Abstract
Statins are known for their anticancer effects, and many studies have shown the effectiveness of statins for cancer prevention and improvement of cancer-related long-term oncologic outcome. However, their effectiveness on recurrence or survival of non-small cell lung cancer (NSCLC) after curative resection remains unknown. This was a retrospective cohort study that assessed the medical records of patients who were diagnosed with NSCLC and treated with curative resection at a tertiary care hospital between August 2003 and July 2012. The primary outcome was the comparison of postoperative overall survival (OS) and recurrence-free survival (RFS) between the statin group of patients, who were administered statins at least 1 month before the surgery and continued it after the surgery, and the nonstatin group of patients, who were not administered statins. Propensity score (PS) matching was used to balance the 2 groups, and the analysis was performed using a Cox proportional hazards model. In total, 994 patients with NSCLC were included in the final analysis: 135 patients in the statin group and 859 patients in the nonstatin group. After PS matching, there was no significant difference in postoperative recurrence (P = .862) or death (P = .074) between the statin group and the nonstatin group. Similarly, there was no significant difference in postoperative RFS (P = .862) and OS (P = .072) between the 2 groups after PS matching. This study demonstrated that statin administration had no significant association with recurrence or survival after NSCLC treatment.
Keywords: cancer risk, cancer survival, lung cancer, metastasis, NSCLC
Introduction
Lung cancer is one of the most frequent causes of cancer-related deaths worldwide.1 Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancer cases at the time of initial diagnosis, and the standard treatment is curative resection, which is associated with a higher chance of long-term survival.2 However, even after curative resection of NSCLC, long-term survival is reported as <50%, with 33.1% of patients exhibiting recurrence within 2 years.3
Statins are inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which suppresses intramolecular mevalonate synthesis. They are widely used to lower blood cholesterol, and their effectiveness in lowering cardiovascular mortality and morbidity has been confirmed.4 In addition, statins are reported to have potential anticancer effects.5,6 The mechanism by which statins have an anticancer effect is associated with suppressing inflammation, immunomodulation, and angiogenesis.7 More specifically, in lung cancer cells, statins are reported to induce cancer cell apoptosis8 and suppress tumor growth.9 A previous meta-analysis reported no association between statin usage and the prevalence of lung cancer,10 but other studies have reported that statin administration after the diagnosis improved the mortality rate of patients with lung cancer.11,12 Moreover, another study showed that statins improved the survival of patients with stage IV lung cancer.13 However, no study has assessed the effect of statin administration on recurrence-free survival (RFS) or overall survival (OS) in patients with lung cancer after curative resection.
This study aimed to assess the effect of statin administration on RFS and OS of patients with NSCLC after curative resection. We hypothesized that statin administration would improve both OS and RFS after curative resection.
Materials and Methods
This was retrospective observational study approved by the institutional review board (IRB approval number: B-1708/412-126). Because this was a retrospective review of electronic patient medical records, the requirement for informed consent was waived. The medical records of adult patients (≥19 years) who were diagnosed with primary NSCLC and treated with elective curative resection (lobectomy, segmentectomy, wedge resection) between August 2003 and July 2012 were used for the analysis. The exclusion criteria were as follows: (1) intraoperative conversion to pneumonectomy or bilobectomy, (2) pathologic stage M1 or N3, (3) incomplete resection, (4) loss to follow-up within 5 years after the surgery, (5) death within a month from surgical complications, (6) onset of another primary cancer within 5 years after the surgery, and (7) incomplete medical records. Lobectomy with sublobar resection in another lobe was considered lobectomy, and segmentectomy with wedge resection in another lobe was considered segmentectomy. An electronic medical record system has been established since 2003 to maintain and manage patients’ medical records.
Measurements and Outcome
The following information was collected for the study: gender, age, body mass index (kg/m2), American Society of Anesthesiologists classification, histologic type of tumor, surgery type (video-assisted thoracic surgery), preoperative comorbidities (hypertension, diabetes mellitus, stroke, ischemic heart disease), pathologic tumor stage, pathologic lymph node stage, receipt of adjuvant chemotherapy or adjuvant radiotherapy, surgery time (minutes) and anesthesia time (minutes), date of death and/or recurrence, and statin administration. Tumor stage and lymph node stage were determined using American Joint Committee on Cancer guideline, Seventh Edition.14 Preoperative hypertension and diabetes mellitus were considered present if the patient had regular medication to control these conditions before the surgery. Ischemic heart disease included a wide variety of conditions from stable angina to myocardial infarction. The date of death was obtained with approval from the Ministry of the Interior and Safety in South Korea, and the date of recurrence was defined as the date of confirmed diagnosis of recurrence during outpatient clinic follow-up after the surgery.
The statin group was defined as patients who took a constant daily dose of statin (simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, or rosuvastatin) at least 1 month before the surgery and continued it after the surgery. The nonstatin group was defined as patients who had not taken any statin for at least a year prior to the surgery and who were not prescribed a statin after the surgery. The primary outcome of this study was the difference in RFS and OS between the statin group and the nonstatin group. Overall survival was defined as the time period between the surgery date and the date of death, and RFS was defined as the time period between the surgery date and the date of recurrence or death.
Statistical Method
The t test was used to compare continuous variables and the χ2 test for categorical variables. In order to balance the covariates between the 2 groups, the propensity score (PS) matching method was used.15 Then, univariate regression analysis was performed in order to identify the covariates that had individual effects on recurrence and death after lung cancer surgery. After balancing the 2 groups using PS matching with a criterion of P > .1, we applied Cox proportional hazards models. The results of Cox regression analysis before and after applying PS matching are displayed as hazard ratios (HRs) and 95% confidence intervals (CIs). Furthermore, we utilized the log-rank test to compare RFS and OS between the statin group and the nonstatin group after balancing each covariate through Kaplan-Meier estimation after PS matching. All statistical analyses were performed using R software (version 3.3.2 with R packages), and P values <.05 were considered statistically significant.
Results
In total, 1548 patients were diagnosed with NSCLC and treated with elective lung cancer surgery between August 2003 and July 31, 2012. The exclusion criteria were (1) 34 patients due to intraoperative conversion to pneumonectomy; (2) 48 patients due to intraoperative conversion to bilobectomy; (3) 35 patients due to incomplete resection; (4) 82 patients due to follow-up loss within 5 years after the surgery; (5) 3 patients due to death within a month from complications; (6) 49 patients due to pathologic stages N3 or M1; (7) 89 patients due to onset of another primary cancer within 5 years after the surgery; and (8) 131 patients due to lack of information on medication usage before, during, and within a year after the surgery. In the end, 994 patients were included in the final analysis. From this cohort, 135 patients belonged to the statin group and 859 patients belonged to the nonstatin group. After applying PS matching, each group had 133 patients. Table 1 shows the differences in baseline characteristics between the 2 groups. In the total cohort prior to applying PS matching, several variables exhibited differences. However, after applying PS matching, the 2 groups had similar characteristics (P > .1 for all covariates; Table 1).
Table 1.
Baseline Characteristics Before and After Propensity Score Matching.
| Variables | Unmatched Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Statin Group, n = 135 | Nonstatin Group, n = 859 | P Value | Statin Group, n = 133 | Nonstatin Group, n = 133 | P Value | |
| Statin dosage, mg/d, mean (SD) | 18.2 (10) | 0 | <.001 | 18.2 (10) | 0 | <.001 |
| Sex: male, n (%) | 82 (60.7%) | 548 (63.6%) | .515 | 81 (60.9%) | 80 (60.2%) | .900 |
| Age, mean (SD) | 68.5 (7.6) | 62.6 (10.2) | <.001 | 68.6 (7.6) | 67. (8.0) | .182 |
| BMI, mean (SD) | 24.3 (2.7) | 23.8 (2.9) | .046 | 24.3 (2.7) | 24.3 (2.7) | .863 |
| ASA, n (%) | <.001 | .442 | ||||
| 1 | 13 (9.6%) | 255 (29.6%) | 12 (9.0%) | 18 (13.0%) | ||
| 2 | 92 (68.1%) | 525 (61.0%) | 91 (68.4%) | 83 (62.4%) | ||
| 3 | 30 (22.2%) | 81 (9.4%) | 30 (22.6%) | 32 (24.1%) | ||
| Histologic type, % | .694 | .710 | ||||
| Squamous Cell | 33 (24.4%) | 210 (24.4%) | 32 (24.4%) | 32 (24.4%) | ||
| Adenocarcinoma | 87 (64.4%) | 531 (61.8%) | 86 (64.4%) | 84 (63.0%) | ||
| Othersa | 15 (11.1%) | 118 (13.7%) | 15 (11.1%) | 17 (12.6%) | ||
| VATS, n (%) | 33 (24.4%) | 310 (86.4%) | .009 | 32 (24.1%) | 37 (27.8%) | .484 |
| Operation, n (%) | .874 | .311 | ||||
| Lobectomy | 119 (88.1%) | 749 (87.0%) | 118 (88.7%) | 115 (86.5%) | ||
| Segmentectomy, wedge resection | 16 (11.9%) | 112 (13.0%) | 15 (11.3%) | 18 (13.5%) | ||
| Surgery time, minutes, mean (SD) | 177.9 (86.4) | 188.8 (73.8) | .126 | 178.5 (86.7) | 170.4 (61.4) | .401 |
| Hypertension, n (%) | 54 (40.0%) | 138 (16.0%) | <.001 | 54 (40.6%) | 53 (39.8%) | .900 |
| Diabetes mellitus, n (%) | 26 (19.3%) | 57 (6.6%) | <.001 | 26 (19.5%) | 24 (18.0%) | .754 |
| Cerebrovascular disease, n (%) | 16 (11.9%) | 32 (3.7%) | <.001 | 16 (12.0%) | 17 (12.8%) | .852 |
| Ischemic heart disease, n (%) | 27 (20.0%) | 26 (3.0%) | <.001 | 27 (20.3%) | 19 (14.3%) | .195 |
| Tumor, % | .063 | .961 | ||||
| T1 | 79 (58.5%) | 414 (48.1%) | 78 (58.6%) | 77 (57.9%) | ||
| T2 | 48 (35.6%) | 345 (40.1%) | 47 (35.3%) | 46 (34.6%) | ||
| T3 | 6 (4.4%) | 61 (7.1%) | 6 (4.5%) | 7 (5.3%) | ||
| T4 | 2 (1.5%) | 41 (4.8%) | 2 (1.5%) | 3 (2.3%) | ||
| Node, % | .086 | .837 | ||||
| N0 | 110 (81.5%) | 610 (71.0%) | 108 (81.2%) | 106 (79.7%) | ||
| N1 | 14 (10.4%) | 140 (16.3%) | 14 (10.5%) | 17 (12.8%) | ||
| N2 | 11 (8.1%) | 110 (12.7%) | 11 (8.3%) | 10 (7.5%) | ||
| Adjuvant chemotherapy, n (%) | 6 (4.4%) | 76 (8.8%) | .085 | 6 (4.5%) | 8 (6.0%) | .583 |
| Adjuvant radiotherapy, n (%) | 16 (11.9%) | 159 (18.5%) | .060 | 15 (11.3%) | 17 (12.8%) | .706 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; SD, standard deviation; VATS, video-assisted thoracic surgery.
a Others: large cell type, sarcomatoid lung cancer.
Univariate Analysis and Cox Regression Model After PS Matching
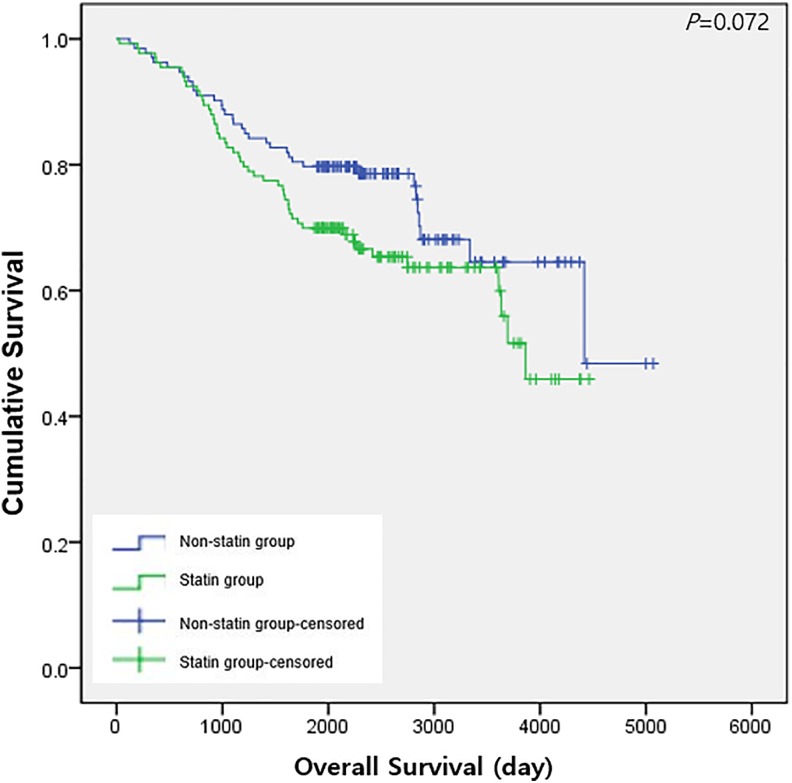
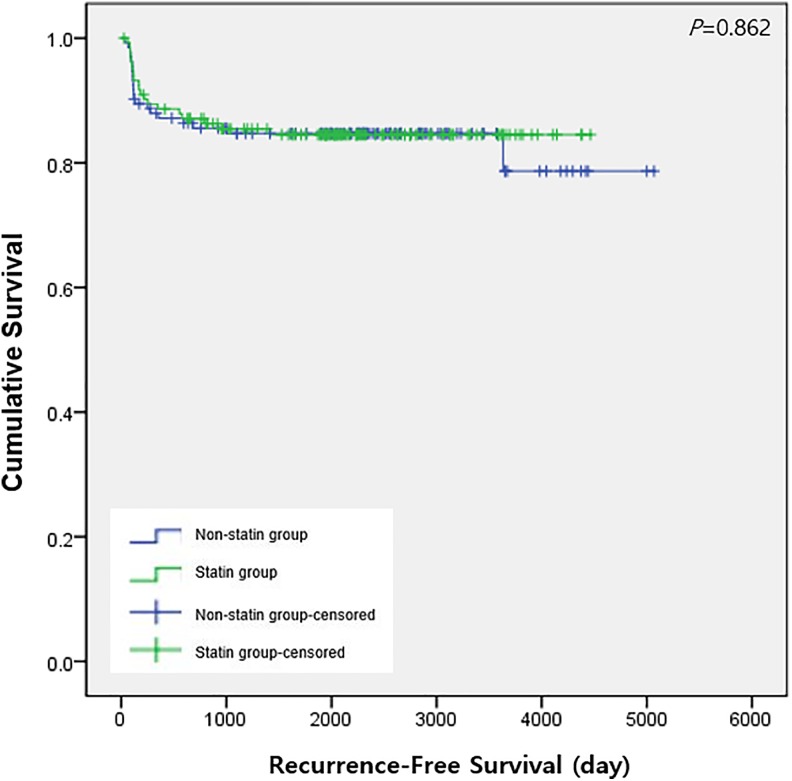
Table 2 shows the outcome of univariate regression analysis to identify the variables associated with death or recurrence after surgery. Table 3 shows the outcomes of a cox regression model of recurrence and death, before and after applying PS matching. The statin group had a significant reduction in recurrence (HR: 0.59, 95% CI: 0.37-0.93, P = 0.024), but no significant difference in death prior to PS matching (P = .745). However, after applying PS matching, Cox regression models showed no significant differences in recurrence (P = .862) and death (P = .074) between the statin group and nonstatin group. Figures 1 and 2 show Kaplan-Meier curves of OS and RFS, respectively, after PS matching; there was no significant difference between the 2 groups for either OS (P = .072) or RFS (P = .862).
Table 2.
Univariate Cox Regression Analysis for Recurrence and Death After Lung Cancer Surgery.
| Variable | Recurrence | 95% Confidence Interval | P Value | Death | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|---|---|---|---|
| HR | Lower | Upper | HR | Lower | Upper | |||
| Female (vs male) | 0.860 | 0.653 | 1.133 | .284 | 0.524 | 0.414 | 0.663 | <.001 |
| Age, years | 1.000 | 0.990 | 1.016 | .654 | 1.050 | 1.034 | 1.058 | <.001 |
| Body mass index, kg/m2 | 1.040 | 0.995 | 1.093 | .082 | 0.929 | 0.894 | 0.965 | <.001 |
| ASA | ||||||||
| 1 | 1.000 | 1.000 | ||||||
| 2 | 1.380 | 1.003 | 1.912 | .048 | 1.430 | 1.102 | 1.862 | .007 |
| 3 | 1.210 | 0.744 | 1.975 | .441 | 2.070 | 1.470 | 2.925 | <.001 |
| Histologic type | ||||||||
| Squamous cell carcinoma | 1.000 | 1.000 | ||||||
| Adenocarcinoma | 0.708 | 0.533 | 0.941 | .018 | 0.549 | 0.437 | 0.689 | <.001 |
| Othersa | 0.268 | 0.146 | 0.495 | <.001 | 0.691 | 0.498 | 0.958 | .027 |
| Type of operation I | ||||||||
| VATS | 1.000 | 1.000 | ||||||
| Open thoracotomy | 2.160 | 1.664 | 2.813 | <.001 | 2.040 | 1.659 | 2.520 | <.001 |
| Type of operation II | ||||||||
| Lobectomy | 1.000 | 1.000 | ||||||
| Segmentectomy | 0.000 | 0.000 | Inf | .992 | 0.322 | 0.133 | 0.780 | .012 |
| Wedge resection | 0.314 | 0.155 | 0.636 | .001 | 0.593 | 0.385 | 0.913 | .018 |
| Hypertension | 1.220 | 0.860 | 1.730 | .266 | 0.844 | 0.610 | 1.167 | .305 |
| Diabetes mellitus | 1.410 | 0.870 | 2.280 | .164 | 1.140 | 0.730 | 1.770 | .570 |
| Stroke history | 0.768 | 0.362 | 1.630 | .492 | 1.080 | 0.622 | 1.888 | .777 |
| IHD history | 0.795 | 0.392 | 1.609 | .523 | 1.100 | 0.653 | 1.842 | .727 |
| Tumor | ||||||||
| 0-1 | 1.000 | 1.000 | ||||||
| 2 | 3.970 | 2.829 | 5.570 | <.001 | 2.070 | 1.641 | 2.623 | <.001 |
| 3 | 7.540 | 4.794 | 11.866 | <.001 | 3.490 | 2.412 | 5.051 | <.001 |
| 4 | 7.930 | 4.765 | 13.213 | <.001 | 5.660 | 3.894 | 8.229 | <.001 |
| Node | ||||||||
| 0 | 1.000 | 1.000 | ||||||
| 1 | 79.500 | 43.758 | 144.413 | <.001 | 2.080 | 1.606 | 2.695 | <.001 |
| 2 | 105.000 | 57.658 | 192.847 | <.001 | 2.920 | 2.236 | 3.816 | <.001 |
| Adjuvant radiotherapy | 3.146 | 2.389 | 4.143 | <.001 | 3.063 | 2.453 | 3.825 | <.001 |
| Adjuvant chemotherapy | 2.299 | 1.135 | 4.660 | .021 | 2.293 | 1.317 | 3.994 | .003 |
| Surgery time, minutes | 1.004 | 1.002 | 1.005 | <.001 | 1.004 | 1.003 | 1.005 | <.001 |
| Anesthesia time, minutes | 1.004 | 1.003 | 1.006 | <.001 | 1.004 | 1.003 | 1.006 | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; IHD, ischemic heart disease; HR, hazard ratio; VATS, video-assisted thoracic surgery.
a Others: large cell type, sarcomatoid lung cancer.
Table 3.
Cox Proportional Hazard Model for Recurrence and Death After Lung Cancer Surgery.
| Model | Recurrence | 95% CI | P Value | Death | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|---|
| HR | Lower | Upper | HR | Lower | Upper | |||
| Before PS matching | ||||||||
| Nonstatin | 1.00 | 1.00 | ||||||
| Statin | 0.59 | 0.37 | 0.93 | .024 | 1.05 | 0.78 | 1.42 | .745 |
| After PS matching | ||||||||
| Nonstatin | 1.00 | 1.00 | ||||||
| Statin | 0.95 | 0.51 | 1.75 | .862 | 1.49 | 0.96 | 2.30 | .074 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PS, propensity score.
Figure 1.
Overall survival after lung cancer surgery between the statin group and the nonstatin group after propensity score matching.
Figure 2.
Recurrence-free survival after lung cancer surgery between the statin group and nonstatin group after propensity score matching.
Discussion
This study demonstrated that statin administration before and after surgery for NSCLC had no effect on postoperative RFS or OS. The outcome of the study is meaningful because we matched several conditions—such as tumor stage, lymph node stage, adjuvant chemotherapy, adjuvant radiotherapy, and underlying diseases—between patients in the statin group and nonstatin group through PS matching. Furthermore, unlike previous studies,11–13 this study assessed the effect of statin administration only in those patients with NSCLC who were treated by curative resection.
The outcomes of this study are different from those of previous studies in which statin use had a positive effect on the long-term outcome of lung cancer.11–13 One of the potential causes of the different results is the difference in the patient cohort. Two previous population-based cohort studies11,12 analyzed all patients, including those with advanced cancer and those who were receiving palliative care, and another previous study focused on a cohort of patients with stage IV advanced lung cancer.13 The difference in the cohort is critically important because the primary treatment option for patients with advanced cancer is chemotherapy rather than surgery. The anticancer effect of statins is reported as a “synergistic” effect when administered in conjunction with chemotherapy.16,17 Therefore, in this study, in which early-stage patients who underwent surgical treatment as the primary treatment option were the majority of the sample cohort, the anticancer effect of statins may not have been as pronounced.
There are a few things to consider when interpreting the outcome of this study. First, lung cancer surgery is usually performed under general anesthesia, and the patients in the statin group were not able to take the drug for 1 to 2 days after surgery. In general, the 24 hours after tumor resection is known as a decisive time period for tumor recurrence, because nonvisible malignant cells proliferate most rapidly during this period.18 Therefore, the absence of statin administration for 1 to 2 days immediately after the surgery may have weakened the anticancer effect of the statin. For this reason, statins are unlikely to have a positive effect on preventing tumor recurrence. In fact, a previous study on a prostate cancer patient cohort demonstrated that statin administration at the time of prostatectomy was not able to significantly reduce tumor recurrence after surgery,19 which is similar to our findings.
However, a prospective cohort study from a Danish group reported that statin administration significantly reduced postoperative tumor recurrence in patients with breast cancer.20 Nevertheless, an important issue needs to be addressed: In that prospective cohort study, only simvastatin—a highly lipophilic statin—significantly reduced tumor recurrence. Previous studies21,22 have demonstrated that lipophilic statins play a critical role in improving cancer outcomes. Moreover, an in vitro study demonstrated that lipophilic statins can induce cancer cell apoptosis more effectively than hydrophilic statins.23 Although still controversial, the outcomes of the present study may have been different if we had only used lipophilic statins, such as simvastatin, which have a stronger anticancer effect.
A final consideration is the sample size. Determining the correct sample size to detect the anticancer effect of statins is quite difficult, and therefore, many studies have been large sample-based population cohort studies.11–13,20 However, this study strictly focused on the long-term outcome after curative resection of NSCLC, and we were forced to match the 2 groups using a relatively smaller sample size. Therefore, the appropriateness of statistical power in this study to demonstrate the anticancer effect of statin—if any—is debatable.
There are few limitations in this study. First, the retrospective design of the study introduced selection bias. Second, because the study was performed in a single center, the results might not be generalizable. Third, as mentioned before, we were not able to separately analyze the effects of lipophilic and hydrophilic statins. Fourth, although the dates of death for the patients were accurate, we were unable to confirm whether the deaths were cancer related. Finally, this study did not show the dose–response relationship of daily statin dose due to the small population of the statin group. Nonetheless, the study is valuable because it is the first study to analyze the effect of statin administration on long-term oncologic outcome after curative resection of NSCLC.
Conclusion
In conclusion, this study showed that statin administration had no significant association with recurrence or survival after surgical treatment of NSCLC. Future, additional, larger sample sized prospective cohort studies are needed to validate the findings of this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Pearson FG. Non-small cell lung cancer: role of surgery for stages I-III. Chest. 1999;116(6 suppl):500S–503S. [DOI] [PubMed] [Google Scholar]
- 3. Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93(6):1813–1820; discussion 1820-1811. [DOI] [PubMed] [Google Scholar]
- 4. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. [DOI] [PubMed] [Google Scholar]
- 5. Platz EA. Epidemiologic musing on statin drugs in the prevention of advanced prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2175–2180. [DOI] [PubMed] [Google Scholar]
- 6. Pocobelli G, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Hampton JM, Egan KM. Statin use and risk of breast cancer. Cancer. 2008;112(1):27–33. [DOI] [PubMed] [Google Scholar]
- 7. Vallianou NG, Kostantinou A, Kougias M, Kazazis C. Statins and cancer. Anticancer Agents Med Chem. 2014;14(5):706–712. [DOI] [PubMed] [Google Scholar]
- 8. Hwang KE, Na KS, Park DS, et al. Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Invest New Drugs. 2011;29(5):945–952. [DOI] [PubMed] [Google Scholar]
- 9. Hanai J, Doro N, Sasaki AT, et al. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227(4):1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Li C, Tao H, et al. Statin use and risk of lung cancer: a meta-analysis of observational studies and randomized controlled trials. PLoS One. 2013;8(10):e77950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang WY, Li CH, Lin CL, Liang JA. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget. 2016;7(27):42208–42215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hung MS, Chen IC, Lee CP, et al. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS One. 2017;12(2):e0171137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin JJ, Ezer N, Sigel K, Mhango G, Wisnivesky JP. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer. 2016;99:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang L, Wang S, Zhou Y, et al. Evaluation of the 7th and 8th editions of the AJCC/UICC TNM staging systems for lung cancer in a large North American cohort. Oncotarget. 2017;8(40):66784–66795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calabro A, Tai J, Allen SL, Budman DR. In-vitro synergism of m-TOR inhibitors, statins, and classical chemotherapy: potential implications in acute leukemia. Anticancer Drugs. 2008;19(7):705–712. [DOI] [PubMed] [Google Scholar]
- 17. Taylor-Harding B, Orsulic S, Karlan BY, Li AJ. Fluvastatin and cisplatin demonstrate synergistic cytotoxicity in epithelial ovarian cancer cells. Gynecol Oncol. 2010;119(3):549–556. [DOI] [PubMed] [Google Scholar]
- 18. Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29(4):517–526. [DOI] [PubMed] [Google Scholar]
- 19. Mondul AM, Han M, Humphreys EB, Meinhold CL, Walsh PC, Platz EA. Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery. J Urol. 2011;185(4):1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahern TP, Pedersen L, Tarp M, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19(1):26–37. [DOI] [PubMed] [Google Scholar]
- 22. Katz MS. Therapy insight: potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol. 2005;2(2):82–89. [DOI] [PubMed] [Google Scholar]
- 23. Kato S, Smalley S, Sadarangani A, et al. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J Cell Mol Med. 2010;14(5):1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]