing Wu at MSK Design and Creative Services for assistance with illustrations and Whitney Underwood for administrative support.
Funding Sources
LWJ is supported by research grants from the National Cancer Institute. JMS, TSN, and LWJ are supported by AKTIV Against Cancer, the Kalvi Trust, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Perry S. Reduction of toxicity in cancer chemotherapy. Cancer Res. 1969;29:2319–2325. [PubMed] [Google Scholar]
- 2.Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 3.Malpas JS, Scott RB. Rubidomycin in acute leukaemia in adults. British medical journal. 1968;3:227–229. doi: 10.1136/bmj.3.5612.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88:168–175. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 5.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Friedman MA, Bozdech MJ, Billingham ME, Rider AK. Doxorubicin cardiotoxicity. Serial endomyocardial biopsies and systolic time intervals. JAMA. 1978;240:1603–1606. doi: 10.1001/jama.240.15.1603. [DOI] [PubMed] [Google Scholar]
- 7.Ramos A, Meyer RA, Korfhagen J, Wong KY, Kaplan S. Echocardiographic evaluation of adriamycin cardiotoxicity in children. Cancer treatment reports. 1976;60:1281–1284. [PubMed] [Google Scholar]
- 8.Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, Sandor G, Benson L, Williams R. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89(5 Pt 1):942–949. [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U, American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Jones L, Muss HB. Cancer Treatment as an Accelerated Aging Process: Assessment, Biomarkers, and Interventions. Am Soc Clin Oncol Educ Book. 2016;35:e516–522. doi: 10.1200/EDBK_156160. [DOI] [PubMed] [Google Scholar]
- 13.Jarden M, Hovgaard D, Boesen E, Quist M, Adamsen L. Pilot study of a multimodal intervention: mixed-type exercise and psychoeducation in patients undergoing allogeneic stem cell transplantation. Bone marrow transplantation. 2007;40:793–800. doi: 10.1038/sj.bmt.1705807. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, 2nd, Douglas PS, Haykowsky Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 16.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan G, Balady GJ. Cardiac rehabilitation 2012: advancing the field through emerging science. Circulation. 2012;125:e369–373. doi: 10.1161/CIRCULATIONAHA.112.093310. [DOI] [PubMed] [Google Scholar]
- 18.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 20.Palomo A, Ray RR, Johnson L, et al. Associations Between Exercise Prior to and Around the Time of Cancer Diagnosis and Subsequent Cardiovascular Events in Women With Breast Cancer: A Women’s Health Initiative (WHI) Analysis. J Am Coll Cardiol. 2017;69(Supplement) [Google Scholar]
- 21.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38:348–351. [PubMed] [Google Scholar]
- 22.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 23.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 24.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 25.Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, Povsic TJ, Peppercorn J, Marcom PK, Blackwell KL, Kimmick G, Turkington TG, Dewhirst MW. Modulation of Circulating Angiogenic Factors and Tumor Biology by Aerobic Training in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Prevention Research. 2013;6:925–937. doi: 10.1158/1940-6207.CAPR-12-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D’Angelo ME. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 27.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 28.Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, Chua N, Tkachuk L, Mackey JR. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist. 2008;13:1012–1020. doi: 10.1634/theoncologist.2008-0017. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli EM, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Taylor JG, 6th, Hannoush H, Goldsmith JC, Gladwin MT, Gordeuk VR, Walk-PHASST Investigators Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124:1452–1460. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 32.Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, Ibrahim JG, Jolly TA, Williams G, Carey LA, Drobish A, Gordon BB, Alston S, Hurria A, Kleinhans K, Rudolph KL, Sharpless NE, Muss HB. Journal of the. Vol. 106. National Cancer Institute; 2014. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer; p. dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C, Dolan LB, Forbes CC, Wooding E, Trinh L, Segal RJ. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. Journal of the National Cancer Institute. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 34.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE, 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 35.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46:909–916. doi: 10.1136/bjsports-2010-082719. [DOI] [PubMed] [Google Scholar]
- 36.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP, Jr, Scott J, Sternfeld B, Yu A, Kushi LH, Caan BJ. Exercise and Risk of Cardiovascular Events in Women With Nonmetastatic Breast Cancer. J Clin Oncol. 2016;34:2743–2749. doi: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 39.Rogers LQ, Courneya KS, Anton PM, Hopkins-Price P, Verhulst S, Vicari SK, Robbs RS, Mocharnuk R, McAuley E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149:109–119. doi: 10.1007/s10549-014-3216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 41.Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, Szczotka A, Courneya KS. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer. 2017;123:4057–4065. doi: 10.1002/cncr.30859. [DOI] [PubMed] [Google Scholar]
- 42.Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, Ferrandino MN, Allen JD, Kenjale AA, Thomas SM, Herndon JE, 2nd, Koontz BF, Chan JM, Khouri MG, Douglas PS, Eves ND. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–855. doi: 10.1016/j.eururo.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, HF-ACTION Investigators Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones LW, Douglas PS, Khouri MG, Mackey JR, Wojdyla D, Kraus WE, Whellan DJ, O’Connor CM. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol. 2014;32:2496–2502. doi: 10.1200/JCO.2013.53.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23:2378–2388. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 46.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 47.West MA, Parry MG, Lythgoe D, Barben CP, Kemp GJ, Grocott MP, Jack S. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg. 2014;101:1166–1172. doi: 10.1002/bjs.9551. [DOI] [PubMed] [Google Scholar]
- 48.West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S, Grocott MP. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth. 2014;112:665–671. doi: 10.1093/bja/aet408. [DOI] [PubMed] [Google Scholar]
- 49.West MA, Asher R, Browning M, Minto G, Swart M, Richardson K, McGarrity L, Jack S, Grocott MP, Perioperative Exercise Testing and Training Society Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg. 2016;103:744–752. doi: 10.1002/bjs.10112. [DOI] [PubMed] [Google Scholar]
- 50.Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA, Shatten C, Hie Kim Y, Whitley J, Serody JS, Shea T, Battaglini C. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone marrow transplantation. 2013;48:1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 51.Herrero F, Balmer J, San Juan AF, Foster C, Fleck SJ, Pérez M, Cañete S, Earnest CP, Lucía A. Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? J Strength Cond Res. 2006;20:535–540. doi: 10.1519/r-18215.1. [DOI] [PubMed] [Google Scholar]
- 52.West MA, Loughney L, Barben CP, Sripadam R, Kemp GJ, Grocott MP, Jack S. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol. 2014;40:1421–1428. doi: 10.1016/j.ejso.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Lakoski SG, Willis BL, Barlow CE, Leonard D, Gao A, Radford NB, Farrell SW, Douglas PS, Berry JD, DeFina LF, Jones LW. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA Oncol. 2015;1:231–237. doi: 10.1001/jamaoncol.2015.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Lane AT, West M, Eves ND, Gradison M, Coan A, Herndon JE, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 56.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 58.Yeh RW, Kramer DB. Decision Tools to Improve Personalized Care in Cardiovascular Disease: Moving the Art of Medicine Toward Science. Circulation. 2017;135:1097–1100. doi: 10.1161/CIRCULATIONAHA.116.024247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leifer ES, Brawner CA, Fleg JL, Kraus WE, Whellan DJ, Piña IL, Keteyian SJ. Are there negative responders to exercise training among heart failure patients? Med Sci Sports Exerc. 2014;46:219–224. doi: 10.1249/MSS.0b013e3182a44164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 61.Leon AS, Gaskill SE, Rice T, Bergeron J, Gagnon J, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Variability in the response of HDL cholesterol to exercise training in the HERITAGE Family Study. Int J Sports Med. 2002;23:1–9. doi: 10.1055/s-2002-19270. [DOI] [PubMed] [Google Scholar]
- 62.Ross R, de Lannoy L, Stotz PJ. Separate Effects of Intensity and Amount of Exercise on Interindividual Cardiorespiratory Fitness Response. Mayo Clin Proc. 2015;90:1506–1514. doi: 10.1016/j.mayocp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 63.Montero D, Lundby C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training. J Physiol. 2017;595:3377–3387. doi: 10.1113/JP273480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones LW, Douglas PS, Khouri MG, Mackey JR, Wojdyla D, Kraus WE, Whellan DJ, O’Connor CM. Safety and Efficacy of Aerobic Training in Patients With Cancer Who Have Heart Failure: An Analysis of the HF-ACTION Randomized Trial. J Clin Oncol. 2014;32:2496–2502. doi: 10.1200/JCO.2013.53.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 66.Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med. 2015;162:325–334. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- 67.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott JM, Nilsen TS, Michalski M, et al. Tolerability, Safety, and Efficacy of Aerobic Training in Pretreated Patients with Metastatic Breast Cancer: A Randomized Clinical Trial. Submitted. doi: 10.1002/cncr.31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koelwyn GJ, Jones LW, Moslehi J. Unravelling the causes of reduced peak oxygen consumption in patients with cancer: complex, timely, and necessary. J Am Coll Cardiol. 2014;64:1320–1322. doi: 10.1016/j.jacc.2014.07.949. [DOI] [PubMed] [Google Scholar]
- 70.Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL, Green DM, Meacham LR, Mulrooney DA, Ness KK, Oeffinger KC, Ronckers CM, Sklar CA, Stovall M, van der Pal HJ, van Dijk IWEM, van Leeuwen FE, Weathers RE, Robison LL, Armstrong GT, Yasui Y. Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer. J Clin Oncol. 2017:JCO2017748673. doi: 10.1200/JCO.2017.74.8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 72.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 73.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 74.ATS/ACCP Statement on cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 75.Khouri MG, Hornsby WE, Risum N, Velazquez EJ, Thomas S, Lane A, Scott JM, Koelwyn GJ, Herndon JE, Mackey JR, Douglas PS, Jones LW. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res Treat. 2014;143:531–539. doi: 10.1007/s10549-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 77.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise Intolerance in HFpEF: Diagnosing and Ranking its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seiler KS, Kjerland GO. Quantifying training intensity distribution in elite endurance athletes: is there evidence for an “optimal” distribution? Scand J Med Sci Sports. 2006;16:49–56. doi: 10.1111/j.1600-0838.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 79.Scott JM, Jones LW, Hornsby WE, Koelwyn GJ, Khouri MG, Joy AA, Douglas PS, Lakoski SG. Cancer therapy-induced autonomic dysfunction in early breast cancer: implications for aerobic exercise training. Int J Cardiol. 2014;171:e50–51. doi: 10.1016/j.ijcard.2013.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad T, Pencina MJ, Schulte PJ, O’Brien E, Whellan DJ, Piña IL, Kitzman DW, Lee KL, O’Connor CM, Felker GM. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64:1765–1774. doi: 10.1016/j.jacc.2014.07.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen JH, Asch SM. Machine Learning and Prediction in Medicine - Beyond the Peak of Inflated Expectations. N Engl J Med. 2017;376:2507–2509. doi: 10.1056/NEJMp1702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bray MS, Hagberg JM, PÉRusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The Human Gene Map for Performance and Health-Related Fitness Phenotypes. Medicine & Science in Sports & Exercise. 2009;41:35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Sun CL, Quinones-Lombrana A, Singh P, Landier W, Hageman L, Mather M, Rotter JI, Taylor KD, Chen YD, Armenian SH, Winick N, Ginsberg JP, Neglia JP, Oeffinger KC, Castellino SM, Dreyer ZE, Hudson MM, Robison LL, Blanco JG, Bhatia S. CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study. J Clin Oncol. 2016;34:863–870. doi: 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouchard C. Exercise genomics–a paradigm shift is needed: a commentary. Br J Sports Med. 2015;49:1492–1496. doi: 10.1136/bjsports-2015-095294. [DOI] [PubMed] [Google Scholar]
- 91.Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. Methods of integrating data to uncover genotype-phenotype interactions. Nat Rev Genet. 2015;16:85–97. doi: 10.1038/nrg3868. [DOI] [PubMed] [Google Scholar]
- 92.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 93.Zarrinpar A, Lee DK, Silva A, Datta N, Kee T, Eriksen C, Weigle K, Agopian V, Kaldas F, Farmer D, Wang SE, Busuttil R, Ho CM, Ho D. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci Transl Med. 2016;8:333ra349. doi: 10.1126/scitranslmed.aac5954. [DOI] [PubMed] [Google Scholar]
- 94.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, Joy AA, Kumar V, Winton TW, Reiman T. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 95.Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th. Champaign, IL: Human Kinetics; 2013. Vol. [Google Scholar]
- 96.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, O’Connor K, Campbell N, Tolaney SM, Mayer EL, Yung R, Freedman RA, Partridge AH, Ligibel JA. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123:1249–1258. doi: 10.1002/cncr.30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh JA, 3rd, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130:573–581. doi: 10.1161/CIRCULATIONAHA.114.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott JM, Armenian S, Giralt S, Moslehi J, Wang T, Jones LW. Cardiovascular disease following hematopoietic stem cell transplantation: Pathogenesis, detection, and the cardioprotective role of aerobic training. Crit Rev Oncol Hematol. 2016;98:222–234. doi: 10.1016/j.critrevonc.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]


