Reproductive barriers are often assumed to arise from fixed genetic differences between species, despite frequent individual variation in the strength of reproductive isolation between populations. Larson et al. report polymorphism...
Keywords: polymorphism, hybrid male sterility, transmission ratio distortion, QTL mapping, introgression
Abstract
Resolving the mechanistic and genetic bases of reproductive barriers between species is essential to understanding the evolutionary forces that shape speciation. Intrinsic hybrid incompatibilities are often treated as fixed between species, yet there can be considerable variation in the strength of reproductive isolation between populations. The extent and causes of this variation remain poorly understood in most systems. We investigated the genetic basis of variable hybrid male sterility (HMS) between two recently diverged subspecies of house mice, Mus musculus domesticus and Mus musculus musculus. We found that polymorphic HMS has a surprisingly complex genetic basis, with contributions from at least five autosomal loci segregating between two closely related wild-derived strains of M. m. musculus. One of the HMS-linked regions on chromosome 4 also showed extensive introgression among inbred laboratory strains and transmission ratio distortion (TRD) in hybrid crosses. Using additional crosses and whole genome sequencing of sperm pools, we showed that TRD was limited to hybrid crosses and was not due to differences in sperm motility between M. m. musculus strains. Based on these results, we argue that TRD likely reflects additional incompatibilities that reduce hybrid embryonic viability. In some common inbred strains of mice, selection against deleterious interactions appears to have unexpectedly driven introgression at loci involved in epistatic hybrid incompatibilities. The highly variable genetic basis to F1 hybrid incompatibilities between closely related mouse lineages argues that a thorough dissection of reproductive isolation will require much more extensive sampling of natural variation than has been commonly utilized in mice and other model systems.
THE evolution of intrinsic hybrid incompatibilities, whereby divergent genomic regions interact negatively in hybrid genomes, is one of the most commonly studied models of speciation [i.e., Bateson–Dobzhansky–Muller incompatibilities or BDMIs, Bateson 1909; Dobzhansky 1937; Muller 1942; reviewed in Maheshwari and Barbash 2011]. Although often viewed as fixed epistatic barriers to gene flow between species, many incompatible alleles are polymorphic within populations, leading to variation in the overall strength of reproductive isolation between populations (Gordon 1927; Patterson and Stone 1952; Forejt and Ivanyi 1974; Reed and Markow 2004; Good et al. 2008b; Scopece et al. 2010; Cutter 2012). Relatively few studies have examined the genetic basis of variation in BDMIs (Christie and Macnair 1987; Vyskočilová et al. 2005; Wright et al. 2013; Sweigart and Flagel 2015; Case et al. 2016) and the evolutionary forces underlying variable incompatibilities remain unexplored in most species.
Hybrid incompatible alleles can arise through any evolutionary process that contributes to genetic divergence (e.g., genetic drift, natural or sexual selection). However, reproductive isolation is expected to evolve more quickly when divergence is driven by selection. Consistent with this, several incompatibility genes show signatures of positive selection or have diverged through antagonistic coevolutionary dynamics (Johnson 2010; Presgraves 2010; Maheshwari and Barbash 2011). Polymorphism is an inevitable phase in the fixation of an allele, but directional selection should fix alleles relatively quickly. Thus, it should be rare that incompatibilities are sampled while polymorphic if positive directional selection drives the evolution of BDMIs. Alternatively, BDMIs could involve a combination of unsorted ancestral variation or modifying loci that segregate neutrally within species (Rieseberg and Blackman 2010; Scopece et al. 2010; Cutter 2012; Matute et al. 2014) or are subject to balancing selection (Cutter 2012). Finally, polymorphic incompatibilities may reflect the breakdown of reproductive barriers due to gene flow between partially isolated populations. Hybrid incompatible alleles are generally assumed to be resistant to introgression (Barton and Hewitt 1985; Harrison 1990; Payseur 2010), but epistatic barriers may quickly erode in the face of gene flow (Bank et al. 2012; Lindtke and Buerkle 2015). Differentiating between these alternatives is crucial to understanding the evolution of reproductive isolation and the nature of species boundaries.
House mice provide a powerful system to understand the causes of polymorphic barriers during the early stages of speciation. There are three major lineages within Mus musculus—M. m. musculus, M. m. domesticus, and M. m. castaneus—that diverged ∼0.35–0.50 MYA (Geraldes et al. 2011) and show partial reproductive isolation primarily due to hybrid male sterility (HMS). However, there appears to be considerable standing genetic variation for the strength of HMS (Britton-Davidian et al. 2005; Vyskočilová et al. 2005; Good et al. 2008b; Turner et al. 2012). For example, crosses between M. m. musculus females and M. m. domesticus males typically yield sterile F1 hybrid males due, in part, to negative interactions between M. m. musculus Chr X and the autosomal gene Prdm9, a DNA binding protein that directs the location of double-strand breaks during recombination (Mihola et al. 2009). PRDM9 binding sites evolve rapidly (Baker et al. 2015), leading to asymmetric binding and autosomal asynapsis that disrupts sex chromosome expression during spermatogenesis (Bhattacharyya et al. 2013; Campbell et al. 2013; Turner et al. 2014; Davies et al. 2016; Larson et al. 2017). Prdm9 appears to be polymorphic for sterile and fertile alleles within both M. m. domesticus and M. m. musculus (Forejt and Ivanyi 1974; Vyskocilová et al. 2009; Flachs et al. 2012), and the strength of Prdm9-associated sterility is variable within M. m. musculus (Bhattacharyya et al. 2014; Flachs et al. 2014; Turner et al. 2014).
There is also variation in the severity of HMS in house mice that is independent of the M. m. musculus X (Good et al. 2008b). For example, crosses between M. m. domesticus females and M. m. musculus males produce sterile or fertile hybrid males dependent on the genotype of the M. m. musculus sire (Vyskočilová et al. 2005; Good et al. 2008b; Bhattacharyya et al. 2014; Flachs et al. 2014). The autosomal variants contributing to HMS in these crosses are unresolved and, aside from the rapid evolution of Prdm9 (Davies et al. 2016), the causes of standing variation for HMS are not clear. One possible factor is that the small effective population sizes of house mice results in strong genetic drift and local inbreeding (Geraldes et al. 2011). Further, M. m. domesticus and M. m. musculus form a narrow hybrid zone in central Europe (Janoušek et al. 2012), which may weaken reproductive barriers through introgression (Turner and Harr 2014).
In this study, we used the genetic variation segregating between two wild-derived inbred strains of M. m. musculus to begin to characterize the genetic architecture of polymorphic barriers between M. m. domesticus females and M. m. musculus males. We found that polymorphic HMS encompasses at least five autosomal regions of the genome. We then used additional genetic crosses, whole genome sequencing of sperm pools, and population genomic analyses to explore the mechanistic and evolutionary drivers contributing to variation at one of these regions on the distal portion of Chr 4. These diverse genetic and genomic experiments further reveal the complex genetic basis of reproductive isolation in this system and demonstrate how these reproductive barriers have shaped introgression among mouse subspecies and the genomic composition of common laboratory strains of mice.
Materials and Methods
Mouse strains and experimental crosses
We focused on two wild-derived inbred strains of M. m. musculus (PWK/PhJ and CZECHII/EiJ, hereafter musculusPWK and musculusCZII) that differ in the degree of HMS when crossed to M. m. domesticus (Good et al. 2008b). The musculusPWK strain was originally isolated near the hybrid zone in Prague, Czechia (50.0216°N, 14.4350°E) and yields weak HMS when crossed to female M. m. domesticus. The musculusCZII was isolated further from the hybrid zone in Bratislava, Slovakia (48.1492°N, 17.1070°E) and produces mostly sterile males when crossed to female M. m. domesticus. We used two wild-derived strains of M. m. domesticus (WSB/PhJ and LEWES/PhJ, hereafter domesticusWSB and domesticusLEW) derived from natural populations in North America (MD, 39.3358°N, 77.3282°W and DE, 39.1453°N, 75.4188°N). Mice were originally purchased from Jackson Laboratory (Bar Harbor, ME). All animal use was approved by the University of Montana (protocol 002–13) and the University of Southern California (protocol 11,394) Institutes for Animal Care and Use Committees.
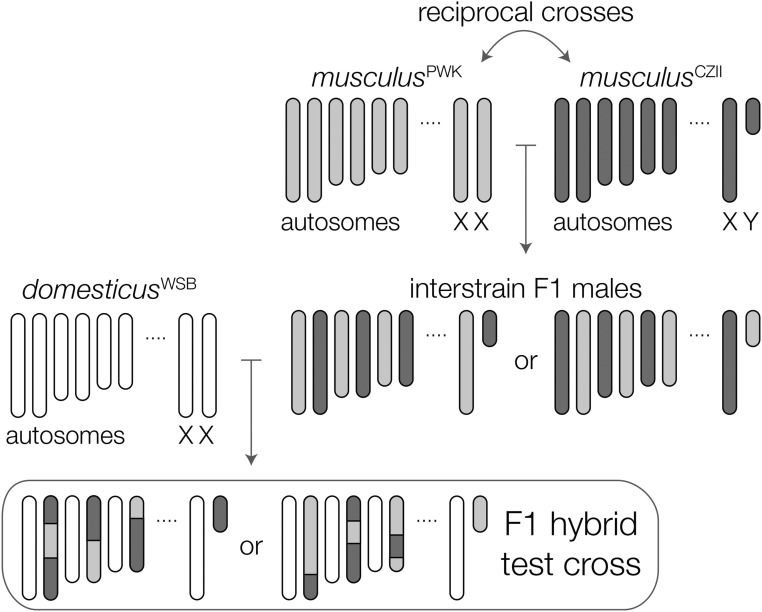
Hybrid incompatibilities underlying F1 hybrid phenotypes usually cannot be mapped because of a lack of genetic variation. However, the existence of polymorphic sterility factors within musculus provides an elegant way to resolve these incompatibilities directly in F1 hybrid males. We first quantified HMS in F1 crosses between female domesticusWSB and either musculusPWK or musculusCZII males. To control for the effects of inbreeding depression in inbred strains, we compared the fertility of these F1 hybrids to M. m. domesticus interstrain F1 males (domesticusWSB × domesticusLEW), evaluating each cross at different time points [60–65, 70–80, and 85–95 days postpartum (dpp)]. We then used an F1 hybrid test cross between domesticusWSB females and interstrain F1 males from reciprocal crosses of musculusPWK and musculusCZII (Figure 1). This design maintains the F1 hybrid genotype, while segregating variation between two different M. m. musculus genomes. Finally, we backcrossed M. m. musculus interstrain F1 males to musculusPWK females to determine if loci on Chr 4 showing strong transmission ratio distortion (TRD) in our hybrid crosses also showed TRD within M. m. musculus.
Figure 1.
Crossing design to map polymorphic HMS loci in musculus. The fertility of F1 hybrids from crosses between M. m. domesticus females and M. m. musculus males depends on the strain of M. m. musculus; hybrids with musculusCZII sires have more severe sterility. We test crossed interstrain M. m. musculus F1s to M. m. domesticus females to map F1 hybrid sterility alleles segregating within M. m. musculus.
Male reproductive phenotypes
We quantified reproductive phenotypes of virgin males weaned in same-sex sibling groups at 21 dpp and housed singly at 45 dpp to mitigate dominance interactions (Snyder 1967). Males were killed using carbon dioxide followed by cervical dislocation at 58–70 dpp (F1 hybrid test cross) or up to 90 dpp (aged F1 males). Following Good et al. (2008b), we measured paired testes (an overall measure of fertility) and seminal vesicles (correlated with serological testosterone levels) relative to body weight. We isolated sperm from caudal epididymides diced in 1 ml of Dulbecco’s PBS (Sigma, St. Louis, MO) and incubated at 37° for 10 m. The proportion of motile sperm and total sperm numbers were estimated from 5 μl suspensions (regular and heat-shocked, respectively) viewed in a Makler counting chamber on a light microscope over a fixed area and observation time. To evaluate sperm morphology, 25 μl sperm suspensions were fixed and stained, and ≥100 intact sperm were visually classified by a single individual (E.L.L.) while blind to genotype. Sperm head morphology were (1) normal with a long apical hook, (2) slightly abnormal (i.e., shortened hook), (3) abnormal (i.e., short hook and rounded shape), or (4) severely abnormal (i.e., amorphous shape). We summarized these categories with a weighted index that ranged from high (3) to low (0) quality sperm (Oka et al. 2004; Good et al. 2008a). Sperm tail morphology were (1) normal, (2) bent at the base of the sperm head, (3) bent in the center of the tail forming a loop, or (4) twisted distally (White et al. 2011).
Genotyping and genome sequencing
We genotyped 468 individuals from our two genetic mapping experiments (i.e., 156 F1 hybrid test cross males and 312 M. m. musculus backcross) and eight reference samples (two of each parent strain and domesticusWSB × musculusCZII F1 hybrids) using double-digest restriction site–associated DNA sequencing (ddRADseq; Peterson et al. 2012), with minor modifications. DNA was extracted from liver tissue using the NucleoSpin Tissue kit (Machery-Nagel, Düren, Germany) and incubated in 5 μl RNAase A (Fisher Scientific, Waltham, MA) at 37° for 15 m. We digested 1 μg of DNA with MspI and SbfI-High-Fidelity enzymes (New England Biolabs, Beverly, MA), ligated unique adaptors, and selected 200–500 bp fragments using a two-step size selection with AMPure XP beads (Agencourt Bioscience, Beverly, MA). Individual libraries were amplified 16 cycles in three 20 μl reactions using Phusion High-Fidelity DNA Polymerase (New England BioLabs), cleaned using AMPureXP, and quantified with a NanoPhotometer (IMPLEN, München, Germany). F1 hybrid libraries were paired-end sequenced on an Illumina HiSeq 2000 at the California Institute for Quantitative Biosciences, University of California, Berkley and on a MiSeq at the IBEST Genomic Resources Core, University of Idaho. The M. m. musculus backcross libraries were single-end sequenced on an Illumina HiSeq 4000 at the University of Oregon Genomics and Cell Characterization Core Facility.
Libraries were checked for intact barcodes, restriction enzyme cut-sites, and demultiplexed using preprocess_radtag_lane.py (Peterson et al. 2012). We used Trimmomatic v0.32 (Lohse et al. 2012) to remove adaptor sequences and low-quality bases and mapped reads to the Genome Reference Consortium mouse build 38 (GRCm38) using BWA-MEM v0.7.10 (Li 2013). We applied the Genome Analysis Tool Kit (GATK) v3.4 (McKenna et al. 2010) to call SNPs (HaplotypeCaller) that we then filtered (minDP 10, maxDP 150, minGQ 20) using VCFtools v0.1.14 (Danecek et al. 2011). We retained biallelic SNPs that were homozygous in the parent references, heterozygous in the F1 hybrid reference, genotyped in ≥95% of individuals, and >1000 bp from other SNPs. We retained individuals that were genotyped in ≥85% of markers and showed normal crossover rates.
We also performed two targeted genotyping assays. Males from the F1 hybrid test cross were genotyped for microsatellite length variants that encompass different Prdm9 alleles. We used modified versions of D17Mit78 (forward: CACAGTGAGTCTGGGCTAGTC, reverse: GCATCTTATGGATTGAAATACGG) and D17Mit261 (forward: CCCTTGCTCTCCTTCATTCA, reverse: AATGCCAAATGGTCAGCC; Copeland et al. 1993) in 10 μl PCR reactions using MangoTaq (Bioline, Luckenwalde, Germany), run at 35 cycles of 94° for 30 sec, 58–48° for 30 sec (decreased by 1° per cycle for the first 10 cycles), and 72° for 1 m. We also expanded the genotyping of our M. m. musculus backcross using diagnostic microsatellites from the middle (D4Mit64: 140.08–141.03 bp) and distal end (D4Mit127: 148.60–151.60 bp) of Chr 4 (Copeland et al. 1993). These markers spanned SNPs genotyped using ddRADseq, and we genotyped 88 mice using both methods to allow cross validation. All fragments were analyzed on an ABI 3130xl at the University of Montana Genomics Core.
Reference genomes have been published for musculusPWK and domesticusWSB (Keane et al. 2011). We generated whole genome shotgun sequences of a female musculusCZII and a female domesticusLEW from NEXTflex DNA sequencing genomic libraries (Bio Scientific, Austin, TX) that were paired-end sequenced on an Illumina HiSeq 2000 at the California Institute for Quantitative Biosciences University of California, Berkley. We generated additional whole genome sequence data from the same musculusCZII female at GENEWIZ (South Plainfield, NJ) using Illumina TruSeq libraries, paired-end sequenced on an Illumina HiSeq 2500. These data were processed as described above.
QTL mapping
We performed QTL mapping in R/qtl v1.40-8 (Broman et al. 2003) with an assumed genotyping error rate of 0.001 and the Carter–Falconer mapping function (Carter and Falconer 1951). For all QTL analyses we used a grid size of 1 cM and 5% genome-wide significance thresholds estimated from 1000 permutations. We used standard interval QTL interval mapping (scanone) with a Haley–Knott regression for normally distributed traits, and nonparametric interval mapping for the proportion of motile sperm, and sperm head and tail morphologies. We used two-dimensional QTL mapping (scantwo) and multiple QTL model selection (stepwiseqtl) to identify additional QTL that may be involved in epistatic interactions. Multiple QTL models were compared using penalized LOD score with thresholds calculated from scantwo permutations. We tested for TRD in our cross using a χ2 test of Mendelian proportions.
Genomic analyses
To investigate the evolutionary history of genomic regions associated with polymorphic sterility, we first analyzed the newly sequenced musculusCZII and domesticusLEW genomes and published genomic data from GRCm38 (domesticusC57), domesticusWSB, musculusPWK, and Mus spretus SPRET/EiJ (Keane et al. 2011). For each genome, we called SNPs using the GATK (HaplotypeCaller) and filtered SNPs with VCFtools (minDP 10, maxDP 150, minGQ 30). We generated a BED file with all SNP positions and used the GATK to re-call genotypes in each genome for our target SNPs (HaplotypeCaller) and filter our VCF files (SelectVariants, minGQ 30, biallelic). We then tested for introgression using the four-taxon D-statistic (Green et al. 2010; Durand et al. 2011) as implemented in dfoil (Pease and Hahn 2015). Here, the D-statistic is the normalized difference in site pattern counts that support a closer relationship between musculusCZII and a focal M. m. domesticus (ABBA, negative D-statistic) or musculusPWK and a focal M. m. domesticus (BABA, positive D-statistic) with variants polarized using M. spretus. We calculated the D-statistic per chromosome and for nonoverlapping 100 kb and 1 Mb windows. We repeated these analyses using three different strains of M. m. domesticus (domesticusC57, domesticusWSB, and domesticusLEW).
Second, we used published genotype data for classic laboratory strains, wild-derived strains (Yang et al. 2011), and wild populations of house mice (Harr et al. 2016) to evaluate genetic structure with principal components analysis using the R package SNPRelate (Zheng et al. 2012). We then used genotype data from 76 classic laboratory strains (Yang et al. 2011) to test for gametic disequilibrium (r2) between candidate sterility regions and SNPs on other chromosomes using PLINK v2.0 (Chang et al. 2015). We restricted these analyses to SNPs between musculusPWK and musculusCZII ≥1 Mb apart with no missing data, minor allele frequencies ≥0.1, and that were also fixed between strains of M. m. musculus (CZECHII, STUS, and STUP) and M. m. domesticus (LEWES, ZALENDE, and PERA) with very low levels of introgression (Yang et al. 2011; Didion and Pardo-Manuel de Villena 2012).
Third, we evaluated phylogenetic discordance using whole exome data from 10 species of Mus (Sarver et al. 2017) and whole genomes from domesticusC57 and domesticusWSB (Keane et al. 2011). We cleaned and mapped reads to species-specific exome-pseudoreferences generated by Sarver et al. (2017). We used RAxML v8.2.3 (Stamatakis 2014) to estimate maximum likelihood phylogenies (rapid bootstrapping and a GTR + Γ model of sequence evolution) for nonoverlapping 100 kb windows, and used these windows to produce a concatenated species tree for each chromosome. We then used ASTRAL v4.10.11 (Mirarab and Warnow 2015) to estimate the species tree while accounting for phylogenetic discordance among individually estimated gene trees. Trees were visualized with FigTree v1.4.3.
Genome-wide assessment of TRD
To test for TRD associated with sperm function, we used low-coverage whole genome sequencing of motile and immotile sperm populations collected from four F1 male M. m. musculus (musculusPWK × musculusCZII). Epididymal sperm were collected from euthanized adult males (106 dpp) in 2 ml Dulbecco’s PBS (equilibrated at 37° and 5% carbon dioxide overnight). We applied 1 ml aliquots of sperm to a Percoll gradient (1 ml layers of 90 and 45% Percoll at 37°; GE Healthcare Life Sciences) and centrifuged (300 g for 13 min) to separate cellular debris (top), immotile sperm (middle), and motile sperm (bottom) (Ng et al. 1992; Phelps et al. 1999). Immotile and motile sperm fractions (400 μl each) were rinsed (1 ml 1.5 M NaCl, centrifuged at 10,000 g for 10 min) and stored at −80°. We purified DNA using the MasterPure Complete DNA purification kit (Epicentre Biotechnologies). Sperm fractions were rinsed in 600 μl of 70% EtOH (centrifuged at 14,000 g for 5 min) and incubated overnight at 55° in 600 μl lysis buffer, 25 μl of 1 M dithiothreitol, and 10 μl of 20 mg/ml proteinase K. We treated samples with RNase A (3 μl, for 30 min at 37°), precipitated the sperm in 200 μl of protein precipitation buffer (centrifuged at 14,000 g for 30 min), and incubated in 600 μl of isopropanol at −80° for 2–3 hr (centrifuged at 14,000 g for 20 min). The pellet was rinsed with 500 μl 75% ethanol (centrifuged at 14,000 g for 10 min) and dried overnight. We then constructed sequencing libraries using the NEBNext Ultra DNA Library Prep Kit for Illumina with Bio Scientific NEXTflex DNA Barcodes and generated 76 bp paired-end sequences on a HiSeq 2000 at the Epigenome Center, University of Southern California.
We conducted all analyses using reads mapped to strain-specific pseudoreferences for musculusCZII or musculusPWK (Sarver et al. 2017). Briefly, for each whole genome (described above), we called SNPs relative to GRCm38 (GATK HaplotypeCaller), hard-filtered our SNPs (maskExtension 5, QD < 2.0, FS > 60.0, MQ < 40.0, MQRankSum < −12.5, ReadPosRankSum < −8.0, QUAL < 30.0, minDP 10, maxDP 150), recalled SNPs that passed filtering at a base-pair resolution in each genome, and used this high-confidence SNP set to inject variants into GRCm38 using the GATK FastaAlternativeReferenceMaker. We trimmed and quality-filtered sperm fraction reads using expHTS (Streett et al. 2015), mapped reads to each pseudoreference using BWA-MEM, and called SNPs using the GATK (HaplotypeCaller). We assigned reads (MQ ≥ 56) that overlapped at least one diagnostic SNP as either musculusPWK or musculusCZII origin, and summarized read counts in 1 Mb sliding windows (step size 0.5 Mb). We tested for TRD in windows with ≥100 reads in all samples using a χ2 test (false discovery rate–corrected P < 0.01; Benjamini and Hochberg 1995) to test the proportions of musculusPWK vs. musculusCZII reads in motile vs. immotile sperm fractions. We considered a window skewed if the proportion of musculusCZII-derived reads significantly differed by at least 0.15 between sperm fractions.
To validate our Percoll method, we repeated our pipeline with experimentally combined normal and heat-shocked (immotile) sperm samples from two predominantly M. m. domesticus inbred strains (C57BL/6J and DBA/2J). We pooled normalized sperm extractions from each strain and then mixed sperm in equal proportions from the two strains, and repeated our experiment to vary which strain was heat shocked. We isolated DNA as described above, and PCR-amplified and Sanger-sequenced through a marker containing a diagnostic SNP.
Data availability
All data are available through NCBI under projects SRP093943 (F1 hybrid test cross RADseq), SRP094878 (M. m. musculus CZECHII/EiJ whole genome sequencing), SRP094877 (M. m. domesticus LEWES/EiJ whole genome sequencing), SRP082237 (sperm pools whole genome sequencing), and SPR102485 (backcross RADseq). Supplemental Material, File S1 contains phenotype data for F1 hybrids and F1 hybrid test cross. File S2 contains microsatellite genotypes for markers inside and outside the Chr 4 TRD region. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6149399.
Results
HMS is polymorphic and polygenic in M. m. musculus
We found that F1 M. m. domesticus × M. m. musculus hybrids had variable fertility that was dependent on the strain of M. m. musculus sires (Table 1), extending previous results (Good et al. 2008b). Compared to fertile M. m. domesticus F1 males (domesticusWSB × domesticusLEW), hybrid males with musculusPWK sires had smaller testes and more abnormal sperm morphologies. Hybrid males with musculusCZII sires were even more severely sterile. These males had smaller testes, lower sperm counts, and a high proportion of abnormal sperm head and tail morphologies compared to hybrid males with musculusPWK sires (Table 1). The fertility of domesticusWSB × musculusPWK hybrids was lower than previously reported from domesticusLEW × musculusPWK crosses (Good et al. 2008b; Campbell et al. 2013; Larson et al. 2017), suggesting that domesticusWSB has sterility factors not present in other strains of M. m. domesticus (Odet et al. 2015). Differences among F1 crosses remained qualitatively consistent as males aged and sperm head morphology actually worsened with age (Figure S1). Therefore, HMS was not due to delayed reproductive maturity, as has been observed in other crosses (Campbell and Nachman 2014; Flachs et al. 2014). Overall, we found that M. m. domesticus × M. m. musculus HMS was dependent on the paternal strain, indicating autosomal and/or Y-linked sterility loci contribute to polymorphic sterility in M. m. musculus.
Table 1. Reproductive phenotypes for M. m. domesticus and hybrid males.
| M. m. domesticus | F1 hybrids | F1 hybrid test cross | |||
|---|---|---|---|---|---|
| domesticusWSB × domesticusLEW | domesticusWSB × musculusPWK | domesticusWSB × musculusCZII | domesticusWSB × musculusPWK × CZII | domesticusWSB × musculusCZII × PWK | |
| Sample size | 21 | 28 | 30 | 78 | 78 |
| Body weight (g) | 17.32 ± 3.1e−01 | 18.26 ± 4.6e−01 | 17.15 ± 2.8e−01 | 18.39 ± 2.8e−01 | 17.87 ± 2.1e−01 |
| Relative paired testis weight (mg/g) | 11.5 ± 2.9e−01 | 7.5 ± 1.5e−01 ▾ | 6.2 ± 1.3e−01 ▾ | 6.6 ± 1.3e−01 ▾ | 6.95 ± 1.6e−01 ▾ |
| Relative paired seminal vesicle weight (mg/g) | 5.25 ± 3.7e−01 | 6 ± 2.2e−01 ▴ | 5.8 ± 1.7e−01 | 5.9 ± 1.4e−01 ▴ | 5.6 ± 1.1e−01 |
| Proportion motile sperm | 0.77 ± 3.1e−02 | 0.83 ± 1.9e−02 | 0.77 ± 3.4e−02 | 0.84 ± 1.6e−02 ▴ | 0.83 ± 2.7e−02 ▴ |
| Sperm count (1 × 106) | 19.4 ± 1.5e+00 | 11.8 ± 1.6e+00 | 5.8 ± 9.6e−01 ▾ | 4.9 ± 4.2e−01 ▾ | 5.7 ± 4.2e−01 ▾ |
| Sperm head morphology index | 2.99 ± 4.7e−03 | 2.84 ± 2e−02 ▾ | 1.14 ± 4.8e−02 ▾ | 2.43 ± 6.6e−02 ▾ | 2.18 ± 7.6e−02 ▾ |
| Proportion normal sperm head attachment | 1 ± 8.8e−04 | 0.99 ± 2.7e−03 ▾ | 0.96 ± 6.2e−03 ▾ | 0.99 ± 2.8e−03 ▾ | 0.98 ± 4.4e−03 ▾ |
| Proportion straight proximal sperm tail | 0.97 ± 6.9e−03 | 0.96 ± 4.6e−03 | 0.99 ± 4.2e−03 ▴ | 0.97 ± 2.5e−03 | 0.96 ± 3e−03 |
| Proportion straight distal sperm tail | 1 ± 3.1e−03 | 1 ± 2.6e−03 | 1 ± 3.4e−03 | 0.99 ± 1.5e−03 ▾ | 0.99 ± 1.9e−03 ▾ |
Only F1 hybrids between 58 and 70 days postpartum (dpp) are included to allow direct comparison with the F1 hybrid test cross. See Figure S1 for reproductive traits across an F1 hybrid’s reproductive lifespan. Traits are summarized as median values (±SE), and arrows represent significant increase (▴) or decrease (▾) in trait values relative to the M. m. domesticus control cross. Values in bold are significantly different between F1 hybrids (Wilcoxon test, false discovery rate–corrected P ≤ 0.05). Trait proportions are polarized so that higher values indicate higher quality reproductive traits.
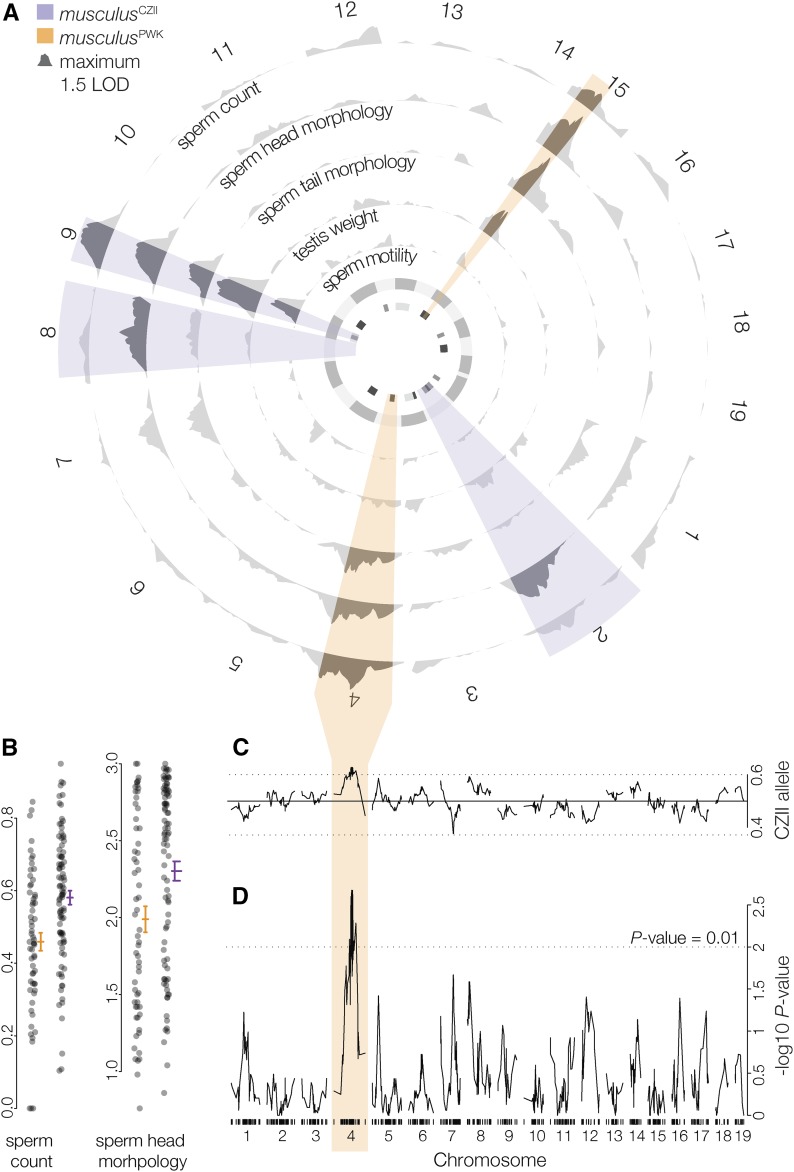
Next, we used an F1 hybrid test cross to generate 156 males (62 litters) that ranged from reproductively normal to mostly sterile. On average these males had smaller testes, lower sperm counts, and more abnormal sperm head and tail morphologies (Table 1). After filtering for coverage, we retained ddRADseq data for 150 males that had between 118,000 and 921,000 uniquely mapped paired reads (median 297,634, total mapped reads of 48.5 million paired reads). We constructed a genetic map using 582 high-quality SNPs between musculusCZII and musculusPWK. Using standard interval mapping we detected two regions of the M. m. musculus genome on Chr 9 and Chr 15 that contributed to multiple sterility phenotypes (Figure 2A), suggesting a shared genetic and/or developmental basis. Chr 9 QTL reduced the fertility of hybrids carrying a musculusCZII allele and Chr 15 QTL reduced the fertility of hybrids carrying a musculusPWK allele (Table 2). We identified two additional QTL on Chr 2 and Chr 8 that contributed to abnormal sperm head morphologies associated with the musculusCZII allele, and QTL on Chr 4 that contributed to lower sperm counts and abnormal sperm head and tail morphologies associated with the musculusPWK allele (Figure 2B). Using two-dimensional QTL mapping, we identified pairs of QTL that additively contributed to sperm count (Chr 4 and Chr 9) and abnormal sperm head morphologies (Chrs 1, 2, 4, 7, 8, 9, and 15). We found no evidence of epistatic interactions (Table S1), although sample sizes were likely too small to detect such effects. Multiple QTL models supported several loci contributing to sperm count and abnormal sperm head morphology, consistent with our single QTL results (Table 3). Neither Chr Y origin nor genotyped Prdm9 alleles were associated with hybrid sterility phenotypes (Table S2).
Figure 2.
QTL for polymorphic HMS in musculus. (A) LOD curves (standard interval mapping) for HMS phenotypes. Highlighted intervals are the maximum LOD intervals (across all traits) on each chromosome for QTL associated with lower fertility in musculusCZII (purple) and musculusPWK (orange). The inner circle is QTL LOD support intervals for previously reported hybrid sterility loci mapped in M. m. domesticus and M. m. musculus F2 crosses (White et al. 2011; dark gray), wild mice from the hybrid zone (Turner et al. 2014; medium gray), and an F1 hybrid test cross (Bhattacharyya et al. 2014; light gray). (B) Normalized sperm count and sperm head morphology index plotted against the genotype of the marker with the largest Chr 4 LOD score. Lines indicate mean trait values (±SE). (C) Frequency of the musculusCZII allele at each marker (Mendelian expectations 0.5:0.5, genome wide average: 0.496:0.504). (D) TRD plotted as the −log10P value from χ2 test for Mendelian segregation per chromosome. Tick marks indicate SNP positions.
Table 2. Polymorphic hybrid sterility QTL detected using standard interval QTL mapping.
| Chr | Position (cM) | LOD score | P-value | Position (Mb) | 1.5 LOD interval (Mb) | %Vara | Effectb | Phenotype means ± SE | ||
|---|---|---|---|---|---|---|---|---|---|---|
| musculusPWK | musculusCZII | |||||||||
| Relative paired testis weight (mg/g) | 9 | 36.49 | 4.79 | 0.001 | 92.81 | 49.08–113.49 | 11.22 | −0.88 | 7.3 ± 0.13 | 6.34 ± 0.15 |
| 15 | 22.74 | 3.12 | 0.023 | 78.25 | 29.87–84.06 | 6.9 | 0.69 | 6.49 ± 0.14 | 7.28 ± 0.15 | |
| Proportion motile spermc | 9 | 35.49 | 3.11 | 0.012 | 88.11 | 73.06–122.96 | 6.42 | −0.1 | 0.84 ± 0.02 | 0.74 ± 0.02 |
| Normalized sperm countd | 4 | 41.82 | 3.25 | 0.031 | 125.29 | 90.72–155.46 | 9.83 | 0.12 | 0.46 ± 0.02 | 0.58 ± 0.02 |
| 9 | 36.49 | 3.03 | 0.051 | 92.81 | 59.96–111.01 | 8.77 | −0.12 | 0.58 ± 0.02 | 0.47 ± 0.02 | |
| Sperm head morphology index | 2 | 75.42 | 3.06 | 0.023 | 174.03 | 148.24–198.11 | 5.03 | −0.29 | 2.37 ± 0.07 | 1.97 ± 0.07 |
| 4 | 67.84 | 2.87 | 0.041 | 154.51 | 4.17–180.22 | 3.62 | 0.25 | 1.95 ± 0.08 | 2.33 ± 0.06 | |
| 8 | 45.05 | 2.86 | 0.042 | 113.45 | 58.94–169.42 | 7.03 | −0.34 | 2.38 ± 0.08 | 2.02 ± 0.07 | |
| 9 | 41.49 | 3.21 | 0.015 | 105.4 | 64.49–122.96 | 5.98 | −0.32 | 2.38 ± 0.07 | 1.96 ± 0.07 | |
| 15 | 22.74 | 4.94 | <0.001 | 78.25 | 44.19–84.06 | 8.48 | 0.38 | 1.95 ± 0.07 | 2.43 ± 0.07 | |
| Proportion normal sperm head attachmentc | 4 | 65.23 | 2.76 | 0.061 | 152.74 | 4.17–180.22 | 5.75 | 0.02 | 0.96 ± 0.004 | 0.98 ± 0.004 |
| 9 | 42.49 | 2.69 | 0.069 | 106.53 | 64.50–122.96 | 3.77 | −0.01 | 0.98 ± 0.004 | 0.96 ± 0.004 | |
| 15 | 22.15 | 4.85 | <0.001 | 77.78 | 71.46–86.98 | 7.13 | 0.02 | 0.96 ± 0.004 | 0.98 ± 0.004 | |
The percent of the phenotypic variance explained by the QTL, calculated using Haley–Knott regression.
The difference between the phenotype averages of the musculusPWK and musculusCZII alleles. A negative effect indicates the musculusCZII allele lowers the reproductive phenotype value. Effects were estimated using Haley–Knott regression.
Phenotypes were analyzed using nonparametric interval mapping and %var and effect were estimated using standard interval mapping.
Square-root transformed sperm count (1 × 106).
Table 3. Polymorphic F1 hybrid sterility QTL detected using multiple QTL mapping.
| Chr | Position (cM) | LOD score | P-value | Position (Mb) | 1.5 LOD interval (Mb) | %Var | Effect ± SEc | ||
|---|---|---|---|---|---|---|---|---|---|
| QTLa | Fullb | ||||||||
| Relative paired testis weight (mg/g) | 9 | 34.65 | 4.71 | <0.001 | 85.99 | 59.96–113.49 | 13.46 | NA | −0.96 ± 0.20 |
| Normalized sperm countd | 4 | 41.82 | 3.7 | <0.001 | 125.29 | 90.72–155.46 | 9.83 | 18.30 | 0.12 ± 0.03 |
| 9 | 34.65 | 3.32 | <0.001 | 85.99 | 59.96–104.82 | 8.77 | −0.12 ± 0.03 | ||
| Sperm head morphology index | 4 | 67.84 | 6.82 | <0.001 | 154.51 | 149.56–155.46 | 12.93 | 45.35 | 0.35 ± 0.08 |
| 8 | 45.05 | 5.08 | <0.001 | 113.45 | 95.68–129.09 | 9.36 | −0.4 ± 0.08 | ||
| 9 | 34.65 | 9.33 | <0.001 | 85.99 | 85.99–85.99 | 18.41 | −0.5 ± 0.08 | ||
| 15 | 22.74 | 5.72 | <0.001 | 78.25 | 76.5–78.25 | 10.65 | 0.42 ± 0.08 | ||
| 4:9 | NA | 3.95 | <0.001 | NA | NA | 7.14 | NA | 0.70 ± 0.16 | |
QTL identified using nonparametric interval mapping were not assessed using multiple QTL mapping.
The percent of the phenotypic variance explained by each QTL.
The percent of the phenotypic variance explained by all terms (e.g., all QTL) in the model.
The difference between the phenotype averages of the musculusPWK and musculusCZII alleles. A negative effect indicates the musculusCZII allele lowers the reproductive phenotype value.
Square-root transformed sperm count (1 × 106).
Hybrid sterility QTL colocalized with TRD on Chr 4
Sterility phenotypes associated with the musculusPWK allele on Chr 4 colocalized with a large region (46.91:153.39 Mb) that had a deficit of musculusPWK alleles at 50 consecutive markers (expected allelic ratio: 50:50, median observed 39.5:60.5, χ2 test, P ≤ 0.05; Figure 2, C and D). The most extreme TRD was observed at 116.01:151.14 Mb (median observed 38.7:61.3, χ2 test, P ≤ 0.001) and was also observed when crosses were parsed by sire (musculusPWK × CZII sire, N = 75, 33.3:66.7; musculusCZII × PWK sire N = 75, 44.3:55.7). Sex ratios were normal in these crosses (females:males 51:49, χ2 test, P = 0.701). The Chr 4 region showing TRD overlapped with QTL for lower sperm count (±1.5 LOD interval 90.72–155.46) and more abnormal sperm head and tail morphology (±1.5 LOD interval 4.17–180.22) in males with the musculusPWK allele. This could be due to chance given that the ±1.5 LOD intervals for all sterility QTL encompassed 19.1% (275.59 cM) of the total genome, although sterility QTL associated with musculusPWK alleles encompassed only 5.5% of the genome (79.16 cM total).
Chr 4 sterility and TRD loci showed unusual patterns of introgression
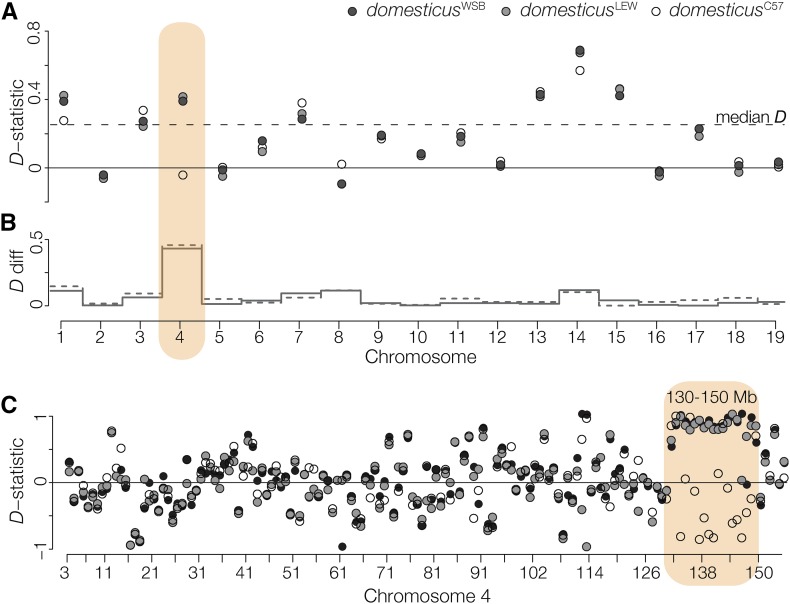
The distal region of Chr 4 showing TRD contained an unusually high density of SNPs between musculusCZII and musculusPWK (hypergeometric test, P < 0.001). Previous work has shown appreciable subspecific introgression into musculusPWK, including a large tract of M. m. domesticus introgression on the distal portion of Chr 4 (Yang et al. 2011). Consistent with this, we found considerable genome-wide introgression between musculusPWK and M. m. domesticus (median D-statistic of 0.253, Figure 3A). Across most chromosomes, D-statistic estimates were similar regardless of which M. m. domesticus strain was used. On Chr 4 we detected introgression between M. m. domesticus (domesticusWSB, domesticusLEW) and musculusPWK, but not between domesticusC57 and musculusPWK. Discordance in the D-statistic among M. m. domesticus strains was localized to a 20 Mb region on the distal end of Chr 4 (130–150 Mb), coincident with the musculusPWK sterility QTL and the region with the strongest TRD (Figure 3B). For simplicity, we will refer to this narrower region as the Chr 4 TRD locus.
Figure 3.
Introgression between M. m. musculus and M. m. domesticus. (A) D-statistics, calculated for each chromosome, testing for introgression between musculusCZII or musculusPWK and M. m. domesticus. The median D-statistic across all M. m. domesticus comparisons is represented by the dashed line. Patterns on Chr 4 vary depending on the strain of M. m. domesticus. (B) The absolute difference in the D-statistic using domesticusWSB (solid gray line) or domesticusLEW (dashed gray line) compared to domesticusC57. (C) D-statistic calculated over 1 Mb nonoverlapping windows localizes discordant introgression to a 20 Mb window on the distal end of Chr 4.
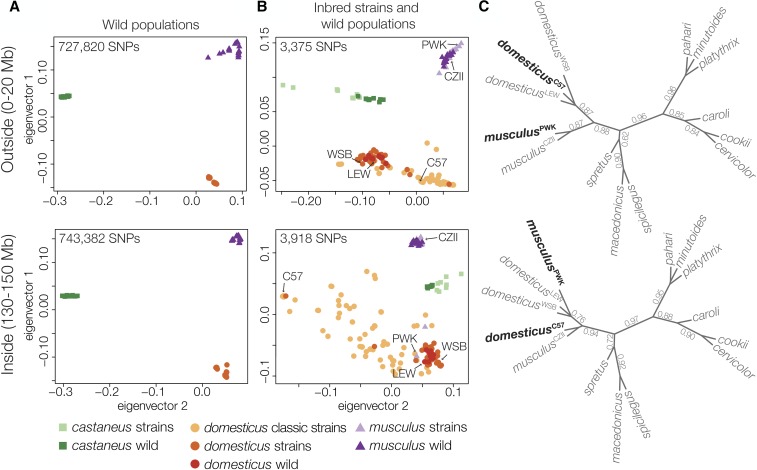
We then contrasted patterns of divergence outside and inside of the Chr 4 TRD locus using other M. m. musculus inbred strains and wild house mice (Yang et al. 2011; Harr et al. 2016). Wild mice strongly clustered by subspecies both outside and inside of the TRD locus (Figure 4A). In contrast, some M. m. musculus wild-derived strains (PWK and PWD) clustered with M. m. domesticus at the TRD region while classic laboratory strains (primarily M. m. domesticus in origin) showed a mosaic of subspecific origins within the Chr 4 TRD locus (Figure 4B). We then estimated gametic disequilibrium between Chr 4 (130–150 Mb) and 1313 autosomal and Y-linked SNPs to test for other genomic regions that may be associated with the Chr 4 TRD locus. Overall, gametic disequilibrium was low (r2: median 0.014, maximum 0.645), and similar to prior estimates (Payseur and Hoekstra 2005). Seven SNPs showed elevated r2 (Table S3), but we did not find any SNPs that had high r2 values across the Chr 4 TRD haplotype. There was no association between Chr 4 and Chr Y, which has either a M. m. musculus or M. m. domesticus origin in the classic strains (Bishop et al. 1985).
Figure 4.
Patterns of discordance outside and inside the Chr 4 TRD locus. (A) Principal components analysis of SNPs from whole genome sequencing of eight wild populations of M. m. musculus, M. m. domesticus, and M. m. castaneus (Harr et al. 2016) and (B) the Mouse Diversity Array for wild mice (dark colors) and classic laboratory and wild-derived strains of mice (light colors) (Yang et al. 2011). There was strong clustering of SNPs in wild populations, both outside and inside the TRD region, but the classic strains showed mixed SNP clustering. (C) Unrooted species trees estimated across 100 kbp windows outside and inside the Chr 4 TRD locus. Branches are annotated with their local quartet scores.
To evaluate the deeper evolutionary origin of the Chr 4 TRD locus, we estimated the phylogenies across 10 species of Mus for Chr 3 (a similar sized chromosome with limited introgression relative to other chromosomes and no TRD) and Chr 4 regions outside and inside the TRD locus. Concatenated trees for Chr 3 and non-TRD Chr 4 (Figure S2) were consistent with previous species tree estimates (Sarver et al. 2017). In contrast, trees from the Chr 4 TRD locus showed reciprocal swapping of M. m. musculus and M. m. domesticus strains, with musculusPWK closest to domesticusWSB and musculusCZII closest to domesticusC57. These conflicting patterns were most apparent using a gene-tree approach to characterize patterns of fine-scale topological discordance across Chr 4 (Figure 4C). We found no other Mus lineages with variant topologies for the Chr 4 TRD region.
TRD was restricted to hybrid crosses and was not associated with sperm motility
Male meiotic drivers often operate through various mechanisms of sperm impairment (Lindholm et al. 2016). In the classic house mouse t complex drive system, heterozygous males show a higher frequency in motile sperm of the sperm killing Chr 17 t haplotype (Lyon 2003). We used whole genome sequencing of sperm pools to test if the higher frequency of the musculusCZII Chr 4 TRD haplotype in the offspring of the F1 hybrid test cross reflected motility differences in the sperm of the M. m. musculus (musculusPWK × musculus CZII) sires. We generated between 40 and 64 million uniquely mapped reads (MQ ≥ 56) from the motile and immotile sperm fractions of four F1 M. m. musculus males. An average of 7 million reads per sample spanned at least one diagnostic SNP. We parsed reads into 5463 overlapping 1 Mb windows and analyzed an average of 4777 windows with ≥100 mapped reads and ≥1 diagnostic SNP (QUAL ≥ 24). No window showed significant skew between motile and immotile sperm pools in any male. In an additional experiment, we confirmed that the Percoll method was effective in separating motile from immotile sperm (Figure S3).
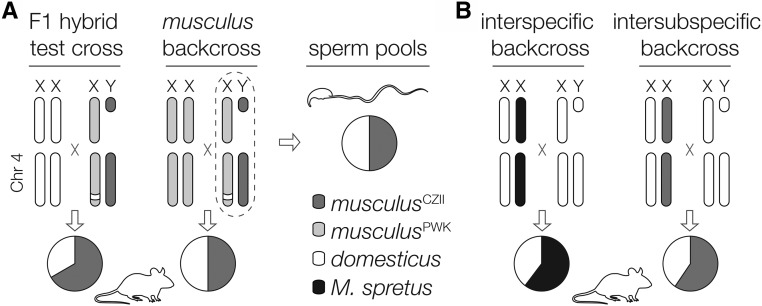
Our sequencing experiment demonstrated that Chr 4 TRD is likely not related to sperm motility and therefore must reflect genotypic differences in sperm competitive interactions (including female choice), fertilization ability, or postzygotic development. To localize the timing of distortion, we crossed the same M. m. musculus sire genotype (musculusPWK × musculus CZII) to musculusPWK females to generate 602 backcross offspring (319 female and 283 male) from 133 litters. We generated ddRADseq libraries for 312 backcross mice. After removing individuals with low coverage, we retained 303 mice that had between 156,791 and 3,315,067 uniquely mapped reads (median of 664,627 per mouse, total of 232,515,877 single reads) and we constructed a genetic map using 358 high-quality SNPs. We found no evidence of TRD on Chr 4 (expected allelic ratio, 50:50; median observed, 50:50) and this pattern held when parsed by sire, sex, or sire and sex. To confirm these results, we genotyped an additional 193 backcross mice using microsatellite markers spanning Chr 4 and still found no evidence of TRD (N = 496 mice; Table 4). Thus, Chr 4 TRD between musculusCZII and musculusPWK alleles was only observed in crosses involving domesticusWSB females (Figure 5A).
Table 4. Summary of genotype frequencies in M. m. musculus backcross.
| Crosses | AA | AB | Total | %AB | P-value | |
|---|---|---|---|---|---|---|
| Region 1 | musculusCZII × PWK sire | 100 | 93 | 195 | 47.7 | 0.614 |
| 140,089,156– | females | 54 | 50 | 106 | 47.2 | 0.695 |
| 141,037,913 bp | males | 46 | 43 | 89 | 48.3 | 0.75 |
| musculusPWK × CZII sire | 144 | 157 | 301 | 52.2 | 0.454 | |
| females | 77 | 83 | 160 | 51.9 | 0.635 | |
| males | 67 | 74 | 141 | 52.5 | 0.556 | |
| Total | 244 | 250 | 496 | 50.4 | 0.787 | |
| Region 2 | musculusCZII × PWK sire | 97 | 97 | 195 | 49.7 | 1 |
| 148,602,050– | females | 53 | 53 | 106 | 50 | 1 |
| 151,609,913 bp | males | 44 | 44 | 89 | 49.4 | 1 |
| musculusPWK × CZII sire | 138 | 162 | 301 | 53.8 | 0.166 | |
| females | 73 | 86 | 160 | 53.8 | 0.303 | |
| males | 65 | 76 | 141 | 53.9 | 0.354 | |
| total | 235 | 259 | 496 | 52.2 | 0.28 |
To test for TRD within M. m. musculus, female musculusPWK were crossed to reciprocal interstrain F1s between musculusCZII and musculusPWK. Offspring were genotyped for two regions inside the introgressed TRD on Chr 4. AA and AB represent offspring genotype.
Figure 5.
Summary of Chr 4 TRD. (A) The musculusPWK haplotype on the distal portion of Chr 4, which is derived from M. m. domesticus, was undertransmitted in the offspring of crosses involving M. m. domesticus females and M. m. musculus males, but not in M. m. musculus backcrosses or the sperm of M. m. musculus males. (B) Two other crosses have reported reduced transmission of the distal portion of Chr 4 derived from M. m. domesticus, but through females. The first was an interspecific cross between F1 females (M. m. domesticus C57BL/6J × M. spretus) and M. m. domesticus C57BL/6J males (TRD ∼73 Mb to end of Chr 4; Ceci et al. 1989), and the second was an interspecific cross between interstrain F1 females (M. m. domesticus C57BL/KsJ × musculusCZII) and M. m. domesticus C57BL/KsJ males (TRD ∼107–138 Mb; Fiedorek and Kay 1994).
Discussion
Polymorphic HMS has a polygenic basis in house mice
Individuals often vary in the degree that they are reproductively isolated from other lineages, but the genetic basis and evolutionary origin of such variation remains poorly understood. In house mice, there is considerable variability in the strength of F1 HMS in crosses using different inbred strains or wild isolates of M. m. musculus and M. m. domesticus (Britton-Davidian et al. 2005; Vyskočilová et al. 2005; Good et al. 2008b; Bhattacharyya et al. 2014). One simple interpretation of these results is that there are one or a few common incompatibilities that are polymorphic within M. m. musculus and/or M. m. domesticus populations. Consistent with this, the only HMS gene yet identified in mammals, Prdm9, appears to be polymorphic for sterile and fertile alleles within both M. m. musculus and M. m. domesticus (Forejt and Ivanyi 1974; Vyskocilová et al. 2009; Flachs et al. 2012). While the evolutionary origin and extent of Prdm9-linked HMS variation remains unclear in natural populations, our results reveal that there is likely to be considerable polymorphism at other HMS loci.
We identified five autosomal regions that contributed to variation in HMS in crosses between M. m. domesticus females and M. m. musculus males, despite sampling just two wild-derived inbred strains of M. m. musculus (Figure 2). F1 hybrid males from crosses between female M. m. domesticus and musculusPWK yield only weak sterility phenotypes, while crosses involving musculusCZII are more severely sterile in both directions of the cross (Table 1; Good et al. 2008b). Surprisingly, considerable variation exists beneath this seemly simple F1 architecture. Sterility loci were associated with both strains; sterility alleles on Chrs 2, 8, and 9 derived from musculusCZII, while musculusPWK sterility variants were mapped to Chr 4 and Chr 15.
F1 HMS variability has been observed in other M. m. musculus strains (Piálek et al. 2008; Bhattacharyya et al. 2014) and in wild M. m. musculus isolated from eastern Czechia (Good et al. 2008b). Thus, the polymorphic HMS that we document here may be relatively widespread within M. m. musculus (Vyskočilová et al. 2005; Good et al. 2008b; Bhattacharyya et al. 2014). Consistent with this, there was some overlap between the HMS loci we identified and HMS QTL from other studies (Figure 2A). Bhattacharyya et al. (2014) used a similar experimental design between domesticusC57 females and M. m. musculus interstrain males (PWD and STUS) to map polymorphic hybrid sterility to Chr 9 (sperm count). Sterility loci were identified on Chr 4 (epididymis weight) in recombinant inbred lines derived from all three M. m. musculus subspecies (Shorter et al. 2017), Chr 4 (testis weight) and Chr 15 (abnormal sperm morphology) were identified in F2 crosses between domesticusWSB and M. m. musculus PWD (White et al. 2011; Turner et al. 2014), and Chr 2 and Chr 9 were associated with low testis weights in wild-caught hybrid mice (Turner and Harr 2014). However, these studies also found sterility QTL on Chrs 1, 2, 3, 5, 6, 10, 12, 13, 14, 17, and 18, which implies that nearly every autosome is linked to some form of HMS. The emerging picture is of an increasingly complex genetic basis to HMS that depends strongly on genotype. Indeed, multiple polymorphic hybrid sterility factors would account for the variable fertility of multigeneration hybrids from the center of the hybrid zone (Turner et al. 2012). Inbred line crosses remain one of the most powerful tools for the genetic dissection of hybrid incompatibilities. However, the common assumption that most incompatibilities reflect fixed differences between lineages appears increasingly tenuous, especially during the early stages of speciation. This realization has the potential to broadly impact important issues in speciation genetics. In addition to the need to incorporate population-level sampling into the design of mapping studies on the genetics of speciation, many theoretical predications on the accumulation of reproductive isolation are based on epistatic models that treat interacting hybrid incompatibilities as fixed within species (e.g., Orr and Turelli 2001; Wang et al. 2013; Lindtke and Buerkle 2015).
The causes of polymorphic reproductive isolation
Several incompatibilities are polymorphic in house mice, but the origins of these variants are unclear. One possible source is introgression at previously fixed incompatibilities. Alleles contributing to hybrid incompatibilities should have restricted introgression relative to the rest of the genome. Indeed, the identification of loci showing restricted gene flow across hybrid zones is a powerful approach to identifying alleles that contribute to reproductive barriers (Barton and Hewitt 1985; Harrison 1990; Payseur 2010). However, gene flow and recombination within a hybrid zone can quickly break down epistatic interactions among BDMIs (Virdee and Hewitt 1994; Shuker et al. 2005; Bank et al. 2012; Lindtke and Buerkle 2015), which could in turn result in polymorphic incompatibilities. The house mouse hybrid zone is wide relative to the dispersal distances of mice. As a result, pure M. m. domesticus and M. m. musculus rarely come into contact and few F1 mice are found in the hybrid zone. The zone is primarily composed of complex, multigeneration hybrids that show extensive variation in the severity of HMS (Janoušek et al. 2012; Turner et al. 2012; Turner and Harr 2014), which likely reflects the partial breakdown of epistatic reproductive barriers.
Several of the common wild-derived strains show appreciable introgression between subspecies of M. m. musculus, including musculusPWK (Yang et al. 2011; Sarver et al. 2017). Four of our polymorphic HMS regions did not colocalize with strong signatures of introgression (results not shown), although gene flow cannot be ruled out at our current mapping resolution. At least one HMS region (Chr 4) did coincide with introgression into musculusPWK (Figure 3), but not necessarily in the direction predicted if HMS polymorphism reflects the partial erosion of reproductive barriers. Hybrid sterility QTL on Chr 4 contributed to low sperm counts and abnormal sperm morphology in males carrying the musculusPWK allele (Figure 2A). Coincident with the Chr 4 HMS QTL, an ∼20 Mb M. m. domesticus haplotype (represented here by domesticusWSB and domesticusLEW) was introgressed into musculusPWK, while a M. m. musculus haplotype (represented by musculusCZII) appears introgressed into domesticusC57 (Figure 3) and some other classic strains (Figure 4, Yang et al. 2011). At least two M. m. musculus strains (PWK and PWD) derived from different localities carry introgressed M. m. domesticus haplotypes. In other words, a M. m. domesticus-derived hybrid sterility locus has introgressed into at least two independent M. m. musculus strains. Transmission of the same introgressed musculusPWK allele was also underrepresented in our hybrid test cross (Figure 2C). Thus, the distal end of Chr 4 shows a propensity to reciprocally introgress between M. m. musculus and M. m. domesticus genomes despite asymmetric TRD and detrimental effects on hybrid fertility.
How can recurrent reciprocal introgression be reconciled with the evolution of HMS and TRD in the same genomic region? Non-Mendelian segregation is common in divergent crosses and can reflect differences in gamete production, fertilization, and zygote survival (Lindholm et al. 2016). For example, sexual selection can lead to TRD when gametes carrying different alleles have contrasting fertilization abilities due to male gamete competition or cryptic female choice (e.g., Fishman et al. 2008). We did not observe TRD in our independent M. m. musculus backcross or distal Chr 4 introgression in wild mice, arguing against simple competitive advantage of the musculusCZII haplotype. In divergent crosses, TRD is often caused by biased transmission of selfish genetic elements (i.e., meiotic drive or segregation distortion; McDermott and Noor 2010; Lindholm et al. 2016) as found, for example, at the R2d2 locus in house mice (Didion et al. 2015, 2016). Drive elements generate intragenomic conflict, which should drive strong counter selection for unlinked drive suppressors. Drive systems coevolve independently in isolated populations, which can lead to sterility when drivers and suppressers are uncoupled in hybrid genomes (Frank 1991; Hurst and Pomiankowski 1991). Male meiotic drivers often act by impairing the development or fertilization capacity of nondriving sperm (Lindholm et al. 2016). We tested this scenario directly and found no TRD between motile and immotile sperm of musculusPWK × CZII males. More broadly, TRD on the distal region of Chr 4 has also been reported in two other divergent crosses: TRD favoring the distal Chr 4 M. spretus allele in crosses between domesticusC57 × M. spretus F1 females and domesticusC57 males (Ceci et al. 1989), and TRD again favoring the musculusCZII distal Chr 4 allele in crosses between domesticusC57BL/KsJ males and domesticusC57BL/KsJ × musculusCZII F1 females (Fiedorek and Kay 1994). Importantly, both crosses reveal reduced transmission of the distal portion of Chr 4 derived from M. m. domesticus through female gametogenesis (Figure 5B). If these patterns reflect a common mechanism, then Chr 4 TRD must act independent of male-specific mechanisms.
Collectively, these results suggest that Chr 4 TRD and introgression are both a consequence of incompatibilities that reduce hybrid embryo viability (postzygotic inviability). In principle, TRD could occur because of a negative interaction between egg (or female reproductive tract) and sperm resulting in reduced fertilization (postmating prezygotic barriers) (Nadeau 2017), although incompatible egg–sperm interactions are often asymmetric (i.e., depend on the parent of origin of gametes; Larson et al. 2012). Chr 4 TRD occurs in crosses involving both male and female M. m. domesticus, with consistent bias against the Chr 4 M. m. domesticus allele when backcrossed to M. m. domesticus (Figure 5). The simplest explanation for this pattern is a two locus BDMI involving a recessive Chr 4 incompatibility derived in the M. m. domesticus lineage. It remains unclear why TRD driven by hybrid inviability in crosses involving M. m. domesticus colocalizes with HMS QTL that manifests in the F1 offspring. It is possible that early-acting hybrid inviability leads to the pleiotropic impairment of other reproductive traits. Alternatively, this region may harbor multiple incompatibilities, which appears to be the case for TRD of polymorphic hybrid incompatibilities in monkeyflowers (Kerwin and Sweigart 2017).
Under an inviability model, introgression at the Chr 4 TRD locus in various classic and wild-derived inbred strains (i.e., musculusPWK, PWD) would reflect different outcomes of selection against particular incompatible allelic pairings. Such epistatic selection should generate linkage disequilibrium between distal Chr 4 and other genomic regions within hybrid genomes. Although our initial scan of genotypes from 76 classic laboratory strains failed to detect these associations (Table S3), multiple genome-wide studies have revealed that selection against other deleterious allelic combinations has shaped the mosaic composition of introgressed laboratory strains (Payseur and Hoekstra 2005; Petkov et al. 2005) and the M. m. domesticus–M. m. musculus hybrid zone (Turner et al. 2012; Turner and Harr 2014). There has been considerable effort to resolve the extent to which various classic and common wild-derived laboratory strains are introgressed, with an emphasis on overall strain genetic purity (Yang et al. 2011; Didion and Pardo-Manuel de Villena 2012). While overall admixture proportions are of some relevance, our results suggest that specific genome-wide patterns of introgression may be strongly shaped by selection with the unexpected result that selection against epistatic BDMIs may facilitate introgression at underlying loci. These results underscore the intricacies of nascent species boundaries during the early stages of speciation when reproductive isolation remains incomplete and genetically variable.
Acknowledgments
We thank Kathleen Tsung, Joseph Dysthe, Brent Young, Nick Schultz, Selene Tyndale, and Charlie Nicolet for assistance with data collection and analysis. We also thank members of the Good laboratory; Ryan Bracewell, Lila Fishman, David Aylor, Bret Payseur, Michael Nachman, Peter Ellis, and two anonymous reviewers for helpful feedback; the Vincent J. Coates Genomics Sequencing Laboratory at the University of California Berkeley, supported by the National Institutes of Health S10 instrumentation grants S10RR029668 and S10RR027303; and the University of Montana Genomics Core, supported by a grant from the M.J. Murdock Charitable Trust. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R01-HD073439 and R01-HD094787 to J.M.G.), the National Institute of General Medical Sciences (R01-GM098536 to M.D.D.), and the National Science Foundation (1146525 to M.D.D.).
Author contributions: J.M.G. conceived the study. J.M.G., E.L.L., and M.D.D. designed the experiments. E.L.L., D.V., C.C., V.S., E.N., and L.P.P. performed experiments and collected data. E.L.L., D.V., B.A.J.S., S.K., L.P.P., M.D.K., and M.D.D. analyzed data. E.L.L. and J.M.G. wrote the manuscript with feedback from the authors.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6149399.
Communicating editor: D. Barbash
Literature Cited
- Baker C. L., Kajita S., Walker M., Saxl R. L., Raghupathy N., et al. , 2015. PRDM9 drives evolutionary erosion of hotspots in Mus musculus through haplotype-specific initiation of meiotic recombination. PLoS Genet. 11: e1004916 10.1371/journal.pgen.1004916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank C., Bürger R., Hermisson J., 2012. The limits to parapatric speciation: dobzhansky–Muller incompatibilities in a continent–island model. Genetics 191: 845–863. 10.1534/genetics.111.137513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N. H., Hewitt G. M., 1985. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 16: 113–148. 10.1146/annurev.es.16.110185.000553 [DOI] [Google Scholar]
- Bateson W., 1909. Heredity and variation in modern lights, pp. 85–101 in Darwin and Modern Science, edited by Seward A. C. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57: 289–300. [Google Scholar]
- Bhattacharyya T., Gregorova S., Mihola O., Anger M., Sebestova J., et al. , 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA 110: E468–E477. 10.1073/pnas.1219126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T., Reifova R., Gregorova S., Simecek P., Gergelits V., et al. , 2014. X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet. 10: e1004088 10.1371/journal.pgen.1004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C. E., Boursot P., Baron B., Bonhomme F., Hatat D., 1985. Most classical Mus musculus domesticus laboratory mouse strains carry a Mus musculus musculus Y chromosome. Nature 315: 70–72. 10.1038/315070a0 [DOI] [PubMed] [Google Scholar]
- Britton-Davidian J., Fel-Clair F., Lopez J., Alibert P., Boursot P., 2005. Postzygotic isolation between the two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory‐bred hybrids. Biol. J. Linn. Soc. Lond. 84: 379–393. 10.1111/j.1095-8312.2005.00441.x [DOI] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. 10.1093/bioinformatics/btg112 [DOI] [PubMed] [Google Scholar]
- Campbell P., Nachman M. W., 2014. X-Y interactions underlie sperm head abnormality in hybrid male house mice. Genetics 196: 1231–1240. 10.1534/genetics.114.161703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P., Good J. M., Nachman M. W., 2013. Meiotic sex chromosome inactivation is disrupted in sterile hybrid male house mice. Genetics 193: 819–828. 10.1534/genetics.112.148635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. C., Falconer D. S., 1951. Stocks for detecting linkage in the mouse, and the theory of their design. J. Genet. 50: 307–323. 10.1007/BF02996226 [DOI] [PubMed] [Google Scholar]
- Case A. L., Finseth F. R., Barr C. M., Fishman L., 2016. Selfish evolution of cytonuclear hybrid incompatibility in Mimulus. Proc. Biol. Sci. 283: 20161493–20161499. 10.1098/rspb.2016.1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci J. D., Siracusa L. D., Jenkins N. A., Copeland N. G., 1989. A molecular genetic linkage map of mouse chromosome 4 including the localization of several proto-oncogenes. Genomics 5: 699–709. 10.1016/0888-7543(89)90111-0 [DOI] [PubMed] [Google Scholar]
- Chang C. C., Chow C. C., Tellier L. C., Vattikuti S., Purcell S. M., et al. , 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaSci 4: 7–16. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P., Macnair M. R., 1987. The distribution of postmating reproductive isolating genes in populations of the yellow monkey flower, Mimulus guttatus. Evolution 41: 571–578. 10.1111/j.1558-5646.1987.tb05827.x [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Gilbert D. J., Eppig J. T., Maltais L. J., et al. , 1993. A genetic linkage map of the mouse: current applications and future prospects. Science 262: 57–66. 10.1126/science.8211130 [DOI] [PubMed] [Google Scholar]
- Cutter A. D., 2012. The polymorphic prelude to Bateson-Dobzhansky-Muller incompatibilities. Trends Ecol. Evol. 27: 209–218. 10.1016/j.tree.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C. A., Banks E., et al. , 2011. The variant call format and VCFtools. Bioinformatics 27: 2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Hatton E., Altemose N., Hussin J. G., Pratto F., et al. , 2016. Re-engineering the zinc fingers of PRDM9 reverses hybrid sterility in mice. Nature 530: 171–176. 10.1038/nature16931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion J. P., Pardo-Manuel de Villena F., 2012. Deconstructing Mus gemischus: advances in understanding ancestry, structure, and variation in the genome of the laboratory mouse. Mamm. Genome 24: 1–20. 10.1007/s00335-012-9441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion J. P., Morgan A. P., Clayshulte A. M. F., Mcmullan R. C., Yadgary L., et al. , 2015. A multi-megabase copy number gain causes maternal transmission ratio distortion on mouse chromosome 2. PLoS Genet. 11: e1004850 10.1371/journal.pgen.1004850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion J. P., Morgan A. P., Yadgary L., Bell T. A., Mcmullan R. C., et al. , 2016. R2d2 drives selfish sweeps in the house mouse. Mol. Biol. Evol. 33: 1381–1395. 10.1093/molbev/msw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T., 1937. Genetics and the Origin of Species. Columbia University Press, New York. [Google Scholar]
- Durand E. Y., Patterson N., Reich D., Slatkin M., 2011. Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 28: 2239–2252. 10.1093/molbev/msr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorek F. T., Kay E. S., 1994. Mapping of PCR-based markers for mouse chromosome 4 on a backcross penetrant for the misty (m) mutation. Mamm. Genome 5: 479–485. 10.1007/BF00369316 [DOI] [PubMed] [Google Scholar]
- Fishman L., Aagaard J., Tuthill J. C., 2008. Toward the evolutionary genomics of gametophytic divergence: patterns of transmission ratio distortion in monkeyflower (Mimulus) hybrids reveal a complex genetic basis for conspecific pollen precedence. Evolution 62: 2958–2970. 10.1111/j.1558-5646.2008.00475.x [DOI] [PubMed] [Google Scholar]
- Flachs P., Mihola O., Simecek P., Gregorova S., Schimenti J. C., et al. , 2012. Interallelic and intergenic incompatibilities of the Prdm9 (Hst1) gene in mouse hybrid sterility. PLoS Genet. 8: e1003044 10.1371/journal.pgen.1003044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachs P., Bhattacharyya T., Mihola O., Piálek J., Forejt J., et al. , 2014. Prdm9 incompatibility controls oligospermia and delayed fertility but no selfish transmission in mouse intersubspecific hybrids. PLoS One 9: e95806 10.1371/journal.pone.0095806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forejt J., Ivanyi P., 1974. Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.). Genet. Res. 24: 189–206. 10.1017/S0016672300015214 [DOI] [PubMed] [Google Scholar]
- Frank S. A., 1991. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution 45: 262–267. 10.1111/j.1558-5646 [DOI] [PubMed] [Google Scholar]
- Geraldes A., Basset P., Smith K. L., Nachman M. W., 2011. Higher differentiation among subspecies of the house mouse (Mus musculus) in genomic regions with low recombination. Mol. Ecol. 20: 4722–4736. 10.1111/j.1365-294X.2011.05285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Dean M. D., Nachman M. W., 2008a A complex genetic basis to X-linked hybrid male sterility between two species of house mice. Genetics 179: 2213–2228. 10.1534/genetics.107.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Handel M. A., Nachman M. W., 2008b Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M., 1927. The genetics of a viviparous top-minnow Platypoecilus; the inheritance of two kinds of melanophores. Genetics 12: 253–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. E., Krause J., Briggs A. W., Maricic T., Stenzel U., et al. , 2010. A draft sequence of the Neandertal genome. Science 328: 710–722. 10.1126/science.1188021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr B., Karakoc E., Neme R., Teschke M., Pfeifle C., et al. , 2016. Genomic resources for wild populations of the house mouse, Mus musculus and its close relative Mus spretus. Sci. Data 3: 160075 10.1038/sdata.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. G., 1990. Hybrid zones: windows on evolutionary process. Oxf. Surv. Evol. Biol. 7: 69–128. [Google Scholar]
- Hurst L. D., Pomiankowski A., 1991. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane’s rule and related phenomena. Genetics 128: 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoušek V., Wang L., Luzynski K., Dufková P., Vyskočilová M. M., et al. , 2012. Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus. Mol. Ecol. 21: 3032–3047. 10.1111/j.1365-294X.2012.05583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. A., 2010. Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet. 26: 317–325. 10.1016/j.tig.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Keane T. M., Goodstadt L., Danecek P., White M. A., Wong K., et al. , 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294. 10.1038/nature10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R. E., Sweigart A. L., 2017. Mechanisms of transmission ratio distortion at hybrid sterility loci within and between Mimulus species. G3 7: 3719–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. L., Hume G. L., Andrés J. A., Harrison R. G., 2012. Post-mating prezygotic barriers to gene exchange between hybridizing field crickets. J. Evol. Biol. 25: 174–186. 10.1111/j.1420-9101.2011.02415.x [DOI] [PubMed] [Google Scholar]
- Larson E. L., Keeble S., Vanderpool D., Dean M. D., Good J. M., 2017. The composite regulatory basis of the large X-effect in mouse speciation. Mol. Biol. Evol. 34: 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997v2. [Google Scholar]
- Lindholm A. K., Dyer K. A., Firman R. C., Fishman L., Forstmeier W., et al. , 2016. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 31: 315–326. 10.1016/j.tree.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Lindtke D., Buerkle C. A., 2015. The genetic architecture of hybrid incompatibilities and their effect on barriers to introgression in secondary contact. Evolution 69: 1987–2004. 10.1111/evo.12725 [DOI] [PubMed] [Google Scholar]
- Lohse M., Bolger A. M., Nagel A., Fernie A. R., Lunn J. E., et al. , 2012. RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 40: W622–W627. 10.1093/nar/gks540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F., 2003. Transmission ratio distortion in mice. Annu. Rev. Genet. 37: 393–408. 10.1146/annurev.genet.37.110801.143030 [DOI] [PubMed] [Google Scholar]
- Maheshwari S., Barbash D. A., 2011. The genetics of hybrid incompatibilities. Annu. Rev. Genet. 45: 331–355. 10.1146/annurev-genet-110410-132514 [DOI] [PubMed] [Google Scholar]
- Matute D. R., Gavin-Smyth J., Liu G., 2014. Variable post-zygotic isolation in Drosophila melanogaster/D. simulans hybrids. J. Evol. Biol. 27: 1691–1705. 10.1111/jeb.12422 [DOI] [PubMed] [Google Scholar]
- McDermott S. R., Noor M. A. F., 2010. The role of meiotic drive in hybrid male sterility. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 1265–1272. 10.1098/rstb.2009.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., et al. , 2010. The genome analysis toolkit: a mapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihola O., Trachtulec Z., Vlcek C., Schimenti J. C., Forejt J., 2009. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323: 373–375. 10.1126/science.1163601 [DOI] [PubMed] [Google Scholar]
- Mirarab S., Warnow T., 2015. ASTRAL-II: coalescent-based species tree estimation with many hundreds of taxa and thousands of genes. Bioinformatics 31: i44–i52. 10.1093/bioinformatics/btv234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., 1942. Isolating mechanisms, evolution and temperature. Biol. Symp. 6: 71–125. [Google Scholar]
- Nadeau J. H., 2017. Do gametes woo? Evidence for their nonrandom union at fertilization. Genetics 207: 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F. L., Liu D. Y., Baker H. G., 1992. Comparison of Percoll, mini-Percoll and swim-up methods for sperm preparation from abnormal semen samples. Hum. Reprod. 7: 261–266. 10.1093/oxfordjournals.humrep.a137628 [DOI] [PubMed] [Google Scholar]
- Odet F., Pan W., Bell T. A., Goodson S. G., Stevans A. M., et al. , 2015. The founder strains of the collaborative cross express a complex combination of advantageous and deleterious traits for male reproduction. G3 (Bethesda) 5: 2671–2683. 10.1534/g3.115.020172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Mita A., Sakurai-Yamatani N., Yamamoto H., Takagi N., et al. , 2004. Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies. Genetics 166: 913–924. 10.1534/genetics.166.2.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. H., Turelli M., 2001. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution 55: 1085–1094. 10.1111/j.0014-3820.2001.tb00628.x [DOI] [PubMed] [Google Scholar]
- Patterson J. T., Stone W. S., 1952. Evolution in the Genus Drosophila. The Macmillan Company, New York. [Google Scholar]
- Payseur B. A., 2010. Using differential introgression in hybrid zones to identify genomic regions involved in speciation. Mol. Ecol. Resour. 10: 806–820. 10.1111/j.1755-0998.2010.02883.x [DOI] [PubMed] [Google Scholar]
- Payseur B. A., Hoekstra H. E., 2005. Signatures of reproductive isolation in patterns of single nucleotide diversity across inbred strains of mice. Genetics 171: 1905–1916. 10.1534/genetics.105.046193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease J. B., Hahn M. W., 2015. Detection and polarization of introgression in a five-taxon phylogeny. Syst. Biol. 64: 651–662. 10.1093/sysbio/syv023 [DOI] [PubMed] [Google Scholar]
- Peterson B. K., Weber J. N., Kay E. H., Fisher H. S., Hoekstra H. E., 2012. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One 7: e37135 10.1371/journal.pone.0037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov P. M., Graber J. H., Churchill G. A., DiPetrillo K., King B. L., et al. , 2005. Evidence of a large-scale functional organization of mammalian chromosomes. PLoS Genet. 1: e33 10.1371/journal.pgen.0010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps M. J., Liu J., Benson J. D., Willoughby C. E., Gilmore J. A., et al. , 1999. Effects of Percoll separation, cryoprotective agents, and temperature on plasma membrane permeability characteristics of murine spermatozoa and their relevance to cryopreservation. Biol. Reprod. 61: 1031–1041. 10.1095/biolreprod61.4.1031 [DOI] [PubMed] [Google Scholar]
- Piálek J., Vyskocilová M., Bímová B., Havelková D., Piálková J., et al. , 2008. Development of unique house mouse resources suitable for evolutionary studies of speciation. J. Hered. 99: 34–44. 10.1093/jhered/esm083 [DOI] [PubMed] [Google Scholar]
- Presgraves D. C., 2010. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 11: 175–180. 10.1038/nrg2718 [DOI] [PubMed] [Google Scholar]
- Reed L. K., Markow T. A., 2004. Early events in speciation: polymorphism for hybrid male sterility in Drosophila. Proc. Natl. Acad. Sci. USA 101: 9009–9012. 10.1073/pnas.0403106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg L. H., Blackman B. K., 2010. Speciation genes in plants. Ann. Bot. (Lond.) 106: 439–455. 10.1093/aob/mcq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver B. A. J., Keeble S., Cosart T., Tucker P. K., Dean M. D., et al. , 2017. Phylogenomic insights into mouse evolution using a pseudoreference approach. Genome Biol. Evol. 9: 726–739. 10.1093/gbe/evx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopece G., Lexer C., Widmer A., Cozzolino S., 2010. Polymorphism of postmating reproductive isolation within plant species. Taxon 59: 1367–1374. [Google Scholar]
- Shorter J. R., Odet F., Aylor D. L., Pan W., Kao C.-Y., et al. , 2017. Male infertility is responsible for nearly half of the extinction observed in the mouse collaborative cross. Genetics 206: 557–572. 10.1534/genetics.116.199596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker D. M., Underwood K., King T. M., Butlin R. K., 2005. Patterns of male sterility in a grasshopper hybrid zone imply accumulation of hybrid incompatibilities without selection. Proc. Biol. Sci. 272: 2491–2497. 10.1098/rspb.2005.3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. L., 1967. Fertility and reproductive performance of grouped male mice, pp. 458–472 in Comparative Aspects of Reproductive Failure, edited by Benirschke K. Springer-Verlag, New York: 10.1007/978-3-642-48949-5_26 [DOI] [Google Scholar]
- Stamatakis A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streett D. A., Petersen K. R., Gerritsen A. T., Hunter S. S., Settles M. L., 2015. expHTS: analysis of high throughput sequence data in an experimental framework. BCB 15: 523–524. 10.1145/2808719.2811442 [DOI] [Google Scholar]
- Sweigart A. L., Flagel L. E., 2015. Evidence of natural selection acting on a polymorphic hybrid incompatibility locus in Mimulus. Genetics 199: 543–554. 10.1534/genetics.114.171819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. M., Harr B., 2014. Genome-wide mapping in a house mouse hybrid zone reveals hybrid sterility loci and Dobzhansky-Muller interactions. eLife 3: e02504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. M., Schwahn D. J., Harr B., 2012. Reduced male fertility is common but highly variable in form and severity in a natural house mouse hybrid zone. Evolution 66: 443–458. 10.1111/j.1558-5646.2011.01445.x [DOI] [PubMed] [Google Scholar]
- Turner L. M., White M. A., Tautz D., Payseur B. A., 2014. Genomic networks of hybrid sterility. PLoS Genet. 10: e1004162 10.1371/journal.pgen.1004162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdee S. R., Hewitt G. M., 1994. Clines for hybrid dysfunction in a grasshopper hybrid zone. Evolution 48: 392–407. 10.1111/j.1558-5646.1994.tb01319.x [DOI] [PubMed] [Google Scholar]
- Vyskočilová M., Trachtulec Z., Forejt J., Pialek J., 2005. Does geography matter in hybrid sterility in house mice? Biol. J. Linn. Soc. Lond. 84: 663–674. 10.1111/j.1095-8312.2005.00463.x [DOI] [Google Scholar]
- Vyskocilová M., Pražanová G., Piálek J., 2009. Polymorphism in hybrid male sterility in wild-derived Mus musculus musculus strains on proximal chromosome 17. Mamm. Genome 20: 83–91. 10.1007/s00335-008-9164-3 [DOI] [PubMed] [Google Scholar]
- Wang R. J., Cécile A., Payseur B. A., 2013. The evolution of hybrid incompatibilities along a phylogeny. Evolution 67: 2905–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. A., Steffy B., Wiltshire T., Payseur B. A., 2011. Genetic dissection of a key reproductive barrier between nascent species of house mice. Genetics 189: 289–304. 10.1534/genetics.111.129171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. M., Lloyd D., Lowry D. B., Macnair M. R., Willis J. H., 2013. Indirect evolution of hybrid lethality due to linkage with selected locus in Mimulus guttatus. PLoS Biol. 11: e1001497 10.1371/journal.pbio.1001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang J. R., Didion J. P., Buus R. J., Bell T. A., et al. , 2011. Subspecific origin and haplotype diversity in the laboratory mouse. Nat. Genet. 43: 648–655. 10.1038/ng.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Levine D., Shen J., Gogarten S. M., Laurie C., et al. , 2012. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 28: 3326–3328. 10.1093/bioinformatics/bts606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available through NCBI under projects SRP093943 (F1 hybrid test cross RADseq), SRP094878 (M. m. musculus CZECHII/EiJ whole genome sequencing), SRP094877 (M. m. domesticus LEWES/EiJ whole genome sequencing), SRP082237 (sperm pools whole genome sequencing), and SPR102485 (backcross RADseq). Supplemental Material, File S1 contains phenotype data for F1 hybrids and F1 hybrid test cross. File S2 contains microsatellite genotypes for markers inside and outside the Chr 4 TRD region. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6149399.