Abstract
Objective:
Obtaining informed consent in pediatric cancer research can be subject to important ethical challenges because of the difficulty in distinguishing between care and research, which are interrelated. Pediatric oncologists also often conduct research, such as clinical trials, on their own patients, which may influence voluntary informed consent. This review aims to determine the ethical issues encountered in obtaining informed consent in pediatric oncology by identifying and summarizing the findings of existing qualitative studies on this topic.
Methods:
A systematic review of qualitative studies was conducted. Medline, Embase, CINAHL, and PubMed were searched using the following terms: (oncolog* or cancer or hematol* or haematol* or leuk* or malign* or neoplasm*) and (child* or adolescent* or minor* or young people or pediatr* or paediatr*) and ethic* or moral*) and (qualitative or interview). Other sources were also mined to identify all relevant studies. The data analysis method used was thematic analysis.
Results:
At the end of the search process, 2361 studies were identified. Duplicates were removed and irrelevant studies were excluded. After screening the full text of the remaining studies against our inclusion and exclusion criteria, 13 studies were included in the qualitative analysis. All studies were qualitative studies using semistructured and structured interviews, qualitative analysis of open-ended questions, and observation of informed consent conferences. Four themes were identified: parental comprehension of the trial and medical terms, influence of parental distress on decision-making, no offer of an alternative treatment, and influence of the doctor–parent relationship.
Conclusion:
Many ethical challenges affect the informed consent process. These challenges may include a lack of parental understanding, the potential influence of treating doctors, and vulnerability because of psychological status. All of these result in parents being unable to give well-informed and voluntary consent. Researchers are encouraged to adopt a stepwise approach during the informed consent process.
Keywords: informed consent, pediatric, decision making, bioethics, qualitative, systematic review
Background
Globally, around 300 000 cases with cancer are diagnosed each year in children and adolescents younger than 19 years.1 However, despite a high survival rate of 80% for most pediatric cancers in Western countries, approximately 80 000 children around the world die from cancer every year.1 Those who survive from cancer are also at risk of the late consequences of aggressive treatments, such as neurocognitive problems (including difficulties with visual function), cardiovascular diseases, and secondary malignant neoplasms (such as breast cancer).2,3 Improving cancer treatment aims not only to increase cure rates but also to minimize the late consequences of treatment.4
Introduction
Oncological research is important, but evidence from studies performed on adults with cancer cannot be extrapolated to children; they are not simply “small adults,” and there are differences in the cancer types, prognoses, and even methods of therapy.5 Because of the rarity of pediatric cancers, it is imperative that as many children with cancer as possible are recruited to these studies. In developed countries, over 70% of children undergoing cancer treatments are enrolled in a study.6 As a result, research is intertwined with care. Most pediatric oncologists are involved in both research and clinical care. As research participants, children are more vulnerable to unethical practices for several reasons, including a lack of understandable language used in the consent, ignorance of their wishes because of their cognitive development, the level of autonomy permitted, and reliance on their family’s decision-making ability. Hence, this dual role of the pediatric oncologist can pose some ethical challenges and influence the informed consent process.
This review aims to systematically integrate existing qualitative studies exploring the experiences of parents, adolescents, health workers, and research coordinators to identify the ethical issues that can negatively influence decision-making in the informed consent process in pediatric oncology. This will synthesize existing information on ethical issues related to informed consent and will help researchers, ethical committee members, and other policy-making stakeholders to implement measures that guarantee that informed consent in research is truly voluntary and informed.
Methods
Search Strategy
A search strategy was constructed using the following key words: (oncolog* or cancer or hematol* or haematol* or leuk* or malign* or neoplasm*) and (child* or adolescent* or minor* or young people or pediatr* or paediatr*) and (ethic* or moral*) and (qualitative or interview). MESH terms relevant to each database were incorporated to improve the sensitivity and specificity of identifying relevant research works. Four databases were searched: Medline, Embase, CINAHL, and PubMed. There were no date or language limitations. The reference lists of relevant studies were also searched to identify applicable research. Duplicate studies were identified and removed. Further irrelevant studies were removed after screening the abstracts of the remaining studies. The full texts of the remaining studies were screened against the inclusion and exclusion criteria, and only those meeting the inclusion criteria were included in the review. Inclusion criteria were research articles in peer-reviewed journals that studied ethical aspects related to pediatric cancer and the use of qualitative methods. Meta-analyses and case reports were excluded. The study selection process was conducted independently by 2 reviewers, and individual selections were discussed and agreed upon. Interrater reliability was examined by double-coding 30% of the selected papers. All conflicts were resolved by careful deliberation against the inclusion and exclusion criteria of this review and agreed in collaboration.
Methodological Quality Assessment
Methodological quality assessment was carried out using the Critical Appraisal Skills Program checklist for qualitative studies.7 The checklist is made up of 10 questions that determine whether the results of a study are valid, what the results were, and if they were locally relevant. Each question was answered with a “yes,” “no,” or “unclear” response. An aggregate score was given to demonstrate the quality of each study. A study with a score <6 was considered to be of poor quality.
Data Extraction and Synthesis
The following data were extracted from each study: demographic data of the participants, themes arising from the qualitative analysis, and study settings. Thematic analysis was chosen as the data-processing method. A process of thematic networking was used to identify initial aspects to which themes could be ascribed. This was achieved by reading the studies several times, and themes were extracted and analyzed to identify the major common themes across the studies.
Results
Search Results
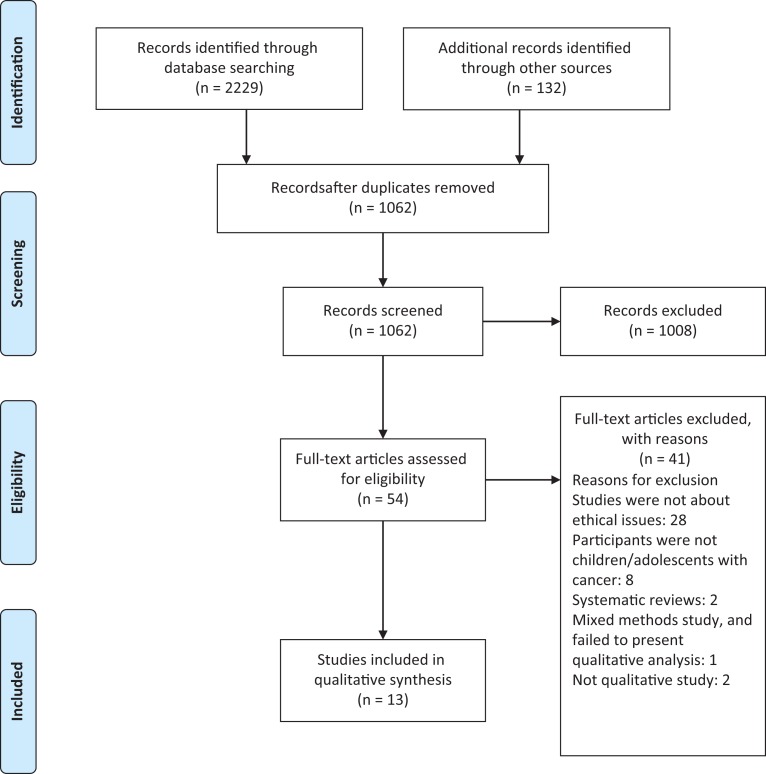
At the end of the search process, 2361 studies were identified from databases and other sources. Of these, 1299 duplicates were removed, and 1062 titles were screened. After screening the abstracts, 1008 studies were excluded because they were clearly irrelevant to the objectives of this study. The full texts of the remaining 54 studies were screened against the inclusion and exclusion criteria, and 41 studies were excluded (Figure 1). Thirteen studies were included in the qualitative analysis (Table 1).
Figure 1.
Flowchart of the selection process (PIRSMA).
Table 1.
Demographics.
| Study Author (Date) | Methods | Sample | Ethnicity | |
|---|---|---|---|---|
| 1. | Dekking et al (2016)18 | Focus groups; semistructured in-depth interviews | 16 pediatric oncologists 4 research ethics committee members 3 research coordinators 17 parents of children with cancer 5 adolescents with cancer | the Netherlands (Dutch) |
| 2. | Byrne-Davis et al (2010)8 | Collection of audio-recordings of consultations between pediatric oncologists and parents to obtain informed consent for clinical trials; semistructured interviews of parents | 20 consultations 30 parents (17 mothers and 13 fathers) | United States |
| 3. | Dekking et al (2015)13 | Focus groups; Semistructured, in-depth interviews | 35 respondents 16 pediatric oncologists 14 parents 2 adolescents 3 research coordinators | the Netherlands |
| 4. | Kupst et al (2003)11 | Semistructured interview | 20 parents of newly diagnosed children | United States |
| 5. | Levi et al (2000)12 | Focus groups | 22 parents of children with cancer | United States |
| 6. | Oppenheim et al (2005)14 | Interview | 1 mother | France |
| 7. | Stevens et al (2002)15 | Qualitative study: interview | 12 mothers | United States |
| 8. | Bartholdson et al (2015)16 | Qualitative analysis of open-ended questions in a questionnaire | 86 doctors, nurses, and nursing aides | Sweden |
| 9. | Eiser et al (2015)10 | Interviews | 50 mothers of children newly diagnosed with cancer | United Kingdom |
| 10. | Chappuy et al (2010)9 | Semidirected interview at 1 and 6 months after consent was sought | First interview: 37 mothers and 14 fathers Second interview: 29 mothers and 10 fathers | France |
| 11. | Chappuy et al (2013)4 | Semidirected interview in response to standardized questions | 40 parents | France |
| 12. | Deatrick et al (2002)17 | Interviews | First interview: 39 English-speaking parents of children Second interview: 52 parents, 10 adolescents, and 22 physicians | United States |
| 13. | De Vries et al (2010)5 | In-depth, semistructured interviews | 15 pediatric hematooncologists | the Netherlands |
Methodological Quality Assessment
All studies were of average to high quality, with quality scores ranging between 6 and 9. Although the composite scores were favorable, there were specific methodological problems that ought to have been addressed. None of the included studies reported on whether the relationship between the researcher and the participants was considered or whether this had any influence on patients’ responses. Half of the included studies failed to state their recruitment strategy; hence, it was unclear how this may have influenced their results. Considering these limitations, the studies appeared to be of average quality. The results of the quality assessment are shown in Table 2.
Table 2.
Included Studies With Quality Assessment (CASP).
| Study Author (Date) | Was There a Clear Statement of the Aims of the Research | Is a Qualitative Methodology Appropriate | Was the Research Design Appropriate to Address the Aims of the Research | Was the Recruitment Strategy Appropriate to the Aims of the Research | Was the Data Collected in a Way That Addressed the Research Issue | Has the Relationship Between Researcher and Participants Been Adequately Considered | Have Ethical Issues Been Taken Into Consideration? | Was the Data Analysis Sufficiently Rigorous? | Is There a Clear Statement of Findings | Is the Research Valuable | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dekking et al (2016)18 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Byrne-Davis et al (2010)8 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Dekking et al (2015)13 | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | 8 |
| Kupst et al (2003)11 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Levi et al (2000)12 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Oppenheim et al (2005)14 | Yes | Yes | Yes | No | Yes | Unclear | Unclear | Unclear | Yes | Yes | 6 |
| Stevens et al (2002)15 | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | 8 |
| Bartholdson et al (2015)16 | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | 7 |
| Eiser et al (2015)10 | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | 8 |
| Chappuy et al (2010)9 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Chappuy et al (2013)4 | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | 8 |
| Deatrick et al (2002)17 | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | 7 |
| De Vries et al (2010)5 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
Abbreviation: CASP, Critical Appraisal Skills Program.
Qualitative Analysis
The 13 studies included in the review represented 496 participants. All studies were qualitative studies using semistructured and structured interviews, qualitative analysis of open-ended questions, and observation of informed consent conferences. The studies were mainly conducted in developed countries between the 2000 and 2016. All studies explored the ethical issues arising from the informed consent process for randomized clinical trials or clinical trials. Most studies were conducted from the perspective of the parents. Other participants included pediatric oncologists, adolescents, and research ethics committee members. After analysis, 4 themes were prominent across most of the studies: (1) parental comprehension of the trial and medical terms, (2) influence of parental distress on decision making, (3) parents were not offered an alternative treatment apart from that of the clinical trial, and (4) influence of the doctor–parent relationship on informed consent. The details of each study are provided in Table 3.
Table 3.
Summary of the Relevant Themes Identified in Each Included Paper.
| Study Author (Date) | Summary of the Relevant Themes | CASP Score | |
|---|---|---|---|
| 1 | Dekking et al (2016)18 | Infringement of autonomy may arise because of emotional distress and the influence of professionals. Deciding on treatment levels and conflicting perspectives constituted a challenge. | 9 |
| 2 | Byrne-Davis et al (2010)8 | Parents did not understand certain aspects of the trial and they were emotionally distressed while informed consent was collected. | 9 |
| 3 | Dekking et al (2015)13 | Parental comprehension and satisfaction in informed consent in pediatric clinical trials. Provided information was appropriate. One-fifth did not realize that their child had been included in a research study. Randomization concept is not well understood. Half of the parents could explain neither the aim of the clinical trial nor the potential benefit of inclusion to their child. Only one-third were aware of alternatives. | 8 |
| 4 | Kupst et al (2003)11 | Forty-five percent did not understand the concept of randomization. Half of the parents could explain neither the aim of the clinical trial nor the potential benefit to their child of inclusion. | 9 |
| 5 | Levi et al (2000)12 | Parents’ situations are interrelated to the decision-making choices, treatment expectations, and interactions with healthcare providers. | 9 |
| 6 | Oppenheim et al (2005)14 | There is ambiguity regarding the categorization of research or treatment, and conflicts appear within the work of the pediatric oncologists. | 6 |
| 7 | Stevens et al (2002)15 | Involvement of the physician in the informed consent process is valuable and has a positive impact. | 8 |
| 8 | Bartholdson et al (2015)16 | There was wide variation in parents’ understanding of the aims, costs, and benefits. Most mothers reported the aim of the trial as being to compare “old” and “new” treatments. | 7 |
| 9 | Eiser et al (2015)10 | Satisfaction with the consent process and with parents’ decisions to enroll children in protocols, but there were significant gaps in parental understanding of clinical trials and of the experimental nature of treatment. Misperceived the notion of randomization. Insufficient or no discussion of the alternatives to enrolling their child on the proposed clinical trial. | 8 |
| 10 | Chappuy et al (2010)9 | Dialogues regarding the diagnosis and treatment options occurred amid tremendous stress; a sense of constraint and lack of control were common. Parents experienced variable degrees of choice regarding their child’s participation in a clinical trial. Parents did not verbalize distinctions between understanding of treatment and research. | 9 |
| 11 | Chappuy et al (2013)4 | Emotion distress and influence of caregivers required to show more respect to the ethical principles including autonomy. | 8 |
| 12 | Deatrick et al (2002)17 | Mothers find themselves in life-and-death circumstances, and this reality alters the entire research enterprise. There is an effect of mothers’ emotional trauma on research enrollment. | 7 |
| 13 | De Vries et al (2010)5 | Clinicians do not always provide adolescents with all available information. | 9 |
Abbreviation: CASP, Critical Appraisal Skills Program.
Parental Comprehension of the Trial and Medical Terms
Parents in 8 of 13 studies did not understand certain aspects of the trial, which led to misconceptions.4,5,8-13 The term “randomization” was commonly misunderstood, and it influenced parents’ perceptions about their child’s treatment.4,9-12 Parents expressed displeasure that randomization was used to determine treatment allocation, perceiving that their child may be allocated to standard care that was regarded as a low-intensity treatment.9,10 Some parents felt uncomfortable that a computer was used to decide the treatment group to which their children were allocated11 and felt disappointed when their children were randomized into the standard care group. This was misinterpreted that they had not been chosen for the trial despite going through a difficult decision-making process. Another issue arising under this theme was the use of complex medical terms during discussions about clinical trial consent. Parents expressed that they were confused when medical jargon was used during these discussions.11
Influence of Parental Distress on Decision-Making
Eight of the reviewed studies indicated that parents were still emotionally distressed when informed consent for clinical trials was collected; this sometimes occurred just a few hours or days after they had been informed that their child had cancer.8,11-17 Parents did not realize there was a difference between care and research and confused giving consent for research with consent for care at the point they permitted their child to participate in the clinical trial. They stated that this period was very distressing, and it influenced their ability to comprehend the information provided about the research protocol. It also diminished their ability to ask questions and seek additional information. Some parents indicated that after giving consent, they realized that they could not recall the information given to them during the discussions on informed consent with the pediatric oncologist.15 This finding was affirmed in a prospective qualitative study, in which, 6 months after informed consent discussions took place, parents had forgotten most of the information they were given.9 Parents were prone to regrets and self-doubt, especially if the outcome of the clinical trial was unfavourable. For instance, in bone marrow transplantation research, mothers tended to worry when they were unsure about the outcome of securing a nonrelated bone marrow donor or initiation of full-body irradiation.15 In 1 study, pediatric oncologists and research coordinators admitted that when informed consent consultations were organized shortly after a parent was notified of their child’s diagnosis, they often felt that parents did not fully understand the details of the study, but they were included in the studies anyway.16
Parents Were Not Offered an Alternative Treatment Apart From the Clinical Trial
Parents indicated that they had no or insufficient information about the alternatives to the proposed treatment of the clinical trial.9,11-15Some parents indicated that they were given only 1 option: the clinical trial.12 With no option, they felt obliged to give consent for the clinical trial. Some parents stated that although options were given, these were very limited. Some parents stated that the decision-making process was easy because they felt they had no choice. They felt they had to accept the clinical trial to save their children.15
Influence of Doctor–Parent Relationship on Informed Consent
Of 13 studies, 11 discussed parents’ perceptions of the influence their relationship with their child’s doctor had on their ability to give consent and provided 2 categories.9,10,12,14,16,17 Some parents felt that their decisions were not influenced by the dual role of the pediatric oncologist as a caregiver and a researcher.16,17 Some parents indicated that they wanted to please the doctor and they trusted the doctor’s judgment; hence, they agreed to participate in the trial. Research coordinators also believed that the dual role of the pediatric oncologist influenced parents’ decisions,17 despite being told they had the right to decline. Across these studies, most parents felt that they would displease their doctor if they refused to join the trial. Parents felt guilty and apologetic to doctors when they refused to allow their children to take part in the study.
Discussion
The aim of this systematic review was to identify the ethical issues arising from qualitative studies on the experiences of parents, health workers, and research coordinators on the informed consent process for pediatric oncology research. The 4 major themes identified illustrated major ethical problems that could negatively influence parents’ decisions about participating in a research study. This can lead to parents giving consent that is not truly informed.
A diagnosis of pediatric cancer is very distressing for the child, their parents, and the entire family.18 Having to make decisions on clinical trials immediately after the diagnosis can add to this distress. In a study on the public perception of cancer in 10 countries, most participants indicated that if they were to be diagnosed with cancer, their greatest concern would be dying from cancer and the cost of treatment.19 It is not surprising that parents felt the timing of the informed consent discussion to be inappropriate. Their distressed state made them more vulnerable and less critical of the research. Parents also verbalized that it affected their ability to comprehend the information given to them by the researchers. When the research yielded unfavorable outcomes, the parents felt guilty, which was unhealthy for their mental state.14 Similarly, in a systematic review, Dupont et al20 identified that the urgency to make a decision to join a clinical trial soon after diagnosis is an aggravating factor for consent.20 In a narrative review, Vries et al5 explored the ethical consequences of merging research and care in pediatric oncology and also reported that parental emotional distress prevents them from fully understanding the research protocols. The urgency of commencing treatment seems to be the reason why early information about the clinical trials is provided.20 Regardless, it is unethical for pediatric oncologists and research coordinators to allow a parent to permit their child to join a study when it is obvious that they do not understand the details of the research. The informed consent process may be made more ethical by offering sufficient and easily understandable information, and giving the patients options; only then can we show real respect for their autonomous choices. Directing parents to the treating doctors’ preferences alone may damage their ability to make choices of their own free will.
Many of the studies we reviewed indicated that parents did not fully understand the trial process or some of the medical/research terms used during informed consent discussions. This finding was also reported in studies by Vries et al5 and Dupont et al.21 Randomization was the most poorly understood factor across the studies included in this review. This lack of understanding led to parents holding misconceptions about the research. Some felt that the informed consent process was a waste of time when their children were randomized into the standard care/control group. One study conducted in France attributed the lack of comprehension to parents’ socioeconomic level and native language.9 Some parents were not able to differentiate between research and care.9 They failed to appreciate the difference between the context and goals of research and treatment. This is commonly reported in research as therapeutic misconceptions.5 As the boundary between research and care continues to blur in pediatric oncology, therapeutic misconceptions will only increase.
The failure to provide treatment alternatives takes away parents’ ability to choose the appropriate course of action for their child, especially in a distressing environment such as pediatric oncology. In such situations, given consent is not truly informed, raising a major ethical concern. This problem may also arise when parents misunderstand the information provided by the research team as a result of their continued stress. Some parents noted that they were barely able to listen to what the doctors were saying because they were in a state of distress and confusion. One study noted that parents felt they had no choice; not because of coercion but because they felt the research protocol to be the best treatment for their child.9 Truly informed consent can be obtained if emotional support is provided, simple language is used, and all alternative therapies are offered, as well as training the staff responsible for collecting informed consent.
The impact of the doctor–parent relationship is widely regarded to be influential in the informed consent process.5,20 Parents’ dependency on their relationship with their child’s physician may not be necessarily negative. Parents felt that the involvement of their child’s physician in the informed consent process was valuable. One parent indicated that if the physician treating her child was not involved in the informed consent process, she would still ask for his opinion before agreeing to join the trial. This trust may lead to unethical practices. In this instance, a treating doctor is more likely to give an objective opinion on the benefits and risks of the trial than if he or she is the researcher. Although the welfare of the study participants is considered by an ethics committee before approval, every informed consent process must be truly informed and parents must adequately understand the benefits and risks of every research study they are asked to join.
This study has limitations. First, although we aimed to understand the experiences of parents, healthcare professionals, adolescents, and research coordinators, most of the included studies focused on parents’ experiences. There is therefore a need for more qualitative studies that explore the experiences of health professionals, research coordinators, and adolescents. Second, all of the included studies were conducted in developed countries in Europe and North America; thus, any ethical issues with the informed consent process arising in countries in Africa, Asia, and Australasia are not represented in this review. It is important that qualitative studies on ethical issues in pediatric oncology are conducted to provide an understanding of the situation in these regions. Despite these limitations, this review has provided valuable information on the ethical issues present in the informed consent process in pediatric oncology.
Conclusions and Recommendations
There are many ethical issues to be addressed in terms of the informed consent process in pediatric oncology research, including the timing of the process, the presentation of all options during the process, therapeutic misconceptions, and the negative influence of the parent–doctor relationship. To address these issues, pediatric oncologists should be very careful during patient recruitment, perhaps recruiting patients being treated by other physicians rather than their own, and to use or develop mechanisms that guarantee that consent obtained is voluntary and well informed. Future studies should explore parents’ thoughts about child assent, in particular that of adolescents, as well the experiences of children and adolescents with the informed consent process and their participation in clinical trials. Researchers should endeavor to ensure that parents understand the study details by systematically asking them to recall the information given to them. To promote understanding, information about research studies should be given in small quantities over varied periods, but should not go beyond the scope of the process of obtaining informed consent or complicate the issue. Obtaining truly informed consent can be achieved through good training on how to deal with such situations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Sanad Children’s Cancer Support Association, Saudi Arabia.
References
- 1. International Statistics (summary of IARC report): American Childhood Cancer Organisation 2016. http://www.acco.org/global-childhood-cancer-statistics/. Accessed April 20, 2018.
- 2. Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52(2):159–164. [DOI] [PubMed] [Google Scholar]
- 3. Dzolganoski B. Clinical trials In: Tomlinson D, Kline NE, eds. Pediatric Oncology Nursing. 2nd ed Germany: Springer; 2010:307–335. [Google Scholar]
- 4. Chappuy H, Bouazza N, Minard-Colin V, et al. Parental comprehension of the benefits/risks of first-line randomised clinical trials in children with solid tumours: a two-stage cross-sectional interview study. BMJ Open. 2013;3(5):e002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Vries MC, Houtlosser M, Wit JM, et al. Ethical issues at the interface of clinical care and research practice in pediatric oncology: a narrative review of parents’ and physicians’ experiences. BMC Med Ethics. 2011;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ablett S, Pinkerton C; United Kingdom Children’s Cancer Study Group. Recruiting children into cancer trials – role of the United Kingdom Children’s Cancer Study Group (UKCCSG). Br J Cancer. 2003;88(11):1661–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green HE. Use of theoretical and conceptual frameworks in qualitative research. Nurse Res. 2014;21(6):34–38. [DOI] [PubMed] [Google Scholar]
- 8. Byrne-Davis LMT, Salmon P, Gravenhorst K, Eden TO, Young B. Balancing high accrual and ethical recruitment in paediatric oncology: a qualitative study of the “look and feel” of clinical trial discussions. BMC Med Res Methodol. 2010;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chappuy H, Baruchel A, Leverger G, et al. Parental comprehension and satisfaction in informed consent in paediatric clinical trials: a prospective study on childhood leukaemia. Arch Dis Child. 2010;95(10):800–804. [DOI] [PubMed] [Google Scholar]
- 10. Eiser C, Davies H, Jenney M, Glaser A. Mothers’ attitudes to the randomized controlled trial (RCT): the case of acute lymphoblastic leukaemia (ALL) in children. Child Care Health Dev. 2005;31(5):517–523. [DOI] [PubMed] [Google Scholar]
- 11. Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. J Pediatr Hematol Oncol. 2003;25(10):787–790. [DOI] [PubMed] [Google Scholar]
- 12. Levi RB, Marsick R, Drotar D, Kodish KD. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J Pediatr Hematol Oncol. 2000;22(1):3–12. [DOI] [PubMed] [Google Scholar]
- 13. Dekking SAS, Van Der Graaf R, Kars MC, Beishuizen A, De Vries MC, Van Delden JJ. Balancing research interests and patient interests: a qualitative study into the intertwinement of care and research in paediatric oncology. Pediatr Blood Cancer. 2015;62(5):816–822. [DOI] [PubMed] [Google Scholar]
- 14. Oppenheim D, Geoerger B, Hartmann O. Ethical issues in pediatric oncology phase I-II trials based on a mother’s point of view. Bull Cancer. 2005;92(11):E57–E60. [PubMed] [Google Scholar]
- 15. Stevens PE, Pletsch PK. Ethical issues of informed consent: mothers’ experiences enrolling their children in bone marrow transplantation research. Cancer Nurs. 2002;25(2):81–87. [DOI] [PubMed] [Google Scholar]
- 16. Bartholdson C, Lützén K, Blomgren K, Pergert P. Experiences of ethical issues when caring for children with cancer. Cancer Nurs. 2015;38(2):125–132. [DOI] [PubMed] [Google Scholar]
- 17. Deatrick JA, Angst DB, Moore C. Parents’ views of their children’s participation in phase I oncology clinical trials. J Pediatr Oncol Nurs. 2002;19(4):114–121. [DOI] [PubMed] [Google Scholar]
- 18. Dekking SAS, Van Der Graaf R, Meeteren AYNS. A qualitative study into dependent relationships and voluntary informed consent for research in pediatric oncology. Paediatr Drugs. 2016;18(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bracken JM. Children With Cancer: A Comprehensive Reference Guide for Parents. New York: Oxford University Press; 2010:329–336. [Google Scholar]
- 20. Ramers-Verhoeven CW, Geipel GL, Howie M. New insights into public perceptions about cancer. Ecancermedicalscience. 2013;7:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dupont JCK, Pritchard-Jones K, Doz F. Ethical issues of clinical trials in paediatric oncology: a systematic review over 10 year developments (2003–2013). Lancet Oncol. 2016;17:e187–e197. [DOI] [PubMed] [Google Scholar]