In this issue of ATVB, Richardson and colleagues investigate the contribution of telomerase in different cell types within the hematopoietic linage to the development of atherosclerosis1. The study raises several interesting points that address an ongoing controversy whether telomere length (TL) is or is not an independent risk factor for development of atherosclerosis and coronary artery disease (CAD).
Telomerase consists of TERT (catalytic subunit) and TERC (RNA component), and is well described as an anti-aging factor. The 2009 Nobel Prize in Medicine or Physiology was awarded to Elizabeth H. Blackburn, Carol W. Greider, and Jack W. Szostak for their discoveries on how chromosomes are protected by telomeres and the enzyme telomerase2. While there are numerous substances that claim to elongate telomeres (mostly seen on late-night television), no drug conferring longevity by telomerase has been discovered. In contrast to the beneficial effects of chromosomal elongation to overall cellular and organismal heath, the nuclear actions of telomerase promote cellular immortality, contributing to the progression, but not development, of cancers3. Not surprisingly the role of telomerase activity (TA) and TL in is mostly studied in the cancer literature4 and TERT inhibition has been explored as a chemotherapeutic. Despite the potential utility of TERT inhibition in cancer, no FDA approved telomerase-inhibitors exist. To further complicate the matter, cellular and subcellular localization appear to contribute to the prevention or increased risk of cancer5, cardiovascular disease6, defects of the endocrine system7 and neurodegenerative disease8, to name a few. In this editorial, we attempt to discuss the growing evidence of tissue specific and subcellular effects of TA and TL and their role in cardiovascular pathology.
Mice are not men - rodents have longer telomeres compared humans (10–100 fold, depending on cell type), and pathologically short telomeres and associated effects are not observed until 3–4 generations of intercrossing homozygote knock-out mice. Telomerase knock-out mice are protected from development of atherosclerosis9 via a not yet identified mechanism. Neither TERT−/− nor TERC−/− mice have obvious signs of cardiovascular disease in early generations, and only in later generations show a progressive increase in systemic blood pressure and large vessel dysfunction10, 11. In contrast to the progressive telomere shortening that take generations to be observed, our recently published work shows endothelial defects in both mouse12 and human13 microvessels that are telomere independent and entirely mediated by TERT. Interestingly, TERC knock-out mice have no endothelial phenotype in early generations but develop large vessel defects in later generations - presumably directly related to telomere shortening10. Work by the Blasco lab14 has elegantly demonstrated that short-term overexpression of TERT increases overall survival and decreases infarct size in mouse models of myocardial infarction. This evidence points toward both traditional and nontraditional roles for TERT in contributing to the cardiovascular phenotype(s) observed in relation to telomerase, with TERC only contributing to the traditional role of telomerase.
Decreased telomere length (TL) has been associated with many diseases in small scale studies, including atherosclerosis and CAD15, 16. Recently several papers suggested that TA, rather than TL, is the important factor associated with disease development/progression17–19. Several large-scale studies for CAD13 and atherosclerosis20 have confirmed this. The free-radical theory of aging postulates that aging occurs due to accumulation of free-radical damage over time, providing at least one reason why aging is the number one risk factor of cardiovascular disease (CVD). TERT, but not TERC, has been shown to protect against mitochondrial-derived reactive oxygen species (ROS) and mtDNA damage21,22, and is a likely cause of telomere independent contributions to the development of CVD. Most studies that link TL to disease development/progression have used peripheral mononuclear blood cells (PBMCs) as surrogate markers to study tissue TL and TA. An obvious downfall of this approach is that PBMCs consist of several different cell types that contribute differently to the development and prevention of disease. Addressing this point, the data of Richardson, et al, explore the effects of TL and TA in primary splenocytes sorted into different subpopulations that could contribute to the development of atherosclerosis1. Presented data shows that telomerase is critical for lymphocyte proliferation but has no role in Treg function if the TL is not critically short. Differences in cellular proliferation during oxidative stress and inflammation (caused by hyperoxia) are due to specific CD4+ population of T cells, but not B cells or monocytes. The reduction in proliferation due to hyperoxia was associated with reduced TA, and pharmacological stimulation of TA stimulated proliferation. Using mouse models, Richardson and colleagues tried to separate out the effects of early loss of telomerase where telomere length is still similar to wild-type (TERT−/−, only observed changes in CD4+ T cell proliferation) from later generations that have critically short telomeres (TERC−/− observed proliferation and activity defects in CD4+ Treg cells). However, by not using the same genetic model, the differences in phenotypic changes could be due to differences in telomere length or differences in non-canonical TERT functions. While the data support a critical role of TA activity in the proliferative response, the authors also did not directly investigate whether TL was changed in the stressed proliferating splenocytes. In line with the greater developing picture, this evidence suggest that atherosclerosis development is affected by both increase in oxidative stress and acceleration of telomere attrition in Tregs via nuclear actions of telomerase.
The complete mechanism of the non-traditional role of TERT has yet to be identified. The work by Richardson et all in this issue1 suggests the matter is more complex than first meets the eye. With existing evidence, telomerase appears to have highly specific roles in different cell types that contribute to the development of CVD, and further work to evaluate contributions of other cell types are warranted and should expand to other diseases. Present evidence supports the notion that increased TA in T cells or endothelial cells can protect against cardiovascular related defects, and small molecule activators such as TA-65 or AGS-499 deserve consideration as therapeutic interventions13, 23–25.
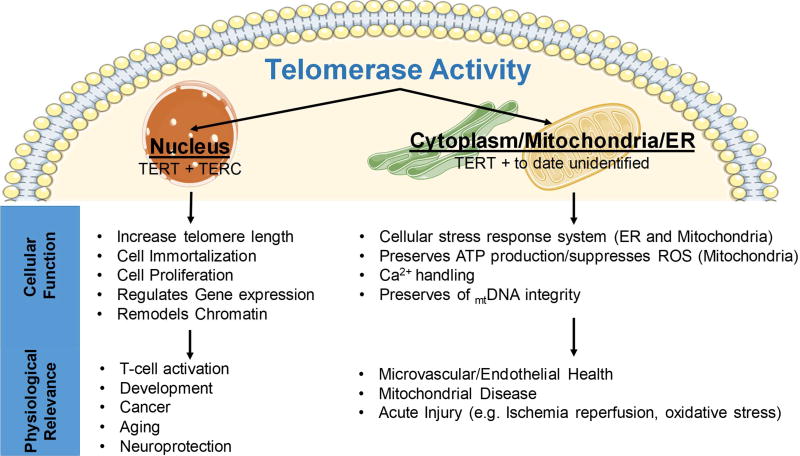
Figure 1.
Subcellular and Tissue Specific Contribution of Telomerase Activity to Cardiovascular Physiology
Acknowledgments
This work was partially supported by NIH grants R01-HL133029 and the Advancing a Healthier Wisconsin Endowment/MCW Redox Biology program (A.M.B.). We thank Dr. Karima Ait-Aissa for her assistance in generation of the figure.
Footnotes
Competing financial interests
The authors report no conflict of interest pertaining to the work presented in this manuscript.
References
- 1.Richardson ATVB. 2018 in press. [Google Scholar]
- 2.Varela E, Blasco M. 2009 nobel prize in physiology or medicine: Telomeres and telomerase. Oncogene. 2010;29:1561. doi: 10.1038/onc.2010.15. [DOI] [PubMed] [Google Scholar]
- 3.Ait-Aissa K, Ebben JD, Kadlec AO, Beyer AM. Friend or foe? Telomerase as a pharmacological target in cancer and cardiovascular disease. Pharmacological Research. 2016;111:422–433. doi: 10.1016/j.phrs.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal P, Ray S, De Dutta K. Activities of human telomerase in cancer development, detection and therapeutics-a review. World Scientific News. 2016;42:87. [Google Scholar]
- 5.Rudolph KL, Chang S, Lee H-W, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 6.Durand MJ, Zinkevich NS, Riedel M, Gutterman DD, Nasci VL, Salato VK, Hijjawi JB, Reuben CF, North PE, Beyer AM. Vascular actions of angiotensin 1–7 in the human microcirculation: Novel role for telomerase. Arterioscler Thromb Vasc Biol. 2016;36:1254–1262. doi: 10.1161/ATVBAHA.116.307518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monickaraj F, Aravind S, Gokulakrishnan K, Sathishkumar C, Prabu P, Prabu D, Mohan V, Balasubramanyam M. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem. 2012;365:343–350. doi: 10.1007/s11010-012-1276-0. [DOI] [PubMed] [Google Scholar]
- 8.Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4:313–329. doi: 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poch E, Carbonell P, Franco S, Diez-Juan A, Blasco MA, Andres V. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein e-null mice. FASEB J. 2004;18:418–420. doi: 10.1096/fj.03-0710fje. [DOI] [PubMed] [Google Scholar]
- 10.Bhayadia R, Schmidt BM, Melk A, Homme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2016;71:161–169. doi: 10.1093/gerona/glv008. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, Blasco MA, Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114:309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. [DOI] [PubMed] [Google Scholar]
- 12.Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, Beyer AM. Telomerase reverse transcriptase protects against angiotensin ii induced microvascular endothelial dysfunction. American Journal of Physiology-Heart and Circulatory Physiology. 2017 doi: 10.1152/ajpheart.00472.2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res. 2016;118:856–866. doi: 10.1161/CIRCRESAHA.115.307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar C, Bernardes de Jesus B, et al. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun. 2014;5:5863. doi: 10.1038/ncomms6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ, West of Scotland Coronary Prevention Study G. Telomere length, risk of coronary heart disease, and statin treatment in the west of scotland primary prevention study: A nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 17.Findeisen HM, Gizard F, Zhao Y, Cohn D, Heywood EB, Jones KL, Lovett DH, Howatt DA, Daugherty A, Bruemmer D. Telomerase deficiency in bone marrow-derived cells attenuates angiotensin ii-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2011;31:253–260. doi: 10.1161/ATVBAHA.110.218545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitroulis D, Katsargyris A, Klonaris C, Avgerinos ED, Fragou-Plemenou M, Kouraklis G, Liapis CD. Telomerase expression on aortic wall endothelial cells is attenuated in abdominal aortic aneurysms compared to healthy nonaneurysmal aortas. J Vasc Surg. 2011;54:1778–1783. doi: 10.1016/j.jvs.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 19.Leeansyah E, Cameron PU, Solomon A, et al. Inhibition of telomerase activity by human immunodeficiency virus (hiv) nucleos(t)ide reverse transcriptase inhibitors: A potential factor contributing to hiv-associated accelerated aging. J Infect Dis. 2013;207:1157–1165. doi: 10.1093/infdis/jit006. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Alvira JM, Fuster V, Dorado B, Soberon N, Flores I, Gallardo M, Pocock S, Blasco MA, Andres V. Short telomere load, telomere length, and subclinical atherosclerosis: The pesa study. J Am Coll Cardiol. 2016;67:2467–2476. doi: 10.1016/j.jacc.2016.03.530. [DOI] [PubMed] [Google Scholar]
- 21.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 22.Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol. 2009;29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez M, Thomas M, Lemos B, DiMarco D, Missimer A, Melough M, Chun O, Murillo A, Alyousef H, Medina-Vera I. Ta-65, a telomerase activator, improves cardiovascular markers in patients with metabolic syndrome. Current pharmaceutical design. 2018 doi: 10.2174/1381612824666180316114832. in press. [DOI] [PubMed] [Google Scholar]
- 24.Harley CB, Liu W, Flom PL, Raffaele JM. A natural product telomerase activator as part of a health maintenance program: Metabolic and cardiovascular response. Rejuv Res. 2013;16:386–395. doi: 10.1089/rej.2013.1430. [DOI] [PubMed] [Google Scholar]
- 25.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO molecular medicine. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]