Abstract
Purpose of Review
The prevalence of end-stage organ disease is increasing among HIV-infected (HIV+) individuals. Individuals with well-controlled HIV on antiretroviral therapy (ART), without active opportunistic infections or cancer, and with specified minimum CD4 cell counts are appropriate transplant candidates. Infectious disease clinicians can improve access to transplantation for these patients and optimize management pre- and post-transplant.
Recent Findings
Clinical trials and registry-based studies demonstrate excellent outcomes for select HIV+ kidney and liver transplant recipients with similar patient and graft survival as HIV-uninfected patients. Elevated allograft rejection rates have been observed in HIV+ individuals; this may be related to a dysregulated immune system or drug interactions. Lymphocyte-depleting immunosuppression has been associated with lower rejection rates without increased infections using national registry data. Hepatitis C virus (HCV) coinfection has been associated with worse outcomes, however improvements are expected with direct-acting antivirals.
Summary
Solid organ transplantation should be considered for HIV+ individuals with end-stage organ disease. Infectious disease clinicians can optimize ART to avoid pharmacoenhancers, which interact with immunosuppression. The timing of HCV treatment (pre- or post-transplant) should be discussed with the transplant team. Finally, organs from HIV+ donors can now be considered for HIV+ transplant candidates, within research protocols.
Keywords: HIV infection, solid organ transplantation, end-stage renal disease, end-stage liver disease, kidney transplant, liver transplant, immunosuppression
Introduction
There are over 1.2 million HIV+ individuals in the US, and approximately 50,000 new infections each year [1,2]. The advent of antiretroviral therapy (ART) in the mid-1990’s fundamentally altered the landscape of HIV transforming it into a chronic, manageable disease [3]. Though obstacles remain in the HIV care continuum, improvements in treatment options have resulted in an increase in life-expectancy and a decrease in the incidence of opportunistic infections (OIs) and AIDS-defining illnesses [4,5]. As a result, chronic diseases now contribute to significant morbidity and mortality for HIV+ individuals [6,7]. These chronic illnesses arise from a number of sources including: common co-morbidities (such as cardiovascular disease, hypertension, and diabetes), co-infections with other chronic viruses (such as hepatitis B and hepatitis C), and long-term effects of HIV-infection (such as continuous ART exposure, inflammation, and cancer). These factors have led to an increase in the burden of end-stage organ disease and organ failure, and thus a corresponding increase in the need for solid organ transplantation (SOT), for HIV+ individuals.
End-stage renal and liver disease in HIV
In the post-ART era, there has been an increase in the prevalence of end-stage renal disease (ESRD) among HIV+ individuals. The prevalence of HIV infection among the 661,000 ESRD patients in the US varies, depending on the underlying prevalence of HIV in the population being measured [8]. Studies from the US and Europe indicate that approximately 0.5% – 1.5% of ESRD patients have HIV [9–12]. Particularly among African Americans, HIV-associated nephropathy (HIVAN) is a leading cause of ESRD and these patients have an increased mortality risk, compared to patients with other causes of ESRD [7,9]. Although HIVAN is decreasing in incidence in the era of effective ART [10,13], HIV+ individuals remain at risk of developing ESRD due to more traditional risk factors, such as hypertension and diabetes. Up to one-third of all HIV+ individuals will develop chronic kidney disease [14–17]. Overall, HIV+ individuals appear to experience an accelerated progression to ESRD. On dialysis, they face a higher risk of death, are less likely to be listed for a kidney transplant (KT), and are less likely to receive a KT, compared to ESRD patients without HIV [18–20].
End-stage liver disease (ESLD) also leads to significant morbidity and mortality among HIV+ individuals [21]. A large international multi-cohort study of HIV+ individuals on ART found that liver disease was the most common cause of non-AIDS related death for these patients [6]. Co-infections with HIV and hepatitis B virus (HBV) and/or hepatitis C virus (HCV) are common in this population. In the US, approximately 20–33% of HIV+ individuals are co-infected with HCV [22]. Not only is co-infection common, it accelerates progression to ESLD [23,24]. In addition, ESLD in HIV+ individuals may develop due to adverse effects of ART [25] or due to factors seen in HIV-uninfected individuals, including alcoholic liver disease and disorders of hepatic fat accumulation (non-alcoholic fatty liver and non-alcoholic steatohepatitis) [26,27]. Recent longitudinal studies of well-characterized HIV+ cohorts have reported non-alcoholic fatty liver disease prevalence estimates of 13–55%, depending on the cohorts described and the biomarkers used [28–31]. Once HIV+ individuals progress to ESLD, they are less likely to receive a liver transplant (LT) and have a greater waitlist mortality risk, compared to HIV-patients with ESLD [32–34].
TREATMENT: HIV+ Kidney and Liver Transplant
Historically, HIV+ individuals were rarely offered organ transplants due to concerns regarding immunosuppression, risk of OIs, and accelerating the progression of HIV disease. Between 2003–2008, several small studies of carefully selected HIV+ KT and LT showed promise [33,35,36]. These initial reports led to a larger prospective NIH-funded trial called the HIV Multisite Transplant Recipient (HIV-TR) Study, conducted at 26 US transplant centers [37–39]. Outcomes from both the HIV-TR cohort and data on HIV+ KT and LT from the Scientific Registry of Transplant Recipients (SRTR) have informed current practice and guidelines for solid organ transplantation in HIV+ individuals. The SRTR data system includes data on all donors, waitlist candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network (OPTN) as previously described [40]. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
HIV+ Kidney Transplant
In the HIV-TR Study, excellent outcomes were reported in 150 HIV KT recipients: 1-year patient and graft survival were 95% and 90%, respectively; 3-year patient and graft survival were 88% and 74%, respectively (Table 1). These rates were superior to rates among HIV− KT recipients >65 years of age in SRTR (which the authors used as a comparison group, based on the fact that older KT recipients are considered higher risk) [37]. These favorable results were confirmed with four-year patient and graft survival rates of 89% and 70%, respectively [39].
Table 1.
Summary of key studies of post-transplant patient and graft survival for HIV+ individuals.
| Study | N | Organ | Patient Survival | Graft Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1-year | 3-year | 5-year | 10-year | 1-year | 3-year | 5-year | 10-year | |||
| Stock, et al. 2010 [37] | 150 | Kidney | 95% | 91% | ND | ND | 90% | 77% | ND | ND |
| Locke, et al. 2015 [41] | 362 | Kidney | 96% | 92% | 88% | 64% | 90% | 82% | 75% | 56% |
| Terrault, et al. 2012 [38] | 89 | Liver | 76% | 60% | ND | ND | 72% | 53% | ND | ND |
| Locke, et al. 2016 [46]* | 180 | Liver | 77% | 62% | 58% | 41% | 73% | 58% | 51% | 35% |
Using SRTR data, Locke et al. reported outcomes in 510 HIV+ KT recipients with HIV-uninfected matched controls and up to 10 years of follow-up [41]. In this analysis, HIV+ KT recipients without HCV co-infection had similar patient and graft survival to HIV− KT recipients up to 10 years post-transplant (Table 1). In another analysis, Locke et al. found that, compared to remaining on dialysis, HIV+ KT was associated with an 80% lower risk of death at 5 years [42]. Finally, Locke et al. studied the association between transplant center experience measures and transplant era on HIV+ KT outcomes. There were no differences in outcomes related to transplant center experience, however HIV+ KT recipients who received transplants after 2008 had 41% and 38% lower risks of death and graft failure, respectively [43].
HIV+ Liver Transplant
In HIV-TR, there were 89 HIV/HCV co-infected LT recipients; 1-year patient and graft survival were 76% and 72%, respectively; 3-year patient and graft survival were 60% and 53%, respectively (Table 1). These survival rates were significantly lower than the comparison group of HCV mono-infected LT recipients (n=325) [38]. Lower survival for HIV/HCV LT recipients compared to HCV-only LT was also seen in a study in Spain of 84 HIV LT recipients [44]. In both studies, reasons for worse survival were hypothesized to be related in part to HCV co-infection. This is supported by outcomes of LT observed in HIV+ individuals without HCV co-infection. For example, a small study (n=20) comparing hepatitis B virus (HBV) mono-versus HIV/HBV co-infected LT recipients showed patient and graft survival were similar: 100% versus 85% in with 4 years of follow-up [45].
Using a cohort in SRTR that included both HIV mono-infected and HIV/HCV co-infected LT recipients (n=180), Locke et al. found that HIV/HCV co-infection was associated with a 2.24-fold higher hazard of death. However, after 2008, the hazard of death for HIV mono-infected LT recipients was equivalent to that in HCV mono-infected LT recipients [46]. Overall, given the high mortality of ESLD, even for HIV/HCV co-infected candidates LT remains a reasonable treatment.
Based on the results of these studies (Table 1), current guidelines state that HIV-infection is no longer considered to be an absolute contraindication for transplantation for individuals who otherwise meet standard transplant criteria. However, HIV+ transplant candidates must have well-controlled HIV and it is recommended that they meet the same inclusion criteria used in the HIV-TR Study. These include: HIV VL <50 copies/mL on ART or an anticipated effective post-transplant ART regimen for patients with ART intolerance/hepatotoxicity, and limited or no history of OIs. HIV+ candidates must also meet minimum CD4 count thresholds of >100/μL for liver candidates and or >200/μL for kidney candidates or for those with a history of OIs [47].
Solid organ transplant in HIV: Beyond Liver and Kidney
Excellent cohort studies of HIV+ individuals have generated data on increased incidence of cardiovascular disease and lung disease in HIV+ individuals [6,48,49]. HIV+ individuals are at risk for heart failure resulting from cardiomyopathy directly related to the virus and metabolic effects of ART as well as increased ischemic cardiovascular disease resulting from traditional risk factors [50]. Non-infectious pulmonary disease such as chronic obstructive pulmonary disease and pulmonary hypertension have also been associated with HIV-infection [51].
There are limited data regarding access to transplantation, and pre- and post-transplant outcomes for HIV+ recipients of heart, lung, pancreas, and intestines. The limited reports of heart transplant recipients with HIV indicate that short-term outcomes are good, but that rejection remains a potential complication [52–56]. There is one published case of a lung transplant recipient with HIV, who was also co-infected with HBV, and survived for at least 2 years post-transplant indicating no major infectious complications or rejection [57]. There are also limited reports of simultaneous liver-kidney [58] and simultaneous kidney-pancreas transplants [59–63] in recipients with HIV, though only 1 published case from the US [64].
For all organ types, the number of HIV+ transplant recipients has increased over time, according to SRTR data (Figure 1). From 2000 to 2016, there has been a >40-fold increase in HIV+ KTs and >10-fold increase in HIV+ LTs. Only 6 lung transplant recipients and 42 heart HIV+ transplant recipients in the US have been reported in the national registry.
Figure 1.
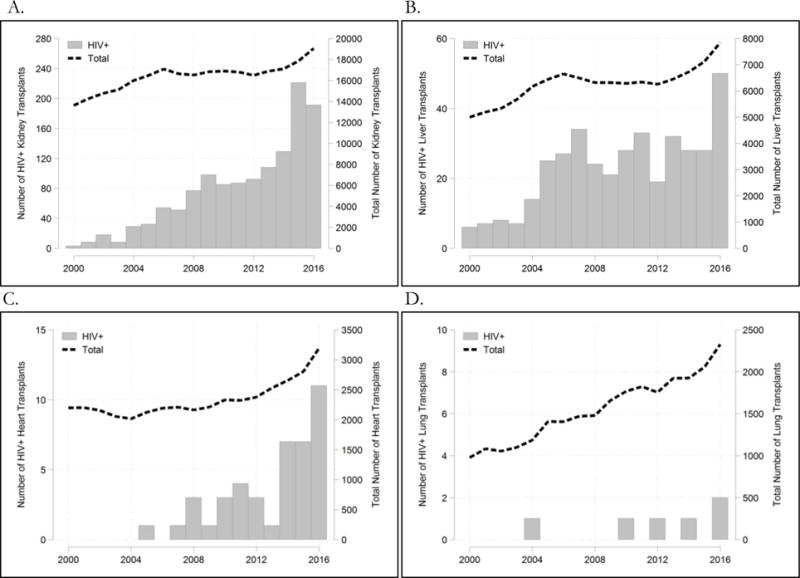
Annual number of HIV+ recipients (y-axis, left) has grown since 2000 for kidney transplants (A), liver transplants (B), and heart transplants (C), but remained rare for lung transplants (D). The total number of annual transplants per organ is shown for reference as a dashed line (y-axis, right).
Post-transplant Management Challenges
Infections including AIDS-Defining Illnesses
HIV+ transplant candidates who had a prior history of an appropriately treated OI were not excluded from the HIV-TR Study [37,38]. Post-transplant survival was not associated with a recipient’s history an OI. At 4 years follow-up, there were 13 cases of AIDS-defining illnesses: cutaneous Kaposi’s sarcoma (n=4), esophageal candidiasis (n=5), bronchial candidiasis (n=1), Pnuemocystis jirovecci pneumonia (n=2), and cryptosporidiosis (n=1) and none of these were cases of an OI recurrence [39]. In HIV-TR, those who received induction immunosuppression with a lymphocyte depleting agent, antithymocyte globulin (ATG), did experience a higher number of infections than HIV+ KT recipients who did not receive ATG, however this higher rate of infection was not associated with a difference in survival.
In a study of 308 HIV KT recipient kidney recipients in the national registry, Kucirka et al. found that post-transplant infections were common, with over 50% of patients experiencing an infection in the first year after transplant. Urinary tract infections were the most common, accounting for about one-third of all infections, followed by sepsis, which occurred in 15–20%, depending on the type of induction immunosuppression. Approximately, 10% of HIV KT recipients had an AIDS-related infection. This was primarily CMV disease which is a frequent complication among all KT recipients, regardless of HIV status. Induction with ATG was not associated with a higher risk of infection [65].
Allograft Rejection
HIV+ transplant recipients do experience an increased incidence of allograft rejection [34, 38, 41, 47, 48]. For HIV+ KT recipients, rates were between 2–4 times higher than HIV− KT recipients. At 1-year post-transplant, 15% and 31% of HIV+ KT recipients had an episode of rejection in HIV-TR and SRTR, respectively [37,66]. At 3 years post-transplant, 41% of HIV+ KT recipients in HIV-TR experienced rejection. For HIV LT, there has also been an elevated rate of rejection reported. In the HIV-TR Study, acute rejection occurred in 39% of the HIV+ LT recipients, with half of the episodes occurring within the first 21 days. There was a 1.6-fold higher risk of allograft rejection, compared to HCV mono-infected recipients (39% vs. 24%) [38].
There are multiple potential mechanisms proposed to explain the observed increased rejection rates. Drug interactions between ART and calcineurin inhibitors may lower overall exposure to immunosuppressant agents, thus guidelines now recommend avoiding these problematic combinations (as will be discussed below) [47]. Chronic viral infection may lead to immune dysregulation with more memory alloreactive T cells [67,68] and there may also be a higher prevalence of HLA alloantibodies in HIV+ individuals [69]. One small study of HIV+ KT recipients showed that HIV was detectable in the allograft, suggesting another potential mechanism for the acute rejection seen in these patients [70,71].
Given the dual priorities of preventing infection and rejection in this population, there has been significant debate over the optimal choice of induction immunosuppression. Induction is generally considered standard of care for KT recipients, but there may be reluctance to use these agents, particularly T-cell depleting regimens such as ATG. A large national registry study of HIV+ KT recipients showed that receipt of ATG was associated with a 2.6-fold lower risk of acute rejection [66]. A follow-up study using Medicare claims data, specifically looking at infection rates by induction type, confirmed that receipt of ATG was associated with a 40% reduction in risk of rejection and was not associated with an increase in the risk of infections compared to no induction [65]. Based on this, the current recommendation for HIV+ individuals undergoing SOT is to treat these patients with the appropriate level and class of immunosuppression based on their overall risk of rejection, independent of their HIV-infection.
Antiretroviral Therapy and Drug Interactions
Calcineurin inhibitors such as tacrolimus and cyclosporine are mainstays of long-term immunosuppression to prevent rejection in transplant recipients. Dosing is typically based on a 12-hour area under the curve (AUC), and in practice, trough levels are closely monitored, assuming a correlation with AUCs [72]. Calcineurin inhibitors are metabolized by the cytochrome P450 enzyme, CYP3A, which is strongly inhibited by antiretroviral class of protease inhibitors and the pharmacoenhancers ritonavir and cobicistat. As a result, with co-administration in HIV+ transplant recipients, tacrolimus doses need to be reduced up to a 50-fold in order to achieve the same target troughs. The pharmacokinetic curve does not show the normal peak-and-trough pattern but instead resembles a flat line with a half-life of up to 20 days [73]. This leads to a lower tacrolimus AUC and may explain, in part, the increased rejection rates observed in HIV transplant studies. This was seen in a recent cohort analysis of HIV+ KT recipients combining registry data and a pharmacy claims database in which receipt of a PI-based regimen as associated with a 1.8-fold increase of allograft loss and a 1.9-fold increase in the risk of death compared to receipt of non-PI regimens [74]. As such, avoiding protease post-transplant in HIV+ recipients has been recommended in clinical practice [47]. Alternatively, if protease use is unavoidable, raising tacrolimus trough levels to achieve an exposure equivalent to HIV-negative recipients has also been suggested [75].
Integrase strand transferase inhibitors are neither inducers nor inhibitors of cytochrome P450 and have been safely administered to HIV+ transplant recipients on calcineurin inhibitors [76]. In addition, the antiretroviral drug maraviroc does not interact with tacrolimus and may be of special interest. Maraviroc works by blocking the CCR5 receptor on T lymphocytes which is used by the most common strains of HIV (R5 tropic strains) as a co-receptor for viral entry. Blockade of the CCR5 receptor not only has anti-HIV activity in individuals with exclusive R5 virus, but also impairs lymphocyte chemotaxis and has anti-inflammatory properties. Notably, about 1% of white populations are homozygous for a 32 base pair deletion in CCR5 that leads to lack of expression of the co-receptor. Renal transplant recipients who are homozygous for this mutation have been observed to have prolonged graft survival [77]. A multisite clinical trial is ongoing to investigate the impact of maraviroc on rejection and other transplant outcomes in HIV+ KT recipients (ClinicalTrials.gov Identifier: NCT02741323).
Hepatitis C Co-Infection and Treatment
HIV/HCV co-infection is associated with worse outcomes after KT and LT, compared to mono-infection with HCV. In the original HIV-TR Study, the 1-year survival for HIV/HCV co-infected KT recipients was 86% compared to 94% among the HIV mono-infected KT recipients (p=.09) and with 4-year follow-up, the hazard of death for HCV/HIV was 1.9-fold higher, though neither difference was statistically significant with less than 20 HIV/HCV KT recipients [37,39]. In the HIV-TR Study, most LT recipients were HIV/HCV co-infected and as discussed above, patient and graft survival were lower than HCV-mono-infected LT recipients [38]. In a larger cohort of HIV LT recipients (n=180), Locke et al. found significantly lower 5- and 10-year patient survival rates of 67% and 29% among HIV/HCV co-infected KT recipients compared to 5- and 10-year patient survival rates of 79% and 56% among matched HCV-mono-infected controls (p < .01) [41]. The advent of direct acting antivirals (DAAs) for HCV will undoubtedly improve outcomes in HIV/HCV co-infected transplant recipients. Although there are no specific trials of DAA to treat HCV in HIV+ transplant recipients, multiple trials in HIV+ individuals [78–82] and trials in HIV-uninfected transplant recipients have shown cure rates >95% [83–86]. One unique challenge in this patient population is whether to eradicate HCV with DAAs pre- or post-transplant. Current guidelines do not address this issue specifically. Candidates may receive organ offers from donors who are HCV+, many of which are high-quality organs from otherwise low-risk donors. Accepting these offers has been shown to reduce time spent waiting for an organ, and has been associated with good post-transplant outcomes [87–89]. However, eradicating HCV with DAAs prior to transplantation can prevent infection of the graft, avoids the potential for drug-drug interactions with post-transplant immunosuppression, and may improve the health and quality of life for patients while on the waitlist. We recommend infectious diseases clinicians discuss this with the transplant team and the patient, weighing these risks and benefits on a case-by-case basis.
Emerging Therapies – HIV+ to HIV+ Organ Transplantation
HIV+ donor organs represent a unique and novel source of organs for HIV+ individuals. Dr. Elmi Muller of the Groote Schuur Hospital, pioneered HIV+ to HIV+ kidney transplantation in Cape Town, South Africa, successfully performing the first HIV+ to HIV+ KTs in the world [90]. Since this initial groundbreaking work, she has reported on outcomes for a total of 27 HIV+ recipients of HIV+ donors with 3- and 5-year graft-failure-free survival of 84% and 74%, respectively [91]. In the US, the use of HIV+ organ donors was outlawed in the 1980’s and thus, a change in federal law was required to allow this. The HIV Organ Policy Equity Act was signed into law in 2013, implemented in 2015, and permits research into HIV+ to HIV+ transplantation [92,93]. Since the passage of the HOPE Act, studies have begun enrolling participants to investigate the potential of HIV+ to HIV+ kidney and transplants and the first US transplants were done at Johns Hopkins Hospital in March 2016 (ClinicalTrials.gov Identifier: NCT02602262) [94]. There have also been case reports of successful HIV+ to HIV+ LTs in Switzerland [95] and the UK [96].
Conclusions
HIV+ individuals are at increased risk for end-stage organ disease and face disproportionate mortality and decreased access to organ transplantation. Kidney and liver transplant offer a benefit for this population and those with well-controlled HIV disease should be referred and considered for transplantation. Partnering with the transplant team, infectious disease physicians can optimize ART to minimize drug interactions, utilize appropriate opportunistic infection prophylaxis, and carefully consider when to treat HCV co-infection, thereby improve pre- and post-transplant outcomes for HIV+ transplant recipients. Finally, trials are ongoing to determine whether the use of organs from HIV+ donors may safely expand donor options for these patients.
Acknowledgments
The data reported here have been supplied in part by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, the Organ Procurement and Transplantation Network (OPTN), or the US Government.
Funding
C.M.D. is supported by the National Cancer Institute grant K23CA177321-01A1.
A.A.S. is supported by the NIDDK grant F30DK116658-01.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Ashton A. Shaffer declares that she has no conflict of interest.
Christine M. Durand has received research grants from Bristol Meyers Squibb, Gilead Sciences, Merck Pharmaceuticals, and Viiv Healthcare, and has served as a scientific advisor for Bristol Meyers Squibb, Gilead Sciences, and Merck Pharmaceuticals.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, Skarbinski J, Higa DH, Prejean J, Frazier EL, Patel R, et al. Vital signs: Hiv diagnosis, care, and treatment among persons living with hiv–united states, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention. CfDCa. Hiv surveillance report, 2014. 26 [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. Hiv outpatient study investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Bradley H, Hu X, Skarbinski J, Hall HI, Lansky A, Centers for Disease C, Prevention Men living with diagnosed hiv who have sex with men: Progress along the continuum of hiv care–united states, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(38):829–833. [PMC free article] [PubMed] [Google Scholar]
- 5.Losina E, Freedberg KA. Life expectancy in hiv. BMJ. 2011;343(d6015) doi: 10.1136/bmj.d6015. [DOI] [PubMed] [Google Scholar]
- 6.Antiretroviral Therapy Cohort C. Causes of death in hiv-1-infected patients treated with antiretroviral therapy, 1996-2006: Collaborative analysis of 13 hiv cohort studies. Clin Infect Dis. 2010;50(10):1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott KC, Trespalacios FC, Agodoa LY, Ahuja TS. Hivan and medication use in chronic dialysis patients in the united states: Analysis of the usrds dmms wave 2 study. BMC Nephrol. 2003;4(5) doi: 10.1186/1471-2369-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, et al. Us renal data system 2015 annual data report: Epidemiology of kidney disease in the united states. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razzak Chaudhary S, Workeneh BT, Montez-Rath ME, Zolopa AR, Klotman PE, Winkelmayer WC. Trends in the outcomes of end-stage renal disease secondary to human immunodeficiency virus-associated nephropathy. Nephrol Dial Transplant. 2015;30(10):1734–1740. doi: 10.1093/ndt/gfv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham AG, Althoff KN, Jing Y, Estrella MM, Kitahata MM, Wester CW, Bosch RJ, Crane H, Eron J, Gill MJ, Horberg MA, et al. End-stage renal disease among hiv-infected adults in north america. Clin Infect Dis. 2015;60(6):941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickel M, Marben W, Betz C, Khaykin P, Stephan C, Gute P, Haberl A, Knecht G, Wolf T, Brodt HR, Geiger H, et al. End-stage renal disease and dialysis in hiv-positive patients: Observations from a long-term cohort study with a follow-up of 22 years. HIV Med. 2013;14(3):127–135. doi: 10.1111/j.1468-1293.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- 12.Atta MG, Fine DM, Kirk GD, Mehta SH, Moore RD, Lucas GM. Survival during renal replacement therapy among african americans infected with hiv type 1 in urban baltimore, maryland. Clin Infect Dis. 2007;45(12):1625–1632. doi: 10.1086/523728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sexton DJ, Reule S, Solid C, Collins AJ, Foley RN. End-stage renal disease from human immunodeficiency virus-associated nephropathy in the united states, 2001 through 2010. JAMA Intern Med. 2014;174(5):809–811. doi: 10.1001/jamainternmed.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for esrd in hiv-infected individuals: Traditional and hiv-related factors. Am J Kidney Dis. 2012;59(5):628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, Anastos K, Klassen PS, Svetkey LP. Predictors of proteinuria and renal failure among women with hiv infection. Kidney Int. 2002;61(1):195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, Tashima KT, Roland M, Franceschini N, Palella FJ, Lennox JL, et al. Guidelines for the management of chronic kidney disease in hiv-infected patients: Recommendations of the hiv medicine association of the infectious diseases society of america. Clin Infect Dis. 2005;40(11):1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, Atta MG, Wools-Kaloustian KK, Pham PA, Bruggeman LA, Lennox JL, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with hiv: 2014 update by the hiv medicine association of the infectious diseases society of america. Clin Infect Dis. 2014;59(9):e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke JE, Mehta S, Sawinski D, Gustafson S, Shelton BA, Reed RD, MacLennan P, Bolch C, Durand C, Massie A, Mannon RB, et al. Access to kidney transplantation among hiv-infected waitlist candidates. Clin J Am Soc Nephrol. 2017;12(3):467–475. doi: 10.2215/CJN.07460716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawinski D, Wyatt CM, Casagrande L, Myoung P, Bijan I, Akalin E, Schroppel B, DeBoccardo G, Sehgal V, Dinavahi R, Lerner S, et al. Factors associated with failure to list hiv-positive kidney transplant candidates. Am J Transplant. 2009;9(6):1467–1471. doi: 10.1111/j.1600-6143.2009.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trullas JC, Cofan F, Barril G, Martinez-Castelao A, Jofre R, Rivera M, Martinez-Ara J, Ros S, Perez I, Moreno A, Miro JM, et al. Outcome and prognostic factors in hiv-1-infected patients on dialysis in the cart era: A gesida/sen cohort study. J Acquir Immune Defic Syndr. 2011;57(4):276–283. doi: 10.1097/QAI.0b013e318221fbda. [DOI] [PubMed] [Google Scholar]
- 21.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with hiv infection. Lancet. 2011;377(9772):1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 22.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of hcv co-infection in people living with hiv: A global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 23.Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, Kirk O, Phillips A, Ledergerber B, Lundgren J, Euro SSG. Influence of hepatitis c virus infection on hiv-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192(6):992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 24.Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on hiv/hcv coinfection. Curr HIV/AIDS Rep. 2013;10(3):226–234. doi: 10.1007/s11904-013-0169-5. [DOI] [PubMed] [Google Scholar]
- 25.Soriano V, Puoti M, Garcia-Gasco P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22(1):1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 26.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: The d:A:D study. Arch Intern Med. 2006;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 27.Salmon-Ceron D, Lewden C, Morlat P, Bevilacqua S, Jougla E, Bonnet F, Heripret L, Costagliola D, May T, Chene G, Mortality study g Liver disease as a major cause of death among hiv infected patients: Role of hepatitis c and b viruses and alcohol. J Hepatol. 2005;42(6):799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ, Jr, Witt MD, Post WS, Thio CL. Risk factors for fatty liver in the multicenter aids cohort study. Am J Gastroenterol. 2014;109(5):695–704. doi: 10.1038/ajg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterling RK, Smith PG, Brunt EM. Hepatic steatosis in human immunodeficiency virus: A prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J Clin Gastroenterol. 2013;47(2):182–187. doi: 10.1097/MCG.0b013e318264181d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, Abu-Asab M, Orenstein A, Engle RE, Hu X, Lempicki R, et al. Nonalcoholic steatohepatitis and hepatic fibrosis in hiv-1-monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis. 2015;60(10):1569–1578. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuille-Lessard E, Lebouche B, Lennox L, Routy JP, Costiniuk CT, Pexos C, Giannakis A, Szabo J, Klein MB, Sebastiani G. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected hiv monoinfected patients. AIDS. 2016;30(17):2635–2643. doi: 10.1097/QAD.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian A, Sulkowski M, Barin B, Stablein D, Curry M, Nissen N, Dove L, Roland M, Florman S, Blumberg E, Stosor V, et al. Meld score is an important predictor of pretransplantation mortality in hiv-infected liver transplant candidates. Gastroenterology. 2010;138(1):159–164. doi: 10.1053/j.gastro.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragni MV, Belle SH, Im K, Neff G, Roland M, Stock P, Heaton N, Humar A, Fung JF. Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis. 2003;188(10):1412–1420. doi: 10.1086/379254. [DOI] [PubMed] [Google Scholar]
- 34.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in hiv-positive than hiv-negative subjects with end-stage liver disease. Liver Transpl. 2005;11(11):1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 35.Stock PG, Roland ME, Carlson L, Freise CE, Roberts JP, Hirose R, Terrault NA, Frassetto LA, Palefsky JM, Tomlanovich SJ, Ascher NL. Kidney and liver transplantation in human immunodeficiency virus-infected patients: A pilot safety and efficacy study. Transplantation. 2003;76(2):370–375. doi: 10.1097/01.TP.0000075973.73064.A6. [DOI] [PubMed] [Google Scholar]
- 36.Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP, Murphy B, et al. Hiv-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8(2):355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 37.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, Millis JM, et al. Outcomes of kidney transplantation in hiv-infected recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, Ragni MV, Barin B, Simon D, Olthoff KM, Johnson L, et al. Outcomes of liver transplant recipients with hepatitis c and human immunodeficiency virus coinfection. Liver Transpl. 2012;18(6):716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roland ME, Barin B, Huprikar S, Murphy B, Hanto DW, Blumberg E, Olthoff K, Simon D, Hardy WD, Beatty G, Stock PG, et al. Survival in hiv-positive transplant recipients compared with transplant candidates and with hiv-negative controls. AIDS. 2016;30(3):435–444. doi: 10.1097/QAD.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: Registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locke JE, Mehta S, Reed RD, MacLennan P, Massie A, Nellore A, Durand C, Segev DL. A national study of outcomes among hiv-infected kidney transplant recipients. J Am Soc Nephrol. 2015;26(9):2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Locke JE, Gustafson S, Mehta S, Reed RD, Shelton B, MacLennan PA, Durand C, Snyder J, Salkowski N, Massie A, Sawinski D, et al. Survival benefit of kidney transplantation in hiv-infected patients. Ann Surg. 2017;265(3):604–608. doi: 10.1097/SLA.0000000000001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locke JE, Reed RD, Mehta SG, Durand C, Mannon RB, MacLennan P, Shelton B, Martin MY, Qu H, Shewchuk R, Segev DL. Center-level experience and kidney transplant outcomes in hiv-infected recipients. Am J Transplant. 2015;15(8):2096–2104. doi: 10.1111/ajt.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miro JM, Montejo M, Castells L, Rafecas A, Moreno S, Aguero F, Abradelo M, Miralles P, Torre-Cisneros J, Pedreira JD, Cordero E, et al. Outcome of hcv/hiv-coinfected liver transplant recipients: A prospective and multicenter cohort study. Am J Transplant. 2012;12(7):1866–1876. doi: 10.1111/j.1600-6143.2012.04028.x. [DOI] [PubMed] [Google Scholar]
- 45.Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, Ragni M, Regenstein FG, Sherman KE, Roland ME, Terrault NA. Virologic and clinical outcomes of hepatitis b virus infection in hiv-hbv coinfected transplant recipients. Am J Transplant. 2010;10(5):1268–1275. doi: 10.1111/j.1600-6143.2010.03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locke JE, Durand C, Reed RD, MacLennan PA, Mehta S, Massie A, Nellore A, DuBay D, Segev DL. Long-term outcomes after liver transplantation among human immunodeficiency virus-infected recipients. Transplantation. 2016;100(1):141–146. doi: 10.1097/TP.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumberg EA, Rogers CC, Practice ASTIDCo Human immunodeficiency virus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):169–178. doi: 10.1111/ajt.12109. [DOI] [PubMed] [Google Scholar]
- 48.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of hiv-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 49.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992;116(2):124–128. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- 50.Bloomfield GS, Leung C. Cardiac disease associated with human immunodeficiency virus infection. Cardiol Clin. 2017;35(1):59–70. doi: 10.1016/j.ccl.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Thao C, Shorr AF, Woods C. Non-infectious pulmonary disorders in hiv. Expert Rev Respir Med. 2017;11(3):209–220. doi: 10.1080/17476348.2017.1288101. [DOI] [PubMed] [Google Scholar]
- 52.Conte AH, Kittleson MM, Dilibero D, Hardy WD, Kobashigawa JA, Esmailian F. Successful orthotopic heart transplantation and immunosuppressive management in 2 human immunodeficiency virus-seropositive patients. Tex Heart Inst J. 2016;43(1):69–74. doi: 10.14503/THIJ-14-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calabrese LH, Albrecht M, Young J, McCarthy P, Haug M, Jarcho J, Zackin R. Successful cardiac transplantation in an hiv-1-infected patient with advanced disease. N Engl J Med. 2003;348(23):2323–2328. doi: 10.1056/NEJMoa022935. [DOI] [PubMed] [Google Scholar]
- 54.Morgan JA, Bisleri G, Mancini DM. Cardiac transplantation in an hiv-1-infected patient. N Engl J Med. 2003;349(14):1388–1389. doi: 10.1056/NEJM200310023491421. author reply 1388-1389. [DOI] [PubMed] [Google Scholar]
- 55.Boignard A, Blanc M, Chavanon O. High-urgency priority heart transplantation in hiv-positive patients on life support: Breaking barriers? J Heart Lung Transplant. 2011;30(8):968–969. doi: 10.1016/j.healun.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Mehdiani A, Petrov G, Akhyari P, Saeed D, Kamiya H, Westenfeld R, Lichtenberg A, Boeken U. Heart transplantation bridged by mechanical circulatory support in a hiv-positive patient. J Card Surg. 2016;31(8):559–561. doi: 10.1111/jocs.12787. [DOI] [PubMed] [Google Scholar]
- 57.Bertani A, Grossi P, Vitulo P, D’Ancona G, Arcadipane A, Nanni Costa A, Gridelli B. Successful lung transplantation in an hiv- and hbv-positive patient with cystic fibrosis. Am J Transplant. 2009;9(9):2190–2196. doi: 10.1111/j.1600-6143.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 58.Di Benedetto F, D’Amico G, De Ruvo N, Cocchi S, Montalti R, Cautero N, Guerrini GP, Ballarin R, Spaggiari M, Tarantino G, Baisi B, et al. Combined liver-kidney transplantation in patients infected with human immunodeficiency virus. Transpl Infect Dis. 2011;13(5):501–506. doi: 10.1111/j.1399-3062.2011.00622.x. [DOI] [PubMed] [Google Scholar]
- 59.Toso C, Berney T, Oberholzer J, Chave JP, Martin PY, Zeender E, Bosco D, Morel P. Kidney-pancreas transplantation in a long-term non-progressor hiv-infected recipient. Am J Transplant. 2003;3(5):631–633. doi: 10.1034/j.1600-6143.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 60.Grossi PA, Righi E, Gasperina DD, Donati D, Tozzi M, Mangini M, Astuti N, Cuffari S, Castelli P, Carcano G, Dionigi G, et al. Report of four simultaneous pancreas-kidney transplants in hiv-positive recipients with favorable outcomes. Am J Transplant. 2012;12(4):1039–1045. doi: 10.1111/j.1600-6143.2011.03906.x. [DOI] [PubMed] [Google Scholar]
- 61.Dalla Gasperina D, Tozzi M, Astuti N, Balsamo ML, Donati D, Rossi A, Dionigi R, Grossi PA. Pulmonary tuberculosis in an hiv- and hepatitis c virus-coinfected kidney-pancreas transplant recipient: A case report. Transplant Proc. 2011;43(4):1206–1209. doi: 10.1016/j.transproceed.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 62.Akhtar MZ, Patel N, Devaney A, Sinha S, Shankar S, Vaidya A, Friend PJ. Simultaneous pancreas kidney transplantation in the hiv-positive patient. Transplant Proc. 2011;43(10):3903–3904. doi: 10.1016/j.transproceed.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 63.Miro JM, Ricart MJ, Trullas JC, Cofan F, Cervera C, Brunet M, Tuset M, Manzardo C, Oppenheimer F, Moreno A. Simultaneous pancreas-kidney transplantation in hiv-infected patients: A case report and literature review. Transplant Proc. 2010;42(9):3887–3891. doi: 10.1016/j.transproceed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Hayes K, Van Sickels N, Buell J, Killackey M, Zhang R, Slakey D, Lukitsch I, Alper A, Jr, Mushatt D, Asad S, Paramesh A. Successful transplantation of hiv patients: The louisiana experience. J La State Med Soc. 2012;164(4):191–193. [PubMed] [Google Scholar]
- 65.Kucirka LM, Durand CM, Bae S, Avery RK, Locke JE, Orandi BJ, McAdams-DeMarco M, Grams ME, Segev DL. Induction immunosuppression and clinical outcomes in kidney transplant recipients infected with human immunodeficiency virus. Am J Transplant. 2016;16(8):2368–2376. doi: 10.1111/ajt.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, Desai NM, Montgomery RA, Segev DL. Immunosuppression regimen and the risk of acute rejection in hiv-infected kidney transplant recipients. Transplantation. 2014;97(4):446–450. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 67.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific cd8 t cells generated in response to viral infections. J Immunol. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 68.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filaci G, Contini P, Brenci S, Lanza L, Scudeletti M, Indiveri F, Puppo F. Increased serum concentration of soluble hla-dr antigens in hiv infection and following transplantation. Tissue Antigens. 1995;46(2):117–123. doi: 10.1111/j.1399-0039.1995.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 70.Canaud G, Dejucq-Rainsford N, Avettand-Fenoel V, Viard JP, Anglicheau D, Bienaime F, Muorah M, Galmiche L, Gribouval O, Noel LH, Satie AP, et al. The kidney as a reservoir for hiv-1 after renal transplantation. J Am Soc Nephrol. 2014;25(2):407–419. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avettand-Fenoel V, Rouzioux C, Legendre C, Canaud G. Hiv infection in the native and allograft kidney: Implications for management, diagnosis, and transplantation. Transplantation. 2017;101(9):2003–2008. doi: 10.1097/TP.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 72.Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, den Hartigh J, Paul LC, de Fijter JW. Auc-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67(6):2440–2447. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 73.Jain AK, Venkataramanan R, Shapiro R, Scantlebury VP, Potdar S, Bonham CA, Pokharna R, Rohal S, Ragni M, Fung JJ. Interaction between tacrolimus and antiretroviral agents in human immunodeficiency virus-positive liver and kidney transplantation patients. Transplant Proc. 2002;34(5):1540–1541. doi: 10.1016/s0041-1345(02)03011-7. [DOI] [PubMed] [Google Scholar]
- 74.Sawinski D, Shelton BA, Mehta S, Reed RD, MacLennan PA, Gustafson S, Segev DL, Locke JE. Impact of protease inhibitor-based anti-retroviral therapy on outcomes for hiv+ kidney transplant recipients. Am J Transplant. 2017 doi: 10.1111/ajt.14419. [DOI] [PubMed] [Google Scholar]
- 75.van Maarseveen EM, Crommelin HA, Mudrikova T, van den Broek MP, van Zuilen AD. Pretransplantation pharmacokinetic curves of tacrolimus in hiv-infected patients on ritonavir-containing cart: A pilot study. Transplantation. 2013;95(2):397–402. doi: 10.1097/TP.0b013e3182734651. [DOI] [PubMed] [Google Scholar]
- 76.Tricot L, Teicher E, Peytavin G, Zucman D, Conti F, Calmus Y, Barrou B, Duvivier C, Fontaine C, Welker Y, Billy C, et al. Safety and efficacy of raltegravir in hiv-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant. 2009;9(8):1946–1952. doi: 10.1111/j.1600-6143.2009.02684.x. [DOI] [PubMed] [Google Scholar]
- 77.Fischereder M, Luckow B, Hocher B, Wuthrich RP, Rothenpieler U, Schneeberger H, Panzer U, Stahl RA, Hauser IA, Budde K, Neumayer H, et al. Cc chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357(9270):1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 78.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, Bhatti L, Gathe J, Ruane PJ, Elion R, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis c in patients co-infected with hiv-1: A randomized trial. JAMA. 2015;313(12):1223–1231. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 79.Younossi ZM, Stepanova M, Sulkowski M, Wyles D, Kottilil S, Hunt S. Patient-reported outcomes in patients co-infected with hepatitis c virus and human immunodeficiency virus treated with sofosbuvir and velpatasvir: The astral-5 study. Liver Int. 2017 doi: 10.1111/liv.13462. [DOI] [PubMed] [Google Scholar]
- 80.Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, Sherman KE, Dretler R, Fishbein D, Gathe JC, Jr, Henn S, et al. Daclatasvir plus sofosbuvir for hcv in patients coinfected with hiv-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 81.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, Marks K, Luetkemeyer A, Baden RP, Sax PE, Gane E, et al. Ledipasvir and sofosbuvir for hcv in patients coinfected with hiv-1. N Engl J Med. 2015;373(8):705–713. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, et al. Efficacy and safety of grazoprevir (mk-5172) and elbasvir (mk-8742) in patients with hepatitis c virus and hiv co-infection (c-edge co-infection): A non-randomised, open-label trial. Lancet HIV. 2015;2(8):e319–327. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 83.Brown RS, Jr, O’Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR, Jr, Stravitz RT, Durand C, Di Bisceglie AM, Kwo P, Frenette CT, et al. Interferon-free therapy for genotype 1 hepatitis c in liver transplant recipients: Real-world experience from the hepatitis c therapeutic registry and research network. Liver Transpl. 2016;22(1):24–33. doi: 10.1002/lt.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr, Gordon F, Levitsky J, Terrault NA, Burton JR, Jr, Xie W, et al. An interferon-free antiviral regimen for hcv after liver transplantation. N Engl J Med. 2014;371(25):2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 85.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Jr, Hassan MA, Sulkowski MS, O’Leary JG, Koraishy F, Galati JS, Kuo AA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis c: Results from the hcv-target study. Hepatology. 2017;66(4):1090–1101. doi: 10.1002/hep.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colombo M, Aghemo A, Liu H, Zhang J, Dvory-Sobol H, Hyland R, Yun C, Massetto B, Brainard DM, McHutchison JG, Bourliere M, et al. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis c virus genotype 1 or 4 infection: A randomized trial. Ann Intern Med. 2017;166(2):109–117. doi: 10.7326/M16-1205. [DOI] [PubMed] [Google Scholar]
- 87.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M, Rakestraw K, Gurakar A, Kuo I, Segev DL, Durand CM. Changes in utilization and discard of hepatitis c-infected donor livers in the recent era. Am J Transplant. 2017;17(2):519–527. doi: 10.1111/ajt.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kucirka LM, Peters TG, Segev DL. Impact of donor hepatitis c virus infection status on death and need for liver transplant in hepatitis c virus-positive kidney transplant recipients. Am J Kidney Dis. 2012;60(1):112–120. doi: 10.1053/j.ajkd.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis c-positive kidneys for hepatitis c-positive recipients. Am J Transplant. 2010;10(5):1238–1246. doi: 10.1111/j.1600-6143.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 90.Muller E, Kahn D, Mendelson M. Renal transplantation between hiv-positive donors and recipients. N Engl J Med. 2010;362(24):2336–2337. doi: 10.1056/NEJMc0900837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muller E, Barday Z, Mendelson M, Kahn D. Hiv-positive-to-hiv-positive kidney transplantation–results at 3 to 5 years. N Engl J Med. 2015;372(7):613–620. doi: 10.1056/NEJMoa1408896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Health R, Services Administration DoH, Human S. Organ procurement and transplantation: Implementation of the hiv organ policy equity act. Final rule. Fed Regist. 2015;80(89):26464–26467. [PubMed] [Google Scholar]
- 93.Boyarsky BJ, Segev DL. From bench to bill: How a transplant nuance became 1 of only 57 laws passed in 2013. Ann Surg. 2016;263(3):430–433. doi: 10.1097/SLA.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 94.Malani P. Hiv and transplantation: New reasons for hope. JAMA. 2016;316(2):136–138. doi: 10.1001/jama.2016.8158. [DOI] [PubMed] [Google Scholar]
- 95.Calmy A, van Delden C, Giostra E, Junet C, Rubbia Brandt L, Yerly S, Chave JP, Samer C, Elkrief L, Vionnet J, Berney T, et al. Hiv-positive-to-hiv-positive liver transplantation. Am J Transplant. 2016;16(8):2473–2478. doi: 10.1111/ajt.13824. [DOI] [PubMed] [Google Scholar]
- 96.Hathorn E, Smit E, Elsharkawy AM, Bramhall SR, Bufton SA, Allan S, Mutimer D. Hiv-positive-to-hiv-positive liver transplantation. N Engl J Med. 2016;375(18):1807–1809. doi: 10.1056/NEJMc1603850. [DOI] [PubMed] [Google Scholar]


