Abstract
Adolescence is a period associated with the initiation and escalation of substance use and is also a time during which substantial changes take place in neural development, personality and behavior. Although rates of substance use between adolescent girls and boys do not differ substantially, there is evidence for sex differences in underlying vulnerability pathways associated with the development of substance use disorder. Here we review sex differences in adolescent brain development and how these differences may contribute to different risk pathways between females and males that emerge during this developmental period. We also discuss methodological considerations in the study of sex differences in brain and behavior and their implications for interpretation. We close by highlighting promising areas for future work.
Introduction
Rates of substance use rise sharply throughout adolescence and peak in young adulthood [1,2]. While rates of past year substance use disorder (SUD) in 12–17 year olds are similar between females (4.5%) and males (4.0%), differences emerge in early adulthood, with higher rates of SUD in males aged 18 or older (10.1%), compared with females (5.7%)[3]. Likewise, sex differences in substance use are small to nonexistent in adolescents but by age 18 men drink more frequently, in larger quantities, and have greater rates of nicotine and marijuana use [4,5]. Therefore, sex differences in substance use vary by age with small differences in adolescence that increase in young adulthood. It is not clear whether this represents true developmental differences or “cohort effects” with current rates in adolescents reflecting changing cultural attitudes [6]: girls have historically had a lower prevalence of nicotine and marijuana use, but this difference has decreased over time [7,8]. Gendered behavior arises from a complex interaction of multiple influences including prevailing culture and social- and individual-level factors; these factors are profoundly different for boys and girls. In human research, it is rarely possible to “control for” the gendered environment and only examine biological sex [9]. We use the term sex in this review with the understanding that in humans it represents both biological sex and sociocultural influences.
Adolescence is not only associated with the initiation and escalation of substance use, but is also a time during which substantial changes in neural development, personality and behavior take place [10]. Models of adolescent brain development propose maturational changes that may contribute to normative increases in substance use during the teen years. However, these models do not explicitly consider sex differences. Although rates of substance use between adolescent girls and boys do not differ substantially, evidence indicates there are sex differences in underlying vulnerability pathways associated with SUD. Externalizing and internalizing problems often precede the initiation of substance use and are associated with a more severe and persistent course of SUD [11]. The externalizing pathway is characterized by rule-breaking, impulsivity, aggression, and sensation-seeking. Males generally show greater externalizing behavior than females [5]. In adolescent males, conduct and attention deficit hyperactivity disorders are most frequently cited as increasing SUD risk [reviewed in 6]. The internalizing pathway is characterized by negative affect, depression, and anxiety [11]. On average, females show greater internalizing behavior than males [12]. Sex differences in depression emerge in puberty with higher rates in females [6], and females have higher prevalence of comorbid depression or anxiety that typically predate SUD onset [13]. Thus, females are more likely to have an internalizing pathway and males more likely to have an externalizing pathway to SUD.
Next, we provide an overview of sex differences in adolescent brain development and discuss how they may contribute to divergent risk pathways (Figure 1). Due to the paucity of information available on sex differences in neurochemical pathways during adolescence in humans, the focus here is on brain structure and function (see [6] for a review of sex differences in neurochemical systems related to addiction in animal models and adult humans). We also consider the interpretation of sex differences in brain and behavior and their dependence upon study methods.
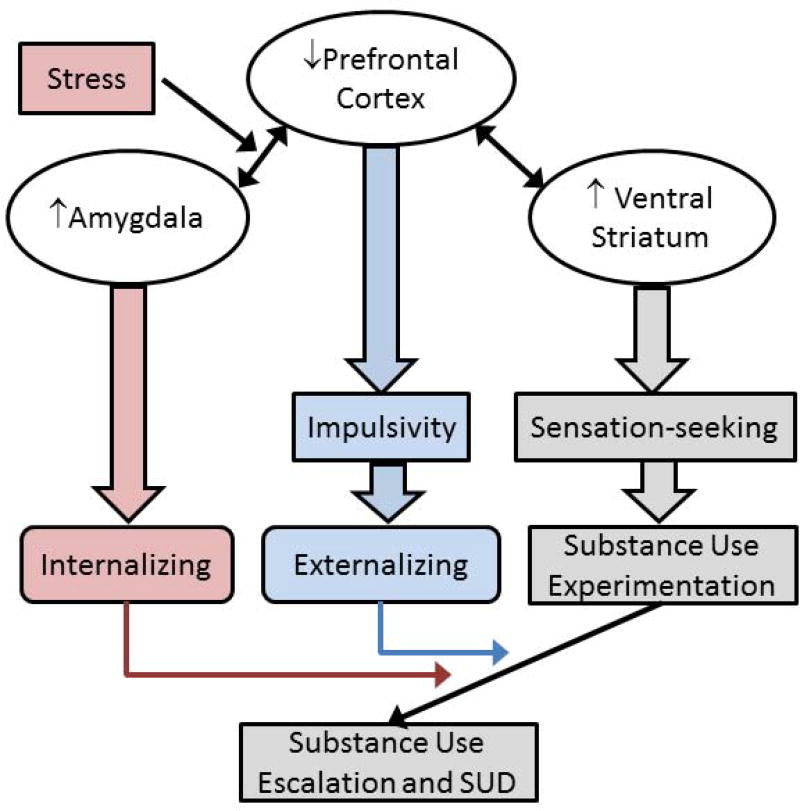
Figure 1.
Theoretical schematic of the influence of sex differences in adolescent brain development on vulnerability pathways to substance use disorder (SUD). Gray indicates pathways proposed to influence girls and boys similarly. This illustrates the suggestion that normative increases in ventral striatal responsivity during adolescence may account for comparable levels of substance use in girls and boys. Blue indicates pathways proposed to be more influential in boys than girls. Weaker inhibitory control due to individual differences in prefrontal cortex development may underlie greater externalizing problems, which in turn influences progression of substance use and development of SUD. Red indicates pathways believed to be more influential in girls than boys. Developmental imbalances between amygdala and prefrontal cortex increase emotional lability, enhancing vulnerability to internalizing problems, which in turn influences progression of substance use and development of SUD. Stress impacts this pathway.
Developmental neuroscience and externalizing behaviors
The “imbalance model” of adolescent brain development proposes a tension between early development of bottom-up subcortical reward circuity, including the ventral striatum, and later-developing top-down prefrontal cortical control circuitry [14]. Normative risk-taking behaviors in adolescence, which include substance use, are believed to reflect high ventral striatum reactivity in the context of rewarding stimuli in absence of a prefrontal control system that can dampen the response [14]. This view is supported by recent research focusing on sensation-seeking, which is underpinned by subcortical motivation circuitry such as the ventral striatum, and impulsivity, which is mediated by cognitive control circuitry in the prefrontal cortex. Longitudinal work demonstrates a linear decrease in impulsivity across adolescence along with a curvilinear association between age and sensation-seeking, which peaks in middle adolescence [15], consistent with the predictions of the imbalance model. Although this pattern holds for both females and males, there are key sex differences at the levels of both brain and behavior.
Prefrontal cortex development plateaus later in males than females although males have consistently greater volume and thickness [16,17]. Trajectories of functional connectivity between left and right dorsolateral prefrontal cortex also differ between females and males, with a pattern suggestive of earlier maturation in females [18]. In the ventral striatum, volumetric change follows a cubic trend (i.e., increasing, then decreasing) for males, but a linear (decreasing) trend for females [19]. Furthermore, functional neuroimaging has shown that adolescent females rely on frontal and striatal regions for inhibitory control more than males and these regions show significantly increased functional maturation in females [20].
The consequences of these brain differences have not been directly investigated, but behavioral studies provide insight. For example, males have lower levels of impulse control and higher levels of sensation-seeking than females, but females reach peak levels of sensation-seeking earlier than males and decline more rapidly thereafter [21]. Furthermore, the decrease in impulsivity is more gradual in males than females. This may have important implications for sex differences in substance use as it has been shown that a slower decline in impulsivity is associated with a more rapid increase in alcohol, marijuana and tobacco use [22]. Furthermore, externalizing behavior problems have been associated with poor prefrontal control [23], so sex differences in prefrontal development and downstream effects on impulsivity may contribute to sex differences in SUD vulnerability.
Trajectories of sensation-seeking, however, are not strongly associated with substance use escalation [22]. Progression in drug use is predicted by an imbalance resulting from heightened reward-seeking and weak executive control whereas heightened reward-seeking balanced by strong executive control is associated with drug experimentation, but not progression [24]. Thus, experimentation with substances may be related to a tendency to seek out rewarding experiences stemming from reward-related circuitry whereas persistent, compulsive use characterizing addiction may be more closely linked to acting without planning or consideration of consequences, stemming from prefrontal circuity. It is possible, then, that normative increases in reward system responsivity during adolescence may account for comparable levels of substance use in girls and boys, whereas greater impulsivity and protracted development of inhibitory control in males may account for greater rates of SUD emerging later in development.
Developmental neuroscience and internalizing behaviors
The triadic model is another influential theory of brain development [25]. It is consistent with the imbalance model with regard to motivated behavior, but also considers the normative increases in emotional intensity and lability that occur in adolescence. Specifically, it proposes that emotional lability reflects heightened emotional responding centered in the amygdala during adolescence due to poor prefrontal cortex modulation [25]. Although this is rarely discussed in relation to SUD risk, it is directly relevant to sex differences in the internalizing vulnerability pathway. Adolescent girls are more likely than boys to endorse coping with negative emotions as a rationale for drinking alcohol [26] and differences in the development of amygdala may affect vulnerability to anxiety and depression. For example, males have greater peak amygdala volume, but they reach this peak later in puberty than females [19]. Larger amygdala volume has been correlated with poorer emotional control in female, but not male, adolescents [27], suggesting that amygdala development may have a larger effect on emotional behavior in females [28].
Adolescent males and females also have different patterns of amygdala functional connectivity, with females exhibiting greater integration between posterior cortex and amygdala regions involved in socio-affective processing [29]. Sex differences in activation patterns across adolescence have also been found, with one study reporting decreasing activity with age in the left amygdala in response to emotional (fearful) faces in females but not males [30] and another reporting decreasing activity in the right amygdala with age to negatively-valenced words in males but not females [31]. These seemingly conflicting reports are difficult to interpret—they suggest a role for lateralization and context (i.e., task demands) in sex differences in emotional processes.
Sex differences in vulnerability to stress may also play an important role in the internalizing pathway. Adolescent girls, but not boys, who abuse alcohol tend to have experienced a high-level of stressful life events [32,33]. Furthermore, interpersonal stress and its cortisol response are more strongly linked to internalizing symptoms in adolescent girls than boys [34]. The prefrontal cortex and amygdala are particularly sensitive to the effects of stress [35]. Females who experienced early life stress had higher cortisol levels in childhood and less connectivity between the amygdala and prefrontal cortex in adolescence, which was associated with greater symptoms of anxiety [36], suggesting that girls are more likely to suffer lasting neural effects of early stress.
The current state of sex differences research in the developmental neuroscience of adolescent SUD
None of the structural imaging studies reported above directly investigated functional consequences of sex differences in the context of SUD vulnerability. A more direct way to understand brain-behavior relationships is through functional neuroimaging techniques such as fMRI. Some sex differences have been reported in healthy adolescents during inhibitory control and emotion processing fMRI studies, as reviewed above, but sample sizes are relatively small, and the tasks and analysis methods differ across studies, which hampers generalizability [37]. One review of fMRI studies comparing reward-related activation between adolescents and adults noted that the majority of studies lack the power to examine sex differences [38]. Furthermore, studies specifically documenting neuro-functional links between age-related sex differences and SUD risk are limited. A recent review of fMRI studies of SUD risk in adolescents found that only 6 of the 18 studies reported analyses of sex and 4 of these were post-hoc analyses in small sample sizes [39].
There has been a recent policy shift at the NIH calling for increased attention to sex in research. This will undoubtedly result in a much-needed increase in research into sex differences in brain functional development and associations with emerging psychopathology, including SUD. However, without appropriate attention to methodological considerations and a framework to interpret differences – as well as similarities – there will be minimal benefit to our understanding [40].
Interpreting sex differences in the developmental neuroscience of adolescent SUD
The interpretation of sex differences largely depends on methodological features of the studies in which the differences were found. When there are sex differences in brain structure or function, but not in fMRI task responses or substance use, findings could reflect equifinality, meaning that similar SUD outcomes have different neurodevelopmental antecedents [41]. This is consistent with emerging literature on sex differences in externalizing and internalizing vulnerability pathways in adolescent girls and boys, and with notions of compensation [42] that parallel interpretations of sex differences in brain size. Boys have larger total brain volumes than girls according to several measures beginning in childhood [43], but potential advantages of this (e.g., more or larger neurons) are offset by other features of the neural architecture in which girls are advantaged; for example, girls have greater inter-hemispheric connectivity than do boys [44]. When there are sex differences in fMRI task responses or substance use, but not in brain structure or function, findings could reflect multifinality, or that the same risk factor can lead to different SUD outcomes [i.e., the same pathway can lead to more than one endpoint; 41]. This is consistent with work reviewed above on the role of stress in the internalizing pathway for girls. Thus, accurate interpretation of the nature of sex differences in substance use relies on holistic considerations of brain structure, function, and behavior, suggesting that future work should include careful consideration of task designs and span levels of analysis.
Another methodological feature that influences the interpretation of sex differences is the way in which sex is handled in statistical analyses. The identification of sex differences is not an a priori aim of many developmental neuroscience or substance use studies. In fact, sex is often considered to be a confound or variable of no interest, and is included as a statistical covariate [45]. This is an especially coarse approach because it only accounts for linear relations in a dichotomous variable, and is based on the assumption that sex does not interact with other variables of interest. If the study of gender is an a priori research aim, however, then differences between girls and boys can simply be identified [46], interactions can be found [31,47], or sex-related processes can be examined separately in girls and boys [48]. Each approach answers different research questions. The first acknowledges individual differences, the second suggests neurodevelopment depends on sex, and the third implies that the neurodevelopment of substance use is a sex-specific process.
Regardless of how sex differences are identified, they reflect the same thing: average differences between girls and boys. Significant differences do not indicate that all boys use substances in one way for one reason, and that all girls use them in another way for a different reason; there is substantial overlap among individuals. Furthermore, individuals who are gendered in one domain may not be gendered in another. For instance, a boy can have “male-typical” amygdala volumes, but “female-typical” patterns of substance use. Thus, it will be important for future research in adolescent neuroscience to adopt methods that account for heterogeneity in SUDs and their antecedents and outcomes within as well as between the sexes. Person-specific connectivity approaches hold promise in this regard; they can map connections between brain regions that are related to sex or unique to an individual [49].
Variation within the sexes also highlights that sex reflects biological and sociocultural influences. Sex differences, therefore, provide insight into the mechanisms underlying brain-behavior associations. One likely mechanism underlying sex differences in the neural bases of adolescent substance use is pubertal hormones. Recent research suggests that pubertal stage at first drink is important. Compared to those whose first drink was after puberty, adolescents who drank during puberty had more alcohol-related problems in adulthood as well as decreased frontal activation during reward anticipation and increased striatal activation to reward presentation [50]. Unfortunately, there is limited consideration of sex in this new line of research, so it is unclear what aspects of puberty (e.g., brain sensitivity to hormones or social experiences) contribute to persisting effects. Future natural experiments that disentangle biological and sociocultural experiences could be illuminating. For instance, individuals with precocious puberty begin pubertal development in late childhood [51], and thus, do not have concurrent adolescent social experiences.
Other natural experiments could be similarly leveraged– to disentangle biological and sociocultural influences on the gendered nature of substance use. For instance, girls with congenital adrenal hyperplasia (CAH) have an XX karyotype, are overwhelmingly reared and identify as female, and (if their condition is well-controlled) have female-typical puberty, but due to a genetic condition, they are exposed to sex atypical levels of prenatal androgens [reviewed in 52]. Compared to unaffected girls, those with CAH have masculinized brain structure, function, and behavior (e.g., activity interests and participation, career interests, and spatial ability). Because the gendered influences of prenatal androgen are separated from those related to genes and socialization in girls with CAH, prenatal androgen is the most likely mechanism underlying the effects. There is little conclusive evidence concerning the neural bases of substance use in girls with CAH, particularly during the adolescent transition, but preliminary evidence suggests that they are more likely than controls to misuse substances, including being nearly three times more likely to receive an alcohol disorder diagnosis [53].
Conclusions
Developmental changes occurring in the prefrontal cortex, ventral striatum and amygdala contribute to increased risk for substance use problems in adolescence through effects on risk-taking behavior and emotional lability. Sex differences in these maturational trajectories lend insight into why girls tend toward an internalizing pathway to SUD and boys tend toward an externalizing pathway. To date, however, few studies have directly probed sex differences in the function of this circuitry as it relates to SUD risk. As the use of sex as a biological (and sociocultural) variable increases in this line of research, it will be important to have a framework for interpreting sex differences. This framework should incorporate methodological considerations including careful task design, multiple levels of analysis and appropriate statistical modeling of sex in light of a priori study hypotheses. Furthermore, person-specific connectivity methods that reflect heterogeneity and natural experiments that disentangle biological and sociocultural experiences hold significant potential for uncovering the gendered mechanisms that underlie sex differences in links between the brain and gendered behavior.
Highlights.
Sex differences exist in underlying risk factors for substance use disorder
Sex differences in brain development may underlie these divergent risk pathways
Interpretation of sex differences depends on study methods
Future research on mechanisms underlying sex differences is needed
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers: R01AA024433, R01AA07065 and U01DA041106 to MMH and K01AA024804 to JEH].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miech RA, Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2016: Volume I, secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- 2.Lipari RN, Ahrnsbrak RD, Pemberton MR, Porter JD. Risk and Protective Factors and Estimates of Substance Use Initiation: Results from the 2016 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration; 2017. [PubMed] [Google Scholar]
- 3.Center for Behavioral Health Statistics and Quality. 2016 National Survey on Drug Use and Health: Detailed Tables. Rockville MD: Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 4.Palmer RHC, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug and Alcohol Dependence. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. J Abnorm Psychol. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Kuhn C. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol Ther. 2015;153:55–78. doi: 10.1016/j.pharmthera.2015.06.003. This review provides a thorough and thoughtful description of the current state of the literature on sex differences in the development of addiction in adolescence. It covers the neurobiology of addiction and integrates current theories of adolescent brain development, drawing from studies using animal models as well as human studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RM, Fairman B, Gilreath T, Xuan Z, Rothman EF, Parnham T, Furr-Holden CDM. Past 15-year trends in adolescent marijuana use: Differences by race/ethnicity and sex. Drug and Alcohol Dependence. 2015;155:8–15. doi: 10.1016/j.drugalcdep.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitel L, Geckova AM, van Dijk JP, Reijneveld SA. Gender differences in adolescent health-related behaviour diminished between 1998 and 2006. Public Health. 2010;124:512–518. doi: 10.1016/j.puhe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Rippon G, Jordan-Young R, Kaiser A, Fine C. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front Hum Neurosci. 2014;8:650. doi: 10.3389/fnhum.2014.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–1072. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- 13.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 14.Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- 15.Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: further evidence for a dual systems model. Dev Psychol. 2011;47:739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- 16.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, Brammer M, Smith A. Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- **21.Shulman EP, Harden KP, Chein JM, Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. J Youth Adolesc. 2015;44:1–17. doi: 10.1007/s10964-014-0116-9. Sex differences in trajectories of sensation-seeking and impulsivity across adolescence were found in a large sample (n=8,270) from the National Longitudinal Study of Youth. Males had higher overall levels of impulsivity and sensation-seeking than females. Both decreased in impulsivity across adolescence but this decrease was more gradual in males. Females reached peak levels of sensation-seeking earlier than males and declined more rapidly thereafter. This work has important implications for understanding sex differences in trajectories of brain development and risk for SUD. [DOI] [PubMed] [Google Scholar]
- 22.Quinn PD, Harden KP. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Dev Psychopathol. 2013;25:223–239. doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zucker RA, Heitzeg MM, Nigg JT. Parsing the Undercontrol/Disinhibition Pathway to Substance Use Disorders: A Multilevel Developmental Problem. Child Dev Perspect. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Dev Psychopathol. 2015;27:901–913. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuntsche E, Muller S. Why do young people start drinking? Motives for first-time alcohol consumption and links to risky drinking in early adolescence. Eur Addict Res. 2012;18:34–39. doi: 10.1159/000333036. [DOI] [PubMed] [Google Scholar]
- 27.Blanton RE, Chaplin TM, Sinha R. Sex differences in the correlation of emotional control and amygdala volumes in adolescents. Neuroreport. 2010;21:953–957. doi: 10.1097/WNR.0b013e32833e7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerslag LR, Gulley JM. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav Brain Res. 2016;298:15–26. doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- *31.Hardee JE, Cope LM, Munier EC, Welsh RC, Zucker RA, Heitzeg MM. Sex differences in the development of emotion circuitry in adolescents at risk for substance abuse: a longitudinal fMRI study. Soc Cogn Affect Neurosci. 2017;12:965–975. doi: 10.1093/scan/nsx021. At-risk adolescent males had a significant decrease to negative vs neutral words in right amygdala and precentral gyrus activation across the ages of 8.5–17.6 years, when compared to at-risk adolescent females of the same age; yet females (and not males) showed a significant increase with age to the subjective experience of internalizing symptomatology. This is the first study to investigate longitudinal sex differences in brain function with respect to risk for substance use disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: Risks and consequences of an adolescent onset and persistent course. Psychol Addict Behav. 2014;28:322–335. doi: 10.1037/a0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks BM, Iacono WG, McGue M. Consequences of an Adolescent Onset and Persistent Course of Alcohol Dependence in Men: Adolescent Risk Factors and Adult Outcomes. Alcoholism, clinical and experimental research. 2010;34:819–833. doi: 10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: sex differences in the role of cortisol reactivity to interpersonal stress. J Clin Child Adolesc Psychol. 2009;38:513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gur RE, Gur RC. Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neurosci Biobehav Rev. 2016;70:159–170. doi: 10.1016/j.neubiorev.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heitzeg MM, Cope LM, Martz ME, Hardee JE. Neuroimaging Risk Markers for Substance Abuse: Recent Findings on Inhibitory Control and Reward System Functioning. Curr Addict Rep. 2015;2:91–103. doi: 10.1007/s40429-015-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Joel D, McCarthy MM. Incorporating Sex As a Biological Variable in Neuropsychiatric Research: Where Are We Now and Where Should We Be? Neuropsychopharmacology. 2017;42:379–385. doi: 10.1038/npp.2016.79. This circumspective provides a concise and thoughtful summary of the current state of research into the relationship between sex and the brain and outlines a framework for defining measurement of sex and interpretation of differences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pine DS, Fox NA. Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology. 2015;66:459–485. doi: 10.1146/annurev-psych-010814-015038. [DOI] [PubMed] [Google Scholar]
- 42.De Vries GJ. Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 43.Ruigrok ANV, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neuroscience and Biobehavioral Reviews. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, et al. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2016;26:4101–4121. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, Thomas K, Stein DJ. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res. 2013;214:1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J Stud Alcohol Drugs. 2015;76:47–56. [PMC free article] [PubMed] [Google Scholar]
- 49.Beltz AM. Connecting Theory and Methods in Adolescent Brain Research. Journal of Research on Adolescence. 2018 doi: 10.1111/jora.12366. [DOI] [PubMed] [Google Scholar]
- *50.Boecker-Schlier R, Holz NE, Hohm E, Zohsel K, Blomeyer D, Buchmann AF, Baumeister S, Wolf I, Esser G, Schmidt MH, et al. Association between pubertal stage at first drink and neural reward processing in early adulthood. Addiction Biology. 2017;22:1402–1415. doi: 10.1111/adb.12413. Pubertal stage at first drink predicted alcohol problems in young adulthood, such that those who first drank at an earlier pubertal stage had increased problems in adulthood; this effect was mediated by brain activation to reward, but sex differences were not examined, highlighting a key area for future research. [DOI] [PubMed] [Google Scholar]
- 51.Neely EK, Crossen SS. Precocious puberty. Current Opinion in Obstetrics & Gynecology. 2014;26:332–338. doi: 10.1097/GCO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 52.Berenbaum SA, Beltz AM. How early hormones shape gender development. Current Opinion in Behavioral Sciences. 2016;7:53–60. doi: 10.1016/j.cobeha.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Engberg H, Butwicka A, Nordenström A, Hirschberg AL, Falhammar H, Lichtenstein P, Nordenskjöld A, Frisen L, Landén M. Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: A total population study. Psychoneuroendocrinology. 2015;60:195–205. doi: 10.1016/j.psyneuen.2015.06.017. Girls and women with CAH (median age 25 years), who have sex-atypical exposure to prenatal androgens but female-typical rearing and identity, had a greater risk of substance use disorder diagnosis than female controls, including nearly 3 times the risk of receiving an alcohol use disorder diagnosis compared to female controls (and about 2 times the risk compared to male controls). This highlights the utility of natural experiments to disentangle biological and sociocultural influences. [DOI] [PubMed] [Google Scholar]