Abstract
All animal oocytes are surrounded by a glycoproteinaceous egg coat, a specialized extra-cellular matrix that serves both structural and species-specific roles during fertilization. Egg coat glycoproteins polymerize into the extracellular matrix of the egg coat using a conserved protein–protein interaction module—the zona pellucida (ZP) domain—common to both vertebrates and invertebrates, suggesting that the basic structural features of egg coats have been conserved across hundreds of millions of years of evolution. Egg coat proteins, as with other proteins involved in reproduction, are frequently found to be rapidly evolving. Given that gamete compatibility must be maintained for the fitness of sexually reproducing organisms, this finding is somewhat paradoxical and suggests a role for adaptive diversification in reproductive protein evolution. Here we review the structure and function of metazoan egg coat proteins, with an emphasis on the potential role their evolution has played in the creation and maintenance of species boundaries.
1. INTRODUCTION
Fertilization, the union of a single sperm and an egg, is essential to metazoan reproduction. The first contact in fertilization is between sperm and the extracellular matrix (ECM) of the egg coat, a maternally derived glycoprotein envelope present in all sexually reproducing animals as well as many asexual metazoans (Conner, Lefievre, Hughes, & Barratt, 2005; Shu, Suter, & Räsänen, 2015; Wong & Wessel, 2006a). Egg coats vary in size from a few tens of microns to over 15cm (Lombardi, 1998) and are called different names in each major vertebrate lineage: the chorion in fish, the vitelline envelope in amphibians, the perivitelline envelope in reptiles and birds, and the zona pellucida (ZP) in mammals (Goudet, Mugnier, Callebaut, & Monget, 2008; Shu et al., 2015). For simplicity, however, we will collectively refer to these terms as the “egg coat” throughout this review.
Despite their varied nomenclature, the overall structure and function of the egg coat are conserved across vertebrates and invertebrates (Goudet et al., 2008; Han et al., 2010). Egg coats mediate fertilization via sperm recognition and binding, establish blocks to polyspermy, and protect the embryo from biotic (e.g., pathogens, predators) and abiotic (e.g., dehydration, UV radiation, salinity, pollutants) threats (Shu et al., 2015). Egg coats affect embryonic performance by providing a dispersal and attachment medium in aquatic taxa, and in viviparous species they protect the developing embryo until the egg coat hatches and implants in the wall of the uterus (Menkhorst & Selwood, 2008; Monne, Han, & Jovine, 2006; Shu et al., 2015). In addition to their conserved function, egg coat ultrastructure is also conserved across metazoans, consisting of a fibrous matrix of conserved components with common protein domains (Goudet et al., 2008; Monne et al., 2006; Monne & Jovine, 2011; Wong & Wessel, 2006a). Notably, the domain composition of egg coat proteins and the number of genes encoding them is more variable in invertebrates (Shu et al., 2015; Wong & Wessel, 2006a) than in vertebrates (Jovine, Darie, Litscher, & Wassarman, 2005; Litscher & Wassarman, 2007; Monne et al., 2006; Wong & Wessel, 2006a).
While the basic structure of the egg coat has been conserved for more than 600 million years (Han et al., 2010; Litscher & Wassarman, 2007; Monne et al., 2006; Monne & Jovine, 2011), the proteins that make up the egg coat, as with many proteins involved in reproduction, are frequently found to be rapidly evolving (Aagaard, Yi, MacCoss, & Swanson, 2006; Findlay & Swanson, 2010; Palumbi, 2009; Turner & Hoekstra, 2008; Vacquier & Swanson, 2011). This rapid evolution of reproductive proteins is somewhat paradoxical: given the fundamentality of fertilization to species propagation, sperm and egg proteins might be expected to be highly conserved to maintain compatibility. However, this rapid evolution suggests a role for positive Darwinian evolution in creating and maintaining species specificity during sperm–egg interaction (Claw & Swanson, 2012; Meslin et al., 2012; Palumbi, 2009; Turner & Hoekstra, 2008).
In this review we will discuss the composition and evolutionary history of metazoan egg coat proteins, with an emphasis on the role these factors play in the structure, function, and evolution of the metazoan egg coat in fertilization.
2. EGG COAT PROTEINS
Animal egg coat proteins share a common polymerization module called the ZP domain (Jovine, Qi, Williams, Litscher, & Wassarman, 2004; Jovine et al., 2005; Litscher & Wassarman, 2014; Wassarman, 2008; Wilburn & Swanson, 2017; Wong & Wessel, 2006a). ZP domain-containing proteins (ZP proteins) are found in the egg coats of all vertebrate taxa (Litscher, Williams, & Wassarman, 2009; Wong & Wessel, 2006a) and some invertebrate species, including gastropod mollusks (Aagaard, Vacquier, MacCoss, & Swanson, 2010; Aagaard et al., 2006; Monne et al., 2006), cephalochordates (Putnam et al., 2008; Xu et al., 2012), and urochordates (Sawada et al., 2002; Yamada, Saito, Taniguchi, Sawada, & Harada, 2009).
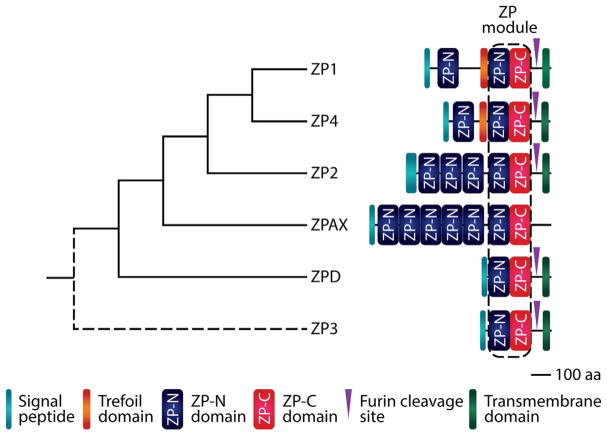
Phylogenetic analyses of ZP proteins suggest that the last common ancestor of vertebrates had at least one ancestral ZP gene, with all major ZP gene subfamilies emerging before the divergence of fish and amphibians ~360 million years ago (Smith, Paton, Hughes, & Burt, 2005; Spargo & Hope, 2003). ZP glycoprotein subfamilies have a historically complicated nomenclature, but recent consensus defines six subfamilies that evolved through gene duplication and pseudogenization: ZP1, ZP2/ZPA, ZP3/ZPC, ZP4/ZPB, ZPAX, and ZPD (Fig. 1) (Claw & Swanson, 2012; Conner et al., 2005; Goudet et al., 2008; Shu et al., 2015; Wong & Wessel, 2006a). ZP4, for instance, is a pseudogene in mouse, ZP1 is a pseudogene in dog, pig, cat, and cow, and ZPD and ZPAX have been pseudogenized or lost in all mammals (Goudet et al., 2008; Meslin et al., 2012; Shu et al., 2015). Such findings suggest that the evolution of ZP genes occurs mainly by gene death, with the accumulation of stop codons and/or insertions/deletions that disrupt reading frame and cause the loss of protein-coding ability (Goudet et al., 2008).
Fig. 1.
Phylogeny and domain structure of zona pellucida (ZP) glycoproteins. ZP3 is thought to be the ancestral ZP gene, but its position in the tree is unknown (as indicated by the dashed line). aa, amino acid. Schematics for ZP1, ZP2, ZP3, and ZP4 are based on the human homologs, and ZPD and ZPAX are based on the homologs from Xenopus tropicalis. Adapted from Callebaut, I., Mornon, J. P., & Monget, P. (2007). Isolated ZP-N domains constitute the N-terminal extensions of zona pellucida proteins. Bioinformatics (Oxford, England), 23(15), 1871–1874. btm265 [pii]; Claw, K. G., & Swanson, W. J. (2012). Evolution of the egg: New findings and challenges. Annual Review of Genomics and Human Genetics, 13, 109–125. doi: 10.1146/annurev-genom-090711-163745; Goudet, G., Mugnier, S., Callebaut, I., & Monget, P. (2008). Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biology of Reproduction, 78(5), 796–806. biolreprod.107.064568 [pii]; Wilburn, D. B., & Swanson, W. J. (2018). Egg, comparative vertebrate. In M. A. Skinner (Ed.), Encyclopedia of reproduction (2nd ed.). Academic Press.
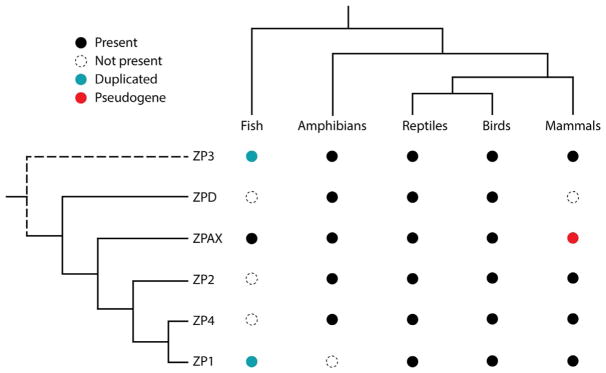
Vertebrate taxa differ in the number and type of ZP proteins incorporated in their egg coat matrix, with anywhere from zero to many copies of each ZP subfamily represented in each lineage (Shu et al., 2015). ZP3, however, is the only universal ZP gene in vertebrates, suggesting that it may be the ancestral gene to all other ZP gene families, in agreement with its more minimal architecture (see Fig. 1) (Goudet et al., 2008; Litscher & Wassarman, 2014; Shu et al., 2015; Wassarman & Litscher, 2016). ZP3 likely duplicated several times hundreds of millions of years ago, giving rise to three to four ZP genes in fish (ZP1, ZP3, ZPAX, variants of ZP1 and ZP3), four to five ZP genes in amphibians (ZP2–4, ZPD, ZPAX), six ZP genes in birds (ZP1–4, ZPD, ZPAX), and three to four ZP genes (ZP1–3, sometimes ZP4) in mammals (Fig. 2) (Wassarman & Litscher, 2016). ZP4 shares a common ancestral gene with ZP1 and is present in rats as well as in humans and other primates but is pseudogenized in the mouse genome (Conner et al., 2005; Goudet et al., 2008; Monne et al., 2006; Wassarman & Litscher, 2016; Wilburn & Swanson, 2016). In summary, ZP1–4 are found in mammals and other vertebrates, ZPD only in amphibians and birds, and ZPAX only in fish, amphibians, and birds (Wassarman & Litscher, 2016).
Fig. 2.
Patterns of ZP gene duplication and loss are highly variable among the major vertebrate lineages; the fish clade comprises teleost fish. Black circle: gene present in genome; dashed circle: gene not present in genome; teal circle: gene duplicated in genome; red circle: gene pseudogenized in genome (Goudet et al., 2008; Meslin et al., 2012). Adapted from Goudet, G., Mugnier, S., Callebaut, I., & Monget, P. (2008). Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biology of Reproduction, 78(5), 796–806. biolreprod.107.064568 [pii]; Shu, L., Suter, M. J., & Räsänen, K. (2015). Evolution of egg coats: Linking molecular biology and ecology. Molecular Ecology, 24(16), 4052–4073. 10.1111/mec.13283; Spargo, S. C., & Hope, R. M. (2003). Evolution and nomenclature of the zona pellucida gene family. Biology of Reproduction, 68(2), 358–362; Wong, J. L., & Wessel, G. M. (2006). Defending the zygote: Search for the ancestral animal block to polyspermy. Current Topics in Developmental Biology, 72, 1–151. S0070-2153(05)72001-9 [pii].
The ZP domain, despite being named for its abundance in mammalian egg coats, is not found solely in reproductive proteins (Jovine et al., 2005; Litscher & Wassarman, 2007, 2014). ZP subfamilies share an ancestral gene with the CUZD1/DMBT1 gene subfamily (CUB and ZP-like domains 1/Deleted in Malignant Brain Tumors 1), which includes proteins that incorporate two domains, the CUB domain and the ZP domain (Goudet et al., 2008). The CUB domain is found almost exclusively in extracellular and plasma membrane-associated proteins, many of which are involved in developmental processes such as embryogenesis and organogenesis (Bork, 1991; Bork & Beckmann, 1993). The CUB domain and ZP domain are also present in two families of proteins involved in sperm–egg recognition: the CUB domain in male spermadhesins, and the ZP domain in female ZP proteins (Goudet et al., 2008; Monne et al., 2006; Topfer-Petersen & Calvete, 1996). CUZD1/DMBT1 proteins are known to be expressed in the female reproductive tract, consistent with a role in fertilization (Goudet et al., 2008).
2.1 ZP Gene Losses Among Vertebrates
Given the diversity of ZP pseudogenes across the vertebrate phylogeny, ZP genes are thought to have evolved mainly through lineage-specific gene losses (Aagaard et al., 2010; Goudet et al., 2008; Meslin et al., 2012). For instance, the presence of both ZP1 and ZP4 in chicken, rat, chimpanzee, and human implies that the gene duplication that permitted the divergence of the ZP1 and ZP4 occurred early in the vertebrate lineage, before the separation of birds and mammals (~310Mya), but after the divergence of fish (Conner et al., 2005; Goudet et al., 2008). ZP4 is a pseudogene in mouse, indicating that the loss of ZP4 occurred after the divergence of mouse and rat (Goudet et al., 2008). In mammals, only primates and rodents have a ZP1 gene, although ZP1 is present as a pseudogene in cow and dog, suggesting that the death of ZP1 in those species happened after the divergence between primate and rodent groups and other mammals (Goudet et al., 2008). The persistence of both ZP1 and ZP4 across the higher vertebrates suggests that there is a functional importance to these paralogs, as both have been retained (Conner et al., 2005).
After the divergence of birds and mammals, the ZPAX and ZPD genes seem to have been lost in mammals but not in birds (Goudet et al., 2008). Loss of ZPAX in mammals is predicted to have occurred before the divergence of humans and monkeys, as similar mutations were observed in human and chimpanzee ZPAX pseudogenes (Goudet et al., 2008).
In fish, the phylogeny of ZP genes is less well known due to both genome and gene duplications, particularly of ZP3: for instance, there are four copies of ZP3 in Oryzias latipes and three in Danio rerio (Goudet et al., 2008; Meslin et al., 2012; Sano et al., 2013; Smith et al., 2005).
The persistence of the ZP2 and ZP3 subfamilies across vertebrate lineages suggests that both genes are functionally significant (Goudet et al., 2008). In fact, the egg coats of all mammals contain ZP2 and ZP3 proteins, along with one or both of the ZP1 and ZP4 proteins (Goudet et al., 2008). These findings may imply that sperm–egg interactions in mammals requires the presence of ZP2 and ZP3 as well as one or both of ZP1 and ZP4 (Goudet et al., 2008).
It has been proposed that the pervasive loss of ZP genes in mammals could be due to taxon-specific differences in selective environments (Goudet et al., 2008; Wong & Wessel, 2006a). ZP genes may be lost in mammals because they no longer play a role in egg coat matrix formation or sperm–egg interactions (Goudet et al., 2008; Meslin et al., 2012; Shu et al., 2015). However, another possibility is that in animals with internal fertilization, embryos no longer develop in external environments and are thus no longer subject to the ecological aspects of natural selection that embryos of animals with external fertilization and/or external development (e.g., fish, amphibians, birds) are subject to (Goudet et al., 2008; Shu et al., 2015; Wong & Wessel, 2006a). This loss of selection on embryonic performance could result in the genes encoding additional structures or functions of egg coats being pseudogenized (Goudet et al., 2008; Shu et al., 2015; Wong & Wessel, 2006a).
2.2 Structure of ZP Proteins
ZP proteins share a common structural organization with four main features: (1) an N-terminal secretory signal peptide (SP) that marks them as secreted proteins; (2) the ZP domain, a conserved sequence of ~260 amino acids including 8 or 10 invariant cysteine residues that adopt two alternative disulfide bond connectivities; (3) a recognition site for members of the pro-protein convertase family of proteolytic enzymes called a consensus furin cleavage site (CFCS); and (4) a C-terminal propeptide (CTP) that includes a single-spanning transmembrane (TM) domain (Jovine et al., 2005; Monne et al., 2006; Wilburn & Swanson, 2017). These elements play crucial roles in the secretion and assembly of ZP subunits (Monne et al., 2006).
3. THE ZP MODULE
All animal egg coat proteins share a common molecular basis, the ZP domain (Jovine et al., 2005; Shu et al., 2015; Wassarman & Litscher, 2016; Wong & Wessel, 2006a). Egg coat subunits polymerize using this conserved structural motif, suggesting that the basic architecture of animal egg coats has been conserved over hundreds of millions of years of evolution (Han et al., 2010; Litscher & Wassarman, 2007; Monne et al., 2006; Monne & Jovine, 2011).
The ZP domain, the structural element that gives ZP proteins their name, was first identified in ZP2 and ZP3 by pattern-based sequence analysis (Bork & Sander, 1992; Wassarman & Litscher, 2016). ZP domains are conserved protein–protein interaction modules comprised of two related immunoglobulin-like domains, ZP-N and ZP-C, that each contain characteristic disulfide bonding patterns (Jovine, Janssen, Litscher, & Wassarman, 2006; Monne & Jovine, 2011). ZP-N (~120 amino acids) and ZP-C (~130 amino acids) both have four conserved cysteine residues present as intramolecular disulfide bonds (Jovine et al., 2005; Wilburn & Swanson, 2016, 2017).
Biochemical data indicate that only ZP-N is required for protein polymerization (Jovine et al., 2006), and many ZP proteins contain tandem arrays of ZP-N repeats that have evolved independently of each other and from their associated ZP-C motifs (Callebaut, Mornon, & Monget, 2007; Swanson et al., 2011; Wilburn & Swanson, 2016, 2017). While the combined ZP-N/ZP-C pair has classically been referred to as the “ZP domain,” ZP-N should be considered a domain of its own independent of ZP-C, and we will use the term “ZP module” as put forth by Bokhove et al. to refer to the combined ZP-N/ZP-C unit (Fig. 3; see also Fig. 1) (Bokhove et al., 2016; Callebaut et al., 2007; Monne & Jovine, 2011; Wilburn & Swanson, 2017).
Fig. 3.
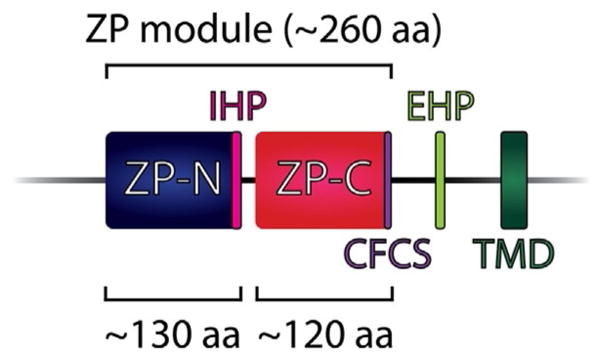
Schematic of the ZP module, which comprises adjacent ZP-N and ZP-C structural domains. The location of other common ZP protein structural features is indicated on the schematic, including the consensus furin cleavage site (CFCS), the trans-membrane domain (TMD) if present, the external hydrophobic patch (EHP), and the internal hydrophobic patch (IHP). The EHP and IHP are involved in ZP protein polymerization. aa, amino acid. Adapted from Jovine, L., Darie, C. C., Litscher, E. S., & Wassarman, P. M. (2005). Zona pellucida domain proteins. Annual Review of Biochemistry, 74, 83–114. doi: 10.1146/annurev.biochem.74.082803.133039; Wassarman, P. M. (2008). Zona pellucida glycoproteins. The Journal of Biological Chemistry, 283(36), 24285–24289. doi: 10.1074/jbc.R800027200.
The ZP module consists of two β sheets whose strands enclose a hydrophobic core comprising 8 or 10 cysteine residues. In Type I ZP modules the hydrophobic core contains eight invariant cysteines, a structure homologous to ZP3. In Type II ZP modules, the hydrophobic core contains 10 invariant cysteines and is homologous to ZP1/ZP2/ZP4 (Bork & Sander, 1992; Claw & Swanson, 2012; Jovine et al., 2005; Monne & Jovine, 2011).
Additional ZP-N domains are present in single or multiple copies at the N-terminus of ZP1, ZP2, ZP4, and ZPAX (see Fig. 1) (Callebaut et al., 2007; Goudet et al., 2008; Jovine, Qi, Williams, Litscher, & Wassarman, 2002; Jovine et al., 2005, 2006; Monne, Han, Schwend, Burendahl, & Jovine, 2008; Wassarman & Litscher, 2016; Wilburn & Swanson, 2017). After the crystal structure of mouse ZP3 ZP-N was solved, the sequences of the other ZP proteins N-terminal to the ZP module were threaded through this three-dimensional structure (Monne et al., 2008). By this analysis, it was determined that the N-terminal regions of ZP1 and ZP4 each contain an additional copy of the ZP-N domain, and the N-terminal region of ZP2 has three additional ZP-N domain repeats in tandem, connected by short linkers (Monne et al., 2008; Monne & Jovine, 2011). ZPAX, too, has several additional ZP-N repeats in its N-terminus—for instance, there are five additional copies in Xenopus tropicalis (Callebaut et al., 2007; Goudet et al., 2008).
3.1 Other Roles for ZP Module-Containing Proteins
The presence of the ZP module is not limited to egg coat proteins and is found in hundreds of extracellular proteins with diverse functions in mammals, amphibians, birds, fish, flies, worms, mollusks, and urochordates (Jovine et al., 2005; Litscher & Wassarman, 2007, 2014). Proteins containing ZP modules are structural components of animal tissues, serve as receptors, mechanotransducers, and antimicrobials, and are involved in cell signaling, differentiation, and morphogenesis (Bork & Sander, 1992; Chung, Zhu, Han, & Kernan, 2001; Fernandes et al., 2010; Heiman & Shaham, 2009; Jovine et al., 2005; Litscher & Wassarman, 2007; Plaza, Chanut-Delalande, Fernandes, Wassarman, & Payre, 2010; Wassarman & Litscher, 2016). ZP module-containing proteins organize and shape highly specialized apical structures in epithelial cells and are involved in the functioning of taste and smell (Wassarman & Litscher, 2016). Examples of ZP module-containing proteins include TGF-β receptor III (betaglycan), uromodulin, tectorin-α and -β, endoglin, vomeroglandin, hensin, cuticlins, oikosins, and mesoglein (Litscher & Wassarman, 2014; Wassarman & Litscher, 2016).
The presence of ZP modules in hundreds of polymeric extracellular proteins in eukaryotes, from jellyfish to humans, suggests that the structure has been conserved through at least 600 million years of evolution (Monne et al., 2006; Wassarman & Litscher, 2016). In fact, a Saccharomyces cerevisiae mating protein called α-agglutinin/Sag1p Ig III adopts a three-dimensional fold similar to ZP-N, so it is possible that the ZP module has been conserved for closer to 1 billion years of evolution (Swanson et al., 2011; Wassarman & Litscher, 2016).
Mutations in ZP module-containing proteins cause severe human pathologies, including deafness, vascular disease, renal disease, cancer, and potentially infertility (Wassarman & Litscher, 2016).
3.2 Not All Egg Coat Proteins Are ZP Proteins
While ZP proteins appear to be the core building blocks of egg coats in vertebrates, the genes encoding egg coat proteins in invertebrates are not as conserved across taxa (Shu et al., 2015; Wong & Wessel, 2006a). Although the egg coats of some marine invertebrates do contain ZP modules, several different egg coat genes are found in other invertebrates. Examples include egg bindin receptor 1 (EBR1) and rendezvin in sea urchins (Vacquier & Swanson, 2011; Wong & Wessel, 2006a); OBi1 in sea stars (Hart, Sunday, Popovic, Learning, & Konrad, 2014); chorion genes in Drosophila (Jagadeeshan & Singh, 2007; Papantonis, Swevers, & Iatrou, 2015), silk moths (Papantonis et al., 2015), other lepidopterans (Carter et al., 2013), and mosquitoes (Marinotti et al., 2014); and the Brownie and Citrus genes in the cockroach Blattella germanica (Irles, Belles, & Piulachs, 2009; Irles & Piulachs, 2011). These genes function as structural components, facilitate sperm–egg interactions, and protect embryos (Shu et al., 2015).
4. SYNTHESIS AND POLYMERIZATION OF ZP PROTEINS
As has been previously noted, the basic molecular structure of the egg coat has been conserved through hundreds of millions of years of evolution. For instance, recombinant mouse egg coat subunits can incorporate into the egg coats of Xenopus oocytes due to their common polymerization domain, the ZP module (Doren et al., 1999; Monne et al., 2006). How do ZP proteins polymerize to form the ECM of the egg coat?
After cleavage of the SP, ZP protein precursors are transported through the endoplasmic reticulum (ER) and the Golgi, remaining bound to the membrane of these organelles by their TM domain (Monne et al., 2006). In the ER/Golgi the ZP proteins form disulfide bonds and are modified with N- and O-linked oligosaccharides (Monne et al., 2006). The membrane-anchored proteins are then packaged into large vesicles (~2μm in diameter), which fuse with the plasma membrane of the oocyte (Monne et al., 2006). After membrane fusion, or potentially prior to fusion within the trans-Golgi, ZP precursors are cleaved at their CFCS (Monne et al., 2006). This C-terminal processing is dependent on the TM domain and releases mature ZP proteins into the perivitelline space where they incorporate into the innermost layer of the growing egg coat via their ZP module (Monne et al., 2006).
ZP modules have been shown to interact with each other directly in the polymerization of ZP proteins (Jovine et al., 2002). The protofilaments formed by ZP proteins are organized in a right-handed double helix with frequent branching, creating a reticular network (Wong & Wessel, 2006a). ZP proteins can interact heterospecifically, permitting a diverse assembly of proteins within the reticular network of protofilaments (Wong & Wessel, 2006a). For instance, both urinary and cochlear ZP proteins can incorporate into the mouse egg coat if the whole ZP module and adjacent C-terminus are intact (Jovine et al., 2002). The autoaggregation and polymerization of ZP proteins are advantageous to ECM formation as additional motifs associated with the ZP module can be incorporated without interfering with matrix assembly (Wong & Wessel, 2006a).
Other structural features of ZP proteins appear to relate to the specific functions of each subunit: cross-links between ZP filaments are thought to be established by the N-terminal region of ZP1 through its trefoil domain, and regions N- and C-terminal to the ZP module of ZP2 and ZP3, respectively, are thought to be involved in sperm–egg interaction (Monne et al., 2006; Monne & Jovine, 2011).
ZP proteins vary across species in their sites of synthesis. In mammals and amphibians, ZP proteins are synthesized solely in the ovary by oocytes and/or follicle cells, whereas in fish and birds ZP proteins are synthesized in the ovary and/or liver in response to estrogen and transported via the bloodstream to the ovary, where they self-assemble around eggs (Jovine et al., 2005; Litscher & Wassarman, 2014; Sano et al., 2013; Wassarman & Litscher, 2016). Consequently, TM domains are not present in fish ZP proteins synthesized by the liver (Hyllner, Westerlund, Olsson, & Schopen, 2001; Sugiyama, Murata, Iuchi, Nomura, & Yamagami, 1999; Wong & Wessel, 2006a). The timing of ZP gene expression in teleosts likely coincides with vitellogenesis, such that soluble ZP proteins lacking a TM domain can be transported to the ovarian follicles along with vitellogenic proteins, limiting ZP protein precipitation in circulation and ensuring the movement of proteins essential to oogenesis (Callard, Riley, & Perez, 1990a, 1990b; Polzonetti-Magni, Mosconi, Soverchia, Kikuyama, & Carnevali, 2004; Schneider, 1996).
In terms of the sites of ZP protein synthesis, it has been proposed that simpler egg coats may contain only oocyte-derived proteins, whereas more elaborate egg coat matrices may require additional contributions from somatic tissue (Wong & Wessel, 2006a). More complex egg coats are often associated with mechanically protective roles, such as resistance to environmental hazards like osmotic shock and desiccation (Wong & Wessel, 2006a). In support of this hypothesis, in animals whose egg coats are very robust, such as fish and birds, ZP proteins are often synthesized in the liver (Bausek, Waclawek, Schneider, & Wohlrab, 2000; Chang, Lu, Lai, Kou, & Huang, 1999; Hyllner et al., 2001; Okumura et al., 2004; Wong & Wessel, 2006a).
Proteins that assemble in the extracellular space must have mechanisms to avoid premature association within the cell as they are synthesized (Monne et al., 2006). ZP protein precursors are stabilized in a soluble, nonpolymerization-competent conformation by two short, conserved motifs: an external hydrophobic patch (EHP) in the C-terminus between the CFCS and the TM domain, if present, and an internal hydrophobic patch (IHP) between the ZP-N and ZP-C of the ZP module (see Fig. 3) (Jovine et al., 2004; Monne et al., 2006). Cleavage of ZP precursors at the CFCS, an event required for secretion of mammalian ZP proteins and incorporation of both fish and mammalian subunits into the inner layer of the growing egg coat, dissociates mature polypeptides from the EHP and activates them for polymerization (Jovine et al., 2004; Litscher, Qi, & Wassarman, 1999; Qi, Williams, & Wassarman, 2002; Sugiyama et al., 1999). Thus, despite fish and mammalian egg coat proteins differing in their sites of synthesis and C-terminal architecture, they share a common assembly mechanism (Monne et al., 2006). Because this mechanism relies on highly conserved elements such as the presence of the EHP/IHP and cleavage at the CFCS, it is likely common to all ZP proteins (Jovine et al., 2005, 2004; Monne et al., 2006).
Notably, in experiments where ZP proteins are truncated just upstream of the TM domain, proteins lacking a TM domain are secreted normally but are not cleaved at the CFCS or incorporated into the egg coat (Jovine et al., 2002, 2004). This suggests that TM domains in ZP proteins are not involved in specific interactions, but help with proper localization and/or topological orientation of nascent proteins for proteolytic processing and assembly (Litscher & Wassarman, 2007). Whereas mammalian ZP precursors lacking a TM domain are not assembled into the egg coat, ZP precursors from fish or birds that lack a TM domain endogenously undergo cleavage at the CFCS and assemble into the egg coat normally upon reaching the ovary (Darie et al., 2005; Sugiyama et al., 1999).
Ultrastructural analyses of egg coats suggest that they consist of filaments of similar dimensions across organisms, but how egg coat subunits are organized into these polymers is less clear (Iconomidou et al., 2000; Jovine et al., 2005; Monne et al., 2006; Nara et al., 2006). Biochemical, electron microscopy, and gene knockout studies are most consistent with a model in which the filaments are a linear repetition of ZP2/ZP3 heterodimers, with the interface between ZP2 and ZP3 running perpendicular to the axis of the filaments (Dean, 2004; Wassarman, 1988; Wassarman & Mortillo, 1991). Filament formation is therefore dependent on the interaction between Type I (ZP3) and Type II (ZP1/ZP2/ZP4) ZP modules (Monne et al., 2006).
ZP1 is thought to be responsible for cross-linking these filaments into a three-dimensional matrix, mediated by its N-terminal trefoil domain (see Fig. 1) (Greve & Wassarman, 1985; Monne et al., 2006; Rankin, Talbot, Lee, & Dean, 1999). In the mammalian egg coat, ZP1 is expressed at much lower levels than the other subunits, so ZP1 (and ZP4, if present) would be incorporated only rarely in place of ZP2 (Monne et al., 2006). By contrast, in fish, the presence of two or more ZP1 homologs (at least one of which is highly expressed (Brivio, Bassi, & Cotelli, 1991)), as well as the lack of a ZP2 homolog, predicts an egg coat with a much higher number of cross-links (Monne et al., 2006). This is in keeping with the significant resistance to mechanical and chemical stress that fish egg coats display, even prior to hardening (Monne et al., 2006). Thus the composition of egg coats in terms of the number of ZP1 homologs they contain may suggest physical properties, by giving an estimate of the number of cross-links (Jovine et al., 2005; Monne et al., 2006). Notably, the formation of additional intra- and intermolecular disulfide bonds has been implicated in the hardening of the mammalian egg coat (Iwamoto et al., 1999; Monne et al., 2006).
5. EGG COAT STRUCTURE
5.1 Mammals
Mouse egg coats are the best understood of all vertebrates, and the most well studied of mammals specifically (Monne et al., 2006; Shu et al., 2015). Mouse egg coats are comprised of homologs of ZP1 (~200kDa), ZP2 (~120kDa), and ZP3 (~83kDa) (Goudet et al., 2008; Litscher & Wassarman, 2007; Wassarman, 2008). These proteins assemble into ~2–3μm long filaments, with a structural repeat of ~150Å, and are cross-linked into a highly porous, 6.5μm thick elastic network (Monne et al., 2006). The egg coats of other mammals, including humans, are thought to have a similar structure with the inclusion of an additional ZP1-like subunit, ZP4 (Goudet et al., 2008; Monne & Jovine, 2011).
By electron microscopy, it was suggested that mouse ZP2 and ZP3 assemble into micron-long polymers which are cross-linked into a three-dimensional matrix by disulfide-bonded ZP1 homodimers (Greve & Wassarman, 1985; Monne & Jovine, 2011; Wassarman & Mortillo, 1991). This model is confirmed by the phenotypes of mice lacking the genes for the individual ZP subunits (Monne & Jovine, 2011). Homozygous ZP1 knockout mice produce an egg coat, but it is loose and insufficiently cross-linked and mutant females are less fertile than wild type, suggesting that ZP1 interconnects ZP fibrils and that a structurally intact egg coat is integral to fertilization (Claw & Swanson, 2012; Rankin et al., 1999; Wassarman & Litscher, 2013). Homozygous knockout female mice lacking either ZP2 or ZP3 fail to construct an egg coat and are infertile, indicating that ZP2 and ZP3 depend on each other for incorporation into the egg coat (Liu et al., 1996; Rankin et al., 1996, 2001; Wassarman & Litscher, 2013, 2016). Mutant female mice with a single ZP3 allele assemble an egg coat and reproduce normally, but their egg coat is less than half the thickness of wild type (Wassarman & Litscher, 2013, 2016; Wassarman, Qi, & Litscher, 1997).
5.2 Birds
The egg coats of birds consist of two layers separated by a thin continuous membrane, with the inner or perivitelline layer representing a 1- to 3.5-μm thick network of fibers analogous to the mammalian egg coat as it mediates the species-specific binding of sperm (Monne et al., 2006). After fertilization, oocytes acquire the continuous membrane (0.1–0.5μm thick) and the outer layer (3–8μm thick), which are thought to be involved in blocks to polyspermy (Ichikawa, Matsuzaki, Hiyama, Mizushima, & Sasanami, 2016; Monne et al., 2006).
As with the mammalian egg coat, the avian perivitelline layer contains several glycoproteins: homologs to ZP1, ZP2, ZP3, ZP4, and ZPD have been found in quail, and genes for ZP1, ZP2, ZP3, ZP4, ZPD, and ZPAX are present in the chicken genome (Goudet et al., 2008; Ichikawa et al., 2016; Meslin et al., 2012; Monne et al., 2006; Serizawa et al., 2011; Smith et al., 2005).
Avian ZP3 is ~32–42kDa, and avian ZP1 exists as both a ~97kDa monomer and a homodimer held together by intermolecular disulfide bonds (Monne et al., 2006). ZP1 is secreted by the liver in response to estrogens and is characterized by a proline/glutamine-rich repeat region N-terminal to the trefoil domain and a short CTP lacking a TM domain, as would be predicted by its liver synthesis (Bausek et al., 2000; Monne et al., 2006; Sasanami, Pan, & Mori, 2003). Chicken ZPD (~42kDa) loosely associates with the perivitelline layer. Together with dimeric ZP1, ZPD has been implicated in sperm activation (Ichikawa et al., 2016; Monne et al., 2006; Okumura et al., 2004). ZP3 is thought to be responsible for sperm binding in the perivitelline layer of both chicken and quail (Ichikawa et al., 2016). Upon binding of sperm, avian ZP1 is degraded and hole-like structures appear in the perivitelline layer (Monne et al., 2006; Takeuchi et al., 2001).
5.3 Amphibians
Amphibians include anurans (frogs), which reproduce by external fertilization in water, and urodeles (salamanders), which reproduce by internal fertilization in the female cloaca (Monne et al., 2006; Watanabe & Onitake, 2002). The ~1μm thick egg coat of Xenopus laevis is the best studied of the anurans and consists of five major ZP glycoproteins that are synthesized by the oocyte: ZP2 (gp69/64), ZP3 (gp43/41), ZP4 (gp37), ZPAX (gp120/112), and ZPD (gp80) (Goudet et al., 2008; Hedrick, 2008; Kubo et al., 1997, 2000, 1999; Lindsay, Wallace, & Hedrick, 2001; Lindsay, Yang, & Hedrick, 2002; Monne et al., 2006; Tian, Gong, & Lennarz, 1999).
Xenopus ZPAX contains a ~600 amino acid N-terminal region and a short CTP lacking a predicted TM domain and is related to ZP2 with its Type II ZP module and absence of a trefoil domain (Monne et al., 2006). In agreement with homology to ZP2, X. tropicalis ZPAX has five additional ZP-N repeats in its N-terminus (Callebaut et al., 2007).
Xenopus ZPD has a simple architecture, consisting of an SP, a ZP module, a CFCS, and a TM domain, and appears to represent a subfamily of its own: although its ZP module sequence is most similar to a Type II ZP module, it lacks 2 of the 10 conserved cysteines (Lindsay et al., 2002; Monne et al., 2006).
Fertilization in urodels has not been as well studied, with the exception of the newt Cynops pyrrhogaster. C. pyrrhogaster eggs can undergo polyspermy, as no fertilization envelope forms after fertilization (Watanabe & Onitake, 2002). A transcriptome assembly from ovary, testis, and oviduct found homologs to all six ZP subfamilies—ZP1, ZP2, ZP3, ZP4, ZPAX, and ZPD—expressed in the ovaries of C. pyrrhogaster (Watanabe & Takayama-Watanabe, 2014). Notably, the authors found six distinct paralogs of ZP3 (Watanabe & Takayama-Watanabe, 2014).
5.4 Teleost Fish
Teleost fish are highly diverse, constituting almost half of the total number of vertebrates (Monne et al., 2006). This diversity is reflected in the architecture of teleost egg coats, which vary in thickness, structure, and number of layers both between and within species (Monne et al., 2006). A single layer of follicle cells surrounds oocytes as they grow (Monne et al., 2006). In response to external signals, follicle cells produce 17β-estradiol to induce synthesis of both egg yolk (vitellogenin) and ZP protein precursors (Monne et al., 2006). In most teleosts, soluble ZP protein precursors are secreted by hepatocytes and travel in the blood to the oocyte for incorporation into the egg coat (Arukwe & Goksoyr, 2003; Sugiyama & Iuchi, 2000). ZP precursors are deposited in the perivitelline space, at the base of long microvilli that stretch from the plasma membrane of the oocyte to the follicle cells (Monne et al., 2006). Egg coat assembly proceeds as the oocyte accumulates egg yolk, resulting in a radially striated structure of helicoidal glycoprotein bundles separated by extended microvilli (Monne et al., 2006).
The egg coats of rainbow trout (Oncorhynchus mykiss) have a thin outer layer and a ~50μm thick inner layer of three major subunits: VEα (~58kDa), VEβ (~52kDa), and VEγ (~47kDa) (Darie, Litscher, & Wassarman, 2008; Litscher & Wassarman, 2007; Monne et al., 2006). Like avian ZP1, these subunits have an N-terminal proline/glutamine-rich repeat region and a short CTP lacking a predicted TM domain (Brivio et al., 1991; Monne et al., 2006). VEα and VEβ are very similar in sequence and contain a trefoil domain immediately before a Type II ZP module, suggesting homology to mammalian ZP1/ZP2/ZP4, whereas VEγ contains a sperm combining site-like sequence C-terminal to its Type I ZP module, suggesting homology to ZP3 (Brivio et al., 1991; Darie, Biniossek, Jovine, Litscher, & Wassarman, 2004; Litscher & Wassarman, 2007; Monne et al., 2006). Rainbow trout egg coats consist of VEα/γ and VEβ/γ heterodimers (Litscher & Wassarman, 2007).
The egg coats of other teleost fish have similar compositions to rainbow trout, although in species such as carp, goldfish, and zebrafish, ZP genes are synthesized in the ovaries and thus contain a predicted TM domain in their CTP (Chang, Hsu, Wang, Tsao, & Huang, 1997; Litscher & Wassarman, 2007; Mold et al., 2001). In medaka, ZP subunits are synthesized by both the liver and oocytes (Kanamori, Naruse, Mitani, Shima, & Hori, 2003). Additionally, ZP3 genes are often duplicated within teleosts: for instance, there are four ZP3 genes in medaka and three in zebrafish (Goudet et al., 2008).
No ZP2 orthologs have been identified in teleosts, despite the classification of some fish egg coat genes as ZP2 (Hughes, 2007). These genes are in fact ZP1 homologs, as evidenced by the presence of a trefoil domain in these proteins (see Fig. 1) (Berois, Arezo, & Papa, 2011; Hughes, 2007). In agreement with the absence of the ZP2 subfamily in teleosts, there are no N-terminal ZP-N repeat regions in teleost ZP proteins, except for some homologs of ZPAX (Hedrick, 2008).
Teleost sperm lack an acrosome, a secretory vesicle that facilitates sperm–egg contact and egg coat dissolution. Instead, sperm reach the egg plasma membrane through the micropyle, a funnel-shaped, narrow channel through the egg coat (Hart & Donovan, 1983; Iwamatsu, Onitake, Matsuyama, Satoh, & Yukawa, 1997; Litscher & Wassarman, 2007; Monne et al., 2006; Wong & Wessel, 2006a). The micropyle attracts sperm by chemotaxis, and its precise diameter prevents polyspermy by permitting one sperm to pass at a time (Amanze & Iyengar, 1990; Lombardi, 1998; Yanagimachi et al., 2013). In contrast to mammals, birds, amphibians, mollusks, echinoderms, and urochordates, teleost sperm do not need to dissolve the egg coat to reach the egg and fuse with its plasma membrane (Hart, 1990; Lombardi, 1998). Therefore, it has been argued that fish egg coat proteins play a purely structural role in fertilization (Hart, 1990; Litscher & Wassarman, 2007; Monne et al., 2006).
The structure of the micropyle has convergently evolved in at least two animal orders with different modes of reproduction, the dipterans and the teleosts (Wong & Wessel, 2006a). In fish the micropyle is formed after the deposition of the egg coat, by the retraction of the cytoplasmic process of a specialized follicle cell called the micropylar cell that extends to the oocyte surface (Hart, 1990; Lombardi, 1998). In zebrafish it has been shown that mutants in the gene bucky ball have an excessive number of follicle cells that develop as micropylar cells, leading to multiple functional micropyles and polyspermic fertilization (Marlow & Mullins, 2008). Micropyle architecture differs across teleost species, from simple channels traversing the egg coat to more elaborate structures with outer sperm catchment areas that funnel sperm into the micropyle (Amanze & Iyengar, 1990; Cherr & Clark, 1986; Hart, 1990; Hart, Pietri, & Donovan, 1984; Yamagami, Hamazaki, Yasumasu, Masuda, & Iuchi, 1992).
After fertilization, teleost egg coats undergo a cortical reaction and secrete a transglutaminase, which hardens the egg coat by introducing cross-links between egg coat subunits, likely via their proline/glutamine-rich repeat regions (Monne et al., 2006; Sugiyama & Iuchi, 2000). The hardened egg coat has a different morphology and protects the developing embryo against environmental hazards and pathogens. Notably, mammalian ZP proteins lack an N-terminal proline/glutamine-rich repeat region, and covalent linkages between ZP subunits have not been detected in mammalian eggs or embryos (Litscher & Wassarman, 2007; Wassarman, 1988).
5.5 Mollusks
Marine invertebrates are some of the first model systems in the study of fertilization, facilitated by their numerous, accessible gametes as an externally fertilizing group (Turner & Hoekstra, 2008). Much of the work has centered on the gastropod abalone (genus Haliotis). The abalone egg coat is known to mediate species specificity in gamete interactions and triggers the sperm acrosome reaction (Lyon & Vacquier, 1999; Monne et al., 2006). This exocytic event releases lysin, a dimeric 16kDa protein that binds to the vitelline envelope receptor for lysin (VERL), a highly repetitive ~1MDa egg coat glycoprotein, ~90% of which is 22 repeats of ~153 residues homologous to the vertebrate ZP-N polymerization domain (Galindo, Moy, Swanson, & Vacquier, 2002; Swanson & Vacquier, 1997; Wilburn & Swanson, 2016). These ZP-N repeats are thought to generate an interlocking network of hydrogen bonds, stabilizing the egg coat supramolecular structure (Wilburn & Swanson, 2016). The VERL protein consists of an SP, the array of 22 tandem ZP-N repeats, a Type II ZP module, a CFCS, and a TM domain (Galindo et al., 2002; Monne et al., 2006).
Lysin dissolves the abalone egg coat through nonenzymatic means, likely competing for hydrogen bonds between VERL repeats and splaying the fibers of the egg coat, allowing sperm to pass (Swanson & Vacquier, 1997; Wilburn & Swanson, 2016). Lysin dissolves conspecific egg coats faster than heterospecific egg coats, suggesting species specificity in its activity (Swanson & Vacquier, 1997; Wilburn & Swanson, 2016). In keeping with this pattern of species specificity, the sequence of lysin homologs from different species of abalone is extremely divergent as a result of strong adaptive evolution (Monne et al., 2006; Yang, Swanson, & Vacquier, 2000). VERL ZP-N repeats 1 and 2 are also rapidly evolving, whereas repeats 3–22 evolve neutrally and are homogenized (>98% identical within a given species) by unequal crossing over and concerted evolution (Galindo, Vacquier, & Swanson, 2003; Monne et al., 2006; Swanson, Aquadro, & Vacquier, 2001; Swanson & Vacquier, 1998; Wilburn & Swanson, 2016). Lysin and VERL experience correlated rates of evolution and display intergenic linkage disequilibrium despite not being physically linked (Clark et al., 2009). This coevolution between a male protein and a female receptor suggests that identification of coevolving proteins in the two sexes could potentially find other interacting fertilization proteins—for instance, mammalian ZP interactors (Clark et al., 2009; Hart et al., 2018).
There are other ZP module-containing proteins in the abalone egg coat in addition to VERL; in fact, at least 30 additional ZP proteins were identified in abalone egg coats by expressed sequence tag sequencing and shotgun proteomics (Aagaard et al., 2010). These additional ZP proteins included a paralog of VERL called VEZP14, which also contains a putative lysin-binding motif and is rapidly evolving (Aagaard et al., 2010).
The gastropod mollusk Tegula, a genus of free-spawning marine snails, also possesses an ortholog to VERL that binds Tegula lysin (Hellberg, Dennis, Arbour-Reily, Aagaard, & Swanson, 2012). Whereas abalone VERL has 22 tandem ZP-N repeats, Tegula VERL contains only one (Hellberg et al., 2012). Of note, however, is the presence of a homogenized array of three to four residues, mainly serine–proline–threonine or serine–proline–threonine–threonine, that is repeated 70 times between Tegula VERL’s ZP-N repeat and its ZP module (Hellberg et al., 2012).
In the basal mollusk chiton, follicle cells surround a gelatinous protective layer around the egg coat called the jelly hull (Buckland-Nicks, Koss, & Chia, 1988; Litscher & Wassarman, 2014; Wong & Wessel, 2006a). These follicle cells shrink on contact with seawater, leaving behind pores in the hull that are almost continuous with pores in the egg coat (Alliegro & Wright, 1983; Buckland-Nicks et al., 1988; Mozingo, Vacquier, & Chandler, 1995; Wong & Wessel, 2006a). These tunnels focus sperm and facilitate access to the egg plasma membrane, somewhat akin to micropyles (Buckland-Nicks, 1993; Wong & Wessel, 2006a).
In the bivalve Unio, a freshwater mussel, the egg coat is attached to the egg plasma membrane solely at the vegetal pole (Focarelli, Renieri, & Rosati, 1988). As the only site of sperm binding and fusion, this region is the functional equivalent of a micropyle (Wong & Wessel, 2006a). The attachment point is a crater on the egg coat comprised exclusively of the sperm-receptive gp273 glycoprotein, marking the only fusogenic region of the egg; the remainder of the egg coat consists of the structural glycoprotein gp180 (Focarelli & Rosati, 1995).
5.6 Sea Urchin
In sea urchin, the egg coat is estimated to contain 25 major glycoproteins (Gache, Niman, & Vacquier, 1983; Longo, 1981; Niman et al., 1984). Two of these have been shown to play a structural role: p160 and rendezvinVL (Wong & Wessel, 2006a). p160 is a 160-kDa protein composed of five CUB domains and a putative TM domain and is predicted to be an integral membrane protein with protein–protein interaction motifs facing the ECM of the egg (Haley & Wessel, 2004). p160 is found at the tips of microvilli, suggesting that it links the egg plasma membrane to the egg coat until fertilization, at which point it is cleaved to separate the two and establish one block to polyspermy (Haley & Wessel, 2004).
RendezvinVL is a splice variant of the oocyte-specific rendezvin gene that is trafficked to the vitelline layer, where it reunites at fertilization with another rendezvin splice variant that associates with cortical granules: specialized organelles that are exocytosed into the perivitelline space between the egg coat and the egg that participate in the slow block to polyspermy (Claw & Swanson, 2012; Wong & Wessel, 2006b). The two splice variants create the fertilization envelope—modifications to the egg coat that form the block to polyspermy in sea urchins—likely via heterologous interactions between their CUB domains (Wong & Wessel, 2006b).
Analogous to lysin and VERL in the abalone and Tegula systems, sea urchins encode a pair of interacting fertilization proteins called bindin and EBR1 (Wilburn & Swanson, 2016). Bindin is the sole protein in sea urchin sperm acrosomes and mediates the species-specific binding of sperm to the egg coat via its receptor, EBR1 (Kamei & Glabe, 2003; Zigler, McCartney, Levitan, & Lessios, 2005). EBR1 has a similar architecture to VERL in that it contains ~19 tandem EBR repeats consisting of alternating CUB and thrombospondin type 1 (TSP-1) domains in Strongylocentrotus franciscanus, with the last 10 EBR repeats being highly conserved and species-specific (Kamei & Glabe, 2003). Strongylocentrotus purpuratus EBR1 has a slightly modified architecture of ~8 EBR repeats that share 88% identity with the S. franciscanus EBR repeat, but an entirely differently species-specific domain consisting of 11 hyalin-like repeats (Kamei & Glabe, 2003). These species-specific domains in S. purpuratus and S. franciscanus EBR1 are known to function in species-specific sperm adhesion (Kamei & Glabe, 2003).
5.7 Insects
The egg coats of flies (dipterans) are composed of two layers: an inner vitelline layer and an outer chorion layer (Degrugillier & Leopold, 1976; Mouzaki, Zarani, & Margaritis, 1991; Pascucci, Perrino, Mahowald, & Waring, 1996; Turner & Mahowald, 1976). The chorion is synthesized by the surrounding follicle cells and protects the egg from desiccation, mechanical stress, and pathogens after it is laid (Papantonis et al., 2015; Wong & Wessel, 2006a).
Dipteran eggs are fertilized internally as the egg travels down the oviduct (Wong & Wessel, 2006a). Sperm are released in a controlled manner from the spermatheca, so the presence of a micropyle in dipterans, as previously noted, is somewhat unexpected (Degrugillier & Leopold, 1976; Mouzaki et al., 1991; Neubaum & Wolfner, 1999; Turner & Mahowald, 1976). It is likely that the importance of the egg coat in preventing desiccation takes precedence, making the micropyle less of a structure whose primary role is to block polyspermy and more one that supports gamete interactions while allowing gas exchange during embryogenesis (Li, Hodgeman, & Christensen, 1996; Wong & Wessel, 2006a).
The Drosophila chorion is composed of ~20 structural proteins synthesized by ovarian follicle cells and contains six major chorion proteins (cp): cp15, cp16, cp18, cp19, cp36, and cp38 (Papantonis et al., 2015; Parks, Wakimoto, & Spradling, 1986). cp36 and cp38 are expressed early in chorion formation (Parks et al., 1986), and cp15, cp16, cp18, and cp19 are expressed late (Griffin-Shea, Thireos, & Kafatos, 1982; Spradling & Mahowald, 1980; Spradling, 1981). These chorion proteins are stabilized in the final egg coat structure by peroxidase-mediated tyrosine cross-links (Konstandi et al., 2005).
Several additional genes in Drosophila are required for the assembly of the chorion, including fs(2)QJ42 and defective chorion 1 (dec-1) (Pascucci et al., 1996). The protein products of these genes are found throughout the egg coat rather than localized at the micropyle, suggesting that they play a role in structural integrity rather than sperm–egg interactions; this hypothesis is supported by the loss-of-function phenotypes associated with these loci (Pascucci et al., 1996; Wong & Wessel, 2006a). Females homozygous for fs(2)QJ42 produce chorions with altered permeability properties, and females who fail to synthesize dec-1 display morphological abnormalities in the chorion layers (Pascucci et al., 1996).
In silk moths, such as the cultivated Bombyx mori and the wild oak silk moth Antheraea polyphemus, the chorion contains more than 100 distinct components by two-dimensional gel electrophoresis (Kafatos et al., 1987). Silk moths possess an additional egg coat structure called the lamellar chorion, which in Bombyx contains unusual cysteine-rich proteins termed high-cysteine (Hc) proteins whose presence is unique to Bombyx (Hamodrakas, Kamitsos, & Papanikolaou, 1984; Nadel & Kafatos, 1980). These Hc proteins give the egg coat enhanced hardness and reduced permeability, likely as an adaptation to prolonged winter diapause (Nadel & Kafatos, 1980). Cysteine-rich proteins have also been found in the chorions of the mosquitoes Aedes aegypti and Anopheles gambiae (Amenya et al., 2010; Marinotti et al., 2014).
In the cockroach B. germanica, two highly abundant chorion genes, Brownie and Citrus, have been recently described (Irles et al., 2009; Irles & Piulachs, 2011). Brownie is expressed in follicle cells, forming a structure called the sponge-like body in a cavity left behind by those cells in late choriogenesis, when Brownie expression is at its highest (Irles et al., 2009). The sponge-like body is a complex structure that combines the micropyle and the aeropyle, a feature of insect eggs that functions in gas exchange (Irles et al., 2009).
Citrus is involved in chorion formation, as females treated with RNAi to Citrus laid fragile eggs showing discontinuous deposition of chorion proteins, resulting in increased permeability (Irles & Piulachs, 2011). Citrus has an SP and is rich in glycine, tyrosine, and proline, as with other insect chorion proteins where these abundances serve structural roles (such as tyro-sine cross-linking) (Irles & Piulachs, 2011). The protein contains 33 repeats of the same motif: 30–40 residues in length, rich in glutamic acid at the N-terminus and glycine, tyrosine, and proline repetitions at the C-terminus (Irles & Piulachs, 2011). The first four N-terminal repeats are the least con-served, and database searches found no homologous proteins or motifs (Irles & Piulachs, 2011). Both Brownie and Citrus show an absence of cysteines and a high concentration of tyrosines, suggesting that they could be cross-linked as has been described in the chorions of dipterans (Irles & Piulachs, 2011; Konstandi et al., 2005).
5.8 Cephalochordates and Urochordates
Cephalochordates, urochordates, and vertebrates represent the three extant groups of chordate animals (Delsuc, Brinkmann, Chourrout, & Philippe, 2006). Ascidians (sea squirts) are mostly hermaphroditic urochordates that produce self-sterile gametes due to the presence of a self-incompatibility system (Monne et al., 2006; Sawada et al., 2004). The ascidian egg coat plays a role in species-specific binding of sperm and egg, participates in the slow block to polyspermy, and prevents self-fertilization in self-sterile species (Lambert & Goode, 1992; Monne et al., 2006).
Ascidian egg coats contain multiple homologs to ZP proteins, most of which possess epidermal growth factor (EGF)-like repeats (Yamada et al., 2009). For instance, the major component of the egg coat in Halocynthia roretzi is HrVC120 (precursor to HrVC70), which consists of an SP, 13 EGF-like repeats, a Type II-like ZP module, a CFCS, and TM domain (Ban, Harada, Yokosawa, & Sawada, 2005; Kurn, Sommer, Bosch, & Khalturin, 2007; Monne et al., 2006; Sawada et al., 2002). HrVC70, the product of proteolytic cleavage of HrVC120 within the last EGF repeat, contains 12 EGF repeats and shows sperm receptor activity (Kurn et al., 2007; Sawada et al., 2004). Because of its sperm-binding capabilities and polymorphic nature, HrVC70 has been proposed as a potential allorecognition molecule mediating self-sterility (Sawada et al., 2004). In Halocynthia aurantium, a homolog to HrVC120 called HaVC130 shows high similarity to HrVC120 but has 14 EGF domain repeats, suggesting that the number of EGF repeats could play a role in species specificity of sperm–egg interactions (Ban et al., 2005).
Ciona intestinalis, another urochordate, has four homologs of ZP proteins called Vc16, Vc20, Vc182, and Vc569 (Kurn et al., 2007). The ZP proteins consist of an SP, a ZP module, and a TM domain; both Vc182 and Vc16 have a CFCS, and all four have varying numbers of EGF repeats: six for Vc569, two for Vc20, one for Vc16, and none for Vc182 (Kurn et al., 2007). All four ZP proteins are expressed in the developing oocyte (Kurn et al., 2007). A more recent proteomic characterization of C. intestinalis egg coats found an additional 7 ZP proteins, bringing the total to 11; all 7 contain single or multiple EGF-like domains (Yamada et al., 2009).
ZP proteins are present in cephalochordate egg coats as well: five were identified in Branchiostoma belcheri by mass spectrometry, termed BbZP1–5 (Xu et al., 2012). Each BbZP has a C-terminal ZP module, and the majority contain a low-density lipoprotein receptor domain and a von Willebrand factor type A domain (Xu et al., 2012). However, none have the EGF-like domain frequently observed in the ZP proteins of urochordates (Xu et al., 2012). Only BbZP1 has a TM domain, and BbZP1, 3, and 4 have CFCSs (Xu et al., 2012). The five BbZPs are synthesized primarily by the developing oocytes (Xu et al., 2012).
Cephalochordate ZP proteins are evolutionary homologs of the ZP1, ZP2, and ZPAX subfamilies, whereas urochordate ZP proteins appear to be more closely related to ZP3 (Xu et al., 2012). Regardless, analyses of cephalochordate and urochordate ZP homologs suggest that vertebrate ZP proteins have an invertebrate origin, or at least arose at the base of chordate evolution (Xu et al., 2012).
6. ZP PROTEINS IN FERTILIZATION
In species-specific mating, gamete recognition ensures that a single sperm fertilizes the egg while preventing polyspermy that can lead to embryo death. The first contact in gamete recognition is mediated by the ECM of the egg coat surrounding ovulated eggs (Baibakov, Boggs, Yauger, Baibakov, & Dean, 2012; Vacquier, 1998). As with the ECM in other tissues, the egg coat ECM is a critical intermediary in cell–cell communication (Wong & Wessel, 2006a). Initial contact with the egg coat triggers a cascade of reactions in sperm, increasing metabolism and motility and initiating the acrosome reaction, releasing the contents of the sperm secretory vesicle into the local environment by exocytosis (Neill & Vacquier, 2004; Okamura & Nishiyama, 1978; Tulsiani, Abou-Haila, Loeser, & Pereira, 1998; Wassarman, 1999). In most animals, these events signify the beginning of successful gamete recognition (Wong & Wessel, 2006a).
The molecules that elicit sperm activation vary significantly among animals, often requiring overlapping receptor–ligand interactions that enhance species specificity (Wong & Wessel, 2006a). Sperm activation results in a shift to chemotactic motility toward the egg coat and initiates the acrosome reaction, exposing additional sperm–egg-binding partners in the form of membrane-associated proteins in the luminal face of the acrosome and soluble proteins released into the local environment (Neill & Vacquier, 2004; Wong & Wessel, 2006a). Some of these soluble proteins aid sperm progression through the egg coat ECM (Wong & Wessel, 2006a). The acrosome reaction thus signifies sperm activation and the successful achievement of initial gamete contact, except in teleost fish whose sperm lack an acrosome (Hart, 1990; Wong & Wessel, 2006a).
It is likely that both ZP2 and ZP3 play important roles in sperm binding. Early experiments in the 1970s demonstrated that solubilized ZP glycoproteins from unfertilized hamster and mouse egg coats could inhibit binding of hamster sperm to ovulated eggs in vitro, suggesting the presence of receptors in the solubilized ZPs that could bind sperm and prevent their interaction with ovulated eggs (Gwatkin & Williams, 1977; Wassarman & Litscher, 2016). In mice, it was found that solubilized ZP3 inhibited sperm binding to ovulated mouse eggs in vitro, whereas solubilized ZP1 and ZP2 did not, suggesting that in mice the egg coat receptor for sperm is ZP3 (Bleil & Wassarman, 1980). ZP3 is able to trigger the acrosome reaction, and acrosome-reacted sperm bind to ZP2, making ZP3 the primary receptor for sperm and ZP2 the secondary receptor (Arnoult, Zeng, & Florman, 1996; Bleil, Greve, & Wassarman, 1988; Bleil & Wassarman, 1983, 1986; Jungnickel, Sutton, Wang, & Florman, 2007; Wassarman & Litscher, 2016).
Over time, it was shown that O-glycans on serine-332 and −334 in the C-terminus of ZP3, located in a so-called sperm combining site, are essential for gamete recognition, and that their postfertilization cleavage could account for another observation in mice: the inability of mouse sperm to bind to solubilized ZPs from two-cell embryos (Baibakov et al., 2012; Chen, Litscher, & Wassarman, 1998; Florman & Wassarman, 1985). However, elimination of glycosyltransferases required for the candidate O-glycans did not affect fertility, and mass spectrometry on native mouse egg coats found no evidence of glycosylation at the two serine residues (Baibakov et al., 2012; Boja, Hoodbhoy, Fales, & Dean, 2003; Williams, Xia, Cummings, McEver, & Stanley, 2007). Furthermore, mutating serine-332 and −334 to preclude modification with O-glycans did not alter sperm recognition or fertility, even if endogenous ZP3 was removed, suggesting that these sites cannot be intrinsically involved in sperm binding (Gahlay, Gauthier, Baibakov, Epifano, & Dean, 2010; Liu, Litscher, & Wassarman, 1995).
Such observations suggest that ZP proteins other than ZP3 may be involved in sperm–egg recognition. Mice lacking ZP1 are fertile, despite reduced fecundity, ruling out an essential role for ZP1 in gamete interactions (Rankin et al., 1999). ZP4 is a pseudogene in mice, and human sperm do not bind to transgenic mouse eggs expressing recombinant human ZP4 (Goudet et al., 2008; Yauger, Boggs, & Dean, 2011). This leaves ZP2 as a candidate.
Although human sperm can bind to the egg coats of Homo sapiens, Gorilla gorilla, and Hylobates lar (gibbon), they do not bind to the egg coats of baboon and rhesus macaque or to other mammals, including mice (Bedford, 1977; Lanzendorf, Holmgren, Johnson, Scobey, & Jeyendran, 1992). Notably, however, replacing mouse ZP2 with human ZP2 is sufficient to permit human sperm to bind to the recombinant mouse egg coat (Baibakov et al., 2012). In this gain-of-function assay, the site of gamete recognition was localized to a domain of ~115 amino acids in the N-terminus of ZP2 (Baibakov et al., 2012).
Following fertilization, the proteolytic cleavage of ZP2 into two fragments of approximately 23 and 90kDa, which remain disulfide bonded, prevents sperm from binding to two-cell mouse embryos (Bleil, Beall, & Wassarman, 1981). The cleavage of ZP2 is catalyzed by ovastacin, an oocyte-specific member of the astacin family of metalloendoproteases that is released into the perivitelline space upon fusion of the egg cortical granules with the plasma membrane (Bleil & Wassarman, 1980; Burkart, Xiong, Baibakov, Jimenez-Movilla, & Dean, 2012; Moller & Wassarman, 1989; Quesada, Sanchez, Alvarez, & Lopez-Otin, 2004). Mutating the cleavage site of ZP2 or eliminating the gene encoding ovastacin leaves ZP2 intact following fertilization, allowing sperm to bind to the egg coat despite cortical granule exocytosis (Burkart et al., 2012; Gahlay et al., 2010).
Taken together, these observations suggest a molecular basis of gamete recognition in which sperm bind to an N-terminal domain of ZP2, followed by egg coat penetration and gamete fusion (Avella, Xiong, & Dean, 2013). After fertilization, ovastacin is released from egg cortical granules, cleaving extracellular ZP2 and eliminating the sperm-binding domain, accounting for the inability of sperm to bind to two-cell embryos (Burkart et al., 2012; Gahlay et al., 2010).
While ZP2 has been historically considered a secondary egg coat receptor that binds acrosome-reacted sperm, there is precedence for ZP2 acting in primary gamete recognition in X. laevis, where its ZP2 homolog gp69/64 inhibits sperm binding to eggs in vitro (Tian, Gong, Thomsen, & Lennarz, 1997). Following fertilization, X. laevis ZP2 is cleaved by a zinc metalloprotease, suggesting that postfertilization proteolysis of ZP2 could apply more generally across vertebrates as a block to polyspermy (Lindsay & Hedrick, 2004).
6.1 ZP-N Repeats in Sperm Binding
In addition to their C-terminal ZP module, some ZP proteins have N-terminal extensions that are thought to be involved in species-specific gamete recognition (Callebaut et al., 2007). These extensions are made up of single or multiple copies of the ZP-N domain, which is related to the N-terminal region of the ZP module. The presence of these ZP-N repeats in ZP1, ZP2, ZP4, and ZPAX suggests that ZP proteins may have evolved around a common ZP-N architecture (Callebaut et al., 2007; Han et al., 2010).
With the exception of some homologs of the ZPAX subfamily, ZP-N repeats are not found in the egg coat proteins of fish, whose ZP proteins play a structural role like their counterparts in other metazoans but are likely not involved in sperm binding due to the presence of the micropyle (Litscher & Wassarman, 2007; Monne & Jovine, 2011). Further, although the ZP module is conserved in hundreds of extracellular proteins unrelated to fertilization, none of these have been shown to possess ZP-N repeats (Jovine et al., 2005). These considerations suggest that ZP-N repeats could have functional roles specific to fertilization, in agreement with what is known about the ZP-N architecture and sperm-binding properties of ZP2 (Avella, Baibakov, & Dean, 2014; Baibakov et al., 2012; Bleil et al., 1988; Callebaut et al., 2007; Gahlay et al., 2010; Monne & Jovine, 2011).
Notably, the mollusk and ascidian egg coat proteins VERL and HrVC70 both contain C-terminal ZP modules preceded by repeats that have been implicated in sperm binding (Ban et al., 2005; Galindo et al., 2002; Sawada et al., 2004; Swanson & Vacquier, 1997). While the 12 repeats of HrVC70 are EGF-like domains, the 22 tandem repeats of abalone VERL are known to adopt a ZP-N fold (Sawada et al., 2004; Swanson et al., 2011; Wilburn & Swanson, 2016).
Furthermore, a ZP-N domain in the N-terminal region of VEZP14, another abalone egg coat protein thought to be involved in sperm binding, facilitated the discovery of a ZP-N-like fold in the Ig III domain of the yeast protein alpha-agglutinin/Sag1p by fold recognition analysis (Aagaard et al., 2010; Swanson et al., 2011). This Sag1p Ig III ZP-N is directly involved in haploid yeast cell interactions during mating, a process that mirrors sperm–egg interactions in higher eukaryotes (Dranginis, Rauceo, Coronado, & Lipke, 2007; Monne & Jovine, 2011). Despite a separation of almost 1 billion years of evolution, gamete recognition in metazoans and mating in yeast appear to share common structural features, potentially mediated by the ZP-N domain (Monne & Jovine, 2011; Swanson et al., 2011).
7. EVOLUTION OF EGG COAT PROTEINS
The rapid evolution of reproductive proteins is a recurring observation among natural populations of animals (Turner & Hoekstra, 2008; Wilburn & Swanson, 2016). Because reproductive proteins regulate an essential process—fertilization—that fundamentally impacts fitness, the rapid divergence characteristic of reproductive proteins is striking and suggests that they may evolve under adaptive evolution (Clark, Aagaard, & Swanson, 2006).
The first evidence for rapid evolution of reproductive proteins came from free-spawning marine invertebrates such as abalone and sea urchins (Kresge, Vacquier, & Stout, 2001; Zigler et al., 2005). Whereas the evolution of gamete interactions in internally fertilizing species can be confounded by physiological and behavioral aspects of mating that may also be under selection, in taxa that release millions of gametes into the external environment for fertilization, gamete interactions can be readily observed and the targets of selection are clear (Clark et al., 2006; Kosman & Levitan, 2014; Turner & Hoekstra, 2008; Vacquier & Swanson, 2011). External fertilization is also thought to be the ancestral mating strategy, providing insight into how gametes in general and gametic compatibility in particular have evolved (Kosman & Levitan, 2014; Ruppert, Fox, & Barnes, 2004).
Since their inception in marine invertebrates, studies on reproductive protein evolution have accumulated in diverse taxa. Evolutionary sequence analyses from insects to vertebrates have found evidence for the rapid, adaptive evolution of reproductive proteins with functionally diverse roles (Turner & Hoekstra, 2008). This rapid evolution of reproductive proteins can contribute to reproductive isolation between diverging taxa, and the evolution of reproductive isolation is integral to the process of speciation (Coyne & Orr, 2004; Hart et al., 2018; Meslin et al., 2012; Turner & Hoekstra, 2008). For instance, rapid evolution may drive changes to amino acids important in sperm–egg interaction, creating or reinforcing reproductive barriers (Meslin et al., 2012).
Rapid evolution can be tested for by comparing the frequency of nucleotide substitutions at codons within genes, usually between species (Wilburn & Swanson, 2016). In the absence of selection, most nucleotide (and thus amino acid) substitutions will occur at a rate that reflects the basal mutation rate. The frequency of synonymous substitutions (dS) provides an estimate of this rate, and under neutral evolution nonsynonymous substitutions (dN) should occur at a similar frequency, leading to a dN/dS ratio of approximately 1. However, since most nonsynonymous substitutions are likely to alter protein structure and negatively impact function, nonsynonymous substitutions should occur less frequently and dN/dS will be less than 1. Sites where nonsynonymous substitutions are disfavored are considered under negative or purifying selection (Goldman & Yang, 1994; Wong, Yang, Goldman, & Nielsen, 2004; Yang, 1998, 2007). Alternatively, in situations where rapid change may be adaptive, nonsynonymous substitutions can occur more frequently than the mutation rate and dN/dS will be greater than 1, reflecting positive Darwinian selection (Claw & Swanson, 2012; Goldman & Yang, 1994; Wilburn & Swanson, 2016).
Numerous evolutionary forces have been implicated in the rapid evolution of reproductive proteins, including pathogen resistance, sperm competition, cryptic female choice, sexual conflict, reinforcement, and avoidance of heterospecific fertilization (Clark et al., 2006; Howard, 1999; Kosman & Levitan, 2014; Swanson & Vacquier, 2002a, 2002b; Turner & Hoekstra, 2008). These mechanisms reflect the potential for selection to act on reproductive proteins at various stages throughout fertilization (Turner & Hoekstra, 2008).
To accomplish fertilization, sperm and egg must encounter foreign molecules in the form of the gametes of the opposite sex (Turner & Hoekstra, 2008). Gametes are additionally exposed to novel environments in the process of fertilization, whether as sperm traveling through the female reproductive tract, or egg and sperm released into the surrounding environment in externally fertilizing species. These exposures render gametes vulnerable to microbial pathogens, making pathogen resistance an exogenous force that can drive the divergence of reproductive proteins (Clark et al., 2006). For instance, microbial attack may impose a constant selective pressure for diversification of gamete surface proteins, necessitating continual adaptation of reproductive proteins to this changing recognition surface (Clark et al., 2006; Vacquier, Swanson, & Lee, 1997).
Interactions between gametes represent another source of selection. In species where females mate with multiple males, females may exhibit cryptic female choice in choosing between sperm of different males (Turner & Hoekstra, 2008). In males, competition among sperm can lead to strong selection to rapidly penetrate and fertilize the egg (Turner & Hoekstra, 2008). Females, however, favor a lower fertilization rate because the larger energy investment of their gametes means polyspermy is more detrimental to female fitness. Consequently, female gamete proteins will evolve to lower the fertilization rate, and sperm proteins will evolve to increase it (Clark et al., 2006). Such opposing optimal fertilization rates can lead to sexual conflict at the gamete level, maintaining polymorphism and leading to a coevolutionary arms race between sperm and egg proteins (Clark et al., 2006; Gavrilets, 2000; Palumbi, 1999; Rice & Holland, 1997; Turner & Hoekstra, 2008).
Heterospecific interactions are also subject to selection. For instance, reinforcement is a selective force driven by the negative consequences of hybridization: if gametes from different species meet and the resulting hybrids have reduced fitness, changes to protein interactions that reduce heterospecific fertilization will be favored, leading to the evolution of reproductive barriers (Kosman & Levitan, 2014; Turner & Hoekstra, 2008). Species that overlap geographically may show only partial gametic incompatibility, so reinforcement is predicted to be strongest in sympatric populations, as opposed to allopatric populations where no heterospecific sperm is present (Dobzhansky, 1940; Kosman & Levitan, 2014; Lessios, 2007).
These and other selective forces acting on reproductive proteins can be challenging to separate and may occur simultaneously in a single species (Coyne & Orr, 2004; Turner & Hoekstra, 2008).
7.1 Reproductive Proteins as Species Barriers
Sperm–egg interaction is a species-specific event mediated by the recognition and binding of complementary molecules on the surface of the egg coat and on the sperm plasma membrane (Tulsiani et al., 1998; Vacquier, 1998; Vieira & Miller, 2006). The importance of the egg coat in maintaining species boundaries is evidenced by the fact that its removal permits heterospecific sperm binding to the egg plasma membrane; for instance, guinea pig, mouse, rat, human, rabbit, goat, dolphin, cattle, horse, and pig sperm can all successfully penetrate hamster oocytes lacking an egg coat (Vieira & Miller, 2006; Yanagimachi & Phillips, 1984).
As the first step in gamete recognition, mutations affecting the interface between sperm and the egg coat can create barriers to fertilization that may ultimately lead to speciation—particularly because sperm and egg coat proteins from ascidians to mollusks to humans are known to diverge rapidly as a result of adaptive evolution (Ban et al., 2005; Galindo et al., 2003; Grayson & Civetta, 2012; Meslin et al., 2012; Metz, Robles-Sikisaka, & Vacquier, 1998; Monne et al., 2006; Smith et al., 2005; Swanson, Nielsen, & Yang, 2003; Swanson & Vacquier, 2002a, 2002b; Vieira & Miller, 2006). Experimental studies have demonstrated that adaptive evolution acting on functional domains within reproductive proteins is sufficient to cause reproductive isolation among closely related species, with implications for the mechanisms of speciation (Clark et al., 2006; Coyne & Orr, 2004; Lyon & Vacquier, 1999; Sainudiin et al., 2005; Turner & Hoekstra, 2008).
In support of this hypothesis, variation in gamete recognition proteins within a species influences reproductive compatibility; protein divergence predicts the likelihood of hybrid fertilization better than neutral genetic markers, and polymorphism in sperm–egg recognition proteins determines reproductive success among individuals within a population (Kosman & Levitan, 2014; Levitan, 2012; Levitan & Ferrell, 2006; Levitan & Stapper, 2010; Palumbi, 1999; Zigler et al., 2005). Gametic compatibility, therefore, influences the effectiveness of reproductive isolation across species boundaries (Evans & Marshall, 2005; Kosman & Levitan, 2014; Palumbi, 1999; Vieira & Miller, 2006).
Furthermore, a comparatively small number of nonsynonymous mutations can alter reproductive protein interactions, with as few as 10 amino acid changes in sea urchins leading to incompatibility (Zigler et al., 2005). Nonsynonymous substitutions occur frequently at sperm–egg-binding sites in many species, indicating that these substitutions may influence gamete interactions (Clark, Findlay, Yi, MacCoss, & Swanson, 2007; Hellberg et al., 2012; Kosman & Levitan, 2014; Lyon & Vacquier, 1999; Springer & Crespi, 2007; Torgerson, Kulathinal, & Singh, 2002).
The species-specific reproduction of externally fertilizing marine invertebrates led to the hypothesis that divergence in gamete recognition proteins could lead to speciation (Coyne & Orr, 2004; Vacquier & Swanson, 2011). In sea urchins, sympatric species with overlapping habitats show the highest rates of sperm bindin evolution and the highest sequence polymorphism, with strong blocks to hybrid fertilization caused by the failure of heterospecific sperm bindin to bind to egg coats (Lessios, 2007; Metz, Kane, Yanagimachi, & Palumbi, 1994; Palumbi & Lessios, 2005; Palumbi & Metz, 1991; Vacquier, 1998; Vacquier & Swanson, 2011). Notably, in the three sea urchin genera that contain allopatric species, bindin has been found to be evolving slowly (Vacquier & Swanson, 2011). These observations provide support for the hypothesis of reinforcement, where the rapid evolution of bindin in sympatric species may be a mechanism to prevent hybridization and reinforce species boundaries (Lessios, 2007; Palumbi & Lessios, 2005; Vacquier & Swanson, 2011; Wilburn & Swanson, 2016). Conversely, in allopatric populations with no overlapping habitats, interspecific hybrids cannot occur, eliminating selective pressure to reinforce differences in gamete recognition proteins and thus slowing the evolutionary rate of bindin (Vacquier & Swanson, 2011).
Gamete recognition shows species specificity in abalone as well (Lyon & Vacquier, 1999; Turner & Hoekstra, 2008). In in vitro egg coat dissolution experiments, significantly more lysin is required to dissolve egg coats in heterospecific pairings than in conspecific pairings, indicating that the interaction of lysin and VERL is the species-specific step in abalone fertilization (Swanson & Vacquier, 1997; Turner & Hoekstra, 2008). Swapping lysin amino acid sequences between two species of abalone to create chimeric proteins determined that the N- and C-termini of lysin—in particular the hypervariable N-terminus, which is always species-unique—mediated species-specific recognition of the abalone egg coat (Lyon & Vacquier, 1999). Notably, a recent NMR solution structure of lysin found that its N- and C-termini are close together in physical space, forming a nexus that also contains most of lysin’s positively selected residues (Wilburn, Tuttle, Klevit, & Swanson, 2018). These regions are thus good candidates for determining which parts of lysin structurally interact with VERL to maintain species boundaries (Turner & Hoekstra, 2008; Wilburn & Swanson, 2016).
8. FINAL COMMENTS
The egg coats surrounding all metazoan eggs are closely related and share common structural features, such as the ZP module. Furthermore, the genes encoding egg coat proteins likely share a common ancestral gene, potentially ZP3 (Goudet et al., 2008; Litscher & Wassarman, 2014; Shu et al., 2015; Wassarman & Litscher, 2016). Despite the deep homology of the egg’s extracellular barrier, however, a frequent observation among egg coat proteins is their rapid evolution.
In humans, approximately 10% of in vitro fertilization (IVF) attempts fail, with no known cause (Liu, Clarke, Martic, Garrett, & Baker, 2001). Such cases of unexplained infertility may be due to incompatibility between sperm–egg recognition proteins, driven by the rapid evolution of reproductive loci (Clark et al., 2006). Correlating this sequence divergence with protein function may help to elucidate the molecular basis of reproductive incompatibilities, with implications for infertility, reproductive isolation, and speciation (Clark et al., 2006; Turner & Hoekstra, 2008; Wilburn & Swanson, 2016).
Anomalies in the structure and thickness of the egg coat are known to affect reproductive fitness, with IVF success being one particularly well-studied aspect; notably, a high degree of sequence variation has been observed in all four human ZP glycoproteins (ZP1–4) in infertile women presenting to IVF clinics (Mannikko et al., 2005; Nayernia et al., 2002; Oehninger et al., 1996; Pokkyla, Lakkakorpi, Nuojua-Huttunen, & Tapanainen, 2011; Sun, Xu, Cao, Su, & Guo, 2005).
On the other hand, ZP glycoproteins are also potential targets for contraception (Paterson, Jennings, Wilson, & Aitken, 2002). Recently, it was shown that the mouse sperm-binding region of ZP2, when conjugated to agarose beads inserted into the uterus of fertile mice, provided a long-acting method of contraception by decoying and binding sperm, preventing their access to the egg (Avella, Baibakov, Jimenez-Movilla, Sadusky, & Dean, 2016). This same construct, when paired with the human ZP2 sperm-binding region, could select for sperm that are better able to bind and penetrate the egg coat, suggesting the suitability of this method for assisted reproductive technologies as well (Avella et al., 2016).
Continued research into the structure, function, and evolution of metazoan egg coat proteins has great potential to contribute to both human health and the field of evolutionary biology.
Acknowledgments
We would like to thank Dr. Damien Wilburn for his insightful comments on the manuscript. This work was supported by NIH grant R01HD076862 (to W.J.S.) and NIH fellowships T32HG000035-22 and F31HD093441 (to E.E.K.).
References
- Aagaard JE, Vacquier VD, MacCoss MJ, Swanson WJ. ZP domain proteins in the abalone egg coat include a paralog of VERL under positive selection that binds lysin and 18-kDa sperm proteins. Molecular Biology and Evolution. 2010;27(1):193–203. doi: 10.1093/molbev/msp221. https://doi.org/10.1093/molbev/msp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard JE, Yi X, MacCoss MJ, Swanson WJ. Rapidly evolving zona pellucida domain proteins are a major component of the vitelline envelope of abalone eggs. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17302–17307. doi: 10.1073/pnas.0603125103. 0603125103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliegro MC, Wright DA. Polyspermy inhibition in the oyster, Crassostrea virginica. The Journal of Experimental Zoology. 1983;227(1):127–137. doi: 10.1002/jez.1402270117. https://doi.org/10.1002/jez.1402270117. [DOI] [PubMed] [Google Scholar]
- Amanze D, Iyengar A. The micropyle: A sperm guidance system in teleost fertilization. Development (Cambridge, England) 1990;109(2):495–500. doi: 10.1242/dev.109.2.495. [DOI] [PubMed] [Google Scholar]
- Amenya DA, Chou W, Li J, Yan G, Gershon PD, James AA, et al. Proteomics reveals novel components of the Anopheles gambiae eggshell. Journal of Insect Physiology. 2010;56(10):1414–1419. doi: 10.1016/j.jinsphys.2010.04.013. https://doi.org/10.1016/j.jinsphys.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult C, Zeng Y, Florman HM. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. The Journal of Cell Biology. 1996;134(3):637–645. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arukwe A, Goksoyr A. Eggshell and egg yolk proteins in fish: Hepatic proteins for the next generation: Oogenetic, population, and evolutionary implications of endocrine disruption. Comparative Hepatology. 2003;2(1):4. doi: 10.1186/1476-5926-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella MA, Baibakov B, Dean J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. The Journal of Cell Biology. 2014;205(6):801–809. doi: 10.1083/jcb.201404025. https://doi.org/10.1083/jcb.201404025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella MA, Baibakov BA, Jimenez-Movilla M, Sadusky AB, Dean J. ZP2 peptide beads select human sperm in vitro, decoy mouse sperm in vivo, and provide reversible contraception. Science Translational Medicine. 2016;8(336):336ra60. doi: 10.1126/scitranslmed.aad9946. https://doi.org/10.1126/scitranslmed.aad9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella MA, Xiong B, Dean J. The molecular basis of gamete recognition in mice and humans. Molecular Human Reproduction. 2013;19(5):279–289. doi: 10.1093/molehr/gat004. https://doi.org/10.1093/molehr/gat004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baibakov B, Boggs NA, Yauger B, Baibakov G, Dean J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. The Journal of Cell Biology. 2012;197(7):897–905. doi: 10.1083/jcb.201203062. https://doi.org/10.1083/jcb.201203062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban S, Harada Y, Yokosawa H, Sawada H. Highly polymorphic vitelline-coat protein HaVC80 from the ascidian, Halocynthia aurantium: Structural analysis and involvement in self/nonself recognition during fertilization. Developmental Biology. 2005;286(2):440–451. doi: 10.1016/j.ydbio.2005.08.004. S0012-1606(05)00523-3 [pii] [DOI] [PubMed] [Google Scholar]
- Bausek N, Waclawek M, Schneider WJ, Wohlrab F. The major chicken egg envelope protein ZP1 is different from ZPB and is synthesized in the liver. The Journal of Biological Chemistry. 2000;275(37):28866–28872. doi: 10.1074/jbc.275.37.28866. 275/37/28866 [pii] [DOI] [PubMed] [Google Scholar]
- Bedford JM. Sperm/egg interaction: The specificity of human spermatozoa. The Anatomical Record. 1977;188(4):477–487. doi: 10.1002/ar.1091880407. https://doi.org/10.1002/ar.1091880407. [DOI] [PubMed] [Google Scholar]
- Berois N, Arezo MJ, Papa NG. Gamete interactions in teleost fish: The egg envelope. Basic studies and perspectives as environmental biomonitor. Biological Research. 2011;44(2):119–124. doi:/S0716-97602011000200. [PubMed] [Google Scholar]
- Bleil JD, Beall CF, Wassarman PM. Mammalian sperm-egg interaction: Fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Developmental Biology. 1981;86(1):189–197. doi: 10.1016/0012-1606(81)90329-8. 0012-1606(81)90329-8 [pii] [DOI] [PubMed] [Google Scholar]
- Bleil JD, Greve JM, Wassarman PM. Identification of a secondary sperm receptor in the mouse egg zona pellucida: Role in maintenance of binding of acrosome-reacted sperm to eggs. Developmental Biology. 1988;128(2):376–385. doi: 10.1016/0012-1606(88)90299-0. 0012-1606 (88)90299-0 [pii] [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Mammalian sperm-egg interaction: Identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. 0092-8674(80)90334-7 [pii] [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Developmental Biology. 1983;95(2):317–324. doi: 10.1016/0012-1606(83)90032-5. 0012-1606(83)90032-5 [pii] [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Autoradiographic visualization of the mouse egg’s sperm receptor bound to sperm. The Journal of Cell Biology. 1986;102(4):1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja ES, Hoodbhoy T, Fales HM, Dean J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. The Journal of Biological Chemistry. 2003;278(36):34189–34202. doi: 10.1074/jbc.M304026200. https://doi.org/10.1074/jbc.M304026200. [DOI] [PubMed] [Google Scholar]
- Bokhove M, Nishimura K, Brunati M, Han L, de Sanctis D, Rampoldi L, et al. A structured interdomain linker directs self-polymerization of human uromodulin. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(6):1552–1557. doi: 10.1073/pnas.1519803113. https://doi.org/10.1073/pnas.1519803113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P. Complement components C1r/C1s, bone morphogenic protein 1 and Xenopus laevis developmentally regulated protein UVS.2 share common repeats. FEBS Letters. 1991;282(1):9–12. doi: 10.1016/0014-5793(91)80433-4. 0014-5793(91)80433–4 [pii] [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain: A widespread module in developmentally regulated proteins. Journal of Molecular Biology. 1993;231(2):539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Bork P, Sander C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Letters. 1992;300(3):237–240. doi: 10.1016/0014-5793(92)80853-9. 0014-5793(92) 80853-9 [pii] [DOI] [PubMed] [Google Scholar]
- Brivio MF, Bassi R, Cotelli F. Identification and characterization of the major components of the Oncorhynchus mykiss egg chorion. Molecular Reproduction and Development. 1991;28(1):85–93. doi: 10.1002/mrd.1080280114. https://doi.org/10.1002/mrd.1080280114. [DOI] [PubMed] [Google Scholar]
- Buckland-Nicks J. Hull cupules of chiton eggs: Parachute structures and sperm focusing devices? The Biological Bulletin. 1993;184(3):269–276. doi: 10.2307/1542445. https://doi.org/10.2307/1542445. [DOI] [PubMed] [Google Scholar]
- Buckland-Nicks J, Koss R, Chia FS. Fertilization in a chiton: Acrosome-mediated sperm-egg fusion. Gamete Research. 1988;21(3):199–212. doi: 10.1002/mrd.1120210302. https://doi.org/10.1002/mrd.1120210302. [DOI] [PubMed] [Google Scholar]
- Burkart AD, Xiong B, Baibakov B, Jimenez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. The Journal of Cell Biology. 2012;197(1):37–44. doi: 10.1083/jcb.201112094. https://doi.org/10.1083/jcb.201112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard IP, Riley D, Perez L. Vertebrate vitellogenesis: Molecular model for multihormonal control of gene regulation. Progress in Clinical and Biological Research. 1990a;342:343–348. [PubMed] [Google Scholar]
- Callard IP, Riley D, Perez L. Vitellogenesis in reptiles as a model for mammalian sex-differentiated hepatic protein synthesis. The Journal of Experimental Zoology. Supplement. 1990b;4:106–111. doi: 10.1002/jez.1402560418. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Mornon JP, Monget P. Isolated ZP-N domains constitute the N-terminal extensions of zona pellucida proteins. Bioinformatics (Oxford, England) 2007;23(15):1871–1874. doi: 10.1093/bioinformatics/btm265. btm265 [pii] [DOI] [PubMed] [Google Scholar]
- Carter JM, Baker SC, Pink R, Carter DR, Collins A, Tomlin J, et al. Unscrambling butterfly oogenesis. BMC Genomics. 2013;14 doi: 10.1186/1471-2164-14-283. 283-2164-14-283. https://doi.org/10.1186/1471-2164-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Hsu CC, Wang SC, Tsao CC, Huang FL. Molecular cloning, structural analysis, and expression of carp ZP2 gene. Molecular Reproduction and Development. 1997;46(3):258–267. doi: 10.1002/(SICI)1098-2795(199703)46:3<258::AID-MRD4>3.0.CO;2-O. https://doi.org/10.1002/(SICI)1098-2795(199703)46:33.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chang YS, Lu LF, Lai CY, Kou YH, Huang FL. Purification, characterization, and molecular cloning of an outer layer protein of carp fertilization envelope. Molecular Reproduction and Development. 1999;54(2):186–193. doi: 10.1002/(SICI)1098-2795(199910)54:2<186::AID-MRD11>3.0.CO;2-G. https://doi.org/10.1002/(SICI)1098-2795(199910)54:23.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chen J, Litscher ES, Wassarman PM. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6193–6197. doi: 10.1073/pnas.95.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherr GN, Clark WH., Jr Induction of the acrosomal reaction in sperm from the white sturgeon, Acipenser transmontanus. Advances in Experimental Medicine and Biology. 1986;207:235–249. doi: 10.1007/978-1-4613-2255-9_14. [DOI] [PubMed] [Google Scholar]
- Chung YD, Zhu J, Han Y, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29(2):415–428. doi: 10.1016/s0896-6273(01)00215-x. S0896-6273(01)00215-X [pii] [DOI] [PubMed] [Google Scholar]
- Clark NL, Aagaard JE, Swanson WJ. Evolution of reproductive proteins from animals and plants. Reproduction (Cambridge, England) 2006;131(1):11–22. doi: 10.1530/rep.1.00357. 131/1/11 [pii] [DOI] [PubMed] [Google Scholar]
- Clark NL, Findlay GD, Yi X, MacCoss MJ, Swanson WJ. Duplication and selection on abalone sperm lysin in an allopatric population. Molecular Biology and Evolution. 2007;24(9):2081–2090. doi: 10.1093/molbev/msm137. msm137 [pii] [DOI] [PubMed] [Google Scholar]
- Clark NL, Gasper J, Sekino M, Springer SA, Aquadro CF, Swanson WJ. Coevolution of interacting fertilization proteins. PLoS Genetics. 2009;5(7):e1000570. doi: 10.1371/journal.pgen.1000570. https://doi.org/10.1371/journal.pgen.1000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claw KG, Swanson WJ. Evolution of the egg: New findings and challenges. Annual Review of Genomics and Human Genetics. 2012;13:109–125. doi: 10.1146/annurev-genom-090711-163745. https://doi.org/10.1146/annurev-genom-090711-163745. [DOI] [PubMed] [Google Scholar]
- Conner SJ, Lefievre L, Hughes DC, Barratt CL. Cracking the egg: Increased complexity in the zona pellucida. Human Reproduction (Oxford, England) 2005;20(5):1148–1152. doi: 10.1093/humrep/deh835. deh835 [pii] [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates, Inc; 2004. [Google Scholar]
- Darie CC, Biniossek ML, Gawinowicz MA, Milgrom Y, Thumfart JO, Jovine L, et al. Mass spectrometric evidence that proteolytic processing of rainbow trout egg vitelline envelope proteins takes place on the egg. The Journal of Biological Chemistry. 2005;280(45):37585–37598. doi: 10.1074/jbc.M506709200. M506709200 [pii] [DOI] [PubMed] [Google Scholar]
- Darie CC, Biniossek ML, Jovine L, Litscher ES, Wassarman PM. Structural characterization of fish egg vitelline envelope proteins by mass spectrometry. Biochemistry. 2004;43(23):7459–7478. doi: 10.1021/bi0495937. https://doi.org/10.1021/bi0495937. [DOI] [PubMed] [Google Scholar]
- Darie CC, Litscher ES, Wassarman PM. Structure, processing, and polymerization of rainbow trout egg vitelline envelope proteins. In: Popescu C, Zamfir AD, Dinca N, editors. Applications of mass spectrometry in life safety. Dordrecht, The Netherlands: Springer; 2008. pp. 23–36. https://doi.org/10.1007/978-1-4020-8811-7_2. [Google Scholar]
- Dean J. Reassessing the molecular biology of sperm-egg recognition with mouse genetics. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2004;26(1):29–38. doi: 10.1002/bies.10412. https://doi.org/10.1002/bies.10412. [DOI] [PubMed] [Google Scholar]
- Degrugillier ME, Leopold RA. Ultrastructure of sperm penetration of house fly eggs. Journal of Ultrastructure Research. 1976;56(3):312–325. doi: 10.1016/s0022-5320(76)90006-x. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. The American Naturalist. 1940;74(753):312–321. https://doi.org/10.1086/280899. [Google Scholar]
- Doren S, Landsberger N, Dwyer N, Gold L, Blanchette-Mackie J, Dean J. Incorporation of mouse zona pellucida proteins into the envelope of Xenopus laevis oocytes. Development Genes and Evolution. 1999;209(6):330–339. doi: 10.1007/s004270050261. 92090330.427 [pii] [DOI] [PubMed] [Google Scholar]
- Dranginis AM, Rauceo JM, Coronado JE, Lipke PN. A biochemical guide to yeast adhesins: Glycoproteins for social and antisocial occasions. Microbiology and Molecular Biology Reviews: MMBR. 2007;71(2):282–294. doi: 10.1128/MMBR.00037-06. 71/2/282 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Marshall DJ. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution; International Journal of Organic Evolution. 2005;59(1):106–112. [PubMed] [Google Scholar]
- Fernandes I, Chanut-Delalande H, Ferrer P, Latapie Y, Waltzer L, Affolter M, et al. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Developmental Cell. 2010;18(1):64–76. doi: 10.1016/j.devcel.2009.11.009. https://doi.org/10.1016/j.devcel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Findlay GD, Swanson WJ. Proteomics enhances evolutionary and functional analysis of reproductive proteins. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2010;32(1):26–36. doi: 10.1002/bies.200900127. https://doi.org/10.1002/bies.200900127. [DOI] [PubMed] [Google Scholar]
- Florman HM, Wassarman PM. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. 0092-8674(85) 90084-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focarelli R, Renieri T, Rosati F. Polarized site of sperm entrance in the egg of a freshwater bivalve, Unio elongatulus. Developmental Biology. 1988;127(2):443–451. doi: 10.1016/0012-1606(88)90330-2. [DOI] [PubMed] [Google Scholar]
- Focarelli R, Rosati F. The 220-kDa vitelline coat glycoprotein mediates sperm binding in the polarized egg of Unio elongatulus through O-linked oligosaccharides. Developmental Biology. 1995;171(2):606–614. doi: 10.1006/dbio.1995.1308. [DOI] [PubMed] [Google Scholar]
- Gache C, Niman HL, Vacquier VD. Monoclonal antibodies to the sea urchin egg vitelline layer inhibit fertilization by blocking sperm adhesion. Experimental Cell Research. 1983;147(1):75–84. doi: 10.1016/0014-4827(83)90272-0. [DOI] [PubMed] [Google Scholar]
- Gahlay G, Gauthier L, Baibakov B, Epifano O, Dean J. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science (New York, NY) 2010;329(5988):216–219. doi: 10.1126/science.1188178. https://doi.org/10.1126/science.1188178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo BE, Moy GW, Swanson WJ, Vacquier VD. Full-length sequence of VERL, the egg vitelline envelope receptor for abalone sperm lysin. Gene. 2002;288(1–2):111–117. doi: 10.1016/s0378-1119(02)00459-6. S0378111902004596 [pii] [DOI] [PubMed] [Google Scholar]
- Galindo BE, Vacquier VD, Swanson WJ. Positive selection in the egg receptor for abalone sperm lysin. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4639–4643. doi: 10.1073/pnas.0830022100. https://doi.org/10.1073/pnas.0830022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403(6772):886–889. doi: 10.1038/35002564. https://doi.org/10.1038/35002564. [DOI] [PubMed] [Google Scholar]
- Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Molecular Biology and Evolution. 1994;11(5):725–736. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- Goudet G, Mugnier S, Callebaut I, Monget P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biology of Reproduction. 2008;78(5):796–806. doi: 10.1095/biolreprod.107.064568. biolreprod.107.064568 [pii] [DOI] [PubMed] [Google Scholar]
- Grayson P, Civetta A. Positive selection and the evolution of izumo genes in mammals. International Journal of Evolutionary Biology. 2012;2012:958164. doi: 10.1155/2012/958164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JM, Wassarman PM. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. Journal of Molecular Biology. 1985;181(2):253–264. doi: 10.1016/0022-2836(85)90089-0. 0022-2836(85)90089-0 [pii] [DOI] [PubMed] [Google Scholar]
- Griffin-Shea R, Thireos G, Kafatos FC. Organization of a cluster of four chorion genes in Drosophila and its relationship to developmental expression and amplification. Developmental Biology. 1982;91(2):325–336. doi: 10.1016/0012-1606(82)90039-2. 0012-1606(82)90039-2 [pii] [DOI] [PubMed] [Google Scholar]
- Gwatkin RB, Williams DT. Receptor activity of the hamster and mouse solubilized zona pellucida before and after the zona reaction. Journal of Reproduction and Fertility. 1977;49(1):55–59. doi: 10.1530/jrf.0.0490055. [DOI] [PubMed] [Google Scholar]
- Haley SA, Wessel GM. Proteolytic cleavage of the cell surface protein p160 is required for detachment of the fertilization envelope in the sea urchin. Developmental Biology. 2004;272(1):191–202. doi: 10.1016/j.ydbio.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Hamodrakas SJ, Kamitsos EI, Papanikolaou A. Laser-Raman spectroscopic studies of the eggshell (chorion) of Bombyx mori. International Journal of Biological Macromolecules. 1984;6(6):333–336. [Google Scholar]
- Han L, Monne M, Okumura H, Schwend T, Cherry AL, Flot D, et al. Insights into egg coat assembly and egg-sperm interaction from the X-ray structure of full-length ZP3. Cell. 2010;143(3):404–415. doi: 10.1016/j.cell.2010.09.041. https://doi.org/10.1016/j.cell.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Hart NH. Fertilization in teleost fishes: Mechanisms of sperm-egg interactions. International Review of Cytology. 1990;121:1–66. doi: 10.1016/s0074-7696(08)60658-0. [DOI] [PubMed] [Google Scholar]
- Hart NH, Donovan M. Fine structure of the chorion and site of sperm entry in the egg of brachydanio. Journal of Experimental Zoology. 1983;227(2):277–296. https://doi.org/10.1002/jez.1402270212. [Google Scholar]
- Hart NH, Pietri R, Donovan M. The structure of the chorion and associated surface filaments in oryzias—Evidence for the presence of extracellular tubules. The Journal of Experimental Zoology. 1984;230(2):273–296. doi: 10.1002/jez.1402300213. https://doi.org/10.1002/jez.1402300213. [DOI] [PubMed] [Google Scholar]
- Hart MW, Stover DA, Guerra V, Mozaffari SV, Ober C, Mugal CF, et al. Positive selection on human gamete-recognition genes. PeerJ. 2018;6:e4259. doi: 10.7717/peerj.4259. https://doi.org/10.7717/peerj.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MW, Sunday JM, Popovic I, Learning KJ, Konrad CM. Incipient speciation of sea star populations by adaptive gamete recognition coevolution. Evolution; International Journal of Organic Evolution. 2014;68(5):1294–1305. doi: 10.1111/evo.12352. https://doi.org/10.1111/evo.12352. [DOI] [PubMed] [Google Scholar]
- Hedrick JL. Anuran and pig egg zona pellucida glycoproteins in fertilization and early development. The International Journal of Developmental Biology. 2008;52(5–6):683–701. doi: 10.1387/ijdb.082580jh. https://doi.org/10.1387/ijdb.082580jh. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137(2):344–355. doi: 10.1016/j.cell.2009.01.057. https://doi.org/10.1016/j.cell.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg ME, Dennis AB, Arbour-Reily P, Aagaard JE, Swanson WJ. The tegula tango: A coevolutionary dance of interacting, positively selected sperm and egg proteins. Evolution; International Journal of Organic Evolution. 2012;66(6):1681–1694. doi: 10.1111/j.1558-5646.2011.01530.x. https://doi.org/10.1111/j.1558-5646.2011.01530.x. [DOI] [PubMed] [Google Scholar]
- Howard DJ. Conspecific sperm and pollen precedence and speciation. Annual Review of Ecology and Systematics. 1999;30(1):109–132. https://doi.org/10.1146/annurev.ecolsys.30.1.109. [Google Scholar]
- Hughes DC. ZP genes in avian species illustrate the dynamic evolution of the vertebrate egg envelope. Cytogenetic and Genome Research. 2007;117(1–4):86–91. doi: 10.1159/000103168. 000103168 [pii] [DOI] [PubMed] [Google Scholar]
- Hyllner SJ, Westerlund L, Olsson PE, Schopen A. Cloning of rainbow trout egg envelope proteins: Members of a unique group of structural proteins. Biology of Reproduction. 2001;64(3):805–811. doi: 10.1095/biolreprod64.3.805. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Matsuzaki M, Hiyama G, Mizushima S, Sasanami T. Sperm-egg interaction during fertilization in birds. The Journal of Poultry Science. 2016;53(3):173–180. doi: 10.2141/jpsa.0150183. https://doi.org/10.2141/jpsa.0150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iconomidou VA, Chryssikos DG, Gionis V, Pavlidis MA, Paipetis A, Hamodrakas SJ. Secondary structure of chorion proteins of the teleostean fish Dentex dentex by ATR FT-IR and FT-Raman spectroscopy. Journal of Structural Biology. 2000;132(2):112–122. doi: 10.1006/jsbi.2000.4307. https://doi.org/10.1006/jsbi.2000.4307. [DOI] [PubMed] [Google Scholar]
- Irles P, Belles X, Piulachs MD. Brownie, a gene involved in building complex respiratory devices in insect eggshells. PLoS One. 2009;4(12):e8353. doi: 10.1371/journal.pone.0008353. https://doi.org/10.1371/journal.pone.0008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irles P, Piulachs MD. Citrus, a key insect eggshell protein. Insect Biochemistry and Molecular Biology. 2011;41(2):101–108. doi: 10.1016/j.ibmb.2010.11.001. https://doi.org/10.1016/j.ibmb.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Onitake K, Matsuyama K, Satoh M, Yukawa S. Effect of micropylar morphology and size on rapid sperm entry into the eggs of the medaka. Zoological Science. 1997;14(4):623–628. https://doi.org/10.2108/zsj.14.623. [Google Scholar]
- Iwamoto K, Ikeda K, Yonezawa N, Noguchi S, Kudo K, Hamano S, et al. Disulfide formation in bovine zona pellucida glycoproteins during fertilization: Evidence for the involvement of cystine cross-linkages in hardening of the zona pellucida. Journal of Reproduction and Fertility. 1999;117(2):395–402. doi: 10.1530/jrf.0.1170395. [DOI] [PubMed] [Google Scholar]
- Jagadeeshan S, Singh RS. Rapid evolution of outer egg membrane proteins in the Drosophila melanogaster subgroup: A case of ecologically driven evolution of female reproductive traits. Molecular Biology and Evolution. 2007;24(4):929–938. doi: 10.1093/molbev/msm009. msm009 [pii] [DOI] [PubMed] [Google Scholar]
- Jovine L, Darie CC, Litscher ES, Wassarman PM. Zona pellucida domain proteins. Annual Review of Biochemistry. 2005;74:83–114. doi: 10.1146/annurev.biochem.74.082803.133039. https://doi.org/10.1146/annurev.biochem.74.082803.133039. [DOI] [PubMed] [Google Scholar]
- Jovine L, Janssen WG, Litscher ES, Wassarman PM. The PLAC1-homology region of the ZP domain is sufficient for protein polymerisation. BMC Biochemistry. 2006;7 doi: 10.1186/1471-2091-7-11. 11-2091-7-11. 1471-2091-7-11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nature Cell Biology. 2002;4(6):457–461. doi: 10.1038/ncb802. https://doi.org/10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):5922–5927. doi: 10.1073/pnas.0401600101. https://doi.org/10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel MK, Sutton KA, Wang Y, Florman HM. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Developmental Biology. 2007;304(1):116–126. doi: 10.1016/j.ydbio.2006.12.023. S0012-1606(06)01458-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos FC, Spoerel N, Mitsialis SA, Nguyen HT, Romano C, Lingappa JR, et al. Developmental control and evolution in the chorion gene families of insects. In: Scandalios JG, Caspari EW, editors. Molecular genetics of development, Advances in genetics. Vol. 24. Academic Press; 1987. pp. 223–242. [DOI] [PubMed] [Google Scholar]
- Kamei N, Glabe CG. The species-specific egg receptor for sea urchin sperm adhesion is EBR1, a novel ADAMTS protein. Genes & Development. 2003;17(20):2502–2507. doi: 10.1101/gad.1133003. https://doi.org/10.1101/gad.1133003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Naruse K, Mitani H, Shima A, Hori H. Genomic organization of ZP domain containing egg envelope genes in medaka (Oryzias latipes) Gene. 2003;305(1):35–45. doi: 10.1016/s0378-1119(02)01211-8. S0378111902012118 [pii] [DOI] [PubMed] [Google Scholar]
- Konstandi OA, Papassideri IS, Stravopodis DJ, Kenoutis CA, Hasan Z, Katsorchis T, et al. The enzymatic component of Drosophila melanogaster chorion is the Pxd peroxidase. Insect Biochemistry and Molecular Biology. 2005;35(9):1043–1057. doi: 10.1016/j.ibmb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kosman ET, Levitan DR. Sperm competition and the evolution of gametic compatibility in externally fertilizing taxa. MHR: Basic Science of Reproductive Medicine. 2014;20(12):1190–1197. doi: 10.1093/molehr/gau069. [DOI] [PubMed] [Google Scholar]
- Kresge N, Vacquier VD, Stout CD. Abalone lysin: The dissolving and evolving sperm protein. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2001;23(1):95–103. doi: 10.1002/1521-1878(200101)23:1<95::AID-BIES1012>3.0.CO;2-C. https://doi.org/10.1002/1521-1878(200101)23:13.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kubo H, Kawano T, Tsubuki S, Kawashima S, Katagiri C, Suzuki A. A major glycoprotein of Xenopus egg vitelline envelope, gp41, is a frog homolog of mammalian ZP3. Development, Growth & Differentiation. 1997;39(4):405–417. doi: 10.1046/j.1440-169x.1997.t01-3-00001.x. [DOI] [PubMed] [Google Scholar]
- Kubo H, Kawano T, Tsubuki S, Kotani M, Kawasaki H, Kawashima S. Egg envelope glycoprotein gp37 as a Xenopus homolog of mammalian ZP1, based on cDNA cloning. Development, Growth & Differentiation. 2000;42(4):419–427. doi: 10.1046/j.1440-169x.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- Kubo H, Matsushita M, Kotani M, Kawasaki H, Saido TC, Kawashima S, et al. Molecular basis for oviductin-mediated processing from gp43 to gp41, the predominant glycoproteins of Xenopus egg envelopes. Developmental Genetics. 1999;25(2):123–129. doi: 10.1002/(SICI)1520-6408(1999)25:2<123::AID-DVG6>3.0.CO;2-3. https://doi.org/10.1002/(SICI)1520-6408(1999)25:23.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kurn U, Sommer F, Bosch TC, Khalturin K. In the urochordate Ciona intestinalis zona pellucida domain proteins vary among individuals. Developmental and Comparative Immunology. 2007;31(12):1242–1254. doi: 10.1016/j.dci.2007.03.011. S0145-305X(07)00063-8 [pii] [DOI] [PubMed] [Google Scholar]
- Lambert CC, Goode CA. Glycolipid linkage of a polyspermy blocking gly-cosidase to the ascidian egg surface. Developmental Biology. 1992;154(1):95–100. doi: 10.1016/0012-1606(92)90051-h. 0012-1606 (92)90051-H [pii] [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Holmgren WJ, Johnson DE, Scobey MJ, Jeyendran RS. Hemizona assay for measuring zona binding in the lowland gorilla. Molecular Reproduction and Development. 1992;31(4):264–267. doi: 10.1002/mrd.1080310407. https://doi.org/10.1002/mrd.1080310407. [DOI] [PubMed] [Google Scholar]
- Lessios HA. Reproductive isolation between species of sea urchins. Bulletin of Marine Science. 2007;81(2):191–208. [Google Scholar]
- Levitan DR. Contemporary evolution of sea urchin gamete-recognition proteins: Experimental evidence of density-dependent gamete performance predicts shifts in allele frequencies over time. Evolution; International Journal of Organic Evolution. 2012;66(6):1722–1736. doi: 10.1111/j.1558-5646.2012.01608.x. https://doi.org/10.1111/j.1558-5646.2012.01608.x. [DOI] [PubMed] [Google Scholar]
- Levitan DR, Ferrell DL. Selection on gamete recognition proteins depends on sex, density, and genotype frequency. Science (New York, NY) 2006;312(5771):267–269. doi: 10.1126/science.1122183. 312/5771/267 (pii) [DOI] [PubMed] [Google Scholar]
- Levitan DR, Stapper AP. Simultaneous positive and negative frequency-dependent selection on sperm bindin, a gamete recognition protein in the sea urchin Strongylocentrotus purpuratus. Evolution; International Journal of Organic Evolution. 2010;64(3):785–797. doi: 10.1111/j.1558-5646.2009.00850.x. https://doi.org/10.1111/j.1558-5646.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- Li J, Hodgeman BA, Christensen BM. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochemistry and Molecular Biology. 1996;26(3):309–317. doi: 10.1016/0965-1748(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Lindsay LL, Hedrick JL. Proteolysis of Xenopus laevis egg envelope ZPA triggers envelope hardening. Biochemical and Biophysical Research Communications. 2004;324(2):648–654. doi: 10.1016/j.bbrc.2004.09.099. S0006-291X(04)02164-3 (pii) [DOI] [PubMed] [Google Scholar]
- Lindsay LL, Wallace MA, Hedrick JL. A hatching enzyme substrate in the Xenopus laevis egg envelope is a high molecular weight ZPA homolog. Development, Growth & Differentiation. 2001;43(3):305–313. doi: 10.1046/j.1440-169x.2001.00577.x. dgd577 [pii] [DOI] [PubMed] [Google Scholar]
- Lindsay LL, Yang JC, Hedrick JL. Identification and characterization of a unique Xenopus laevis egg envelope component, ZPD. Development, Growth & Differentiation. 2002;44(3):205–212. doi: 10.1046/j.1440-169x.2002.00635.x. 635 [pii] [DOI] [PubMed] [Google Scholar]
- Litscher ES, Qi H, Wassarman PM. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry. 1999;38(38):12280–12287. doi: 10.1021/bi991154y. bi991154y [pii] [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Egg extracellular coat proteins: From fish to mammals. Histology and Histopathology. 2007;22(3):337–347. doi: 10.14670/HH-22.337. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Evolution, structure, and synthesis of vertebrate egg-coat proteins. Trends in Developmental Biology. 2014;8:65–76. [PMC free article] [PubMed] [Google Scholar]
- Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Molecular Reproduction and Development. 2009;76(10):933–941. doi: 10.1002/mrd.21046. https://doi.org/10.1002/mrd.21046. [DOI] [PubMed] [Google Scholar]
- Liu DY, Clarke GN, Martic M, Garrett C, Baker HW. Frequency of disordered zona pellucida (ZP)-induced acrosome reaction in infertile men with normal semen analysis and normal spermatozoa-ZP binding. Human Reproduction (Oxford, England) 2001;16(6):1185–1190. doi: 10.1093/humrep/16.6.1185. [DOI] [PubMed] [Google Scholar]
- Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, et al. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(11):5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Litscher ES, Wassarman PM. Transgenic mice with reduced numbers of functional sperm receptors on their eggs reproduce normally. Molecular Biology of the Cell. 1995;6(5):577–585. doi: 10.1091/mbc.6.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi J. Comparative vertebrate reproduction. Boston: Kluwer Academic Publishers; 1998. [Google Scholar]
- Longo FJ. Effects of concanavalin A on the fertilization of sea urchin eggs. Developmental Biology. 1981;82(1):197–202. doi: 10.1016/0012-1606(81)90443-7. [DOI] [PubMed] [Google Scholar]
- Lyon JD, Vacquier VD. Interspecies chimeric sperm lysins identify regions mediating species-specific recognition of the abalone egg vitelline envelope. Developmental Biology. 1999;214(1):151–159. doi: 10.1006/dbio.1999.9411. [DOI] [PubMed] [Google Scholar]
- Mannikko M, Tormala RM, Tuuri T, Haltia A, Martikainen H, Ala-Kokko L, et al. Association between sequence variations in genes encoding human zona pellucida glycoproteins and fertilization failure in IVF. Human Reproduction (Oxford, England) 2005;20(6):1578–1585. doi: 10.1093/humrep/deh837. deh837 [pii] [DOI] [PubMed] [Google Scholar]
- Marinotti O, Ngo T, Kojin BB, Chou SP, Nguyen B, Juhn J, et al. Integrated proteomic and transcriptomic analysis of the Aedes aegypti eggshell. BMC Developmental Biology. 2014:14. doi: 10.1186/1471-213X-14-15. https://doi.org/10.1186/1471-213X-14-15. [DOI] [PMC free article] [PubMed]
- Marlow FL, Mullins MC. Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Developmental Biology. 2008;321(1):40–50. doi: 10.1016/j.ydbio.2008.05.557. https://doi.org/10.1016/j.ydbio.2008.05.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkhorst E, Selwood L. Vertebrate extracellular preovulatory and post-ovulatory egg coats. Biology of Reproduction. 2008;79(5):790–797. doi: 10.1095/biolreprod.108.068551. https://doi.org/10.1095/biolreprod.108.068551. [DOI] [PubMed] [Google Scholar]
- Meslin C, Mugnier S, Callebaut I, Laurin M, Pascal G, Poupon A, et al. Evolution of genes involved in gamete interaction: Evidence for positive selection, duplications and losses in vertebrates. PLoS One. 2012;7(9):e44548. doi: 10.1371/journal.pone.0044548. https://doi.org/10.1371/journal.pone.0044548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz EC, Kane RE, Yanagimachi H, Palumbi SR. Fertilization between closely related sea urchins is blocked by incompatibilities during sperm-egg attachment and early stages of fusion. The Biological Bulletin. 1994;187(1):23–34. doi: 10.2307/1542162. https://doi.org/10.2307/1542162. [DOI] [PubMed] [Google Scholar]
- Metz EC, Robles-Sikisaka R, Vacquier VD. Nonsynonymous substitution in abalone sperm fertilization genes exceeds substitution in introns and mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(18):10676–10681. doi: 10.1073/pnas.95.18.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold DE, Kim IF, Tsai CM, Lee D, Chang CY, Huang RC. Cluster of genes encoding the major egg envelope protein of zebrafish. Molecular Reproduction and Development. 2001;58(1):4–14. doi: 10.1002/1098-2795(200101)58:1<4::AID-MRD2>3.0.CO;2-P. https://doi.org/10.1002/1098-2795(200101)58:13.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Moller CC, Wassarman PM. Characterization of a proteinase that cleaves zona pellucida glycoprotein ZP2 following activation of mouse eggs. Developmental Biology. 1989;132(1):103–112. doi: 10.1016/0012-1606(89)90209-1. 0012-1606(89)90209-1 [pii] [DOI] [PubMed] [Google Scholar]
- Monne M, Han L, Jovine L. Tracking down the ZP domain: From the mammalian zona pellucida to the molluscan vitelline envelope. Seminars in Reproductive Medicine. 2006;24(4):204–216. doi: 10.1055/s-2006-948550. https://doi.org/10.1055/s-2006-948550. [DOI] [PubMed] [Google Scholar]
- Monne M, Han L, Schwend T, Burendahl S, Jovine L. Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats. Nature. 2008;456(7222):653–657. doi: 10.1038/nature07599. https://doi.org/10.1038/nature07599. [DOI] [PubMed] [Google Scholar]
- Monne M, Jovine L. A structural view of egg coat architecture and function in fertilization. Biology of Reproduction. 2011;85(4):661–669. doi: 10.1095/biolreprod.111.092098. https://doi.org/10.1095/biolreprod.111.092098. [DOI] [PubMed] [Google Scholar]
- Mouzaki DG, Zarani FE, Margaritis LH. Structure and morphogenesis of the eggshell and micropylar apparatus in the olive fly, Dacus oleae (diptera: Tephritidae) Journal of Morphology. 1991;209(1):39–52. doi: 10.1002/jmor.1052090105. https://doi.org/10.1002/jmor.1052090105. [DOI] [PubMed] [Google Scholar]
- Mozingo NM, Vacquier VD, Chandler DE. Structural features of the abalone egg extracellular matrix and its role in gamete interaction during fertilization. Molecular Reproduction and Development. 1995;41(4):493–502. doi: 10.1002/mrd.1080410412. https://doi.org/10.1002/mrd.1080410412. [DOI] [PubMed] [Google Scholar]
- Nadel MR, Kafatos FC. Specific protein synthesis in cellular differentiation: IV. The chorion proteins of Bombyx mori and their program of synthesis. Developmental Biology. 1980;75(1):26–40. doi: 10.1016/0012-1606(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Nara M, Yonezawa N, Shimada T, Takahashi K, Tanokura M, Yumoto F, et al. Fourier transform infrared spectroscopic analysis of the intact zona pellucida of the mammalian egg: Changes in the secondary structure of bovine zona pellucida proteins during fertilization. Experimental Biology and Medicine (Maywood, NJ) 2006;231(2):166–171. doi: 10.1177/153537020623100206. 231/2/166 [pii] [DOI] [PubMed] [Google Scholar]
- Nayernia K, Adham IM, Shamsadin R, Muller C, Sancken U, Engel W. Proacrosin-deficient mice and zona pellucida modifications in an experimental model of multifactorial infertility. Molecular Human Reproduction. 2002;8(5):434–440. doi: 10.1093/molehr/8.5.434. [DOI] [PubMed] [Google Scholar]
- Neill AT, Vacquier VD. Ligands and receptors mediating signal transduction in sea urchin spermatozoa. Reproduction (Cambridge, England) 2004;127(2):141–149. doi: 10.1530/rep.1.00085. https://doi.org/10.1530/rep.1.00085. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Current Topics in Developmental Biology. 1999;41:67–97. doi: 10.1016/s0070-2153(08)60270-7. S0070-2153 (08)60270-7 [pii] [DOI] [PubMed] [Google Scholar]
- Niman HL, Hough-Evans BR, Vacquier VD, Britten RJ, Lerner RA, Davidson EH. Proteins of the sea urchin egg vitelline layer. Developmental Biology. 1984;102(2):390–401. doi: 10.1016/0012-1606(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Hinsch E, Pfisterer S, Veeck LL, Kolm P, Schill WB, et al. Use of a specific zona pellucida (ZP) protein 3 antiserum as a clinical marker for human ZP integrity and function. Fertility and Sterility. 1996;65(1):139–145. doi: 10.1016/s0015-0282(16)58041-8. S0015-0282(16)58041-8 [pii] [DOI] [PubMed] [Google Scholar]
- Okamura F, Nishiyama H. The passage of spermatozoa through the vitelline membrane in the domestic fowl, Gallus gallus. Cell and Tissue Research. 1978;188(3):497–508. doi: 10.1007/BF00219787. [DOI] [PubMed] [Google Scholar]
- Okumura H, Kohno Y, Iwata Y, Mori H, Aoki N, Sato C, et al. A newly identified zona pellucida glycoprotein, ZPD, and dimeric ZP1 of chicken egg envelope are involved in sperm activation on sperm-egg interaction. The Biochemical Journal. 2004;384(Pt 1):191–199. doi: 10.1042/BJ20040299. https://doi.org/10.1042/BJ20040299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi SR. All males are not created equal: Fertility differences depend on gamete recognition polymorphisms in sea urchins. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(22):12632–12637. doi: 10.1073/pnas.96.22.12632. https://doi.org/10.1073/pnas.96.22.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi SR. Speciation and the evolution of gamete recognition genes: Pattern and process. Heredity. 2009;102(1):66–76. doi: 10.1038/hdy.2008.104. https://doi.org/10.1038/hdy.2008.104. [DOI] [PubMed] [Google Scholar]
- Palumbi SR, Lessios HA. Evolutionary animation: How do molecular phylogenies compare to Mayr’s reconstruction of speciation patterns in the sea? Proceedings of the National Academy of Sciences of the United States of America. 2005;102(Suppl 1):6566–6572. doi: 10.1073/pnas.0501806102. 0501806102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi SR, Metz EC. Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra) Molecular Biology and Evolution. 1991;8(2):227–239. doi: 10.1093/oxfordjournals.molbev.a040642. [DOI] [PubMed] [Google Scholar]
- Papantonis A, Swevers L, Iatrou K. Chorion genes: A landscape of their evolution, structure, and regulation. Annual Review of Entomology. 2015;60:177–194. doi: 10.1146/annurev-ento-010814-020810. https://doi.org/10.1146/annurev-ento-010814-020810. [DOI] [PubMed] [Google Scholar]
- Parks S, Wakimoto B, Spradling A. Replication and expression of an X-linked cluster of Drosophila chorion genes. Developmental Biology. 1986;117(1):294–305. doi: 10.1016/0012-1606(86)90372-6. 0012-1606 (86)90372-6 [pii] [DOI] [PubMed] [Google Scholar]
- Pascucci T, Perrino J, Mahowald AP, Waring GL. Eggshell assembly in Drosophila: Processing and localization of vitelline membrane and chorion proteins. Developmental Biology. 1996;177(2):590–598. doi: 10.1006/dbio.1996.0188. [DOI] [PubMed] [Google Scholar]
- Paterson M, Jennings ZA, Wilson MR, Aitken RJ. The contraceptive potential of ZP3 and ZP3 peptides in a primate model. Journal of Reproductive Immunology. 2002;53(1–2):99–107. doi: 10.1016/s0165-0378(01)00105-x. S016503780100105X [pii] [DOI] [PubMed] [Google Scholar]
- Plaza S, Chanut-Delalande H, Fernandes I, Wassarman PM, Payre F. From A to Z: Apical structures and zona pellucida-domain proteins. Trends in Cell Biology. 2010;20(9):524–532. doi: 10.1016/j.tcb.2010.06.002. https://doi.org/10.1016/j.tcb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Pokkyla RM, Lakkakorpi JT, Nuojua-Huttunen SH, Tapanainen JS. Sequence variations in human ZP genes as potential modifiers of zona pellucida architecture. Fertility and Sterility. 2011;95(8):2669–2672. doi: 10.1016/j.fertnstert.2011.01.168. https://doi.org/10.1016/j.fertnstert.2011.01.168. [DOI] [PubMed] [Google Scholar]
- Polzonetti-Magni AM, Mosconi G, Soverchia L, Kikuyama S, Carnevali O. Multihormonal control of vitellogenesis in lower vertebrates. Academic Press; 2004. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453(7198):1064–1071. doi: 10.1038/nature06967. https://doi.org/10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Qi H, Williams Z, Wassarman PM. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Molecular Biology of the Cell. 2002;13(2):530–541. doi: 10.1091/mbc.01-09-0440. https://doi.org/10.1091/mbc.01-09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V, Sanchez LM, Alvarez J, Lopez-Otin C. Identification and characterization of human and mouse ovastacin: A novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. The Journal of Biological Chemistry. 2004;279(25):26627–26634. doi: 10.1074/jbc.M401588200. https://doi.org/10.1074/jbc.M401588200. [DOI] [PubMed] [Google Scholar]
- Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development (Cambridge, England) 1996;122(9):2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- Rankin TL, O’Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development (Cambridge, England) 2001;128(7):1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development (Cambridge, England) 1999;126(17):3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- Rice WR, Holland B. The enemies within: Intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific red queen. Behavioral Ecology and Sociobiology. 1997;41(1):1–10. https://doi.org/10.1007/s002650050357. [Google Scholar]
- Ruppert E, Fox R, Barnes R. Invertebrate zoology: A functional evolutionary approach. 7. Brooks Cole; 2004. [Google Scholar]
- Sainudiin R, Wong WSW, Yogeeswaran K, Nasrallah JB, Yang Z, Nielsen R. Detecting site-specific physicochemical selective pressures: Applications to the class I HLA of the human major histocompatibility complex and the SRK of the plant sporophytic self-incompatibility system. Journal of Molecular Evolution. 2005;60(3):315–326. doi: 10.1007/s00239-004-0153-1. https://doi.org/10.1007/s00239-004-0153-1. [DOI] [PubMed] [Google Scholar]
- Sano K, Kawaguchi M, Watanabe S, Nagakura Y, Hiraki T, Yasumasu S. Inferring the evolution of teleostean zp genes based on their sites of expression. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2013;320(5):332–343. doi: 10.1002/jez.b.22507. https://doi.org/10.1002/jez.b.22507. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Pan J, Mori M. Expression of perivitelline membrane glycoprotein ZP1 in the liver of Japanese quail (Coturnix japonica) after in vivo treatment with diethylstilbestrol. The Journal of Steroid Biochemistry and Molecular Biology. 2003;84(1):109–116. doi: 10.1016/s0960-0760(03)00008-6. S0960076003000086 [pii] [DOI] [PubMed] [Google Scholar]
- Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, et al. Extra-cellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1223–1228. doi: 10.1073/pnas.032389499. https://doi.org/10.1073/pnas.032389499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Tanaka E, Ban S, Yamasaki C, Fujino J, Ooura K, et al. Self/non-self recognition in ascidian fertilization: Vitelline coat protein HrVC70 is a candidate allorecognition molecule. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15615–15620. doi: 10.1073/pnas.0401928101. 0401928101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider WJ. Vitellogenin receptors: Oocyte-specific members of the low-density lipoprotein receptor supergene family. In: Jeon KW, editor. International review of cytology. Vol. 166. Academic Press; 1996. pp. 103–137. [DOI] [PubMed] [Google Scholar]
- Serizawa M, Kinoshita M, Rodler D, Tsukada A, Ono H, Yoshimura T, et al. Oocytic expression of zona pellucida protein ZP4 in Japanese quail (Coturnix japonica) Animal Science Journal = Nihon Chikusan Gakkaiho. 2011;82(2):227–235. doi: 10.1111/j.1740-0929.2010.00830.x. https://doi.org/10.1111/j.1740-0929.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- Shu L, Suter MJ, Räsänen K. Evolution of egg coats: Linking molecular biology and ecology. Molecular Ecology. 2015;24(16):4052–4073. doi: 10.1111/mec.13283. https://doi.org/10.1111/mec.13283. [DOI] [PubMed] [Google Scholar]
- Smith J, Paton IR, Hughes DC, Burt DW. Isolation and mapping the chicken zona pellucida genes: An insight into the evolution of orthologous genes in different species. Molecular Reproduction and Development. 2005;70(2):133–145. doi: 10.1002/mrd.20197. https://doi.org/10.1002/mrd.20197. [DOI] [PubMed] [Google Scholar]
- Spargo SC, Hope RM. Evolution and nomenclature of the zona pellucida gene family. Biology of Reproduction. 2003;68(2):358–362. doi: 10.1095/biolreprod.102.008086. [DOI] [PubMed] [Google Scholar]
- Spradling AC. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981;27(1 Pt 2):193–201. doi: 10.1016/0092-8674(81)90373-1. 0092-8674(81)90373-1 [pii] [DOI] [PubMed] [Google Scholar]
- Spradling AC, Mahowald AP. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Crespi BJ. Adaptive gamete-recognition divergence in a hybridizing mytilus population. Evolution; International Journal of Organic Evolution. 2007;61(4):772–783. doi: 10.1111/j.1558-5646.2007.00073.x. EVO073 [pii] [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Iuchi I. Molecular structure and hardening of egg envelope in fish. Recent Research Developments in Comparative Biochemistry & Physiology. 2000;1(1):139–161. [Google Scholar]
- Sugiyama H, Murata K, Iuchi I, Nomura K, Yamagami K. Formation of mature egg envelope subunit proteins from their precursors (choriogenins) in the fish, Oryzias latipes: Loss of partial C-terminal sequences of the choriogenins. Journal of Biochemistry. 1999;125(3):469–475. doi: 10.1093/oxfordjournals.jbchem.a022310. [DOI] [PubMed] [Google Scholar]
- Sun YP, Xu Y, Cao T, Su YC, Guo YH. Zona pellucida thickness and clinical pregnancy outcome following in vitro fertilization. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics. 2005;89(3):258–262. doi: 10.1016/j.ijgo.2005.02.012. S0020-7292(05)00157-8 [pii] [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Aagaard JE, Vacquier VD, Monne M, Al S, Hosseini H, et al. The molecular basis of sex: Linking yeast to human. Molecular Biology and Evolution. 2011;28(7):1963–1966. doi: 10.1093/molbev/msr026. https://doi.org/10.1093/molbev/msr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Aquadro CF, Vacquier VD. Polymorphism in abalone fertilization proteins is consistent with the neutral evolution of the egg’s receptor for lysin (VERL) and positive Darwinian selection of sperm lysin. Molecular Biology and Evolution. 2001;18(3):376–383. doi: 10.1093/oxfordjournals.molbev.a003813. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Molecular Biology and Evolution. 2003;20(1):18–20. doi: 10.1093/oxfordjournals.molbev.a004233. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The abalone egg vitelline envelope receptor for sperm lysin is a giant multivalent molecule. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):6724–6729. doi: 10.1073/pnas.94.13.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. Concerted evolution in an egg receptor for a rapidly evolving abalone sperm protein. Science (New York, NY) 1998;281(5377):710–712. doi: 10.1126/science.281.5377.710. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nature Reviews Genetics. 2002a;3(2):137–144. doi: 10.1038/nrg733. https://doi.org/10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Swanson W, Vacquier V. Reproductive protein evolution. Annual Review of Ecology and Systematics. 2002b;33:161–179. https://doi.org/10.1146/annurev.ecolsys.33.010802.150439. [Google Scholar]
- Takeuchi Y, Cho R, Iwata Y, Nishimura K, Kato T, Aoki N, et al. Morphological and biochemical changes of isolated chicken egg-envelope during sperm penetration: Degradation of the 97-kilodalton glycoprotein is involved in sperm-driven hole formation on the egg-envelope. Biology of Reproduction. 2001;64(3):822–830. doi: 10.1095/biolreprod64.3.822. [DOI] [PubMed] [Google Scholar]
- Tian J, Gong H, Lennarz WJ. Xenopus laevis sperm receptor gp69/64 glycoprotein is a homolog of the mammalian sperm receptor ZP2. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):829–834. doi: 10.1073/pnas.96.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Gong H, Thomsen GH, Lennarz WJ. Gamete interactions in Xenopus laevis: Identification of sperm binding glycoproteins in the egg vitelline envelope. The Journal of Cell Biology. 1997;136(5):1099–1108. doi: 10.1083/jcb.136.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topfer-Petersen E, Calvete JJ. Sperm-associated protein candidates for primary zona pellucida-binding molecules: Structure-function correlations of boar spermadhesins. Journal of Reproduction and Fertility. Supplement. 1996;50:55–61. [PubMed] [Google Scholar]
- Torgerson DG, Kulathinal RJ, Singh RS. Mammalian sperm proteins are rapidly evolving: Evidence of positive selection in functionally diverse genes. Molecular Biology and Evolution. 2002;19(11):1973–1980. doi: 10.1093/oxfordjournals.molbev.a004021. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR, Abou-Haila A, Loeser CR, Pereira BM. The biological and functional significance of the sperm acrosome and acrosomal enzymes in mammalian fertilization. Experimental Cell Research. 1998;240(2):151–164. doi: 10.1006/excr.1998.3943. S0014-4827(98)93943-X [pii] [DOI] [PubMed] [Google Scholar]
- Turner LM, Hoekstra HE. Causes and consequences of the evolution of reproductive proteins. The International Journal of Developmental Biology. 2008;52(5–6):769–780. doi: 10.1387/ijdb.082577lt. https://doi.org/10.1387/ijdb.082577lt. [DOI] [PubMed] [Google Scholar]
- Turner FR, Mahowald AP. Scanning electron microscopy of Drosophila embryogenesis: 1. The structure of the egg envelopes and the formation of the cellular blastoderm. Developmental Biology. 1976;50(1):95–108. doi: 10.1016/0012-1606(76)90070-1. [DOI] [PubMed] [Google Scholar]
- Vacquier VD. Evolution of gamete recognition proteins. Science (New York, NY) 1998;281(5385):1995–1998. doi: 10.1126/science.281.5385.1995. [DOI] [PubMed] [Google Scholar]
- Vacquier VD, Swanson WJ. Selection in the rapid evolution of gamete recognition proteins in marine invertebrates. Cold Spring Harbor Perspectives in Biology. 2011;3(11):a002931. doi: 10.1101/cshperspect.a002931. https://doi.org/10.1101/cshperspect.a002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier VD, Swanson WJ, Lee YH. Positive darwinian selection on two homologous fertilization proteins: What is the selective pressure driving their divergence? Journal of Molecular Evolution. 1997;44(Suppl 1):S15–22. doi: 10.1007/pl00000049. [DOI] [PubMed] [Google Scholar]
- Vieira A, Miller DJ. Gamete interaction: Is it species-specific? Molecular Reproduction and Development. 2006;73(11):1422–1429. doi: 10.1002/mrd.20542. https://doi.org/10.1002/mrd.20542. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Zona pellucida glycoproteins. Annual Review of Biochemistry. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. https://doi.org/10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Mammalian fertilization: Molecular aspects of gamete adhesion, exocytosis, and fusion. Cell. 1999;96(2):175–183. doi: 10.1016/s0092-8674(00)80558-9. S0092-8674(00)80558-9 [pii] [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Zona pellucida glycoproteins. The Journal of Biological Chemistry. 2008;283(36):24285–24289. doi: 10.1074/jbc.R800027200. https://doi.org/10.1074/jbc.R800027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM, Litscher ES. Biogenesis of the mouse egg’s extracellular coat, the zona pellucida. Current Topics in Developmental Biology. 2013;102:243–266. doi: 10.1016/B978-0-12-416024-8.00009-X. https://doi.org/10.1016/B978-0-12-416024-8.00009-X. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Litscher ES. Chapter thirty-one—A bespoke coat for eggs: Getting ready for fertilization. In: Wassarman PM, editor. Current topics in developmental biology: Vol. 117, Essays on developmental biology, Part B. Academic Press; 2016. pp. 539–552. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Mortillo S. Structure of the mouse egg extracellular coat, the zona pellucida. International Review of Cytology. 1991;130:85–110. doi: 10.1016/s0074-7696(08)61502-8. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Qi H, Litscher ES. Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proceedings of the Biological Sciences. 1997;264(1380):323–328. doi: 10.1098/rspb.1997.0046. https://doi.org/10.1098/rspb.1997.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Onitake K. The urodele egg-coat as the apparatus adapted for the internal fertilization. Zoological Science. 2002;19(12):1341–1347. doi: 10.2108/zsj.19.1341. https://doi.org/10.2108/zsj.19.1341. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Takayama-Watanabe E. In silico identification of the genes for sperm-egg interaction in the internal fertilization of the newt Cynops pyrrhogaster. The International Journal of Developmental Biology. 2014;58(10–12):873–879. doi: 10.1387/ijdb.140193aw. https://doi.org/10.1387/ijdb.140193aw. [DOI] [PubMed] [Google Scholar]
- Wilburn DB, Swanson WJ. From molecules to mating: Rapid evolution and biochemical studies of reproductive proteins. Journal of Proteomics. 2016;135:12–25. doi: 10.1016/j.jprot.2015.06.007. https://doi.org/10.1016/j.jprot.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilburn DB, Swanson WJ. The “ZP domain” is not one, but likely two independent domains. Molecular Reproduction and Development. 2017;84(4):284–285. doi: 10.1002/mrd.22781. https://doi.org/10.1002/mrd.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilburn DB, Tuttle LM, Klevit RE, Swanson WJ. Solution structure of sperm lysin yields novel insights into molecular dynamics of rapid protein evolution. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(6):1310–1315. doi: 10.1073/pnas.1709061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. Journal of Cell Science. 2007;120(Pt 8):1341–1349. doi: 10.1242/jcs.004291. jcs.004291 [pii] [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM. Defending the zygote: Search for the ancestral animal block to polyspermy. Current Topics in Developmental Biology. 2006a;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. S0070-2153(05) 72001-9 [pii] [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM. Rendezvin: An essential gene encoding independent, differentially secreted egg proteins that organize the fertilization envelope proteome after self-association. Molecular Biology of the Cell. 2006b;17(12):5241–5252. doi: 10.1091/mbc.E06-07-0634. https://doi.org/10.1091/mbc.E06-07-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WS, Yang Z, Goldman N, Nielsen R. Accuracy and power of statistical methods for detecting adaptive evolution in protein coding sequences and for identifying positively selected sites. Genetics. 2004;168(2):1041–1051. doi: 10.1534/genetics.104.031153. 168/2/1041 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li G, Cao L, Wang Z, Ye H, Chen X, et al. Proteomic characterization and evolutionary analyses of zona pellucida domain-containing proteins in the egg coat of the cephalochordate, Branchiostoma belcheri. BMC Evolutionary Biology. 2012;12 doi: 10.1186/1471-2148-12-239. 239-2148-12-239 https://doi.org/10.1186/1471-2148-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada L, Saito T, Taniguchi H, Sawada H, Harada Y. Comprehensive egg coat proteome of the ascidian Ciona intestinalis reveals gamete recognition molecules involved in self-sterility. The Journal of Biological Chemistry. 2009;284(14):9402–9410. doi: 10.1074/jbc.M809672200. https://doi.org/10.1074/jbc.M809672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami K, Hamazaki TS, Yasumasu S, Masuda K, Iuchi I. Molecular and cellular basis of formation, hardening, and breakdown of the egg envelope in fish. International Review of Cytology. 1992;136:51–92. doi: 10.1016/s0074-7696(08)62050-1. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Cherr G, Matsubara T, Andoh T, Harumi T, Vines C, et al. Sperm attractant in the micropyle region of fish and insect eggs. Biology of Reproduction. 2013;88(2):47. doi: 10.1095/biolreprod.112.105072. https://doi.org/10.1095/biolreprod.112.105072. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Phillips DM. The status of acrosomal caps of hamster spermatozoa immediately before fertilization in vivo. Gamete Research. 1984;9(1):1–19. https://doi.org/10.1002/mrd.1120090102. [Google Scholar]
- Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Molecular Biology and Evolution. 1998;15(5):568–573. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. msm088 [pii] [DOI] [PubMed] [Google Scholar]
- Yang Z, Swanson WJ, Vacquier VD. Maximum-likelihood analysis of molecular adaptation in abalone sperm lysin reveals variable selective pressures among lineages and sites. Molecular Biology and Evolution. 2000;17(10):1446–1455. doi: 10.1093/oxfordjournals.molbev.a026245. [DOI] [PubMed] [Google Scholar]
- Yauger B, Boggs NA, Dean J. Human ZP4 is not sufficient for taxon-specific sperm recognition of the zona pellucida in transgenic mice. Reproduction (Cambridge, England) 2011;141(3):313–319. doi: 10.1530/REP-10-0241. https://doi.org/10.1530/REP-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler KS, McCartney MA, Levitan DR, Lessios HA. Sea urchin bindin divergence predicts gamete compatibility. Evolution; International Journal of Organic Evolution. 2005;59(11):2399–2404. [PubMed] [Google Scholar]