Abstract
Assessment of nitric oxide (NO) dynamics in immune cells, commonly measured using NO surrogates such as inducible nitric oxide synthase (iNOS) rather than NO itself, has been effective in understanding pathophysiology across a wide range of diseases. Although the intracellular measurement of NO is now feasible, many technical issues remain unresolved. The principle aim of our study was to determine the effect of storage time of whole blood on nitric oxide (NO) level expression in leukocytes. This is important because immune cells remain chemically dynamic even after they are removed from the circulation, and the impact of storage time must be known to optimally quantify the effect of a disease or condition on NO dynamics in circulating leukocytes. We measured NO levels using the fluorescent probe, diaminofluorescein (DAF-2DA), and flow cytometry in monocytes, neutrophils, and natural killer cells from healthy subjects immediately after blood draw (Time 0) and 30, 60, and 120 min following the blood draw. There was no significant difference among the 4 study time points in NO (DAF-2) levels, though there was wide intra-subject variability at all time points. Using LPS stimulation, we compared iNOS (the more traditional surrogate marker of NO dynamics) with NO (by DAF-2) in natural killer cells and monocytes and, we found no difference in the response patterns. In summary, we did find that within a 2-hour interval from blood draw to sample processing, there was a remarkably wide intra-subject variability in expression of intracellular NO (DAF-2) in leukocytes of healthy individuals at baseline and over time. The mechanism(s) for these differences are not known but could clearly confound efforts to detect changes in NO metabolism in white blood cells. We speculate that rapid pulsatility of NO could explain the wide variability seen.
Keywords: Nitric oxide, Flow cytometry, Healthy leukocytes, Inducible nitric oxide, Subject variability
1. Introduction
Measuring intracellular nitric oxide (NO) in leukocytes is increasingly explored in disease pathophysiology, diagnosis, and as a prognostic indicator. Direct measures of NO have previously been difficult to make in cells due to the short NO half-life and low NO concentrations [1]. Direct measurement of intracellular NO became possible with the development of a fluorescent probe, diaminofluorescein (DAF-2DA) [2]. Previous studies have utilized DAF-2DA with flow cytometry to measure intracellular NO in different clinical settings including optimization of concentration and incubation time [3–7]. To our knowledge, the effect of whole blood storage duration on NO detection as measured by flow cytometry, which can have substantial impacts on intracellular inflammatory mediators, has not been previously described. Furthermore, it is not known how stable NO detection in leukocytes is over time. These are important factors to consider when developing protocols to use flow cytometry to detect intracellular NO in leukocytes of healthy and disease states.
The purpose of our study was to establish whether time before processing of blood exerted any effect on NO (DAF-2) detection in leukocytes. We hypothesized that with storage of whole blood for up to 2 h, there would be an increase in NO (DAF-2) detection as measured by flow cytometer in granulocytes, monocytes, and natural killer (NK) cells in healthy adults. This was based on previous studies demonstrating activation of neutrophils in whole blood over time during storage [8,9] as well as reactive oxygen species production increasing over time at room temperature [10].
The second aim was to measure both inducible nitric oxide synthase (iNOS) and NO (DAF-2) using flow cytometry, and to determine whether iNOS increased in a similar manner as NO when stimulated with LPS in monocytes and NK cells. iNOS is involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism.
2. Methods
2.1. Subjects
Fifteen healthy males (21–48 years old) were recruited for this study from the Institute for Clinical and Translational Science (ICTS) Normal Blood Donor Program. Participants were screened for infectious agents and were excluded for this study if they were on any prescribed medication or had taken any over the counter medications over the past 48 h. Donors were consented with the approval of the University of California, Irvine, Institutional Review Board. Whole blood was drawn into 4 × 10 ml separate EDTA vacutainers and taken to the laboratory within 30 min of blood draw for all experiments.
2.2. Time experiments and loading with diaminofluorescein (DAF-2DA) (baseline, 30, 60, and 120 min post blood draw)
Blood from ten healthy males was used for this experiment. Intracellular NO levels were determined using conversion of the colorless DAF-2DA, a membrane-permeable indicator for NO (Sigma, MO, USA) to the green fluorescent DAF-2. Whole blood was drawn into vacutainers that were opened only to be used at the specified time. Blood was loaded with 10 μL DAF-2 DA (5 mM stock in DMSO, final concentration 50 μM) per 1 ml of whole blood for 30 min at 37 °C either immediately after blood draw (Time 0) or after sitting at room temperature for 30, 60, and 120 min. Blood was ready to be used for cell surface marker staining.
2.3. Surface marker staining and processing of DAF-2DA loaded samples
100 μl of DAF-2DA loaded blood was aliquoted into 12 × 75 mm tubes for leukocyte surface marker staining for monocytes (CD14-AF647, Biolegend, CA, USA, M5E2 clone), granulocytes (CD16 PE, Biolegend, 3G8 clone), and natural killer cells (CD56 PECy3, Biolegend, MEM-188 clone) antibodies used per manufacturer’s instructions. Briefly, tubes with NO (DAF-2DA) and surface antibodies and controls were incubated for 30 min in the dark, and red blood cells were lysed for 8 min in the dark with 3 ml RBC lysis buffer (0.15 M Ammonium Cl, 10 mM Potassium Bicarbonate, 0.1 mM EDTA). Tubes were centrifuged at 300 × g for 10 min, supernatant was carefully removed, and cell pellet washed 2 × with 4 mL of wash solution (1XPBS-no Ca or Mg, 1% heated inactive fetal calf serum, 0.02% NaN3) and centrifuged again as above. Cells were resuspended in 500 μL of 1 × phosphate buffer saline (1XPBS Irvine Scientific, Irvine, CA, USA). Samples were read on the BD C6 Accuri flow cytometer as described in Section 2.6.
2.4. Lipopolysaccharide (LPS)
1 ml of blood from 5 healthy males was aliquoted into 2 tubes, one without LPS and one with LPS (1 mg/ml). Tubes were placed for 2 h in a 5% CO2 incubator at 37 °C before both tubes were loaded with DAF-2A as described in Section 2.2. Blood was ready to be used for cell surface marker staining.
2.5. Intracellular nitric oxide synthase (iNOS) detection
A series of experiments were performed to detect simultaneous iNOS and NO (DAF-2) in leukocytes, with and without LPS stimulation. Samples were processed as above, with NO (DAF-2) and surface marker staining, in addition to determining intracellular iNOS levels. Briefly, cells and controls were resuspended in 100 μL of permeabilization buffer (eBioscience) with the anti-iNOS antibody (ABCAM, clone 4E5) and tubes were placed on ice for 20 min in the dark. All iNOS samples were run in parallel with isotype controls (ABCAM, mouse IgG1 isotype #ab91353), which was used to determine background negative control staining. Both iNOS and isotype-incubated cells were washed, centrifuged, and resuspended in 100 μL of 1 × PBS with a secondary rat monoclonal anti-mouse IgG1 antibody labeled with phycoerythrin (PE) (ABCAM, clone SB77e), incubated on ice for 15 min, washed, centrifuged, and resuspended in 500 μL 1 × PBS prior to being read on the flow cytometer.
2.6. Flow cytometry and gating strategies
All samples were collected on the ACCURI C6 cytometer (BD California, USA) equipped with a 488 nm, 640 nm lasers with 530/ 30,585/40,670LP, and 675/25 filter sets. Briefly, 100 μL of sample was collected identifying singlets and forward side scatter (FSC) and side scatter (SSC) was used to identify specific cell populations (neutrophils, monocytes and lymphocytes) (see Fig. 1 for gating strategy). Surface markers delineated specific populations granulocytes (CD16 PE) on FL-2, or NK cells (CD56 PEcy3) on FL-3 or monocytes (CD14 AF647) on FL-4 and NO (DAF-2) positive cells always displayed on the FL-1 (FITC channel). We included 7-amino-actinomycin D (7-AAD) in a subset of the samples to evaluate the % of viable cells over time and found less than 1% dead cells in our leukocyte populations. Data was analyzed using the Accuri C6 plus software.
Fig. 1.
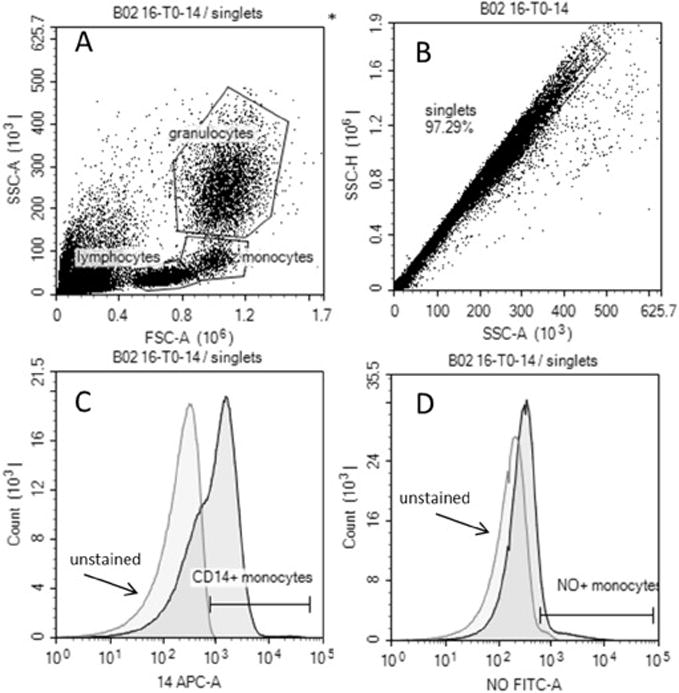
Representative gating strategy: An example at 0 min identifies leukocyte subpopulations using forward side (FSC) and side scatter (SSC) (A), singlets (B), CD14 positive were identified (C) and used to identify NO positive CD14 cells (D). CD14 positive cells were back-gated on scatterplot to confirm they were monocytes (A).
2.7. Statistical analysis
We present data for both mean fluorescence intensity (MFI) and percent of NO (DAF-2)/iNOS positive cells as both approaches are used in flow cytometry studies. Individual data are shown in Figs. 2 and 3 and summary statistics presented are median and interquartile range. The linear mixed model, a statistical model appropriate for repeated measurements from the same study subject, was applied to examine the effect of time and LPS on MFI of NO (DAF-2) activity and percent of NO (DAF-2) positive cells in monocytes, granulocytes, and NK cells. The linear mixed model was also used to examine the effect of LPS on iNOS positive cells in monocytes and NK cells. All analysis was performed using SAS 9.4 (Cary, NC) and the significance level was set at 0.05.
Fig. 2.
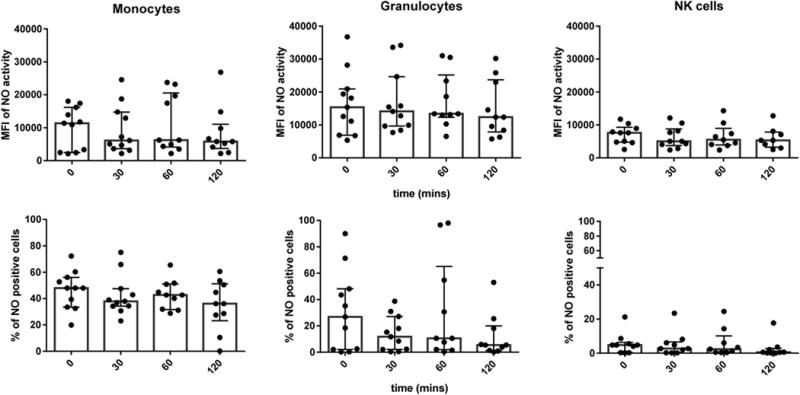
The effect of time on observed NO activity in different leukocyte subtypes. The mean fluorescence intensity (MFI) of NO (DAF-2) activity in monocytes, granulocytes, and NK cells is shown at 0, 30, 60, and 120 min post blood is presented in the top figures. The percent of double positive cells (positive for NO and surface marker) in monocytes, granulocytes, and NK cells is shown at 0, 30, 60, and 120 min post blood draw in the bottom figures. The results are expressed in bar graphs showing the median with interquartile range and includes scatter of all individual points. No significant difference in NO activity was observed between time points in leukocyte subtypes.
Fig. 3.
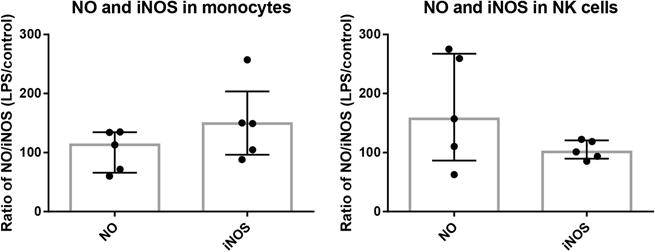
Observed percent of NO (DAF-2) positive cells and iNOS positive cells in monocytes and NK cells with and without LPS stimulation. No significant difference was observed between control and LPS stimulation in monocytes and NK cells. Bar graphs represent the median % and interquartile range of NO (DAF-2) positive and iNOS positive cells.
3. Results
3.1. NO level in response to time
There was no significant difference among the 4 study time points for MFI of NO (DAF-2) activity (all p > 0.5) or percent of positive NO (DAF-2) cells (all p > 0.1) in any of the leukocyte subtypes (Fig. 2). The median MFI from all time points was 6447 (IQR 3474–16495) in monocytes, 13,257 (IQR 9256–23711) in granulocytes, and 5323 (IQR 4061–8552) in NK cells. The median percent of double positive cells, positive for NO (DAF-2) and leukocyte cell surface marker, was 41.4% (IQR 31.7–51.9%) in monocytes, 9.5% (IQR 1.9–36.1%) in granulocytes, and 2.4% (IQR 0.3–6.0%) in NK cells.
3.2. NO and iNOS in monocytes and NK cells
In monocytes, the median percent of NO (DAF-2) positive cells pre-stimulation was 29.9% (IQR 24.0–34.6%) and post LPS stimulation was 39.4% (IQR 16.7–42.7%). In NK cells, the median percent of NO (DAF-2) positive cells pre-stimulation was 7.4% (IQR 3.7–12.0%) and post LPS stimulation was 10.1% (IQR 6.0–17.7%). In monocytes, the median percent of iNOS positive cells was 0.9% (IQR 0.1–1.4%) pre-stimulation and 0.9% (IQR 0.2–1.7%) post LPS stimulation. In NK cells, the median percent of iNOS positive cells was 3.2% (IQR 0.9–3.7%) pre-stimulation and 3.0% (IQR 1.0–3.6%) post LPS stimulation. There was no significant difference in percent of NO (DAF-2) positive cells (all p > 0.5) or percent of iNOS positive cells (all p > 0.3) before and after LPS stimulation in either monocytes or NK cells (Fig. 3).
4. Discussion
We found that in 4 different time points over a 2-hour period there was a wide range of variability in NO (DAF-2) levels with no consistent pattern of NO level change over time. The variation over time may be due to physiologic changes that occur in the cells naturally. Egger et al. found individual variations in polymorphonuclear leukocyte (granulocyte) function with storage of blood samples [10], which suggests that storage alone may cause variation possibly due to cells being activated and deactivated naturally or through the processing stages. The half-life of NO is seconds to minutes depending on its concentration and environment [11], thus our studies are a virtual snapshot of a molecule that has a rapid flux throughout the cell. The concentration of NO is dependent on both its rate of appearance and disappearance. It is possible that either of these rates may vary widely and therefore elude easy recognition of patterns of NO detection.
Another possible mechanism is that NO appearance and disappearance from leukocytes is pulsatile. We speculate that NO detection at baseline in leukocytes may be produced in a pulsatile manner, which is why we see variation in NO levels taken at 4 separate time points. Depending on the rate of the pulsatile waveform, a clear pattern may not be able to be determined at the time intervals we sampled (time 0, 30, 60, and 120 min). More frequent measurements and/or multiple blood sampling from a single individual would be necessary to determine if it is pulsatile in nature.
NO is produced from three types of nitric oxide synthase (NOS). Neuronal NOS (nNOS) and endothelial NOS (eNOS) are known to be constitutively expressed and produce low amounts of NO in a pulse like manner, while iNOS produces NO in immune and inflammatory cells at high concentrations when activated [11]. Saluja et al. found constitutive expression of all 3 NOS mRNA isoforms in monocytes and lymphocytes from healthy subjects using real time PCR however studies show that monocytes produce little to no NO in cell culture models due to epigenetic silencing [12,13]. If iNOS is constitutively expressed like nNOS and eNOS, it may also produce NO in a similar pulsatile manner. The immune system may have a population of cells that are always activated so as to respond rapidly to a stimulus, therefore leading to a cycle of cells activating and deactivating to maintain homeostasis and prevent damage. This could be due to pulsatile production of NO that cannot be seen when randomly sampling a group of cells. A pulsatile production of NO was measured at baseline in mouse gut over 80 s [14], suggesting that NO measurements need to be taken over seconds to minutes to see the pulsatile pattern of production.
Intracellular NO levels in leukocytes using flow cytometry have been evaluated in different disease states. Detection of NO in monocytes and neutrophils of septic patients was found to be higher compared to healthy controls [3]. In contrast, patients with chronic granulomatous disease were found to have half the level of NO detected in their neutrophils compared to controls [5]. In neutrophils of patients with Kawasaki disease, higher NO detection was seen compared to other febrile illnesses and afebrile controls [6]. Detection of NO in leukocytes can vary not only based on underlying disease but also with timing of the illness and treatment. In the same study of patients with Kawasaki disease, NO levels in neutrophils were also found to significantly decrease after IVIG treatment and were significantly higher in the early days of the illness [6]. These studies demonstrate the potential value of measuring intracellular NO in leukocytes in various disease states. The detection of intracellular NO using flow cytometry has been used to further understand the pathophysiology of Kawasaki disease, correlated clinical outcomes in septic patients, and to monitor immunologic status in chronic graph nephropathy patients on immunosuppressive therapy [3,6,7].
When looking at intracellular NO (DAF-2) levels in leukocytes from healthy individuals, we found wide individual variation both over time and among subjects at baseline. There is evidence that baseline NO levels are different in healthy people who are sedentary versus those who exercise regularly [15], therefore variations may be partly related to the overall lifestyle of the different subjects.
The second aim of the study was to examine intracellular NO (DAF-2) along with the iNOS protein in different leukocyte subtypes using flow cytometry and to determine if iNOS changes in a parallel way to NO with LPS stimulation. We found low percent levels of iNOS in monocytes and NK cells measured by flow cytometry. We did not find a significant increase in percent of NO (DAF-2) positive cells or percent of iNOS positive cells after LPS stimulation. Previous studies have reported a lack of iNOS protein expression in NK cells from unstimulated healthy donors [16] and iNOS detection in monocytes being dependent on the technique used in healthy controls [17]. Disease states, like inflammatory bowel disease, reveal a significant increase in iNOS detection in monocytes compared to healthy controls [18]. Therefore, iNOS, like NO, may not be easily detected in leukocytes from healthy subjects as compared to leukocytes from disease states.
Our study is limited by a small sample size. Blood manipulation that occurs with processing samples can potentially activate leukocytes and therefore cause variability in intracellular NO detection, but this risk is equal for all sample sizes if processed the same way. Expression of NO was measured using flow cytometry with no additional confirmation using other methods; to our knowledge, there are no other methods of measuring intracellular NO directly. While our study examined the presence of intracellular NO, we did not examine the source of the NO production. Finally, there are technical factors that can contribute to the variability of NO detection by DAF probes including NO-independent chemical reactions with DAFs, such as with hydrogen peroxide [19,20], that may contribute to the fluorescence detected or leakage of the DAF dye from the permeabilization step. However, we used appropriate controls for each sample in each experiment to address factors that could influence NO detection.
5. Conclusion
We found substantial variability but no consistent pattern of NO (DAF-2) detection in leukocytes from healthy subjects over a 2-hour time window from blood draw to processing. We speculate that NO may be produced in a pulsatile manner which cannot be seen with the time intervals sampled in this study. Multiple frequent sampling of NO may be needed to determine a more accurate estimation of the pattern of NO in leukocytes. The behavior of iNOS is distinct from the pattern of NO, thus iNOS cannot be reliably used as a surrogate for NO patterns in circulating leukocytes. Therefore, when using flow cytometry to detect intracellular NO to shed light on pathophysiology, clinical outcomes, or monitoring of various disease states, one should be aware that there is great individual variation that may be seen in leukocytes of healthy individuals.
Acknowledgments
This work was in part supported by National Institutes of Health (P01HD048721) and the PERC Systems Biology Research Fund.
Abbreviations
- DAF-2DA
diaminofluorescein
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- MFI
mean fluorescence intensity
- NK
natural killer
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Luss H, Nussler NC, Beger HG, Nussler AK. Expression and detection of inducible nitric oxide synthase in experimental models of inflammation. Methods A Companion Methods Enzym. 1996;10:51–60. doi: 10.1006/meth.1996.0078. [DOI] [PubMed] [Google Scholar]
- 2.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indications: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 3.Santos SS, Brunialti MKC, Rigato O, Machado FR, Silva E, Salomao R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. SHOCK. 2012;38:18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar A, Saha P, Mandala G, Mukhopadhayay D, Roy S, Singh SK, Das S, Goswami RP, Saha B, Kumar D, Das P, Chatterjee M. Monitoring of intracellular nitric oxide in leishmaniasis: its applicability in patients with visceral leishmaniasis. Cytom Part A. 2011;79A:35–45. doi: 10.1002/cyto.a.21001. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji S, Iharada A, Taniuchi S, Hasui M, Kaneko K. Increased production of nitric oxide by phagocytic stimulated neutrophils in patients with chronic granulomatous disease. J Pediatr Hematol Oncol. 2012;34:500–502. doi: 10.1097/MPH.0b013e3182668388. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura K, Tatsumi K, Tharada A, Tsuji S, Tateiwa A, Teraguchi M, Ogino H, Kaneko K. Increased nitric oxide production by neutrophils in early stage of Kawasaki disease. Eur J Pediatr. 2009;168:1037–1041. doi: 10.1007/s00431-008-0872-1. [DOI] [PubMed] [Google Scholar]
- 7.Schachnik NCC, Peruhype-Magalhaes V, Paula GMM, Lucas F, Jr, Freitas VM, Martins-Filho OA, Dusse LMS. Intracellular nitric oxide assessment in whole blood leukocytes by flow cytometry: optimization and applicability to monitor patients with chronic graft nephropathy. J Immuno Methods. 2009;343:103–111. doi: 10.1016/j.jim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Saxton JM, Pockely AG. Effect of ex vivo storage on human peripheral blood neutrophil expression of CD 11b and the stabilizing effects of Cyto-Chex. J Immuno Methods. 1998;214:11–17. doi: 10.1016/s0022-1759(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 9.Macey M, McCarthy D, Azam U, Milne T, Golledge P, Newland A. Ethylenediaminetetraacetic acid plus citrate-theophylline-adenosine-dipyridamone (EDTA-CTAD): a novel anticoagulant for the flow cytometric assessment of platelet and neutrophil activation ex vivo in whole blood. Cytom Part B Clin Cytom. 2003;52B:30–40. doi: 10.1002/cyto.b.10001. [DOI] [PubMed] [Google Scholar]
- 10.Egger G, Kukovetz EM, Hayn M, Fabjan JS. Changes in polymorphonuclear leukocyte function of blood samples induced by storage time, temperature and agitation. J Immuno Methods. 1997;206:61–71. doi: 10.1016/s0022-1759(97)00085-9. [DOI] [PubMed] [Google Scholar]
- 11.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 12.Saluja R, Jyoti A, Chatterjee M, Habib S, Verma A, Mitra K, Barthwal MK, Bajpai VK, Dikshit M. Molecular and biochemical characterization of nitric oxide synthase isoforms and their intracellular distribution in human peripheral blood mononuclear cells. Biochimica Biophysica Acta. 2011;1813:1700–1707. doi: 10.1016/j.bbamcr.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Gross TJ, Kremens K, Powers LS, Brink B, Knutson T, Domann FE, Philibert RA, Milhem MM, Monick MM. Epigenetic silencing of the human NOS2Gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J Immunol. 2014:2326–2338. doi: 10.4049/jimmunol.1301758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefano GB, Zhu W, Cadet P, Bilfinger TV, Mantione K. Morphine enhances nitric oxide release in the mammalian gastrointestinal tract via the μ opiate receptor subtype: a hormonal role for endogenous morphine. J physiology Pharmacol. 2004;55:279–288. [PubMed] [Google Scholar]
- 15.Jenkins NT, Landers RQ, Prior SJ, Soni N, Spangeburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide and superoxide in circulating CD34+ and CD34-cells. J Appl Physiol. 2011;111:929–937. doi: 10.1152/japplphysiol.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuke K, Burd PR, Horvath-Arcidiacono JA, Hori K, Mostowski H, Bloom ET. Human NK cells express endothelial nitric oxide synthase, and nitric oxide protects them from activation-induced cells death by regulating expression of TNF-α. J Immunol. 1999;163:1473–1480. [PubMed] [Google Scholar]
- 17.Weinberg JB, Misukonis MA, Shami PJ, Masson SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, Haney AF, Granger DL. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 18.Jijkstra G, Zandvoort MJH, Muller Kobold AC, de Jager-Krikken A, Heeringa P, van Goor H, van Dullemen HM, Cohen Tervaert JW, van de Loosdrecht A, Moshage H, Jansen PLM. Increased expression of inducible nitric oxide synthase in circulating monocytes from patients with active inflammatory bowel disease. Scand J Gastroenterol. 2002;5:546–554. doi: 10.1080/00365520252903099. [DOI] [PubMed] [Google Scholar]
- 19.Rümer S, Krischke M, Fekete A, Mueller MJ, Kaiser WM. DAF-fluorescence without NO: elicitor treated tobacco cells produce fluorescing DAF-derivatives not related to DAF-2 triazol. Nitric Oxide. 2012 Aug 15;27(2):123–135. doi: 10.1016/j.niox.2012.05.007. http://dx.doi.org/10.1016/j.niox.2012.05.007. Epub 2012 Jun 6. [DOI] [PubMed] [Google Scholar]
- 20.Ruemer S, Krischke M, Fekete A, Lesch M, Mueller MJ, Kaiser WM. Methods to detect nitric oxide in plants: are DAFs really measuring NO? Methods Mol Biol. 2016;1424:57–68. doi: 10.1007/978-1-4939-3600-7_6. http://dx.doi.org/10.1007/978-1-4939-3600-7_6. [DOI] [PubMed] [Google Scholar]


