Abstract
Background
We recently demonstrated the acceptability and feasibility of a randomized, double-blind choline supplementation intervention for heavy drinking women during pregnancy. In this paper, we report our results relating to the efficacy of this intervention in mitigating adverse effects of prenatal alcohol exposure on infant growth and cognitive function.
Methods
69 Cape Coloured (mixed ancestry) heavy drinkers in Cape Town, South Africa, recruited in mid-pregnancy, were randomly assigned to receive a daily oral dose of either 2 g of choline or placebo from time of enrollment until delivery. Each dose consisted of an individually wrapped packet of powder that, when mixed with water, produced a sweet tasting grape-flavored drink. The primary outcome, eyeblink conditioning (EBC), was assessed at 6.5 months. Somatic growth was measured at birth, 6.5, and 12 months; recognition memory and processing speed on the Fagan Test of Infant Intelligence, at 6.5 and 12 months.
Results
Infants born to choline-treated mothers were more likely to meet criterion for conditioning on EBC than the placebo group. Moreover, within the choline arm, degree of maternal adherence to the supplementation protocol strongly predicted EBC performance. Both groups were small at birth, but choline-treated infants showed considerable catch-up growth in weight and head circumference at 6.5 and 12 months. At 12 months, the infants in the choline treatment arm had higher novelty preference scores, indicating better visual recognition memory.
Conclusions
This exploratory study is the first to provide evidence that a high dose of choline administered early in pregnancy can mitigate adverse effects of heavy prenatal alcohol exposure on EBC, postnatal growth, and cognition in human infants. These findings are consistent with studies of alcohol-exposed animals that have demonstrated beneficial effects of choline supplementation on classical conditioning, learning, and memory.
Keywords: fetal alcohol spectrum disorders, fetal alcohol syndrome, prenatal alcohol exposure, choline supplementation, eyeblink conditioning, growth
Introduction
Prenatal alcohol exposure (PAE) has been linked to a broad range of deficits in growth and neurobehavioral function, collectively referred to as fetal alcohol spectrum disorders (FASD), which comprise the most common preventable cause of neurodevelopmental disabilities worldwide. Prevalence estimates range from 1.1–5.0% in the US (May et al., 2018) to 13.6–20.9% in South Africa (May et al., 2013). Fetal alcohol-related effects are seen on pre- and postnatal growth (Jacobson et al., 1994a,b; Carter et al., 2016a; Day et al., 2002), IQ (Jacobson et al., 2004; Mattson et al., 1997; Streissguth et al., 1990), and learning and memory (Mattson and Roebuck, 2002; Lewis et al., 2015). In infancy, PAE has been linked to poorer performance on the Bayley Scales of Infant Development (BSID; Streissguth et al., 1980; J. Jacobson et al., 1993) and recognition memory and slower information processing (S. Jacobson et al., 1993; Kable and Coles, 2004).
We have identified a strikingly consistent effect of PAE on eyeblink conditioning (EBC), a culturally-neutral, Pavlovian paradigm that involves contingent temporal pairing of a conditioned stimulus (e.g., auditory tone) with an unconditioned stimulus (e.g., air puff) (Jacobson et al., 2008). In our Cape Town, South Africa, cohort, not a single child with full FAS met criterion for conditioning at age 5 years, compared with 75% of non-exposed controls. Two-thirds of the other heavily exposed children also failed to meet criterion for conditioning. PAE-related EBC impairment was seen again in these children at 10 years (Cheng et al., 2017), in a second Cape Town cohort at 11 years (Jacobson et al. 2011a), and in an independent study of school-age children in the U.S. (Coffin et al., 2005).
The neural circuitry involved in EBC has been documented across a range of animal species and across the life-span (Woodruff-Pak and Steinmetz, 2000). By 5 months postpartum, typically-developing human infants reach the same terminal level in delay EBC as adults (Herbert et al., 2003). Heavy alcohol exposure during the equivalent of the 3rd trimester of pregnancy in humans disrupts EBC in weanling rats (Stanton and Goodlett, 1998) and adults, a deficit that is mediated by dose-dependent cell loss and altered neural activity in the deep cerebellar nuclei (Green et al., 2002). Binge exposure during this period in rodents is also associated with loss of Purkinje and granule cells in the cerebellum (Hamre and West, 1993). Neuroimaging studies of humans, using functional MRI (Cheng et al., 2017), diffusion tensor imaging (Fan et al., 2015), and magnetic resonance spectroscopy (du Plessis et al., 2015), have documented adverse effects of PAE on structure and function of the neural network known to support EBC.
We have been conducting longitudinal research on children and mothers recruited during pregnancy in a Cape Coloured (mixed ancestry) community in Cape Town since 1999 (Jacobson et al., 2008). Despite widely disseminated public health advisories, including educational brochures and posters in antenatal clinics, information about risks of pregnancy drinking provided by nurse/midwives, and numerous psychosocial community-based interventions, heavy drinking during pregnancy continues to be highly prevalent in this community (May et al., 2013). Given the limited effectiveness of these interventions, there is a critical need for pharmacological and/or nutritional treatments that may mitigate the teratogenic effects of alcohol.
Laboratory studies have shown that choline supplementation in rats has remarkable potential to mitigate effects of PAE on a range of behavioral outcomes, including working memory, spatial learning and hyperactivity, and reversal learning (Thomas et al., 2000, 2004, 2007), and trace fear conditioning (Wagner and Hunt, 2006). The beneficial effects of choline supplementation early in development persisted even after treatment was completed, indicating long-lasting changes in central nervous system organization and development. Thomas and Tran (2012) have demonstrated that choline supplementation also mitigates alcohol’s effects on trace EBC. The deficit in acquisition of conditioned responses (CRs) seen in alcohol-exposed rats was not seen in the alcohol-exposed group treated with choline, which performed as well as non-exposed controls. Choline was administered during the equivalent of the 3rd trimester of pregnancy in these studies. When administered earlier in pregnancy, treatment is even more effective in enhancing cognition in typically developing rats (Meck et al., 1989) and can mitigate effects on brain and body weight, reflexes, and motor coordination in alcohol-exposed rats (Thomas et al. 2009).
Choline, an essential nutrient that is a precursor to the neurotransmitter acetylcholine, plays an important role in cell membrane integrity, trans-membrane signaling, and lipid and cholesterol transport and metabolism (Zeisel and Niculescu, 2006). It also serves as a methyl-group donor needed for homocysteine metabolism and DNA methylation, a critical mechanism in epigenetic processes that have been implicated in alcohol teratogenesis (Zeisel, 2011). Choline is derived from dietary intake, principally eggs, liver, wheat germ, and milk, and from endogenous synthesis, catalyzed by the enzyme phosphatidylethanolamine-N-methyltransferase (PEMT; Resseguie et al., 2007). A common SNP variant (rs12325817) in the promoter region of the PEMT gene confers a markedly higher risk for choline deficiency (da Costa et al., 2006). Choline dietary intake in pregnant women is often much lower than the 450 mg/d recommended by the Institute of Medicine (IOM, 2006). U.S. National Health and Nutrition Examination Survey data show that only 7% of women achieve the adequate intake (AI) level for choline (Chester et al., 2011). In the U.S., the lowest quartile of choline intake in women of reproductive age is 25–50% of the AI (Wallace et al., 2014), and in developing countries, including South Africa, choline intake is even lower (Gossell-Williams et al., 2005; Carter et al., 2017).
In a previous paper (Jacobson et al., submitted), we have reported findings from a pilot, randomized placebo-controlled trial demonstrating the acceptability and feasibility of a maternal choline supplementation intervention for heavy drinking women during pregnancy. In this paper, we report our results relating to the efficacy of this intervention. The aims of this study were to assess (1) the efficacy of prenatal choline supplementation in mitigating adverse effects of PAE on our primary outcome, EBC, and (2) efficacy in mitigating deficits in three secondary outcomes—pre- and postnatal growth restriction, recognition memory, and information processing speed. We also examined the effects of choline on FASD diagnosis and the degree to which choline supplementation is particularly effective in women with choline deficient diets and in women who carry the rs12325817 variant of the enzyme PEMT, which limits endogenous choline synthesis.
Methods
Trial Design
Women were randomly assigned to either choline supplementation or placebo using a randomization list with variable blocks of 2 and 4 subjects and 1:1 allocation ratio generated by a biostatistician not otherwise involved in the trial. All investigators and research staff remained blind to the participants’ treatment groups throughout the trial.
Participants
Recruitment occurred between April 2012 and September 2014; the last infant was born in January 2015. 69 women from the Cape Coloured (mixed ancestry) community participated in the trial. Procedures for recruitment, ascertainment of maternal alcohol consumption during pregnancy, and randomization are detailed in Jacobson et al. (submitted). Briefly, maternal alcohol consumption was assessed using a timeline follow-back (TLFB) interview. Heavy drinking during pregnancy was defined as an average of at least 2 standard drinks (1.0 oz absolute alcohol (AA))/day or 2 or more incidents of binge drinking (4 or more drinks/occasion). Maternal exclusionary criteria were weeks gestation at recruitment >23; age <18 years; HIV or syphilis infection; multiple-gestation pregnancy; pharmacologic treatment for a serious pre-existing medical condition (e.g., diabetes, hypertension, epilepsy, or cardiac problems); use of methamphetamine, a popular drug at the time of recruitment; plans to leave Cape Town before study completion; and dietary choline intake ≥1.5g/day, assessed prior to randomization using a quantitative choline food frequency questionnaire (QFFQ) developed for this study (Carter et al., submitted). Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, seizures, very low birth weight (<1500 g), and gestational age (GA) <32 weeks.
Human subjects approval was obtained from the Wayne State University, University of Cape Town (UCT) Faculty of Health Sciences, and Columbia University Medical Center Institutional Review Boards, and the South African Medicines Control Council. Informed consent was obtained from each mother and the father, if available. An independent data safety monitoring board (DSMB), comprised of a developmental psychologist, obstetrician, neonatologist, and statistician, met with SWJ, RCC, CDM, and JLJ via telephone/Skype prior to the initiation of the trial to review the research plan and every 9 months thereafter to review the progress of the trial and any study-related adverse events.
Treatment Protocol
Each participant was instructed to take 2 daily doses (1 in the morning, 1 in the evening) from time of enrollment until delivery. Each choline supplement dose consisted of 1.25g choline bitartrate, which contained 1g of bioavailable choline cation. The daily choline dose of 2g was chosen in consultation with SZ to maximize potential benefit while being well within the parameters for maternal and fetal safety during pregnancy based on the tolerable upper intake level (UL) for choline determined by the Institute of Medicine Food and Nutrition Board (IOM, 2006) (see Jacobson et al., submitted). Each dose consisted of an individually wrapped packet of powder that, when mixed with 8 oz of water, results in a sweet tasting, slightly fizzy, grape-flavored drink. The choline supplement and placebo were indistinguishable in terms of taste, smell, and appearance. When the participant was given the packets, CDM cautioned her that taking the drink mix would not make it safe to drink alcohol during pregnancy. At the initiation of the trial, the mother was given a box with a 1-month supply of drink packets. At the end of each month, the nurse collected the box (with used and unused packets), replacing it with a new 1-month supply, and number of packets used was tabulated in our laboratory. Adherence was good-to-excellent (median doses taken=74.0%; interquartile range=53.9–88.7) for most participants, and poor adherence (≤33.0%) was relatively rare (11.3% of participants). Plasma choline concentrations increased significantly more in the choline supplementation arm over the course of the trial than in the placebo arm (group by treatment phase interaction, t(105)=3.36, p=0.001; see Fig. 4 in Jacobson et al. (in press).
Maternal Assessments
The TLFB interview was re-administered at 4- and 12-weeks following randomization, and data from the three interviews were averaged to provide continuous measures of alcohol consumption during pregnancy. Alcohol abuse and/or dependence was diagnosed based on DSM-IV criteria using the Structured Clinical Interview for DSM-IV. Extensive information was also collected regarding sociodemographic background and illicit drug use. Nutritional status was assessed using a multiple-pass 24-hr dietary recall interview. Adequate dietary caloric intake was defined as average daily nutrient intake (adjusted using the Nutrition Research Council [NRC] method, Dodd et al., 2006) below the estimated energy requirement (Prentice et al., 1996) for energy or, for nutrients, the estimated average requirement (EAR) or, where no EAR is available, the AI (Institute of Medicine, 2006). Dietary choline intake was assessed on the QFFQ prior to initiation of and twice during the trial. Adequate choline intake, defined as ≥450 mg/day (IOM, 2006), was determined from average intake reported on the three QFFQs. Maternal whole blood samples were genotyped for the PEMT SNP rs12325817, using real-time PCR performed on an Eppendorf Realplex 4.0 (Eppendorf North America, Westbury, NY, USA).
Sample Attrition
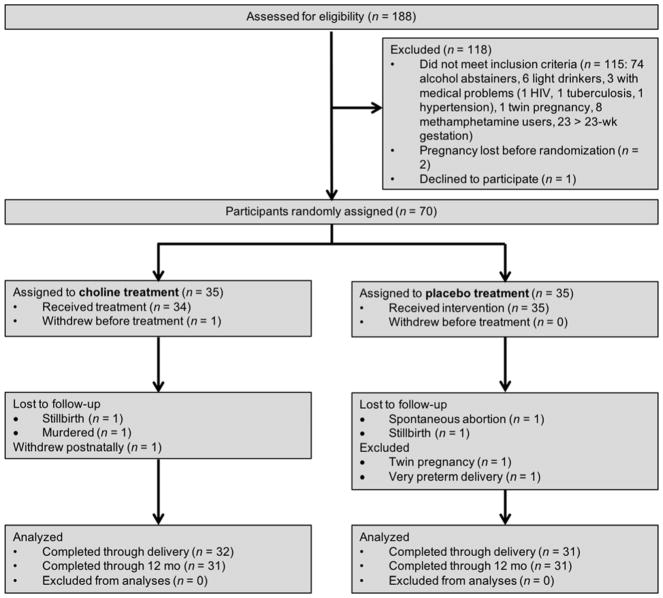
A flow diagram of the progression of participants through the trial is presented in Figure 1. 70 women were randomly assigned to condition, but 1 withdrew from the study prior to initiating treatment. Of the 69 in the trial, there were 4 non-study-related fetal deaths (1 spontaneous abortion, 2 stillbirths, 1 fetus whose mother was murdered during pregnancy); 2 women who met a priori exclusionary criteria were removed from the sample (1 twin pregnancy diagnosed after randomization, 1 very preterm delivery (<29 wk gestation)); and 1 woman withdrew after delivery but prior to the 6.5-month infant assessments. In this paper we present data on the 62 infants (31 choline, 31 placebo) who were assessed during the trial.
Figure 1.
Flow diagram of the progression of participants through the trial
Infant Assessments
EBC
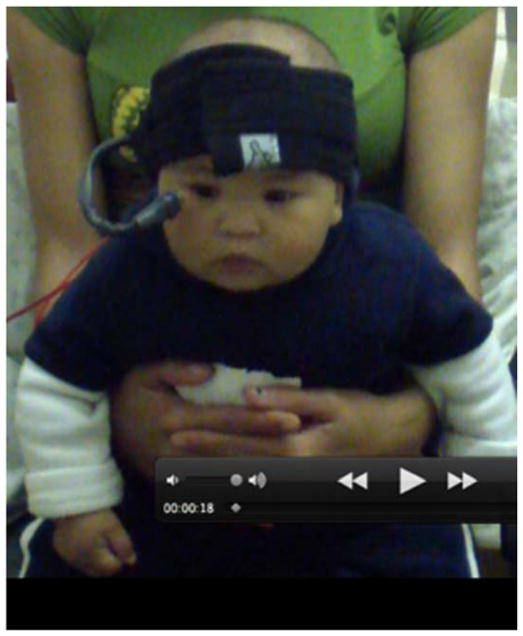
EBC was assessed at 6.5 months (with correction for GA in cases of preterm birth (GA<37 weeks)), using the procedure developed by Ivkovich et al. (1999) and Herbert et al. (2003), in which the infant is entertained by a research assistant using a visual display of brightly colored moving objects and toys while being administered the EBC trials. We used the same commercially available human EBC system (San Diego Instruments, Model #2325-0145-W) from our two previous studies with older children (Jacobson et al., 2008, 2011a). The infant wore a headband which supported a flexible plastic tube that delivered an air puff to the right eye, at a distance of ~2.5 cm (Fig. 2). Eyelid closure was measured with a photodiode placed at the corner of the right eye. Above the head, ~45 cm to either side, two 7 Ohm speakers delivered a 1-kHz, 80-dB tone. The interface units generated the auditory conditioned stimulus (CS) and air puff unconditioned stimulus (US), processed the eyeblink signal, and integrated the peripheral devices with the personal computer.
Figure 2.
Headband supporting tube that (1) delivers air puff to right eye and (2) photodiode that measures right eyelid closure, at a distance of ~2.5 cm.
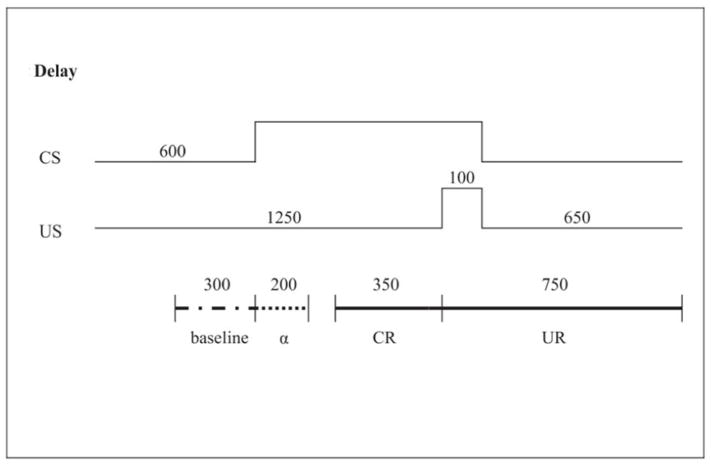
Each session consisted of 51 trials of the delay EBC procedure, in which the air puff was administered simultaneously during the last 100ms of a 750ms presentation of the tone (Fig. 3). The intertrial interval ranged from 8–16s, with an average of 1 trial every 12s. Every fifth trial in each block of 10 was an air puff-alone trial to test for somatosensory responsiveness. Every 10th trial was a tone-alone trial to test for conditioned responding in the absence of the subsequent air puff. In this paradigm the infant must learn to adjust the timing of the anticipatory blink, which occurred optimally between 300 and 650ms after the onset of the tone. Eyeblinks within 350ms prior to the air puff onset were considered CRs (Herbert et al., 2003). Responses that occurred in the first 200ms after the tone onset were coded as alpha or startle responses (see Jacobson et al., 2008). EBC sessions were administered on 3 days. Time between visits did not differ between groups: choline (M=4.0 days between visits) vs. placebo (M=4.5), t=1.22, p=0.226.
Figure 3.
CS and US onset and offset times (2000 milliseconds total) and data recording windows for CR and UR. CS, conditioned stimulus (tone); US, unconditioned stimulus (air puff); CR, conditioned response (blink to CS); UR, unconditioned response (blink to US); a, startle response.
Preprocessing of the data was similar to our previous reports with children (Jacobson et al., 2008; 2011a) with modifications to address movement and other signal-to-noise integrity artifacts that arise when studying EBC in infants (Ivkovich et al., 1999). Sessions in which at least 22 trials provided usable data were considered acceptable. Of the 62 infants assessed at 6.5 months, 11 were excluded (6 choline, 5 placebo) due to excessive movement (noisy data) and/or problems with sensor placement (e.g., sensor became misaligned during the session so that the air puff and photodiode sensor were no longer properly aligned with the eye). An infant met criterion for conditioning if s/he exhibited CRs in at least 50% of the trials administered during at least one of the three sessions. Data were excluded from an additional three infants who failed to meet this criterion in the first two sessions and did not provide usable data for the third session (i.e., did not have the opportunity to demonstrate whether they would have conditioned during the final session). There was no difference between the number of infants excluded from the EBC analyses in the two treatment arms, χ2(1) = 1.48, p = 0.224.
Infant growth
Birth weight, length, and head circumference were obtained from medical records. GA was based on early gestation ultrasound, which was available for 85.7% of the participants, or date of last menstrual period. Infant weight, length, and head circumference were measured at 6.5 and 12 months by research staff trained by RCC using standard WHO protocols; weight, length, and head circumference z-scores and percentiles were calculated using WHO norms, which adjust for age and sex (de Onis et al., 2004).
Fagan Test of Infant Intelligence (FTII)
Visual recognition memory was assessed at 6.5 and 12 months on the FTII (Fagan and Singer, 1983). The infant, seated on the mother’s lap, is first shown two identical photographs and then a novel photograph paired with the familiar one. The normative response, preference for the novel stimulus, indicates ability to recall the familiar stimulus and discriminate it from the novel one. Infant fixation was recorded on a computer, and novelty preference was computed by dividing duration of time looking at the novel stimulus by total time looking at the paired familiar and novel stimuli for each of the 10 problems. Mean length of look, a measure of information processing speed (Colombo et al., 1991), was computed for each problem by dividing the total duration looking time by the number of looks. A pattern of short looks is believed to reflect more rapid and efficient processing of information. Usable FTII data were available for 53 infants (85.5%) at 6.5 months and 52 (83.9%) at 12 months (e.g., fussiness, inattention).
FASD diagnosis
In 2016 and 2017 we organized diagnostic clinics, in which the infants were examined for growth and anomalies independently by HEH and one of four other experienced dysmorphologists, using the revised Institute of Medicine guidelines (Hoyme et al., 2005). All 69 mothers had confirmed heavy alcohol consumption during pregnancy. Infants with at least 2 of the 3 principal dysmorphic features (flat philtrum and thin vermilion [4 or 5 on the Astley and Clarren (2000) lip-philtrum guide] and short palpebral fissures (<10th percentile); microcephaly (<10th percentile); and weight or length restriction (<10th percentile) were diagnosed with FAS. A diagnosis of PFAS required presence of 2 of the 3 facial anomalies and microcephaly or weight or length restriction. The other infants were classified as heavily exposed nonsyndromal (HE). The dysmorphologists, SWJ, RCC, CDM, and JLJ met daily in case conferences following the clinic to reach consensus regarding which infants met criteria for FAS or PFAS diagnoses.
Data Analysis
Statistical analyses were performed using SPSS software (v.22; IBM, Armonk, NY). Demographic background and exposure for the 62 infants whose data are reported in this paper were compared using t-tests and χ2 analysis. χ2 was used to compare the number of infants who met criterion for conditioning in the choline and placebo arms, and mixed model repeated measures regression was used to compare changes in percent CRs across the three EBC sessions in the two groups. Pearson correlation was used to examine the relation of protocol adherence to percent CRs in Session 3 for the choline and placebo arms separately. T-tests were used to compare anthropometric measures between treatment arms during the newborn period; analysis of covariance, to compare treatment arms on the 6.5- and 12-month measures after adjustment for their corresponding anthropometric measures at birth. Mixed model repeated measures regression was used to compare growth trajectory percentiles between groups from birth through 12 months. T-tests were used to compare treatment arms on the FTII measures. χ2 was used to compare incidence of FAS or PFAS in the two groups. Two-way analysis of variance (ANOVA; choline vs. placebo X adequate vs. inadequate choline intake during pregnancy) was used to test the hypothesis that the effects of the choline treatment would be stronger in infants born to mothers with inadequate choline intake during pregnancy. Two-way ANOVA (choline vs. placebo X presence vs. absence of the maternal PEMT polymorphism rs12325817) was used to test the hypothesis that treatment effects would be stronger in infants born to mothers with the polymorphism.
Results
Sample Characteristics
The treatment and placebo arms did not differ in social class or maternal education, verbal and nonverbal intellectual competence, perceived stress, depression, or nutritional status (Table 1). About 30% of both groups were poorly nourished based on their dietary caloric intake, and more than 70% did not meet the AI for dietary choline intake. There were no group differences in alcohol consumption, which was very heavy for both groups at time of conception (≈10.0 standard drinks/occasion on an average of 2–3 days/week). Women in both arms continued to drink heavily across pregnancy (≈8–9 drinks/occasion) but reduced their frequency to about 1–2 days/week. Both groups smoked about ¼ pack/day on average, and cigarettes/day was only slightly higher among those in the choline group. Use of illicit drugs other than marijuana was rare; none of the women reported using cocaine, heroin, or methaqualone. Although women who used methamphetamine were excluded at time of recruitment, 4 reported using it later in pregnancy (2 choline, 2 placebo). There were no group differences in weeks gestation at time of booking, initial screening, or randomization or duration of choline supplementation. Weeks gestation at randomization ranged from 8.6–26.0 (Median=20.0); duration of choline supplementation ranged from 9–27 weeks (Median=18.6). The groups differed on sex of infant, with more girls in the choline and more boys in the placebo arm. There were no group differences in infant age at the 6.5- and 12-month assessments.
Table 1.
Comparison of maternal sociodemographic characteristics and pregnancy alcohol and drug use (N = 62)
| Treatment group
|
t or χ2 | ||
|---|---|---|---|
| Choline (n = 31) |
Placebo (n = 31) |
||
| Maternal characteristics | |||
| Maternal age at delivery | 26.6 | 27.0 | 0.33 |
| (5.6) | (5.3) | ||
| Socioeconomic statusa | 20.7 | 20.8 | 0.06 |
| (8.4) | (7.5) | ||
| Education (years) | 9.1 | 9.4 | 0.74 |
| (1.9) | (1.7) | ||
| Marital status (% married) | 29.0 | 35.5 | 0.30 |
| Parity | 2.4 | 2.7 | 1.06 |
| (2.8) | (0.9) | ||
| Gravidity | 2.9 | 2.7 | 0.46 |
| (1.9) | (1.4) | ||
| Peabody Picture Vocabulary Test IQ | 46.5 | 46.9 | 0.15 |
| (7.7) | (9.6) | ||
| Raven Progressive Matrices | 20.4 | 22.3 | 0.81 |
| (11.4) | (12.3) | ||
| Beck Depression Inventory | 9.0 | 9.8 | 0.37 |
| (9.2) | (9.2) | ||
| Life Events (number) | 7.5 | 8.3 | 0.73 |
| (3.4) | (4.5) | ||
| Perceived Life Stress | 28.9 | 28.8 | 0.02 |
| (18.5) | (24.6) | ||
| Nutritional status | |||
| Dietary caloric intake (kJ/day)b | 9319.9 | 10201.6 | 1.01 |
| (2916.8) | (3875.3) | ||
| Inadequate dietary caloric intake (%) | 29.4 | 30.3 | 0.01 |
| Rate of gestational weight gain (kg/wk) | 0.4 | 0.4 | 0.58 |
| (0.3) | (0.3) | ||
| Inadequate gestational weight gain (%) | 58.8 | 54.5 | 0.13 |
| Dietary choline intake (mg/day)c | |||
| Baseline | 368.5 | 370.9 | 0.04 |
| (221.5) | (261.0) | ||
| Post-treatment (average) | 252.5 | 290.7 | 1.22 |
| (132.3) | (113.4) | ||
| Inadequate dietary choline intake (%) | 73.5 | 71.4 | 0.04 |
| Alcohol during pregnancy | |||
| Oz absolute alcohol (AA)/day at conception | 1.8 | 1.6 | 0.55 |
| (1.8) | (0.9) | ||
| Oz AA/drinking occasion at conception | 4.5 | 5.1 | 0.87 |
| (3.0) | (2.8) | ||
| Frequency of drinking at conception (days/week) | 2.5 | 2.1 | 1.12 |
| (1.2) | (1.0) | ||
| Oz AA/day across pregnancy | 0.8 | 0.8 | 0.17 |
| (0.9) | (0.7) | ||
| Oz AA/drinking occasion across pregnancy | 4.1 | 4.3 | 0.27 |
| (2.3) | (2.4) | ||
| Frequency of drinking across pregnancy (days/week) | 1.3 | 1.3 | 0.03 |
| (0.9) | (0.8) | ||
| History of alcohol abuse and/or dependence (%) | 19.0 | 17.0 | 0.24 |
| Cigarettes/day during pregnancy | 6.4 | 4.2 | 2.19* |
| (4.1) | (3.7) | ||
| Marijuana during pregnancy (days/month) | 3.5 | 1.0 | 1.63 |
| (7.7) | (3.6) | ||
| Methamphetamine during pregnancy (days/month) | 0.3 | 0.01 | 1.23 |
| (1.3) | (0.03) | ||
| Weeks gestation at 1st antenatal visit | 15.7 | 14.9 | 0.67 |
| (4.2) | (4.8) | ||
| Weeks gestation at initial screening | 17.3 | 17.4 | 0.11 |
| (4.5) | (4.3) | ||
| Weeks gestation at randomization | 19.3 | 19.8 | 0.53 |
| (3.8) | (3.8) | ||
| Treatment duration (days) | 131.9 | 128.6 | 0.58 |
| (28.3) | (17.0) | ||
| Breastfeeding duration (wk) | 38.3 | 39.3 | 0.22 |
| (19.1) | (18.1) | ||
| Infant characteristics | |||
| Sex (% male) | 32.3 | 61.3 | 5.25* |
| Age at testing (months) | |||
| 6.5 months | 6.6 | 6.7 | 1.01 |
| (0.4) | (0.5) | ||
| 12 months | 12.1 | 12.1 | 0.35 |
| (0.3) | (0.3) | ||
Values are mean (standard deviation)
Hollingshead (2011) Scale
Based on multiple pass 24-hr dietary recall interviews, quantified using FoodFinder3®. 4.18 kJ = 1 kcal.
Based on choline-indicated food frequency questionnaire (QFFQ), adjusted for daily caloric intake
p < 0.05
Eyeblink Conditioning
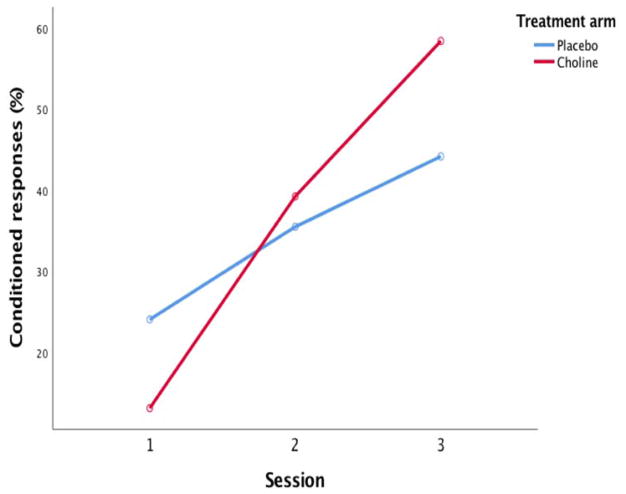
Among the 48 infants with usable EBC data, a larger proportion of the choline group met criterion for conditioning compared with the placebo group, although the effect fell short of conventional levels of statistical significance (Table 2). When the four infants whose mothers’ adherence was very poor (<20%) were excluded from the analysis, the effect was significant despite the smaller sample size; two-thirds of choline-treated infants met criterion for conditioning, compared with only one-third of the placebo group. When percent CRs in the two arms was examined across the three sessions, there was a main effect for session, F(2,60)=32.66, p<0.001, but not for treatment, F(1,30)=0.19, p>0.20 (Fig. 4). The infants in the choline arm showed a significantly greater increase in percent CRs across the three sessions than the placebo group (group by session interaction, F(2,60)=4.85, p<0.01.
Table 2.
Effects of choline supplementation on eyeblink conditioning
| Whole sample
|
Adherencea > 20%
|
|||||
|---|---|---|---|---|---|---|
| Choline | Placebo | Total | Choline | Placebo | Total | |
|
|
|
|||||
| Fail | 9 | 17 | 26 | 6 | 17 | 23 |
| 40.9% | 65.4% | 54.2% | 33.3% | 65.4% | 52.3% | |
| Pass | 13 | 9 | 22 | 12 | 9 | 21 |
| 59.1% | 34.6% | 45.8% | 66.7% | 34.6% | 47.7% | |
| Total | 22 | 26 | 48 | 18 | 26 | 44 |
| 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |
|
|
|
|||||
| χ2 = 2.88; p = 0.090 | χ2 = 4.38; p = 0.036 | |||||
Adherence to supplementation protocol
Figure 4.
Percent CRs across the three eyeblink conditioning sessions by treatment group.
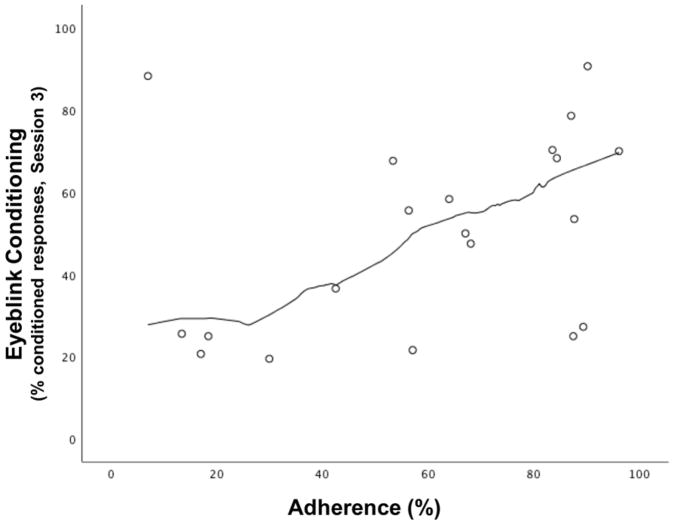
The relation between protocol adherence and percent CRs in Session 3 for the choline treatment group is shown in Figure 5. An analysis of Mahalanobis distance identified one bivariate maternal outlier (p<0.007) with very poor adherence and high percent CRs. When that outlier was removed from the analysis, there was a clear linear relation between degree of adherence and conditioning by Session 3, r=0.63, p<0.01. By contrast, adherence was not related to percent CRs in the placebo group, r=−0.14, p>0.20.
Figure 5.
Relation of protocol adherence (% packets used) in the choline-treated group to percent CRs during eyeblink conditioning session 3.
Gestational Age and Somatic Growth
The majority of the women (93.7%) had full-term pregnancies, and GA did not differ between groups (Table 3). Although low birthweight (<2500 g) was common (26.1%), the incidence (choline 25.0%, placebo 32.3%) was similar between arms, χ2(1)=0.41, p=0.524. There were also no significant group differences in birth size. With the exception of length in the placebo group, birth percentile measures were markedly below the 50th percentile, likely due to the high levels of alcohol to which this cohort was exposed.
Table 3.
Effects of choline supplementation on gestational age and somatic growth
| Treatment group
|
t or Fa | d | ||
|---|---|---|---|---|
| Choline
|
Placebo
|
|||
| (n = 31) | (n = 31) | |||
| Birth | ||||
| Gestational age (weeks) | 38.8 | 38.9 | 0.28 | 0.05 |
| (1.6) | (2.5) | |||
| Weight (g) | 2852.7 | 2844.2 | 0.06 | 0.02 |
| (450.9) | (658.0) | |||
| Weight-for-age z-scoreb | −1.0 | −1.1 | 0.21 | 0.05 |
| (1.1) | (1.5) | |||
| Weight-for-age percentileb | 25.9 | 28.3 | 0.32 | 0.08 |
| (25.2) | (31.6) | |||
| Length (cm) | 47.2 | 48.9 | 1.82† | 0.49 |
| (3.3) | (3.7) | |||
| Length-for-age z-scoreb | −1.2 | −0.4 | 1.61 | 0.32 |
| (1.8) | (2.0) | |||
| Length percentileb | 30.6 | 47.1 | 1.75† | 0.45 |
| (34.2) | (38.7) | |||
| Head circumference (cm) | 32.6 | 33.0 | 1.00 | 0.24 |
| (1.5) | (1.9) | |||
| Head circumference-for-age z-scoreb | −1.2 | −1.0 | 0.66 | 0.14 |
| (1.2) | (1.5) | |||
| Head circumference percentileb | 23.8 | 30.9 | 0.99 | 0.25 |
| (24.4) | (31.6) | |||
| 6.5 months | ||||
| Weight (kg) | 7.5 | 7.3 | 0.80 | 0.19 |
| (1.0) | (1.1) | |||
| Weight-for-age z-scoreb | −0.4 | −0.8 | 2.81† | 0.26 |
| (1.2) | (1.3) | |||
| Weight percentile | 43.8 | 27.1 | 5.76* | 0.52 |
| (32.4) | (31.5) | |||
| Length (cm) | 64.9 | 64.3 | 1.04 | 0.24 |
| (2.6) | (2.5) | |||
| Length-for-age z-scoreb | −1.1 | −1.7 | 6.28* | 0.56 |
| (1.1) | (1.2) | |||
| Length percentile | 23.7 | 15.3 | 2.23 | 0.36 |
| (23.4) | (23.0) | |||
| Head circumference (cm) | 42.6 | 42.2 | 1.45 | 0.29 |
| (1.5) | (1.3) | |||
| Head circumference-for- age z-scoreb | −0.3 | −0.9 | 5.95* | 0.61 |
| (1.2) | (1.0) | |||
| Head circumference percentileb | 41.9 | 25.1 | 5.62* | 0.59 |
| (30.9) | (26.1) | |||
| 12 months | ||||
| Weight (kg) | 9.0 | 8.6 | 1.48 | 0.31 |
| (1.4) | (1.2) | |||
| Weight-for-age z-scoreb | −0.4 | −0.8 | 2.76 | 0.25 |
| (1.4) | (1.2) | |||
| Weight percentileb | 43.5 | 27.0 | 5.13* | 0.53 |
| (33.7) | (28.3) | |||
| Length (cm) | 71.9 | 71.0 | 1.66 | 0.30 |
| (3.2) | (2.8) | |||
| Length-for- age z-scoreb | −1.1 | −1.7 | 5.28* | 0.54 |
| (1.2) | (1.1) | |||
| Length percentileb | 22.2 | 12.4 | 3.45† | 0.45 |
| (23.2) | (20.0) | |||
| Head circumference (cm) | 44.7 | 44.4 | 1.88 | 0.19 |
| (1.7) | (1.4) | |||
| Head circumference-for-age z-scoreb | −0.4 | −0.9 | 3.54† | 0.47 |
| (1.3) | (0.9) | |||
| Head circumference percentileb | 39.2 | 25.1 | 4.00* | 0.50 |
| (32.6) | (23.8) | |||
Values are mean (standard deviation) for birth measures. For 6.5 and 12 months, values are mean (standard deviation), adjusted for birth size. Z-scores and percentiles are adjusted for age and sex.
T-tests were used to compare the birth measures; analysis of covariance, to compare the 6.5- and 12-month measures after adjustment for their corresponding birth size measures.
Percentiles based on WHO norms (de Onis et al., 2004)
p < 0.10
p < 0.05
Among infants in the placebo arm, z-scores and percentiles for weight, length, and head circumference decreased from birth to 6.5 months (Table 3), which is consistent with our previous studies in Detroit (Jacobson et al. 1994a; Carter et al., 2013) and Cape Town (Carter et al., 2012) that found worsening of fetal alcohol growth restriction in the 1st year of life. By contrast, infants in the choline arm demonstrated considerable catch-up growth in weight and head circumference by 6.5 months, which continued to be evident at 12 months. In repeated measures analyses examining the percentiles, the choline group showed significantly greater increases across this age period than the placebo group in weight, F(2,116)=4.85, p=0.009, and head circumference, F(2,116)=5.40, p=0.006. Group differences were not significant for the raw weight (kg), length (cm), and head circumference (cm) measures, presumably because the choline arm contained a higher proportion of females, who tend to be smaller than males.
Fagan Test of Infant Intelligence
Infants in the choline treatment arm performed more optimally on the FTII than placebo infants at 12 months, with higher novelty preference scores, indicating better visual recognition memory function (Table 4). The Cohen’s d measure indicates a moderate-to-large effect size. The moderate effect size for the processing speed measure suggests that this effect might be significant in a larger sample.
Table 4.
Effects of choline supplementation on FTII
| Choline
|
Placebo
|
t | d | |||||
|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | |||
| Recognition memorya | ||||||||
| 6.5 months | 26 | 61.2 | 6.5 | 27 | 61.4 | 6.7 | 0.13 | 0.04 |
| 12 months | 25 | 63.5 | 6.4 | 27 | 59.1 | 7.5 | 2.23* | 0.62 |
| Information processing speedb | ||||||||
| 6.5 months | 26 | 1.8 | 0.4 | 27 | 1.8 | 0.4 | 0.55 | 0.12 |
| 12 months | 25 | 2.0 | 0.5 | 27 | 2.1 | 0.4 | 1.00 | 0.28 |
Values are mean (standard deviation),
Percent novelty preference = (duration looking at novel stimulus/time looking at familiar + novel stimuli) X 100
Duration looking time (s)/number of looks to stimuli during familiarization
p < 0.05
FASD Diagnosis
Within the choline arm, 8 infants met criteria for FAS and 2 for PFAS (32.3%), compared with 5 who met criteria for FAS and 2 for PFAS (22.6%) in the placebo arm, χ2(1)=0.73, p=0.393.
Impact of Maternal Dietary Choline Status
Only 9.7% (3 choline-treated, 3 placebo) of the 62 infants assessed in this study were born to mothers whose dietary choline intake during pregnancy was adequate (>450 mg/day), and none exceeded the UL of 3.5 g/day. 21.6% of the infants (6 choline-treated, 5 placebo) were born to mothers who carry the rs12325817 variant of the enzyme PEMT, which reduces endogenous choline synthesis. The six outcomes on which the choline and placebo groups differed were examined. None of the choline group by maternal PEMT allele interactions were significant (all ps>0.20).
DISCUSSION
The aim of this trial was to assess the efficacy of a high dose choline supplementation initiated early in pregnancy in mitigating effects of heavy PAE on EBC and other infant developmental outcomes known to be affected by alcohol exposure. Infants born to choline-treated mothers were more likely to meet criterion for EBC conditioning and showed greater increases in CRs across the three training sessions, compared with infants whose mothers received placebo. Moreover, within the choline arm, degree of maternal adherence to the supplementation protocol was a strong predictor of infant EBC performance. We selected EBC as our primary outcome based on evidence that it is a highly sensitive indicator of fetal alcohol impairment in humans (Jacobson et al., 2008, 2011a; Coffin et al., 2005), it is impaired in laboratory animals with PAE (Stanton and Goodlett, 1998; Green et al., 2002), and ethanol-induced EBC impairment can be mitigated by choline supplementation in the animal model (Thomas and Tran, 2012). Given that by 5 months of age, human infants reach the same terminal levels of conditioning as adults (Herbert et al., 2003), EBC provides an excellent early biobehavioral marker (Jacobson et al., 2011b) of a PAE-related deficit that can be used for evaluating the efficacy of a micronutrient intervention in pregnancy.
Pre- and/or postnatal growth restriction was identified clinically as a critical feature of FAS when the syndrome was first described (Jones and Smith, 1973) and is a key element in the diagnosis of FAS and PFAS (Hoyme et al., 2005). The finding that choline treatment mitigated postnatal but not fetal growth restriction is consistent with evidence that the effects on growth restriction during these two developmental periods are mediated by different mechanisms (Jacobson et al., 1994a; Carter et al. 2016b; Middaugh and Boggan, 1991). We have previously shown that growth trajectory pattern predicts severity of fetal alcohol-related cognitive impairment (Carter et al., 2016a). Correlations between PAE and cognitive function in childhood were significantly stronger in infants with both pre- and postnatal growth restriction than in infants demonstrating postnatal catch-up growth. Our finding that choline treatment mitigated adverse effects of PAE on postnatal growth thus provides some evidence from infancy that choline supplementation may have potential to protect against a range of alcohol-related cognitive deficits that do not become evident until childhood, although beneficial effects in childhood will need to be confirmed in follow-up studies.
On the FTII, infants in the choline arm exhibited better visual recognition memory than those in the placebo group, as indicated by preferential looking at the novel stimulus. A larger sample would be needed to evaluate the effects on processing speed. By contrast to classical tests of infant development, such as the Bayley Scales, FTII performance has been repeatedly shown to predict intellectual function in childhood (Kavšek, 2004). Whereas Bayley assessments rely heavily on sensorimotor manipulation of objects through 12 months, the FTII assesses basic information-processing skills involving encoding and retrieval (Bornstein and Sigman, 1987). The predictive validity of the FTII is consistent with the assumption that the neural integrity and elementary cognitive processing ability necessary for later more complex cognitive function are already present in infancy and that the processes by which infants distribute their attention during visual processing of novel and familiar processes are essentially the same as those used by older children and adults.
Choline supplementation did not alter the incidence of FAS or PFAS. Mouse model studies have demonstrated that the dysmorphic craniofacial features required for these diagnoses are formed during the early embryonic period (Sulik, 2005). We did not predict that this treatment would protect against emergence of these anomalies, since the intervention was not initiated until the 2nd trimester for all but three study participants, who were randomized at the end of the 1st trimester.
Despite numerous studies showing positive effects in rodents, no clear evidence of beneficial effects of choline supplementation was seen in three previous human trials, two of which assessed postnatal supplementation in childhood. In a pediatric study, Wozniak et al. (2013) administered 500mg/day choline (2–2.5 times AI) or placebo to 60 children (2.5–5 yr) for 9 months. Protocol adherence was confirmed by the finding that higher serum choline and betaine concentrations were found in the choline supplemented group. Positive effects were not seen on the primary outcome, the Mullen Scales of Early Learning (Wozniak et al., 2015). Elicited imitation was assessed as a secondary outcome. There was no effect of choline on immediate imitation, which assesses initial memory encoding, and an effect on delayed imitation that was seen only in the younger group (2.5–4.0 yr) may have been due to a marginal baseline difference between the groups and/or a ceiling effect. In another pediatric study, Nguyen et al. (2016) administered 625 mg/day (1.7–2.5 times AI) to 55 children (ages 5–10 yr) with FASD for 6 weeks. No effects were seen on memory, executive function, attention, or hyperactivity in relation to mean dietary choline intake despite good adherence to the treatment protocol.
In a study of 367 infants in the Ukraine conducted by Chambers and colleagues, two groups (alcohol-consuming women; abstainers and women who drank minimally) were randomly assigned to receive a choline supplement (750 mg/day (1.7 times AI)) or placebo during pregnancy. No beneficial effect of the choline supplement was seen on newborn growth, after adjustment for smoking, or on BSID-II cognitive or psychomotor performance at 6 months (Coles et al., 2015). However, adherence estimates were uncertain since they were based on maternal report (Kable et al., 2015), and significant increases were not seen in the serum choline levels of the mothers in the choline-treated group during the course of the intervention (Coles et al., 2015). Data on cardiac orienting responses (ΔHR) were collected on a subset of these infants during auditory and visual habituation/dishabituation learning paradigms administered at 12 months (Kable et al., 2015). No choline effects were seen on the auditory task. On the visual task, which examines aspects of cognitive function similar to those assessed in the FTII, choline supplementation resulted in greater ΔHR (amplitude and latency) during habituation for all infants (i.e., when the alcohol-exposed and control infants were pooled) and on amplitude only for the infants with no PAE during dishabituation.
Several features of our intervention distinguish our protocol from those used in the previous human studies. First, the choline dose was higher (2g/day)—4.4 times AI—albeit well below the tolerable UL for choline specified by the Food and Nutrition Board (IOM, 2006). The efficacy of this high dose is consistent with the beneficial effects reported for the high doses used in the animal studies. For example, Thomas and Tran (2012) administered 100mg/kg bodyweight, which would be the equivalent of 6g/day in a 60-kg woman. Although it is difficult to compare between-species differences in dose, in one study the human effective dose in the brain was found to be underestimated by 36% using rat data (Tolvanen et al., 2010). If the 2g/day dose in our study were increased by 36%, the effective dose would be 2.7, still considerably below the dose used in the Thomas et al. rodent studies. Second, the treatment was initiated much earlier in development than in the pediatric studies and possibly earlier than in the Ukraine study. Our data are thus consistent with findings from laboratory animal studies suggesting that choline supplementation may be more effective when administered earlier in development (Meck et al., 1989; Thomas et al., 2009). Another strength of this trial was the use of culture-free assessments (EBC, Fagan, anthropometry) that permit generalizability to other populations.
Several studies of effects of ethanol with laboratory animals have demonstrated beneficial effects of choline supplementation on working memory and learning (Thomas et al., 2000, 2004, 2007), and conditioning (Wagner and Hunt, 2006; Thomas and Tran, 2012). Although these effects were initially attributed to increased release of acetylcholine in the hippocampus, the amount of choline that accumulates in the fetal brain following maternal supplementation does not appear to be sufficient to enhance acetylcholine release (Garner et al., 1995). Zeisel and colleagues have suggested two alternative mechanisms: (1) Choline is a potent source of biologically labile methyl-donor groups, which play an important role in DNA methylation, a critical mechanism in epigenetic processes leading to altered gene expression (Zeisel and Niculescu, 2006). Through changes in gene expression, choline may enhance a range of embryological and fetal developmental processes, including neuronal precursor cell proliferation, differentiation, and apoptosis (Wang et al., 2016). Gestational choline supplementation has been shown to prevent PAE-modulation of histone and DNA methylation in hypothalamic proopiomelanocortin neurons (Bekdash et al., 2013) and to reduce hyper-methylation associated with PAE in the hippocampus and prefrontal cortex (Otero et al., 2012). (2) Choline’s role as a precursor to two cell membrane constituents (phosphatidylcholine, sphingomyelin) and to cell signaling factors (platelet activating factor, sphingosylphosphocholine) has also been suggested as a mechanism contributing to its neuroprotective properties (Zeisel and Niculescu, 2006).
In their laboratory animal study on effects of postnatal choline supplementation on EBC, Thomas and Tran (2012) examined two groups of rats during adulthood—one group trained on delay EBC, the procedure used with the infants in our study; the other, on trace EBC. In delay, the CS precedes, overlaps, and co-terminates with the onset of the US, whereas in trace, there is a brief stimulus-free “trace interval” between the offset of the CS and the onset of the US. In our study, we administered delay conditioning because, although learning rates are slower, typically-developing infants demonstrate patterns of acquisition for delay that are indistinguishable from adults in terms of asymptote and timing of response, whereas trace conditioning during infancy is at best rudimentary (Herbert et al., 2003). Thomas and Tran found that, although performance of adult rats exposed to ethanol early in development was significantly impaired on both tasks, choline supplementation mitigated the effect on trace but not delay conditioning. They suggested that the selective deficits in trace conditioning that they observed were likely related to hippocampal dysfunction and that choline did not attenuate effects on delay because it is more dependent on the functional integrity of the cerebellum and less on the hippocampus. However, although animals can perform delay EBC even after the hippocampus has been removed, an intact hippocampus has been found to modulate delay conditioning in both animals and humans (e.g., Solomon et al., 1993; Cheng et al., 2017). The hippocampus may actually be more involved in delay conditioning during early development, as hippocampal injury has been shown to impair delay conditioning in weanling and juvenile rats (Ivkovich and Stanton, 2001). Thomas and Tran’s failure to find the beneficial effect of choline on delay conditioning seen in our study may be related to differences in the period of exposure to alcohol or choline, in the age when EBC assessments were performed, or a difference between rodent models and humans.
In the U.S., choline supplementation is not part of standard prenatal care and dietary choline intake in pregnancy is often inadequate at rates similar to those seen in Cape Town, which can increase the risk of delivering an infant with a birth defect (see Shaw et al., 2004). PAE can further disturb the metabolism of choline and other methyl donors, thereby potentially exacerbating dietary inadequacy (Zeisel, 2011). The American Medical Association has recently issued a policy recommendation for inclusion of choline in prenatal supplements (https://wire.ama-assn.org/ama-news/ama-backs-global-health-experts-calling-infertility-disease).
Zeisel (2011) has suggested that choline supplementation is likely to be most effective in pregnant women with choline deficiency, based on findings in the animal model (Thomas et al., 2000; Idrus et al., 2017). Choline deficiency can be caused by either inadequate dietary choline intake and/or the presence of the rs12325817 variant of the enzyme PEMT, which reduces endogenous choline synthesis. We found a lower incidence of this polymorphism in the Cape Coloured population compared with the prevalence reported in a Chapel Hill, NC, sample (da Costa et al., 2006). Given the small number of women with adequate choline intake and/or the PEMT variant, our sample lacked power to determine whether choline supplementation is differentially effective in these women. It should be noted that the high prevalence of inadequate choline intake in this population (>88%; Carter et al., 2017) and in this sample (>70%) may have enhanced the efficacy of the choline supplementation, although, as noted, dietary choline deficiency is also found in 93% of U.S. women (NHANES survey, Chester et al., 2011; also see Wallace et al., 2014).
Limitations
One limitation of this study was small sample size; the findings need to be confirmed in a larger trial. In addition to the large effect sizes on EBC, postnatal growth, and 12-month recognition memory, the moderate effect size on processing speed suggests another effect that warrants examination in a larger sample. Although there is a greater likelihood of subject loss in infant studies, due to movement artifact, fussiness, etc., compared to older children, our subject loss was somewhat lower than in other comparable studies (e.g., Herbert et al., 2003; Taylor and Herbert, 2013). A multi-site trial will be needed to assess generalizability to other populations, including those from differing ethnic backgrounds and populations that do not have the high incidence of poor nutrition found in this sample. Whether the choline supplementation prevents or repairs fetal alcohol-related impairment to brain structure and function is not clear and needs to be examined using animal models and in human neuroimaging studies. It is also not clear whether choline supplementation specifically targets alcohol-related impairment or improves development in all infants. A human study in which both alcohol-consuming and abstaining mothers are randomly assigned to receive choline at high doses or placebo early in pregnancy is needed to address this question.
Conclusions
This exploratory study is the first to provide evidence that a high dose of choline administered early in pregnancy can mitigate adverse effects of heavy PAE on EBC, postnatal growth, and cognition in human infants. These findings are consistent with studies of ethanol-exposed animals that have demonstrated beneficial effects of choline supplementation on classical conditioning, learning, and memory. Given that psychosocial interventions are often ineffective with heavy drinking pregnant women, the evidence that early choline supplementation can mitigate alcohol effects on the developing fetus provides an important breakthrough in treatment of maternal drinking during pregnancy, which is often intractable to intervention. This supplementation program was effective even among highly disadvantaged, poorly educated heavy drinking women. These findings have important public health implications since FASD continue to be a leading cause of intellectual disabilities, and infant outcomes showing positive effects in this trial are predictive of cognitive function in childhood. Follow-up of this cohort will be important to determine whether the positive effects of choline supplementation persist into childhood.
Acknowledgments
Funding:
Grants from the NIH/National Institute on Alcohol Abuse and Alcoholism R21AA020332 and R01AA016781 (to SWJ) and K23AA020516 (to RCC); National Institute of Diabetes and Digestive and Kidney Diseases R01DK115380 and P30DK056350 (to SHZ) and K24DK104676 and 2P30DK040561 (to CPD); and from the Lycaki-Young Fund of Michigan (to SWJ and JLJ).
We thank the members of the Data Safety Monitoring Board: Sydney Hans, Ph.D. (Chair), a developmental psychologist with expertise in assessment of long-term effects of teratogenic exposures, University of Chicago; Marylou Behnke, M.D., a neonatologist with expertise on teratogenic exposures, University of Florida; Judette Louis, M.D., M.P.H., an obstetrician with expertise in fetal alcohol-related pregnancy complications, Case Western Reserve University; and Cynthia Arfken, Ph.D., a statistician with expertise in alcohol abuse research, Wayne State University. We thank Kristine Lukasik, Balchem Corporation, New Hampton, NY, who conducted the regular quality control analyses for the choline supplement; Rocco Anzaldi, R.Ph., a senior research pharmacist at Children’s Hospital Boston, who supervised the construction of the randomization list and the labeling and shipment of the choline packets to UCT, and Wynand Smythe, PhD, BPharm, Division of Clinical Pharmacology, UCT, who retained the randomization list in case of an adverse medical event including spontaneous abortion, miscarriage or stillbirths requiring unblinding for review by the Data Safety Monitoring Review Board. We thank Marjanne Senekal, Ph.D., who developed the quantitative choline food frequency questionnaire in collaboration with RCC, Sharmilah Booley, MSc, Baheya Najaar, M.Sc., and Lori Bechard, R.D., Ph.D., and Monika Uys, Ph.D., Catherine Day, R.D., and Nicola Cooper, the dieticians who administered the 24-hr recall interviews that were processed by Dr. Bechard. We thank Susan Fawcus, M.D., Head of Department of Obstetrics, Mowbray Maternity Hospital and the nursing staff at the Hanover Park and Retreat Midwife Obstetric Units, where the mothers were recruited. We also thank the dysmorphologists Greetje De Jong, M.D., Prachi Shah, M.D., H Bezuidenhout, M.D., and E Krzesinski, M.D., who assisted HEH at our 2016 and 2017 FASD diagnostic clinics. We thank our research nurses Maggie September, Beverly Arendse, and Patricia O’Leary, for their help recruiting the cohort and conducting home visits involving adherence monitoring during the intervention; our project driver Patricia Solomon; and our UCT research staff Landi Meiring, M.A., Stacey Hall, M.A., and Sivenesi Subramoney, M.A., for their help conducting the infant assessments. We are also extremely grateful to the mothers and infants for their participation in this trial.
Footnotes
The authors declare no competing financial interests.
References
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in b-endorphin-producing POMC neurons of the hypothalamus. Alcohol Clin Exp Res. 2013;37:1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. In: Oates J, Sheldon S, editors. Cognitive Development in Infancy. Erlbaum; Hillsdale, NJ: 1987. pp. 249–284. [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Jiang H, Meintjes EM, Jacobson SW, Duggan C. Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol Clin Exp Res. 2012;36:1973–1982. doi: 10.1111/j.1530-0277.2012.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Sokol RJ, Avison MJ, Jacobson SW. Fetal alcohol-related growth restriction from birth to young adulthood and moderating effects of maternal prepregnancy weight. Alcohol Clin Exp Res. 2013;37:452–462. doi: 10.1111/j.1530-0277.2012.01940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal alcohol growth restriction and cognitive impairment. Pediatr. 2016a;138:e20160775. doi: 10.1542/peds.2016-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Wainwright H, Molteno CD, Georgieff M, Dodge NC, Warton F, Meintjes EM, Jacobson JL, Jacobson SW. Alcohol, methamphetamine, and marijuana exposure have distinct effects on human placental development. Alcohol Clin Exp Res. 2016b;40:753–764. doi: 10.1111/acer.13022. [DOI] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Dodge NC, Bechard L, Meintjes EM, Molteno CD, Duggan C, Jacobson JL, Jacobson SW. Maternal alcohol use and nutrition during pregnancy: Diet and anthropometry. Alcohol Clin Exp Res. 2017;41:2114–2127. doi: 10.1111/acer.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Meintjes EM, Stanton ME, Dodge NC, Pienaar M, Warton CM, Desmond JE, Molteno CD, Peterson BS, Jacobson JL, Jacobson SW. Functional MRI of human eyeblink classical conditioning in children with fetal alcohol spectrum disorders. Cerebral Cortex. 2017;27:3752–3767. doi: 10.1093/cercor/bhw273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester D, Goldman J, Ahuja J, Moshfegh A. Dietary Intakes of Choline. What We Eat in America, NHANES 2007–2008. 2011 [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD CIFASD. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Maternal Child Health J. 2015;19:2605–2614. doi: 10.1007/s10995-015-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: are short lookers faster processors or feature processors? Child Dev. 1991;62:1247–1257. [PubMed] [Google Scholar]
- da Costa K-A, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25:S15–26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, Tooze JA, Krebs-Smith SM. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Dietetic Assoc. 2006;106(10):1640–1650. doi: 10.1016/j.jada.2006.07.011. [DOI] [PubMed] [Google Scholar]
- du Plessis L, Jacobson SW, Molteno CD, Robertson FC, Peterson BS, Jacobson JL, Meintjes EM. Neural correlates of cerebellar-mediated timing during finger tapping in children with fetal alcohol spectrum disorders. NeuroImage Clin. 2015;7:562–570. doi: 10.1016/j.nicl.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. Adv Inf Res. 1983;2:31–78. [Google Scholar]
- Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, Stanton ME, Peterson BS, Jacobson JL, Jacobson SW. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Human Brain Mapp. 2015;36:2470–2482. doi: 10.1002/hbm.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125:2851–2858. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J. 2005;54:355–359. doi: 10.1590/s0043-31442005000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, May PA, Kalberg WO, Kodituwakku P, Gossage JG, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrus NM, Breit KR, Thomas JD. Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 2017;59:43–52. doi: 10.1016/j.ntt.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes, in Series Dietary Reference Intakes. National Academies Press; Washington, DC: 2006. [Google Scholar]
- Ivkovich D, Collins KL, Eckerman CO, Krasnegor NA, Stanton ME. Classical delay eyeblink conditioning in 4-and 5-month-old human infants. Psychol Sci. 1999;10:4–8. [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiol Learning Memory. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin M. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res. 1993;17:174–183. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. J Pediatr. 1994a;124:757–764. doi: 10.1016/s0022-3476(05)81371-x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994b;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011a;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorder. Neuropsychol Rev. 2011b;21:148–166. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489–496. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pederson TL, Chambers CD CIFASD. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol. 2015;49:647–656. doi: 10.1016/j.alcohol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavšek M. Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. Applied Dev Psychol. 2004;25:369–393. [Google Scholar]
- Lewis CE, Thomas KGF, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA. 2018;319:474–482. doi: 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Boggan WO. Postnatal growth deficits in prenatal ethanol-exposed mice: characteristics and critical periods. Alcohol Clin Exp Res. 1991;15:919–926. doi: 10.1111/j.1530-0277.1991.tb05189.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. 2016;104:1683–1692. doi: 10.3945/ajcn.116.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36:1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Spaaij CJ, Goldberg GR, Poppitt SD, Totton M, Swann D, Black AE. Energy requirements of pregnant and lactating women. Euro J Clin Nutr. 1996;50:S82–110. [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltranferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Groccia-Ellison ME, Flynn D, Mirak J, Edwards KR, Dunehew A, Stanton ME. Disruption of human eyeblink conditioning after central cholinergic blockade with scopolamine. Behav Neurosci. 1993;107:271–279. doi: 10.1037//0735-7044.107.2.271. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22:270–275. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC, Herman CS. Effects of maternal alcohol, nicotine, and caffeine use during pregnancy on infant mental and motor development at eight months. Alcohol Clin Exp Res. 1980;4:152–164. doi: 10.1111/j.1530-0277.1980.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med. 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Taylor G, Herbert JS. Eye tracking infants: Investigating the role of attention during learning on recognition memory. Scandinavian J Psychol. 2013;54(1):14–19. doi: 10.1111/sjop.12002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolvanen T, Yli-Kerttula T, Ujula T, Autio A, Lehikoinen P, Minn H, Roivainen A. Biodistribution and radiation dosimetry of [11C]choline: a comparison between rat and human data. Eur J Nucl Med Mol Imaging. 2010;37:874–883. doi: 10.1007/s00259-009-1346-z. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wallace TC, McBurney M, Fulgoni VL. Multivitamin/mineral supplement contribution to micronutrient intakes in the United States, 2007–2010. J Am College Nutr. 2014;33:94–102. doi: 10.1080/07315724.2013.846806. [DOI] [PubMed] [Google Scholar]
- Wang Y, Surzenko N, Friday WB, Zeisel SH. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J. 2016;30:1566–1578. doi: 10.1096/fj.15-282426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning: Volumes I—Applications in Humans and II—Applications in Animals. Kluwer Academic Publishers; Boston, MA: 2000. [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res. 2013;33:897–904. doi: 10.1016/j.nutres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2015;102:1113–1125. doi: 10.3945/ajcn.114.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel S. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol. 2011;44:185–191. doi: 10.1007/s12035-011-8165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]