Short abstract
Background
Epithelial-myoepithelial carcinoma (EMC) is a rare tumor of the major and minor salivary glands. Sinonasal EMC is extremely uncommon and hitherto not described within the frontal or ethmoid sinuses.
Objective
To present a novel sinonasal subsite and review the literature regarding sinonasal EMC.
Methods
A case of frontoethmoidal EMC was presented. A medical literature data base was queried from January 1, 1950, to August 8, 2017, for all reports of sinonasal EMC.
Results
A 69-year-old man underwent combined open and endoscopic craniofacial resection of a right frontoethmoidal EMC, a previously undescribed primary location for this tumor. A comprehensive review of the literature revealed 13 additional cases of sinonasal EMC.
Conclusion
EMC is an uncommon neoplasm typically found in the major salivary glands; occurrence in the nose or paranasal sinuses is extremely rare. EMC often follows an indolent clinical course, although, in a minority of cases, particularly in large tumors with nuclear atypia, more aggressive behavior may be observed.
Epithelial-myoepithelial carcinoma (EMC) is an extremely rare neoplasm that typically occurs in the major salivary glands, predominantly the parotid,1 although it has also been reported in the external auditory canal,2,3 lacrimal gland,4–6 nasopharynx,7,8 palate,9,10 floor of the mouth,11 buccal mucosa,12 base of the tongue,13,14 larynx,15 subglottis,16,17 hypopharynx,18 trachea,19 lung,20–23 liver,24 and Bartholin gland.25 EMC was first described in 197226 and is histologically a biphasic tumor that consists of clear-staining myoepithelial cells surrounding epithelial-lined ductal cells.27
EMC of the major salivary glands demonstrates a female preponderance and commonly follows an indolent clinical course. In the largest cohort study to date, which consisted of 246 patients with salivary EMC, Vazquez et al.1 reported an overall 5-year disease-specific survival of 91.3%, with distant metastasis observed in only 4.5% of patients. A tumor size of >4 cm and high-grade histology (6.5% of patients) were found to be associated with increased mortality. Treatment consisted of surgery, with adjuvant radiation administered to 39% of patients, although no survival benefit was found with the addition of radiation therapy.1
Sinonasal EMC is exceedingly rare, with a total of 13 individual cases described within the English language literature (Table 1). Within the paranasal sinuses, this neoplasm was previously reported only in the maxillary region.28–31 The current study presented a novel case of EMC that originated in the anterior ethmoid sinus and extended into the frontal recess, followed by a review of the available literature regarding the diagnosis and management of sinonasal EMC.
Table 1.
Published cases of epithelial-myoepithelial carcinoma that occurred within the nose and paranasal sinuses
| Study | Age, y/Sex | Presentation | Site of Origin | Primary Tumor | Metastasis | Surgical Approach | Adjuvant Radiation | Outcome |
|---|---|---|---|---|---|---|---|---|
| Current study, 2018 | 69/male | Hyposmia, epistaxis for 2 y | Anterior ethmoid | 3.6 cm, extension to frontal sinus | None | Open and endoscopic craniofacial resection | 66 Gy to resection site and bilateral cervical lymph nodes | No recurrence at 8 mo |
| Amita et al.,36 2016 | 60/female | Nasal obstruction, epistaxis, facial swelling, vision change, headache for 3 mo | Nasal cavity, unspecified | Unspecified | Distant (bilateral lung) | None | Not available | Distant metastasis at initial diagnosis; referred for radiotherapy and lost to follow-up |
| Flam et al.,37 2015 | 63/male | Epiphora for 2 y, epistaxis for 1 y | Nasal cavity lateral to inferior turbinate | 1.6 cm, obstruction of nasolacrimal duct | None | Endoscopic medial maxillectomy | No | No recurrence at 12 mo |
| Patra et al.,29 2012 | 50/male | Cheek swelling for 7 y, nasal obstruction and epistaxis for 3 mo | Maxillary sinus | 8 cm, erosion into the orbit, soft tissues of the face, and the oral cavity | Contralateral cervical lymph node (4 mo after initial resection) | Lateral rhinotomy and/or sublabial approach for primary tumor; selective neck dissection for recurrence in contralateral neck | 30 Gy after initial resection; unspecified dose to the neck after recurrence | Initial recurrence at 4 mo, treated with neck dissection and adjuvant radiotherapy; no further recurrence at 24 mo |
| Chung et al.,38 2013 | 48/female | Nasal obstruction for “several months” | Nasal cavity, unspecified | 5 cm, extension to nasopharynx, hard palate, alveolus | None | Unspecified | Unspecified | Unspecified |
| Park et al.,32 2011 | 36/female | Unspecified | Inferior turbinate | 0.5 cm | Distant (bone) | Endoscopic | No | Recurrence at 15 mo in contralateral nasal cavity |
| Medial maxillectomy for recurrence in contralateral inferior turbinate | 60 Gy after recurrence | Distant metastasis at 22 mo | ||||||
| Kuran et al.,30 2008 | 54/female | Facial swelling for 6 mo | Maxillary sinus (bilateral) | 6.5 and 4.5 cm, extension to hard palate and nasal cavity | None | Partial maxillectomy | No | No recurrence at 30 mo |
| Yamanegi et al.,39 2008 | 70/female | Epistaxis for 3 mo | Inferior turbinate | 3.6 cm, confined to nasal cavity | None | Unspecified | No | No recurrence at 12 mo |
| Pradhan et al.,40 2007* | 29/male | Facial swelling, epistaxis, and nasal obstruction for 6 mo | Unknown | Recurrent tumor of nasal cavity that extended to maxillary sinus | None | Lateral rhinotomy | No | Unspecified |
| Lee et al.,41 2000 | 22/male | Nasal obstruction for 1 y | Inferior turbinate | 3 cm, extension to maxillary sinus and soft palate | None | Partial maxillectomy | 55 Gy | No recurrence at 40 mo |
| Jin et al.,42 1999 | 61/female | Nasal obstruction and epistaxis for 2 mo | Posterior nasal cavity | 4 cm, extension to nasopharynx | None | Unspecified | No | No recurrence at 20 mo |
| Sunami et al.,28 1999 | 65/female | Nasal obstruction for 1 y | Maxillary sinus | 7 cm, erosion into hard palate and nasal cavity | None | Unspecified | No | No recurrence at 24 mo |
| Harada et al.,43 1996 | 56/male | Epistaxis for 2 y, nasal obstruction for ”several months” | Nasal septum | Unspecified size, no extension or bony erosion | None | Endoscopic | No | No recurrence at 7 mo |
| Fonseca et al.,31 1993 | 74/female | Unspecified | Maxillary sinus | Unspecified | None | Unspecified | Unspecified | Died of locally recurrent disease at 252 mo |
*A sinonasal mass had been excised 18 mo before presentation, with no other history available.
CASE REPORT
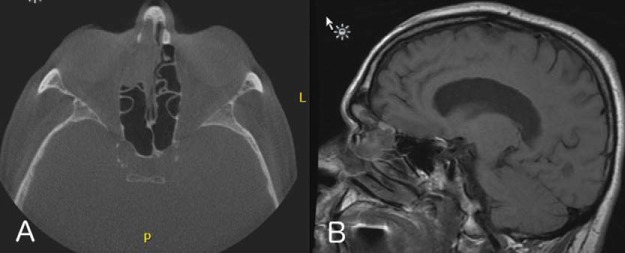
A 69-year-old man reported progressive hyposmia and self-limiting right-sided epistaxis of 2 years’ duration. The patient had been treated with broad-spectrum antibiotics on multiple occasions for presumed recurrent acute sinusitis. He subsequently was evaluated by an otolaryngologist at a different facility who obtained preoperative computed tomography and magnetic resonance imaging, which revealed a 3.6-cm enhancing mass of the right anterior ethmoid region with associated obstruction of the right frontal sinus (Fig. 1). The patient subsequently underwent septoplasty and bilateral functional endoscopic sinus surgery by the outside surgeon, with subtotal resection of the noted right frontoethmoidal mass. The final pathology report was consistent with EMC (Fig. 2).
Figure 1.
Preoperative imaging. (A) Computed tomography, demonstrating a mass in the right anterior ethmoid sinus, with thinning of the lamina papyracea. (B) Sagittal T1-weighted magnetic resonance image, showing this mass as well as associated frontal sinus obstruction.
Figure 2.
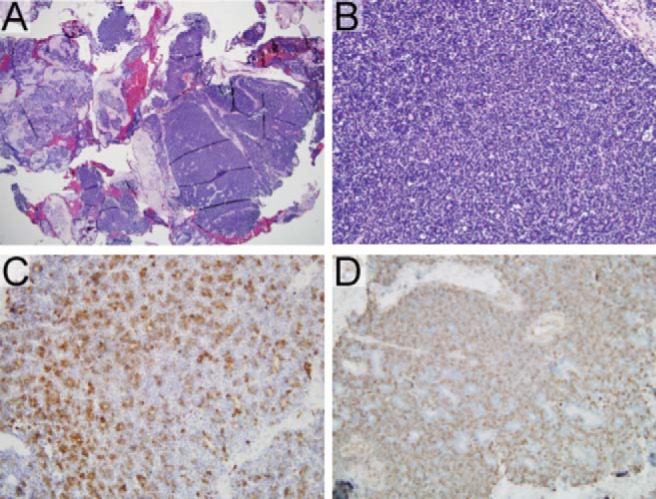
Epithelial-myoepithelial carcinoma. (A) A specimen, demonstrating a densely cellular, multinodular lesion amid background salivary gland tissue (hematoxylin and eosin, original magnification ×20). (B) A specimen, demonstrating small ductal structures within the lesion (hematoxylin and eosin, original magnification ×200). The inner layers of cells are columnar, with granular eosinophilic cytoplasm; surrounding these are layers of cells with indistinct borders, vesicular nuclei, and clear-cell changes in a subset. There is little cytologic atypia or appreciable mitotic activity in either population. (C) Epithelial inner lining cells (pan-cytokeratin immunostain, original magnification ×200). (D) A smooth-muscle actin immunostain is positive in the surrounding myoepithelial cells (smooth-muscle actin immunostain, original magnification ×200).
After this initial procedure, the patient was referred to the multidisciplinary skull base program at the University of North Carolina at Chapel Hill for further management of residual tumor. He underwent combined open and endoscopic craniofacial resection with pericranial flap reconstruction of the anterior skull base. The patient was discharged on postoperative day 7 after removal of nasal packing. He recovered well from the procedure, with no evidence of neurologic complication or cerebrospinal fluid leak. The final pathologic examination showed residual EMC of the right anterior septum and frontal sinus with negative margins. Consensus was reached at the multidisciplinary head and neck tumor board to treat with adjuvant radiation (64.8 Gy) to the tumor site and ipsilateral neck beginning 6 weeks after surgery. The patient currently had no evidence of residual or recurrent disease 8 months after completion of radiotherapy.
METHODS
Clinical data were reviewed after obtaining approval from the institutional review board of the University of North Carolina at Chapel Hill. Surgical specimens underwent standard hematoxylin and eosin staining. Immunohistochemical studies were performed on tissue sections from the primary specimen with antibodies against smooth-muscle actin and pan-cytokeratin (AE1/AE3). Brown staining within a blue background was interpreted as a positive result.
A comprehensive literature review of the MEDLINE data base from January 1, 1950, to August 8, 2017, was performed by using combinations of the search terms “epithelial,” “myoepithelial,” “epithelial-myoepithelial,” “paranasal sinus,” “sinus,” “nasal,” and “nose.” Reference sections of identified articles were searched for additional relevant articles. Only those articles published in English were included in this review.
RESULTS
Thirteen relevant English-language case reports28–32,36–43 were identified (Table 1) for a total of 14 patients with sinonasal EMC, including the current study patient. An additional article reported a heterogeneous series of 61 EMC tumors, including 6 that occurred within the “sinonasal mucoserous glands.”44 Unfortunately, no specific information regarding the sinonasal subsite, pathology, or outcome was provided for this subgroup, and, therefore, these patients were not included in the current review. Of the 14 total patients with sinonasal EMC, 8 (57.1%) were women, with an average age of 54.1±15.7 years. Presenting symptoms were available for 12 of 14 patients, with the most common being nasal obstruction and epistaxis. (Fig. 3). Facial swelling, epiphora, hyposmia, headache, and unilateral vision loss were also reported as initial symptoms (Fig. 3). The duration of symptoms before diagnosis ranged from 2 months to 7 years.
Figure 3.
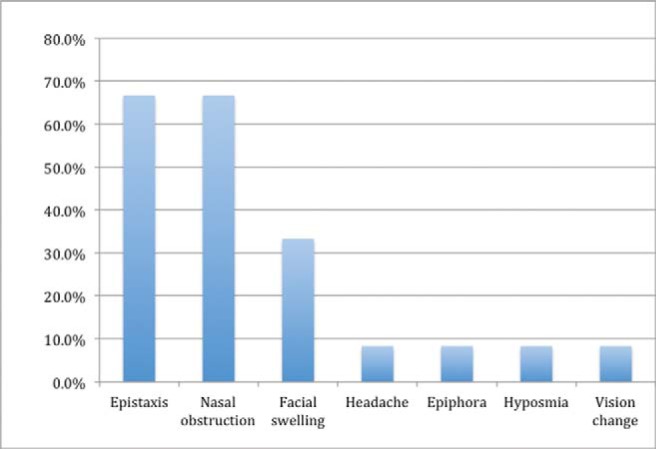
Frequency of presenting symptoms of sinonasal epithelial-myoepithelial carcinoma.
The maxillary sinus and the inferior turbinate were the most common location for these neoplasms (Fig. 4). All the lesions were unifocal at the time of presentation, with the exception of one patient who presented with EMC of the bilateral maxillary sinuses.30 Tumor size ranged from 0.5 to 8.0 cm at the time of surgical resection. Tumor resection was performed through a variety of open and endoscopic approaches, and consisted of at least a partially endoscopic procedure in four of eight patients (50%). Of the patients who underwent primary tumor resection, 3 of 11 (27.3%) received adjuvant radiation. One patient who presented with distant metastasis to the lung was treated with primary radiation therapy alone.
Figure 4.
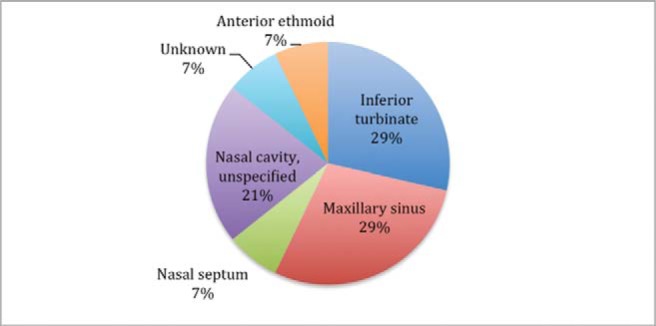
Site of tumor origin.
Eleven patients (including the current study patient) had clinical outcome data available; the average follow-up was 41.0±70.6 months. When excluding a dramatic outlier who was reported to have died of locally recurrent disease 252 months after the initial diagnosis, the average follow-up was 19.9±10.4 months. Recurrent disease occurred in 3 of 11 patients (27.3%): 1 patient developed regional metastasis to the contralateral cervical lymph nodes 4 months after the initial resection29; a second patient developed local recurrence in the contralateral nasal cavity at 15 months, followed by distant bony metastases at 22 months32; and a third patient was reported to have died from local disease 252 months after diagnosis. The calculated mortality rate was 1 of 11 (9.1%), regional metastasis was 1 of 11 (9.1%), and local recurrence was 2 of 11 (18.2%) at 41.0±70.6 months.
DISCUSSION
The differential diagnosis of sinonasal malignancy is broad; common tumors of the anterior skull base in adults include squamous cell carcinoma, adenocarcinoma, and adenoid cystic carcinoma. A multitude of rarer neoplasms of epithelial, mesenchymal, neuroectodermal, and lymphoid origin may also occur in the ethmoid region, including sinonasal undifferentiated carcinoma, mucosal melanoma, and olfactory neuroblastoma.33 An uncommon tumor in any location, EMC is especially rare in the nose and paranasal sinuses; the current study described, to our knowledge, the first reported case within the frontal-ethmoid region. A comprehensive English-language literature review identified 13 other patients with EMC of the sinonasal cavity.
A preponderance of cases of EMC arise within the major salivary glands, typically the parotid, and thus the majority of data regarding the natural history of this neoplasm comes from series of patients with salivary tumors.1 The slight female preponderance (57.1%) and average age (54.1±15.7 years) of the patients with sinonasal EMC were consistent with the largest case series of EMC of the major salivary glands, which also reported a female preponderance (57.3%) and average age of 63.8±15.4 years.1
An early case series of EMC of the major and minor salivary glands reported a 50% recurrence and 40% mortality rate for 22 patients with this tumor.31 More than 2 decades later, a query of the Surveillance, Epidemiology, and End Results data base provided a more optimistic prognosis for salivary EMC; based on 246 patients, the overall survival rates were 91.3% at 60 months, 90.2% at 120 months, and 80.7% at 180 months.1 Of the 11 patients with sinonasal EMC and adequate follow-up data available, survival at an average of 41.0±70.6 months was 90.9%. The spread to regional lymph node basins and spread to distant metastasis were each seen in 1 of 11 (9.1%) of the study population compared with 22.0 and 4.47% for salivary EMC, respectively.1 Due to the scarcity of this tumor, the sample size for sinonasal EMC was unavoidably low, which made reliable estimation of mortality difficult. Nevertheless, the concordance seen for survival between populations of salivary and sinonasal EMC supported the conclusion that, in general, EMC is a low-grade neoplasm that follows a relatively indolent clinical course.
In a small proportion of cases, EMC exhibits more aggressive characteristics and decreased overall survival. Vazquez et al.1 identified a tumor size of >4 cm and high-grade histology as independent predictors of increased mortality for salivary EMC at 180 months. Seethala et al.27 reported positive margins, angiolymphatic invasion, necrosis, and myoepithelial anaplasia as predictors of decreased disease-free survival. In their population of 45 patients with follow-up data, 5-and 10-year disease-specific survivals were 93.5 and 81.8%, respectively; only three patients died of disease, but all had positive margins, angiolymphatic invasion, and necrosis on initial resection, with the eventual development of either regional or distant metastases.27 In an earlier study, of 22 patients with salivary EMC, Fonseca and Soares31 identified nuclear atypia to be associated with an unfavorable prognosis.
A similar pattern emerges from analysis of sinonasal EMC: most patients have no evidence of recurrent or metastatic disease, yet outliers exist. Park et al.32 reported a 36-year-old woman with a 0.5-cm EMC of the inferior turbinate that recurred in the contralateral nasal cavity at 15 months following initial surgical resection and ultimately resulted in distant metastasis at 22 months following initial surgical resection, despite clear resection margins and adjuvant radiation therapy after excision of the locally recurrent tumor. Although the primary tumor was small, unusually high-grade histology was noted, with significant nuclear atypia, necrosis, and elevated mitotic count.32 Information regarding histologic grade was not available for other patients with sinonasal EMC, but high-grade lesions reasonably warrant more aggressive management and surveillance.
The treatment approach to sinonasal EMC varied across case reports, with most patients undergoing a combination of open and/or endoscopic resection, with or without adjuvant radiation. The goal of primary surgery for EMC is margin-negative resection, with avoidance of major morbidity. The role for adjuvant radiation in salivary EMC is not established, although it may be recommended for patients with high-risk clinical or histologic features. Postoperative radiotherapy was recommended for the current study patient due to previous incomplete tumor resection by an outside surgeon. The role of chemotherapy in the management of advanced EMC has not been adequately studied, although several case reports have described its effective use for pulmonary metastasis from EMC of the major salivary glands.34,35 Given the overall rarity of EMC, it is unlikely that adequately powered studies will be available to assess the comparative value of treatment options, and decisions regarding modalities will need to be made by multidisciplinary tumor boards by taking patient factors and available data into account.
CONCLUSION
EMC of the nose and paranasal sinuses is a very rare tumor, with 14 reported cases in the English language medical literature. To our knowledge, this was the first report of EMC within the frontoethmoidal region. Data are sparse, but, in general, EMC follows an indolent clinical course, although, in a minority of cases, particularly in large tumors with nuclear atypia, more aggressive behavior may be observed. Treatment of nonmetastatic disease consists of surgical excision with clear margins if possible and may include adjuvant radiation.
ETHICAL APPROVAL
This study was approved by the IRB at the University of North Carolina at Chapel Hill.
STATEMENT OF HUMAN AND ANIMAL RIGHTS
This article does not contain any studies with human or animal subjects.
STATEMENT OF INFORMED CONSENT
There are no human subjects in this article and informed consent is not applicable.
REFERENCES
- 1.Vazquez A, Patel TD, D’Aguillo CM, et al. Epithelial-myoepithelial carcinoma of the salivary glands: An analysis of 246 cases. Otolaryngol Head Neck Surg. 2015; 153:569–574. [DOI] [PubMed] [Google Scholar]
- 2.Lee JW, Myung NH, Suh MW. Epithelial-myoepithelial carcinoma of external auditory canal evolving from pleomorphic adenoma. Korean J Audiol. 2012; 16:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong SK, Goh EK, Chon KM, Lee IW. Epithelial-myoepithelial carcinoma in the external auditory canal. Otolaryngol Head Neck Surg. 2008; 139:598–599. [DOI] [PubMed] [Google Scholar]
- 4. Venkatesulu BP, Pathy S, Vallonthaiel AG, Chawla B. Epithelial-myoepithelial carcinoma of lacrimal gland from an ex pleomorphic adenoma. BMJ Case Rep. 2015:pii:bcr2015210795. [DOI] [PMC free article] [PubMed]
- 5.Avdagic E, Farber N, Katabi N, Shinder R. Carcinoma ex pleomorphic adenoma of the lacrimal gland with epithelial-myoepithelial carcinoma histologic type. Ophthal Plast Reconstr Surg. 2016; 33(suppl. 1):S136–S138. [DOI] [PubMed] [Google Scholar]
- 6.Singh G, Sharma MC, Agarwal S, et al. Epithelial-myoepithelial carcinoma of the lacrimal gland: A rare case. Ann Diagn Pathol. 2012; 16:292–297. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Park SE, Bae HG, et al. Epithelial-myoepithelial carcinoma of the nasopharynx: A case report and review of the literature. Oncol Lett. 2015; 10:927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imate Y, Yamashita H, Endo S, et al. Epithelial-myoepithelial carcinoma of the nasopharynx. ORL J Otorhinolaryngol Relat Spec. 2000; 62:282–285. [DOI] [PubMed] [Google Scholar]
- 9.Cherian S, Kulkarni R, Bhat N. Epithelial-myoepithelial carcinoma in the hard palate: A case report. Acta Cytol. 2010; 54(suppl):835– 839. [PubMed] [Google Scholar]
- 10.Teppo H, Paronen I. Epithelial-myoepithelial carcinoma in minor salivary gland of the hard palate. J Craniofac Surg. 2008; 19:1689–1691. [DOI] [PubMed] [Google Scholar]
- 11.Mohanty S, Pathak H. Epithelial-myoepithelial carcinoma of floor of mouth: A case report with cytological, histological and immunohistochemical correlation. Natl J Maxillofac Surg. 2014; 5:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima FJ, Porto DE, Cavalcante JR, et al. Epithelial-myoepithelial carcinoma of high grade transformation: The case report in the buccal mucosa. Open Dent J. 2012; 6:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters P, Repanos C, Earnshaw J, et al. Epithelial-myoepithelial carcinoma of the tongue base: A case for the case-report and review of the literature. Head Neck Oncol. 2010; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumai Y, Ogata N, Yumoto E. Epithelial-myoepithelial carcinoma in the base of the tongue: A case report. Am J Otolaryngol. 2006; 27:58– 60. [DOI] [PubMed] [Google Scholar]
- 15.Moukarbel RV, Kwan K, Fung K. Laryngeal epithelial-myoepithelial carcinoma treated with partial laryngectomy. J Otolaryngol Head Neck Surg. 2010; 39:E39–E41. [PubMed] [Google Scholar]
- 16.Oh HJ, Do NY, Kee KH, Park JH. Epithelial-myoepithelial carcinoma arising from the subglottis: A case report and review of the literature. J Med Case Rep. 2016; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikaelian DO, Contrucci RB, Batsakis JG. Epithelial-myoepithelial carcinoma of the subglottic region: A case presentation and review of the literature. Otolaryngol Head Neck Surg. 1986; 95:104–106. [DOI] [PubMed] [Google Scholar]
- 18.Guan M, Cao X, Wang W, Li Y. Epithelial-myoepithelial carcinoma of the hypopharynx: A rare case. Oncol Lett. 2014; 7:1978–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konoglou M, Cheva A, Zarogoulidis P, et al. Epithelial-myoepithelial carcinoma of the trachea—A rare entity case report. J Thorac Dis. 2014; 6(suppl. 1):S194–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arif F, Wu S, Andaz S, Fox S. Primary epithelial myoepithelial carcinoma of lung, reporting of a rare entity, its molecular histogenesis and review of the literature. Case Rep Pathol. 2012; 2012:319– 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westacott LS, Tsikleas G, Duhig E, et al. Primary epithelial-myoepithelial carcinoma of lung: A case report of a rare salivary gland type tumour. Pathology. 2013; 45:420– 422. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld A, Schwartz D, Garzon S, Chaleff S. Epithelial-myoepithelial carcinoma of the lung: A case report and review of the literature. J Pediatr Hematol Oncol. 2009; 31:206– 208. [DOI] [PubMed] [Google Scholar]
- 23.Fulford LG, Kamata Y, Okudera K, et al. Epithelial-myoepithelial carcinomas of the bronchus. Am J Surg Pathol. 2001; 25: 1508–1514. [DOI] [PubMed] [Google Scholar]
- 24.Tsuneyama K, Hoso M, Kono N, et al. An unusual case of epithelial-myoepithelial carcinoma of the liver. Am J Surg Pathol. 1999; 23:349–353. [DOI] [PubMed] [Google Scholar]
- 25.McCluggage WG, Aydin NE, Wong NA, Cooper K. Low-grade epithelial-myoepithelial carcinoma of bartholin gland: Report of 2 cases of a distinctive neoplasm arising in the vulvovaginal region. Int J Gynecol Pathol. 2009; 28:286–291. [DOI] [PubMed] [Google Scholar]
- 26.Donath K, Seifert G, Schmitz R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts. Epithelial-myoepithelial carcinoma of the intercalated ducts [in German]. Virchows Arch A Pathol Pathol Anat. 1972; 356:16–31.[Mismatch] [PubMed] [Google Scholar]
- 27.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: A review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007; 31:44–57. [DOI] [PubMed] [Google Scholar]
- 28.Sunami K, Yamane H, Konishi K, et al. Epithelial-myoepithelial carcinoma: An unusual tumor of the paranasal sinus. ORL J Otorhinolaryngol Relat Spec. 1999; 61:113–116. [DOI] [PubMed] [Google Scholar]
- 29.Patra SK, Panda NK, Saikia UN. Epithelial-myoepithelial carcinoma of the maxillary sinus: A rare case. Laryngoscope. 2012; 122:1579–1581. [DOI] [PubMed] [Google Scholar]
- 30.Kuran G, Sagit M, Akin I, et al. Bilateral epithelial-myoepithelial carcinoma: An extraordinary tumor of the paranasal sinuses. Skull Base. 2008; 18:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonseca I, Soares J. Epithelial-myoepithelial carcinoma of the salivary glands. A study of 22 cases. Virchows Arch A Pathol Anat Histopathol. 1993; 422:389–396. [DOI] [PubMed] [Google Scholar]
- 32.Park JO, Jung CK, Sun DI, Kim MS. An unusual presentation of aggressive epithelial-myoepithelial carcinoma of the nasal cavity with high-grade histology. J Laryngol Otol. 2011; 125(12):1286–1289. [DOI] [PubMed] [Google Scholar]
- 33.Ho AS, Zanation AM, Ganly I. Malignancies of the paranasal sinus In Flint PW, Haughey BH, Lund VJ, eds. Cummings Otolaryngology—Head and Neck Surgery, 6th ed Philadelphia: Saunders Elsevier; 2015:1176–1201. [Google Scholar]
- 34.Pierard S, Gregoire V, Weynand B, Machiels JP. Epithelial-myoepithelial carcinoma of the submandibular gland with symptomatic lung metastases treated with chemotherapy. Eur Arch Otorhinolaryngol. 2006; 263:1158–1160. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki H, Ota Y, Aoki T, Kaneko A. Lung metastases of epithelial-myoepithelial carcinoma of the parotid gland successfully treated with chemotherapy: A case report. J Oral Maxillofac Surg. 2013; 71:220–226. [DOI] [PubMed] [Google Scholar]
- 36.Amita K, Vijayshankar S, Abhishek MG, Kumari A. Cytomorphologic attributes of epithelial myoepithelial carcinoma of nasal cavity—A rare tumor with unusual clinical presentation. J Clin Diagn Res. 2016; 10:ED10–ED12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flam JO, Brook CD, Sobel R, et al. Nasal epithelial myoepithelial carcinoma: An unusual cause of epiphora, a case report and review of the literature. Allergy Rhinol (Providence). 2015; 6:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung HJ, Lee BH, Hwang YJ, Kim SY. Epithelial-myoepithelial carcinoma in nasal cavity with bony destruction: A case report. J Korean Soc Radiol. 2013; 69:265–268. [Google Scholar]
- 39.Yamanegi K, Uwa N, Hirokawa M, et al. Epithelial-myoepithelial carcinoma arising in the nasal cavity. Auris Nasus Larynx. 2008; 35:408– 413. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan SA, Khannan R, Hazarika B, Desai M. Sinonasal epithelial-myoepithelial carcinoma—A rare entity. Indian J Otolaryngol Head Neck Surg. 2007; 59:168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HM, Kim AR, Lee SH. Epithelial-myoepithelial carcinoma of the nasal cavity. Eur Arch Otorhinolaryngol. 2000; 257:376– 378. [DOI] [PubMed] [Google Scholar]
- 42.Jin XL, Ding CN, Chu Q. Epithelial-myoepithelial carcinoma arising in the nasal cavity: A case report and review of literature. Pathology. 1999; 31:148–151. [DOI] [PubMed] [Google Scholar]
- 43.Harada H, Kashiwagi SI, Fujiura H, et al. Epithelial-myoepithelial carcinoma–A report of a case arising in the nasal cavity. J Laryngol Otol. 1996; 110(4):397–400. [DOI] [PubMed] [Google Scholar]
- 44.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: A review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007; 31:44–57. [DOI] [PubMed] [Google Scholar]