Short abstract
Background
Due to the proximity of the maxillary sinus and ethmoid sinuses to the orbit, inflammatory processes that originate in the sinonasal region have the potential to extend into the orbit.
Objective
We presented a case of ptosis and restrictive strabismus of the medial rectus muscle.
Methods
A case report with a literature review of possible diagnoses.
Results
Biopsy, imaging, and laboratory evaluation by otolaryngology, ophthalmology, and rheumatology services were unable to identify the cause of the fibrosis after 22 months of follow-up. A response to oral steroids indicated an inflammatory process.
Conclusion
Unilateral mechanical restriction of the medial rectus muscle is a rare complication of nasal disease. Inflammatory processes and iatrogenic injury are known to cause fibrosis of surrounding tissue. We presented a unique case of medial rectus fibrosis that did not meet the diagnostic criteria of recognized etiologies.
Strabismus is a defect in conjugate vision and a major cause of diplopia. Strabismus may occur secondary to neurologic deficits or impairment of the extraocular muscles (EOM), including mechanical restriction. Known causes of mechanical EOM restriction include orbital fracture, iatrogenic injury, thyroid-associated ophthalmopathy (Graves’ disease), congenital disorders, orbital tumors, and orbital inflammation.1 Although uncommon, autoimmune diseases also have been described as a cause of mechanical EOM restriction.2 We presented a case of a patient with mechanical restriction of the left medial rectus and accompanying ptosis and diplopia due to an unknown inflammatory condition.
CASE PRESENTATION
A 75-year-old woman presented to our clinic with diplopia, left-sided excessive lacrimation, a history of chronic rhinosinusitis, and redness of the ipsilateral eye. The patient’s medical history was significant for glioma resection and radiation therapy 8 years before presentation, without evidence of recurrence. After several years, she began experiencing excessive tearing of the left eye, followed by generalized redness. These symptoms began approximately 2 to 3 years before presentation. Diplopia began 6 weeks before presentation. She subsequently underwent a limited functional endoscopic sinus surgery (ESS) at a different facility for suspected infection. Pathology results were nonspecific. On our examination, the patient had strabismus, generalized redness, and moderate ptosis of the left eye, with an otherwise nonfocal neurologic examination result. According to the patient, ptosis and redness worsened over the past year. She had slight exotropia of the left eye and did not have pain or a history of orbital trauma.
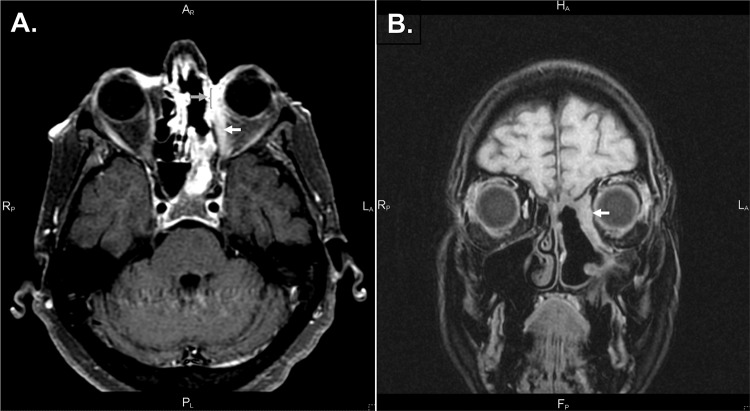
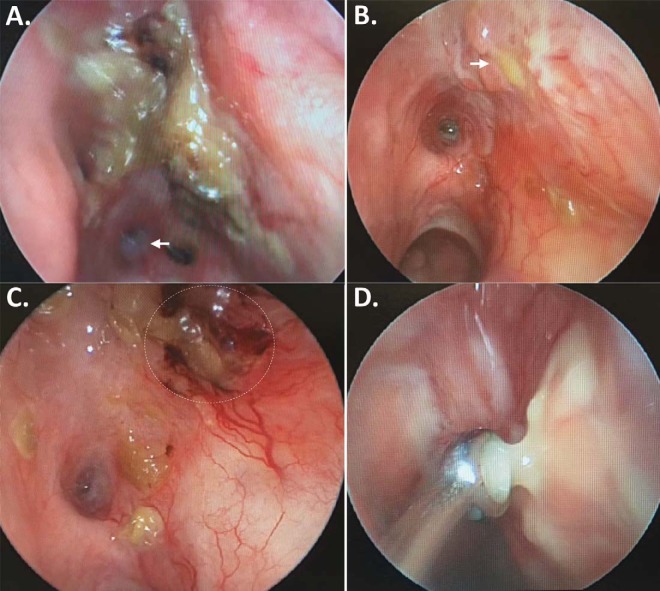
A review of previous imaging demonstrated a chronic inflammatory change in the left medial orbital wall and sinuses for several years before presentation. The last computerized tomography (CT) before the operation at a different facility documented erosion of the left medial orbital wall, with loss of the fat plane separating the wall from the medial rectus, and again identified a soft-tissue density in the medial orbit (Fig. 1). Magnetic resonance imaging after the procedure revealed dehiscence of the left lamina papyracea, enlargement of the ipsilateral medial rectus, thickening of nasal mucosa along the medial orbital wall, and a lesion of the left sphenoid sinus (Fig. 2). A CT detected decalcification of the left lamina papyracea. Nasal endoscopy demonstrated crusting and a bulging of the medial orbital wall, with purulent discharge into the nasal cavity (Fig. 3). Cultures from nasal mucosal swab and tissue biopsy were positive for pansensitive Candida tropicalis. Symptoms failed to resolve after treatment with fluconazole.
Figure 1.
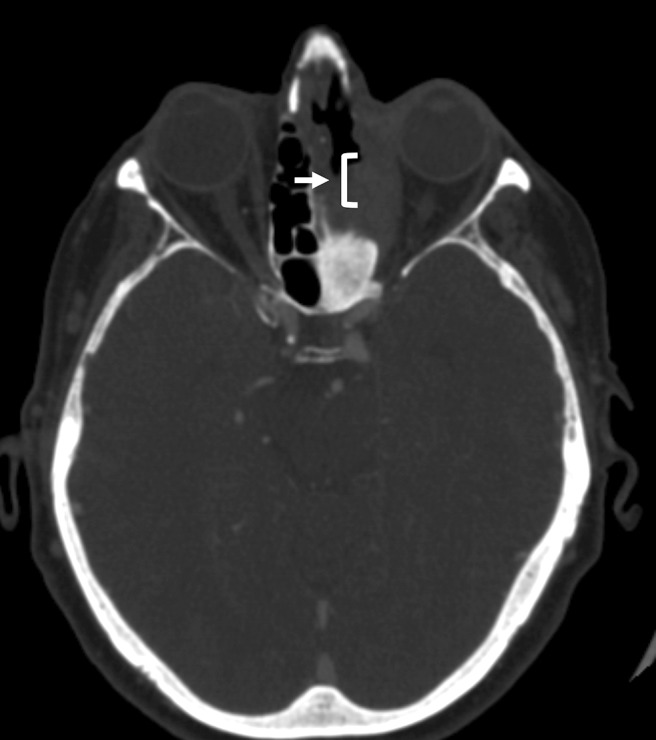
Computed tomography with contrast. Soft-tissue density, with erosion of the left medial orbital wall and loss of the fat plane between the medial orbital wall and the medial rectus muscle (white arrow and bracket).
Figure 2.
T1-weighted magnetic resonance imaging with contrast, fat saturation sequence. (A) T1 fat saturation with contrast; dehiscence of the left lamina papyracea (gray arrow and bracket); enlargement of medial left medial rectus along the entire length of muscle (white arrow). (B) Thickening of the left medial orbital wall, obscuring the medial rectus.
Figure 3.
Left nasal endoscopy. (A) Crusting of the left medial orbital wall; the opening to the sphenoid sinus is visible at the bottom left of the image (arrow); (B) Purulent drainage can be seen coming from the medial orbital wall (arrow); (C) Irritation after crust removal; (D) Purulent drainage sampled for culture.
The patient was referred to an ophthalmologist for evaluation of new onset strabismus. Ocular motility examination detected diplopia and strabismus of the left eye on horizontal and upward gazes. Downward gaze was intact (Fig. 4). Inflammation from retrobulbar infection was thought to be the most likely cause that limited ocular motility. After 3 months of continued symptoms, the patient underwent joint surgery performed by otolaryngology and ophthalmology to address the identified disease and to further evaluate the orbital condition. A left-sided total ethmoidectomy, left maxillary antrostomy, left frontal sinusotomy, and left sphenoidectomy were performed to drain orbital infection and treat persistent rhinosinusitis. A dacryocystorhinostomy was performed to address excessive lacrimation. A forced duction test to investigate the cause of strabismus had findings consistent with mechanical restriction of the medial rectus. Pockets of pus were found around the lamina papyracea. Drainage of the pus revealed fibrotic tissue covering the medial rectus.
Figure 4.
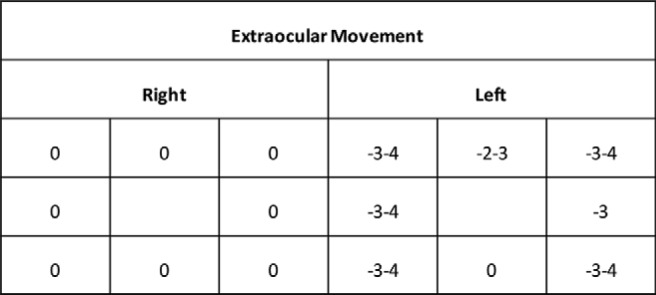
Extraocular muscle function testing, showing a normal right eye; the left eye shows restriction on abduction, adduction, and upward gaze; no abnormality on downward gaze was observed. The ratings are based on 9-point scale, from +4 to —4: zero represents normal movement; negative scores indicate limitations in movement, in which —1 is a 25% deficit, —2 is a 50% deficit, —3 is a 75% deficit, and —4 represents no movement; and positive scores denote movement beyond normal, in which +1 is a 25% increase, +2 is a 50% increase, +3 is a 75% increase and +4 is a 100% increase (from Ref. 30).
Culture of the orbital lesion was positive for multiple drug-resistant coagulase-negative Staphylococcus, Haemophilus influenzae, and Acinetobacter baumannii. Surgical pathology found evidence of acute and chronic sinusitis, necrosis, and poorly formed granulomata, with plasma cell infiltration in the left nasal contents and left cribriform region biopsy specimens. Serum analysis revealed significantly elevated C-reactive protein levels, erythrocyte sedimentation rate, and monocyte count but normal levels of angiotensin-converting enzyme (ACE), antineutrophil cytoplasmic antibody (ANCA), thyroid-stimulating hormone, and immunoglobulin. The patient was referred to the infectious disease service for treatment of orbital and nasal infections, and the rheumatology service for autoimmune evaluation. The infection resolved; however, the patient’s restriction remained. Results of a chest radiography performed for evaluation of autoimmune disease were unremarkable. Immunosuppressive therapy was initiated with methotrexate to evaluate autoimmune disease. Due to the patient’s recent fungal infection, methotrexate was selected rather than systemic corticosteroids as a more conservative approach to immunosuppression. A 6-month course of methotrexate reduced erythrocyte sedimentation rate and C-reactive protein to normal levels but failed to improve her orbital symptoms.
A subsequent biopsy was performed 17 months after presentation; results of analysis of the biopsy specimen found chronic inflammation, fibrosis, ulceration, and multinucleated giant cells. An elevated number of immunoglobulin G4 (IgG4) positive plasma cells was identified, but the ratio of IgG4-to-IgG cells was not elevated. Eosinophils were not prominent. Results of fungal and acid-fast stains were negative. In addition, the samples did not demonstrate evidence of obliterative phlebitis. After the clearance of the infection and failure of the methotrexate trial, the patient was subsequently placed on a trial of systemic corticosteroids, which significantly improved her symptoms, including diplopia.
DISCUSSION
Restrictive strabismus can result from orbital fracture, iatrogenic injury, thyroid-associated ophthalmopathy, congenital disorders, orbital tumors, or orbital inflammation.1 The presented case lacked clinical hall-marks of known causes of restrictive strabismus. The late age of onset of strabismus, at 75 years, rendered congenital disorders, e.g., congenital EOM fibrosis, very unlikely. A normal thyroid-stimulating hormone level, lack of lid retraction, and an absence of constitutional symptoms of thyroid disease ruled out thyroid-associated ophthalmopathy. With a history of brain glioma and previous radiation, tumor recurrence, second primary, or radiation sequelae were high on the differential diagnosis. However, an orbital tumor was excluded because of the absence of neoplastic histology on the biopsy specimen. Thus, delayed radiation fibrosis and autoimmune-mediated orbital inflammation remained as possible etiologies of the medial rectus restriction in our patient. These remained highest on our differential diagnosis when considering her response to oral steroids.
Orbital entry during ESS can cause adhesion formation between the EOM and surrounding structures.3 Huang et al.4 describe four patterns of EOM injury after ESS. Our patient’s chronic inflammation of the medial orbit and lamina papyracea with loss of the fat plane could have increased the likelihood of iatrogenic injury to the medial rectus similar to pattern III or caused adhesions between the medial orbital wall and the muscle. Iatrogenic injury itself is unlikely to account for persistent granulomatous inflammation of the orbital medial wall seen in the case. It is possible, however, that the unidentified inflammatory disease did not directly cause fibrosis of the muscle but only caused the granulomatous inflammation identified on the biopsy specimen.
Orbital inflammation, another cause of restrictive strabismus, can result in compression or fibrosis of orbital structures by chronic inflammation secondary to inflammatory2 or neoplastic disease.1,5 Orbital inflammatory disease (OID) encompasses orbital cellulitis, noninfectious idiopathic orbital inflammation (IOI), and the orbital manifestations of autoimmune disorders.5 Orbital cellulitis is caused by bacterial extension from maxillary or ethmoid sinuses and typically presents acutely with painful ophthalmoplegia, fever, and proptosis.5 Although our patient did not present with an acute onset of diffuse orbital cellulitis, we could not exclude the possibility of medial rectus fibrosis secondary to local, chronic orbital cellulitis.
Noninfectious OID may display diffuse orbital involvement or involve a single EOM. IOI, also called orbital pseudotumor, diffusely affects the orbit, is generally limited to the eye and the orbital tissue, presents most often with acute deep pain in the orbit with associated headache, acute or chronic onset strabismus, or periorbital swelling, and can affect one or both eyes.6 Histologic findings include granulomatous inflammation, eosinophilia, and sclerosis.7 A case of an infiltrative sclerotic mass that involved the EOM and bony destruction was reported, which may be a similar mechanism to the patient in our presentation.8
Orbital myositis, a subtype of IOI that affects a single EOM, is the most common nonthyroid cause of orbital muscle disease and can be idiopathic or secondary to another inflammatory disease.9 It tends to involve the horizontal recti muscles, followed by the vertical and oblique muscles.10 Orbital myositis typically presents with pain during eye movement, restriction of the involved muscle, proptosis, diplopia, and conjunctival hyperemia.10 Sinusitis and upper respiratory tract infections can be complicated by orbital myositis.10,11 Corticosteroids and other agents, e.g., methotrexate, have been shown to improve the symptoms of IOD.6 Our patient did not respond to methotrexate but did improve with oral steroids. Despite response to steroids, noninfectious IOD is unlikely in this case because of the chronic onset of her diplopia and the lack of painful ophthalmoplegia, eosinophilia, and acute onset typically observed in OID.
Autoimmune disease has also been implicated in restrictive strabismus. Sarcoidosis, eosinophilic angiocentric fibrosis (EAF), and granulomatosis with polyangiitis (GPA) (formerly known as Wegener granulomatosis) can involve the orbit and the nasal cavity. Ocular concerns and orbital involvement are the presenting symptoms for 38% of patients with sarcoidosis.12 Orbital symptoms of sarcoidosis include palpable mass, swelling, globe displacement, diplopia, and ptosis.13 Pulmonary disease and elevated ACE levels are found in most patients.14 A biopsy specimen that demonstrates noncaseating granuloma is required for the diagnosis of sarcoidosis.13,14 A diagnosis is typically made by age 40 years, although Stadnyk et al.15 reported an atypical onset in patients ages > 65 years, with marked respiratory and cutaneous symptoms. Despite granulomatous inflammation of the median orbital wall, our patient’s advanced age, normal ACE levels and absence of pulmonary and cutaneous symptoms would render this an atypical presentation of sarcoidosis.
EAF and GPA, by contrast, may present with local or systemic disease. EAF is an IgG4-related sclerosing disease (IgG4-RSD), which produces tumefactive lesions of the sinonasal tract and the orbit with fibrosis.16,17 A diagnosis of IgG4-RSD is made by the histologic findings of dense lymphoplasmacytic infiltrate, “onion-skin” fibrosis, and obliterative phlebitis.18 Elevated serum IgG4 is also suggestive of IgG4-RSD.18 Obliterative phlebitis is often absent in EAF, but eosinophilia is present within the lesion.16–18 Although IgG4-RSD is known to involve the orbit, sinonasal tract, and, rarely, the EOM,19–21 EAF and other IgG4-RSD are unlikely due to our patient’s unelevated serum IgG4 level, normal IgG4-to-IgG plasma cell ratio, and lack of both eosinophilic infiltrate and obliterative phlebitis on biopsy specimens.
Sinusitis and orbital inflammation are common presenting symptoms of GPA.2,22 Ocular manifestations are seen in ∼50% of patients.22,23 Nasolacrimal duct obstruction due to sinus inflammation is also commonly reported.23 The contiguous form of GPA spreads from the paranasal sinuses through the medial orbital wall to the orbit. Woo et al.2 reported that 17 of 20 patients with an orbital mass also had sinus disease; 3 of 15 patients with diplopia also had direct infiltration of the EOM. An elevated ANCA value is observed in GPA, with a sensitivity of 80% in patients with limited disease.24 Vasculitis or granulomatous inflammation is seen in 75 to 85% of cases.25 Sinonasal manifestations include nasal obstruction, nasal septum perforation, bony erosion or formation of the sinuses, and nasolacrimal duct obstruction.22
The American College of Rheumatology classification criteria for GPA26 require at least two of the following: abnormal urinary sediment, abnormal findings on chest radiograph, oral ulcers or nasal discharge, granulomatous inflammation on biopsy specimen. Our patient presented with chronic nasolacrimal duct disease, nasal discharge, and granuloma and necrosis on a biopsy specimen of nasal mucosa, which was consistent with GPA. The unremarkable chest radiograph and unelevated ANCA levels can be seen in the limited form of GPA. Methotrexate has been reported as a treatment option for GPA.27–29 Despite a response to steroids, the diagnosis of GPA was difficult to confirm in our case due to a normal ANCA value, failure of methotrexate treatment, and the inability to rule out other causes of orbital granulomatous inflammation, e.g., orbital cellulitis.
The presentation of restrictive strabismus due to fibrosis of the medial rectus reported in this patient did not fit a single diagnostic category. A factor that impeded diagnosis was that the most likely conditions, atypical GPA, IOI, and sarcoidosis, are diagnoses of exclusion. We postulated that an inflammatory process may have originated in the medial orbital wall, extended into the orbit, and led to fibrosis of the medial rectus. Inflammation may have damaged the superior division of the oculomotor nerve that supplies the levator palpebrae superioris and superior tarsal muscle, which resulted in the observed ipsilateral ptosis. Such a chronic inflammatory process would be consistent with an atypical, painless presentation of medial rectus restriction. Fibrosis from iatrogenic injury with underlying autoimmune disease, although unlikely given the onset of initial symptoms before surgery at an outside facility, could also account for granulomas and eleveated erythrocyte sedimentation rate and C-reactive protein levels . Improvement of orbital symptoms with corticosteroids supported the diagnosis of an autoimmune or inflammatory process such as atypical GPA, IOI, or IgG4-RSD.
CONCLUSION
We presented a case of unilateral mechanical restriction of the medial rectus muscle, with accompanying ptosis and strabismus, most likely due to a condition of unknown inflammatory origin. The differential diagnosis of orbital inflammatory conditions must include commonly known autoimmune diseases as well as atypical GPA, IOI, or IgG4-RSD.
ETHICAL APPROVAL
Not applicable.
STATEMENT OF HUMAN AND ANIMAL RIGHTS
We declare that all research was performed in adherence to ethical standards.
STATEMENT OF INFORMED CONSENT
Not applicable.
References
- 1.Metz HS. Restrictive factors in strabismus. Surv Ophthalmol. 1983; 28:71–83. [DOI] [PubMed] [Google Scholar]
- 2.Woo TL, Francis IC, Wilcsek GA, Coroneo MT, McNab AA, Sullivan TJ. Australasian orbital and adnexal Wegener’s granulomatosis. Ophthalmology. 2001; 108:1535–1543. [DOI] [PubMed] [Google Scholar]
- 3.Dunya IM, Salman SD, Shore JW. Ophthalmic complications of endoscopic ethmoid surgery and their management. Am J Otolaryngol. 1996; 17:322–331. [DOI] [PubMed] [Google Scholar]
- 4.Huang CM, Meyer DR, Patrinely JR, Soparkar CNS, Dailey RA, Maus M, et al. Medial rectus muscle injuries associated with functional endoscopic sinus surgery: Characterization and management. Ophthal Plast Reconstr Surg. 2003; 19: 25–37. [DOI] [PubMed] [Google Scholar]
- 5.Lutt JR, Lim LL, Phal PM, Rosenbaum JT. Orbital inflammatory disease. Semin Arthritis Rheum. 2008; 37:207–222. [DOI] [PubMed] [Google Scholar]
- 6.Espinoza GM. Orbital inflammatory pseudotumors: Etiology, differential diagnosis, and management. Curr Rheumatol Rep. 2010; 12:443–447. [DOI] [PubMed] [Google Scholar]
- 7.Yuen SJ, Rubin PA. Idiopathic orbital inflammation: Distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003; 121:491–499. [DOI] [PubMed] [Google Scholar]
- 8.Zborowska B, Ghabrial R, Selva D, McCluskey P. Idiopathic orbital inflammation with extraorbital extension: Case series and review. Eye (Lond). 2006; 20:107–113. [DOI] [PubMed] [Google Scholar]
- 9.Lacey B, Chang W, Rootman J. Nonthyroid causes of extraocular muscle disease. Surv Ophthalmol. 1999; 44:187–213. [DOI] [PubMed] [Google Scholar]
- 10.Montagnese F, Wenninger S, Schoser B. “Orbiting around” the orbital myositis: Clinical features, differential diagnosis and therapy. J Neurol. 2016; 263:631–640. [DOI] [PubMed] [Google Scholar]
- 11.Dylewski JS, Drummond R, Townsend T. Orbital myositis complicating sinusitis. Can J Infect Dis. 2001; 12:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978; 86:648–655. [DOI] [PubMed] [Google Scholar]
- 13.Mavrikakis I, Rootman J. Diverse clinical presentations of orbital sarcoid. Am J Ophthalmol. 2007; 144:769–775. [DOI] [PubMed] [Google Scholar]
- 14.Prabhakaran VC, Saeed P, Esmaeli B, Sullivan TJ, McNab A, Davis G, et al. Orbital and adnexal sarcoidosis. Arch Ophthalmol. 2007; 125:1657–1662. [DOI] [PubMed] [Google Scholar]
- 15.Stadnyk AN, Rubinstein I, Grossman RF, Baum GL, Hiss Y, Solomon A, Rosenthal T. Clinical features of sarcoidosis in elderly patients. Sarcoidosis. 1988; 5:121–123. [PubMed] [Google Scholar]
- 16.Deshpande V, Khosroshahi A, Nielsen GP, Hamilos DL, Stone JH. Eosinophilic angiocentric fibrosis is a form of IgG4-related systemic disease. Am J Surg Pathol. 2011; 35:701–706. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer J, Andrews P, Lund VJ. Eosinophilic angiocentric fibrosis of the nose and sinuses. J Laryngol Otol. 2014; 128:1071–1077. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama T, Nishida Y, Ugi S, Ishida M, Nishio Y, Ohji M. A case of extraocular muscle swelling due to IgG4-related sclerosing disease. Jpn J Ophthalmol. 2011; 55:315–317. [DOI] [PubMed] [Google Scholar]
- 20.Faramarzi M, Dadgarnia MH, Moghimi M, Sharouny H, Behniafard N. Nasal eosinophilic angiocentric fibrosis with orbital extension. Head Neck Pathol. 2015; 9:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang CH, Mady LJ, Mirani NM, Baredes S, Eloy JA. Sinonasal eosinophilic angiocentric fibrosis: A systematic review. Int Forum Allergy Rhinol. 2014; 4:745–752. [DOI] [PubMed] [Google Scholar]
- 22.Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocular complications of Wegener’s granulomatosis. Ophthalmology. 1983; 90:279–290. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992; 116:488–498. [DOI] [PubMed] [Google Scholar]
- 24.Harman LE, Margo CE. Wegener’s granulomatosis. Surv Ophthalmol. 1998; 42:458–480. [DOI] [PubMed] [Google Scholar]
- 25.Pakrou N, Selva D, Leibovitch I. Wegener’s granulomatosis: Ophthalmic manifestations and management. Semin Arthritis Rheum. 2006; 35:284–292. [DOI] [PubMed] [Google Scholar]
- 26.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990; 33:1101–1107. [DOI] [PubMed] [Google Scholar]
- 27.Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis). J Autoimmun. 2014; 48–49:94–98. [DOI] [PubMed] [Google Scholar]
- 28.Holle JU, Gross WL, Holl-Ulrich K, et al. Prospective long-term follow-up of patients with localised Wegener’s granulomatosis: Does it occur as persistent disease stage? Ann Rheum Dis. 2010; 69:1934–1939. [DOI] [PubMed] [Google Scholar]
- 29.De Groot K, Rasmussen N, Bacon PA, Tervaert JWC, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005; 52:2461–2469. [DOI] [PubMed] [Google Scholar]
- 30.Rowe F. Clinical Orthoptics. Chichester U.K: Wiley Blackwell Science, 2012. [Google Scholar]