Abstract
Patients with chronic heart failure with reduced ejection fraction (HFrEF) benefit from medical and device therapies targeting sudden cardiac death (SCD). Contemporary estimates of SCD risk after hospitalization for HF are limited. We describe the incidence, timing, and clinical predictors of SCD following hospitalization for HFrEF (≤40%) in the EVEREST trial. Multiple logistic regression analyses tested >30 baseline covariates (including treatment randomization, demographics, comorbid conditions, natriuretic peptides, EF, medical and device therapies) to identify predictors of 1-year SCD. Of the 4,024 (97%) trial patients discharged alive, there were 268 (7%) SCD and 703 (17%) non-SCD deaths during median 9.9 months follow-up. Implantable cardioverter-defibrillator use at baseline was 14.5%. Estimates of SCD at 1-, 3-, 6-, and 12-months were 0.8%, 2.3%, 4.1%, and 7.4%, respectively. Most patients were readmitted prior to SCD (n=147, 55%). Male sex, black race, diabetes mellitus, and angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use were potential predictors of 1-year SCD following hospitalization for HFrEF (all P<0.10), however this final model demonstrated poor discrimination (C-statistic 0.57). In conclusion, in EVEREST, patients hospitalized for HFrEF faced risks of 1-year post-discharge SCD of 7%, which accrued gradually over time, and were balanced with high competing risks of non-sudden death (17%). Traditional clinical characteristics fail to adequately predict SCD risk. Further data are needed to identify patients at greatest relative risk for SCD (compared with non-SCD) after hospitalization for HFrEF.
Keywords: acute heart failure trial, hospitalization, risk prediction, sudden cardiac death
Sudden cardiac death (SCD) due to ventricular arrhythmia represents an important mode of death in patients with chronic heart failure with reduced ejection fraction (HFrEF) (1). The overall rates of SCD have substantially decreased over the past 2 decades, related in part to effective implementation of medical and device therapies (2). Despite these advances, contemporary data suggest that nearly 40% of deaths among those with symptomatic chronic HFrEF are attributable to SCD (1). Hospitalization for heart failure (HF) serves as a marker of disease progression and may shift the relative distribution of cause-specific deaths (with greater proportion of worsening HF-related deaths) (3,4). After periods of worsening HF, identification of patients at highest relative risk of SCD and timing of application of SCD preventative strategies have proved challenging (5). Contemporary estimates of SCD risk after hospitalization for HF and in patients with more advanced HF are limited. Accordingly, we aimed to describe the incidence, timing, and clinical predictors of SCD following hospitalization for HFrEF in the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) trial.
METHODS
The study design and primary results of EVEREST have been previously described (3). Briefly, EVEREST was a multicenter, global, randomized, double-blind, placebo-controlled trial of oral tolvaptan (vasopressin V2 receptor antagonist) in patients hospitalized for worsening chronic HFrEF (≤40%), New York Heart Association (NYHA) III and IV symptoms, and ≥2 signs of HF at time of randomization. Relevant exclusion criteria included comorbidities with life expectancy <6 months, end-stage HF, significant valvular disease, acute myocardial infarction (MI), serum potassium >5.5 mEq/dL, serum creatinine >3.5 mg/dL, and those requiring hemodialysis or ultrafiltration. Cause-specific events were independently adjudicated by a blinded clinical events committee. SCD was defined as an unexpected death in a previously stable patient with recent human contact. If the patient was out of contact for 24 hours to 1 week, the event was considered a presumed SCD (4). In our analysis, non-SCD encompassed all deaths not classified as SCD.
All patients discharged alive in either treatment arm of EVEREST were included. Continuous variables are expressed as mean ± standard deviation if normally distributed and median (25th – 75th percentiles) if not normally distributed. χ2 tests and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively. Incidence rates of SCD at 1-, 3-, 6-, and 12-months post-discharge were estimated using the Kaplan-Meier method and by cumulative incidence function (accounting for non-SCD competing risks). Time from last known medical contact to SCD was also calculated. To identify independent predictors SCD after HF hospitalization, multiple logistic regression analyses tested >30 discharge covariates (to correspond to roughly 1 covariate per 10 events) using stepwise backward selection. The covariate set was consistent with prior EVEREST analyses (6–9) and included treatment randomization, demographic characteristics, medical history (prior HF hospitalization, atrial fibrillation, coronary artery disease, prior MI, diabetes mellitus, ischemic HF etiology, hypertension, chronic obstructive pulmonary disease, chronic kidney disease, NYHA class IV), vital signs and anthropomorphic measures, laboratory and diagnostic testing (QRS duration, ejection fraction [EF], serum sodium, serum potassium, estimated glomerular filtration rate, blood urea nitrogen [BUN], natriuretic peptides), and discharge therapies (including angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEi/ARB), β-blockers, mineralocorticoid receptor antagonists, amiodarone, digoxin, and implantable cardioverter defibrillators [ICD]). Model discrimination (ability to discriminate patients who did or did not experience SCD) was assessed using C-statistics and model calibration (agreement between observed and expected SCD events) was tested using the Hosmer-Lemeshow (H–L) goodness-of-fit test (10). All statistical analyses were performed with SAS v9.3 (SAS Institute, Cary, NC).
RESULTS
Of the 4,133 patients enrolled in EVEREST, 109 died in hospital (12 SCD, 97 non-SCD) and were excluded. The remaining 4,024 (97%) were discharged alive and were included in final analysis. Table 1 describes the discharge clinical profiles of patients who experienced SCD (n=268, 7%), non-SCD (n=703, 17%), or remained alive (3,053, 76%) during median follow-up of 9.9 months (5.3 to 16.1 months). Baseline ICD use overall was 14.5%; patients who experienced SCD had lower rates of baseline ICD use compared with patients who died of non-SCD (13% vs. 23%; P=0.001). Of the 583 patients with ICDs, 6% died of SCD and 27% died of non-SCD death. Of the 3,439 patients without an ICD, 7% died of SCD and 16% died of non-SCD death. Compared with patients who experienced non-SCD, patients with SCD tended to be younger, male, with higher body mass indexes, enrolled from Eastern Europe, carry lower rates of diabetes mellitus and chronic kidney disease, and have fewer physical exam findings of HF (P<0.05 for all comparisons; Table 1). Ischemic HF etiology (67% vs. 68%), prior MI (53% vs. 54%), and mean left ventricular EF (25.1±7.7% vs. 25.4±8.2%) did not significantly vary in patients who died of SCD or non-SCD, respectively (P≥0.60 for all comparisons).
Table 1.
Clinical Profiles of EVEREST Patients by Post-Discharge Cause-Specific Death Status
| Characteristic | Sudden Cardiac Death (n=703) |
Non-Sudden Cardiac Death (n=703) |
P | Alive (n=3,053) | Overall Pa |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 65.1±12.6 | 69±11.8 | <0.001 | 65±11.6 | <0.001 |
| Men | 221 (82.5%) | 535 (76.1%) | 0.03 | 2247 (73.6%) | 0.004 |
| Black | 28 (10.4%) | 58 (8.3%) | 0.28 | 217 (7.1%) | 0.10 |
| White | 221 (82.5%) | 591 (84.1%) | 0.55 | 2624 (85.9%) | 0.17 |
| Asian | 1 (0.4%) | 0 (0%) | 0.28 | 8 (0.3%) | 0.36 |
| Hispanic | 13 (4.9%) | 37 (5.3%) | 0.80 | 149 (4.9%) | 0.90 |
| Other race | 6 (2.2%) | 17 (2.4%) | 0.87 | 62 (2%) | 0.80 |
| Eastern Europe | 104 (38.8%) | 167 (23.8%) | <0.001 | 1319 (43.2%) | <0.001 |
| Western Europe | 31 (11.6%) | 111 (15.8%) | 0.10 | 401 (13.1%) | 0.11 |
| North America | 88 (32.8%) | 313 (44.5%) | 0.001 | 818 (26.8%) | <0.001 |
| South America | 45 (16.8%) | 112 (15.9%) | 0.75 | 515 (16.9%) | 0.83 |
| History | |||||
| Prior hospitalization for heart failure | 218 (82.0%) | 602 (85.8%) | 0.14 | 2340 (77.0%) | <0.001 |
| Ischemic etiology of heart failure | 178 (66.9%) | 468 (67.6%) | 0.83 | 1953 (64.8%) | 0.32 |
| New York Heart Association class IV | 115 (42.9%) | 336 (47.8%) | 0.17 | 1099 (36.1%) | <0.001 |
| Prior myocardial infarction | 143 (53.4%) | 382 (54.3%) | 0.78 | 1497 (49.1%) | 0.03 |
| Coronary artery diseaseb | 190 (70.9%) | 510 (72.8%) | 0.56 | 2133 (69.9%) | 0.33 |
| Hypertensionb | 191 (71.3%) | 469 (66.7%) | 0.17 | 2201 (72.1%) | 0.02 |
| Hyperlipidemiab | 131 (48.9%) | 355 (50.6%) | 0.64 | 1468 (48.4%) | 0.59 |
| Diabetes mellitus | 101 (37.7%) | 321 (45.7%) | 0.03 | 1133 (37.1%) | <0.001 |
| Chronic kidney disease | 82 (30.6%) | 311 (44.3%) | <0.001 | 668 (21.9%) | <0.001 |
| Implantable cardioverter-defibrillator | 35 (13.1%) | 159 (22.6%) | 0.001 | 389 (12.7%) | <0.001 |
| Chronic obstructive pulmonary disease | 30 (11.2%) | 108 (15.4%) | 0.10 | 267 (8.8%) | <0.001 |
| Peripheral arterial disease | 60 (22.4%) | 168 (24%) | 0.60 | 613 (20.1%) | 0.06 |
| Discharge Findings | |||||
| Dyspnea | 65 (24.3%) | 188 (27.1%) | 0.39 | 566 (18.7%) | <0.001 |
| Pedal edema | 112 (41.9%) | 355 (51.1%) | 0.01 | 1115 (36.8%) | <0.001 |
| Jugular venous distension ≥10 cm | 14 (5.2%) | 65 (9.4%) | 0.04 | 118 (3.9%) | <0.001 |
| Rales | 69 (25.8%) | 219 (31.5%) | 0.09 | 581 (19.2%) | <0.001 |
| Systolic blood pressure (mmHg), mean ± SD | 112.6±16.4 | 110.6±16.9 | 0.09 | 117.1±17 | <0.001 |
| Body mass index (kg/m2), median (25th, 75th percentiles) | 27.8 (24.4–31.5) | 26.7 (23.6–30.8) | 0.03 | 28.3 (25–32.3) | <0.001 |
| Ejection fraction (%), mean ± SD | 25.1±7.7 | 25.4±8.2 | 0.60 | 28.3±7.9 | <0.001 |
| QRS duration (msec), mean ± SD | 132±35.2 | 137±35.2 | 0.05 | 123.7±34.2 | <0.001 |
| Serum sodium (mg/dL), mean ± SD | 139.7±5.2 | 139±5.5 | 0.08 | 140.5±4.9 | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73 m2), mean ± SDc | 53.6±21.2 | 45.9±20.8 | <0.001 | 56.5±20.6 | <0.001 |
| Creatinine (mg/dL), mean ± SD | 1.5±0.5 | 1.6±0.6 | <0.001 | 1.4±0.5 | <0.001 |
| Blood urea nitrogen (mg/dL), mean ± SD | 33.2±18.1 | 41.2±22 | <0.001 | 29.9±14.9 | <0.001 |
| B-type natriuretic peptide (pg/mL), mean ± SD | 1051.6±1268.1 | 1274.9±1371.1 | 0.07 | 791.4±2721.2 | 0.002 |
| Albumin (mg/dL), mean ± SD | 3.8±0.5 | 3.6±0.5 | <0.001 | 3.9±0.5 | <0.001 |
| Discharge Medications | |||||
| Angiotensin-converting enzyme inhibitor/Angiotensin II receptor blocker | 213 (79.5%) | 528 (75.1%) | 0.15 | 2674 (87.8%) | <0.001 |
| Beta-blocker | 186 (69.4%) | 463 (65.9%) | 0.30 | 2367 (77.7%) | <0.001 |
| Spironolactone | 178 (66.4%) | 399 (56.8%) | 0.006 | 1879 (61.7%) | 0.01 |
| Digoxin | 140 (52.2%) | 373 (53.1%) | 0.82 | 1400 (45.9%) | 0.001 |
| Diuretics | 251 (93.7%) | 664 (94.5%) | 0.63 | 2832 (92.9%) | 0.34 |
| Statins | 94 (35.1%) | 232 (33%) | 0.54 | 1079 (35.4%) | 0.48 |
| Tolvaptan | 139 (51.9%) | 348 (49.5%) | 0.51 | 1535 (50.3%) | 0.80 |
| Amiodarone | 57 (21.3%) | 179 (25.5%) | 0.17 | 498 (16.3%) | <0.001 |
Compares across all 3 groups
Patient-reported medical history
Estimated based on the abbreviated Modification of Diet in Renal Disease equation
Abbreviations: SD = standard deviation
Kaplan-Meier estimates of SCD at 1-, 3-, 6-, and 12-months were 0.8%, 2.3%, 4.1%, and 7.4%, respectively; estimates using cumulative incidence function yielded similar rates (Figure 1). Kaplan-Meier estimates of 1-year non-SCD was 17%. After 1 year, there were 41 SCD events, the last occurring 26 months post-discharge.
Figure 1.
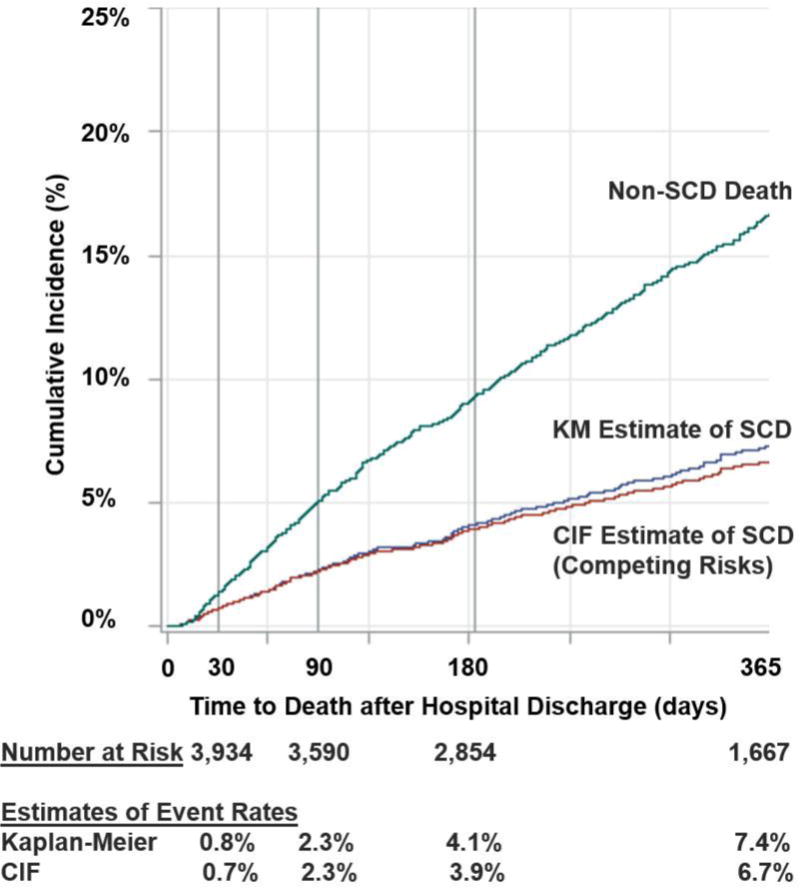
Estimates of Sudden Cardiac Death (SCD) and Non-Sudden Death at 1-year After Hospitalization for Heart Failure. Kaplan-Meier and cumulative incidence function (CIF, to account for competing risks of non-sudden death) estimates of SCD are shown.
Over half of patients who ultimately experienced SCD were readmitted prior to SCD (n=147, 55%). The majority of rehospitalizations were for HF (n=83, 56% of rehospitalizations), and 42 died during readmission of SCD. Two other patients died on the same day as a planned or unplanned outpatient visit. Of the 224 SCDs that occurred outside a healthcare facility, 172 died suddenly a mean 17 days after scheduled/unscheduled visits, and 52 died suddenly 20 days (exponentiated mean given right skew) after discharge.
The final multiple logistic regression model identified 4 potential predictors of SCD at 1-year post-HF hospitalization: male sex (adjusted odds ratio [aOR]: 1.56, 95% confidence interval [CI]: 1.11–2.21; P= 0.01), black race (aOR: 1.51, 95% CI: 0.96–2.37; P=0.07), discharge prescription of ACEi/ARB (aOR: 1.45, 95% CI 0.95–2.21, P=0.08), and diabetes mellitus (aOR: 1.26, 95% CI: 0.96–1.65; P=0.09). This final prediction model demonstrated acceptable fit (H–L statistic P=0.27), but poor discrimination (C-statistic 0.57).
DISCUSSION
We report the incidence, timing, and clinical predictors of SCD following hospitalization in patients with chronic HFrEF and NYHA class III–IV functional class. Our analysis highlights several key findings: 1) 1-year risk of post-discharge SCD (~7%) is approximately double the annualized rate of SCD observed in trials of chronic HFrEF (1,2); 2) patients with advanced HFrEF experience high competing risks of non-SCD (~17% at 1 year); 3) more than 50% of patients are readmitted prior to ultimately experiencing SCD and many die during this readmission or within a month of last medical contact; 4) baseline utilization of ICD therapy was low in this global HFrEF clinical trial; and 5) traditional clinical characteristics fail to adequately predict SCD risk following hospitalization for HFrEF. Overall, our findings highlight the unmet need to critically evaluate risk of SCD after hospitalization for advanced HFrEF.
Few studies have evaluated risks of SCD in patients hospitalized for HF. It is noteworthy that the EVEREST population was an exceptionally sick cohort with NYHA III–IV symptoms, which in part accounts for the high competing risks of non-SCD. However, even in recent trials of patients hospitalized for HF with broader inclusion and with both preserved and reduced EF, trajectory of post-discharge SCD risk was similar: rates of SCD within 1 month <1% (11) and ~2–4% within 6 months (12). Compared with these prior trial-based analyses, EVEREST enrolled only patients with worsening chronic HFrEF patients, captured more than twice the number of SCD events (n=268), and adjudicated events beyond the immediate post-discharge window (median 9.9 months). Prolonged follow-up in EVEREST revealed that SCD risk accumulated gradually, without an initial period of heightened risk after discharge.
Our analysis highlights inherent challenges in identifying at-risk patients for SCD in clinical practice and adjudicating SCD in trials of advanced HF. Most contemporary clinical definitions of SCD require a period of clinical stability prior to unexpected SCD (13). However, over half of the EVEREST population that experienced SCD were readmitted prior to SCD (commonly for HF), many dying during this readmission. Indeed, most patients were evaluated in a healthcare context within a month of SCD. Taken together, these data suggest that patients who ultimately died suddenly were clinically tenuous prior to their death, which may present challenges for distinguishing specific modes of death. Adjudication is subject to some degree of subjectivity related to the relative perceived contribution of progressive, worsening HF to the patient’s death. Populations such as those enrolled in EVEREST with more advanced HF may be less likely to have their death declared as sudden and unexpected. Furthermore, adjunctive rhythm information is rarely available to corroborate final adjudication. Indeed, certain events may not be related to ventricular tachyarrhythmias including major systemic events (massive pulmonary embolism, acute aortic syndrome, etc.) or non-shockable rhythms (asystole or pulseless electrical activity) in the early post-discharge time-frame (14). As such, SCD identified in trials of advanced HF may reflect available clinical information (or lack thereof) rather than a specific, targetable pathophysiological process. Classification of arrhythmic SCD remains an important issue to address in upcoming HF trials, regardless of EF (13).
The benefits of ICD therapy are well-established in many settings in HFrEF. However, our study brings attention to the low rates of use of ICDs at baseline (<15%) in this global trial of patients with worsening chronic HF. Although this may partially be related to the high proportion of patients with advanced HF, ICD therapy utilization was especially low in certain geographic regions (namely, Eastern Europe), which correspondingly have higher relative rates of SCD (15), and this low utilization was similar even in trials of chronic HFrEF (1). As such, appropriate application of ICD therapy continues to require attention.
Our data support current guidelines that note uncertainty regarding the appropriateness of de novo implantation of ICDs in patients with advanced HF, in whom an ICD may prolong a period of frequent hospitalizations and poor health-related quality of life (16,17). It is plausible that select patients with advanced HF may derive benefit from SCD preventative efforts, while avoiding unnecessary and costly therapy in those who may succumb to competing risks of death. Unfortunately, our models poorly discriminated patients who do or do not experience SCD. Certain parameters such as ischemic HF etiology or left ventricular EF did not differ by cause-specific deaths, which contrasts with studies of chronic HF (18). Although natriuretic peptides were elevated in patients who died, these biomarkers did not differ by cause-specific death and may be more specific for non-sudden modes of death (such as worsening HF). Cardiac biomarkers on injury (not collected in our study) may have potential in SCD risk prediction. The limited predictive ability of traditional risk factors may be related to the modest number of SCD events and selection of high-risk patients with advanced HF in EVEREST.
Future efforts are essential to deeply phenotype the group at specific risk for SCD and low competing risks of non-sudden death. Robust and well-calibrated models (19) are required in patients with advanced HFrEF, perhaps incorporating clinical factors such as frequency and recentness of hospitalizations. There is an enduring need to investigate risk-based ICD implantation strategies, beyond reliance on left ventricular EF alone, including leveraging adjunctive imaging and biomarker data (20,21). At present, given limitations of current risk prediction tools and highly dynamic patient trajectories in advanced HF, treatment decisions regarding ICDs should be individualized and aligned with patient and caregiver preferences.
This post hoc analysis is subject to several limitations. Despite multivariable modeling, unmeasured confounders likely remain present. We did not have access to adjudication forms, including availability of autopsies or presence and burden of ICD shocks. Our models did not account for time-varying covariates, such as changes in medications or readmissions in follow-up. The modest number of captured SCD events limited the robustness of our prediction models.
Patients hospitalized for worsening chronic HFrEF and NYHA class III–IV functional class face substantial risks of SCD and competing risks of non-sudden deaths at 1 year. SCD rates accrue gradually and there does not appear to be an immediate period of heightened post-discharge vulnerability. Our data support current guidelines regarding the cautious use of ICDs in patients with advanced HF and recurrent hospitalizations. This analysis also brings attention to low use of ICDs (<15%) and challenges with adjudication of SCD even in the context of carefully-conducted global clinical trials. Further data are needed to identify patients at greatest relative risk for SCD (compared with non-SCD) after hospitalization for HFrEF to better target SCD prevention strategies.
Acknowledgments
In memory of Dr. Mihai Gheorghiade, who passed away in August 2017.
FUNDING
Otsuka Inc. (Rockville, Maryland) provided financial and material support for the EVEREST trial. HS conducted all final analyses for this manuscript with funding from the Center for Cardiovascular Innovation (Northwestern University Feinberg School of Medicine, Chicago, IL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr. Vaduganathan is supported by the NHLBI (T32HL007604).
Dr. Mentz receives research support from Amgen, AstraZeneca, BMS, GSK, Gilead, Novartis, Otsuka, and ResMed; and honoraria from Thoratec.
Dr. Greene is supported by the NHLBI (T32HL069749-14) and a HFSA/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis.
Dr. Udelson reports consulting fees from GE Healthcare, Ironwood Pharmaceuticals, Lantheus Medical Imaging, and Stealth, and has served on study committees sponsored by GSK and Pfizer.
Dr. Swedberg reports receiving honoraria/consulting fees from Amgen, AstraZeneca, Novartis, Pfizer, and Servier, Vifor and research grants from Amgen and Servier.
Dr. O’Connor reports consulting fees from Novella and Amgen; ownership/partnership/principal in Biscardia, LLC; and research support from Otsuka, Roche Diagnostics, BG Medicine, Critical Diagnostics, Astellas, Gilead, GE Healthcare, and ResMed.
Dr. Butler has received research support from the NIH and EU; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, CVRx, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, Vifor, and ZS Pharma.
Dr. Zannad has received grant funding from Novartis, BG Medicine, and Roche Diagnostics; served on a board for Boston Scientific; and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed.
All other authors have no conflicts to declare related to the contents of this manuscript.
References
- 1.Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 2.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 3.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Pina I, Roth S, Sackner-Bernstein JD, Traver B, Cook T, Gheorghiade M. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849 e841. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 5.DeFilippis EM, Butler J, Vaduganathan M. Waiting Period Before Implantable Cardioverter-Defibrillator Implantation in Newly Diagnosed Heart Failure With Reduced Ejection Fraction: A Window of Opportunity. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene SJ, Vaduganathan M, Wilcox JE, Harinstein ME, Maggioni AP, Subacius H, Zannad F, Konstam MA, Chioncel O, Yancy CW, Swedberg K, Butler J, Bonow RO, Gheorghiade M. The prognostic significance of heart rate in patients hospitalized for heart failure with reduced ejection fraction in sinus rhythm: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study With Tolvaptan) trial. JACC Heart Fail. 2013;1:488–496. doi: 10.1016/j.jchf.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Mentz RJ, Greene SJ, Ambrosy AP, Vaduganathan M, Subacius HP, Swedberg K, Maggioni AP, Nodari S, Ponikowski P, Anker SD, Butler J, Gheorghiade M. Clinical profile and prognostic value of anemia at the time of admission and discharge among patients hospitalized for heart failure with reduced ejection fraction: findings from the EVEREST trial. Circ Heart Fail. 2014;7:401–408. doi: 10.1161/CIRCHEARTFAILURE.113.000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Greene SJ, Ambrosy AP, Mentz RJ, Subacius HP, Chioncel O, Maggioni AP, Swedberg K, Zannad F, Konstam MA, Senni M, Givertz MM, Butler J, Gheorghiade M. Relation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan trial) Am J Cardiol. 2014;114:1713–1721. doi: 10.1016/j.amjcard.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Marti CN, Mentz RJ, Greene SJ, Ambrosy AP, Subacius HP, Fonarow GC, Chioncel O, Bazari H, Maggioni AP, Zannad F, Konstam MA, Sato N, Gheorghiade M, Butler J. Serum Osmolality and Postdischarge Outcomes After Hospitalization for Heart Failure. Am J Cardiol. 2016;117:1144–1150. doi: 10.1016/j.amjcard.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med. 2015;34:1659–1680. doi: 10.1002/sim.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pokorney SD, Al-Khatib SM, Sun JL, Schulte P, O’Connor CM, Teerlink JR, Armstrong PW, Ezekowitz JA, Starling RC, Voors AA, Velazquez EJ, Hernandez AF, Mentz RJ. Sudden cardiac death after acute heart failure hospital admission: insights from ASCEND-HF. Eur J Heart Fail. 2018 doi: 10.1002/ejhf.1078. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E, Metra M. Effect of serelaxin on mode of death in acute heart failure: results from the RELAX-AHF study. J Am Coll Cardiol. 2014;64:1591–1598. doi: 10.1016/j.jacc.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017;69:556–569. doi: 10.1016/j.jacc.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosy AP, Fudim M, Chioncel O. Sudden cardiac death following admission for acute heart failure: adding insult to injury. Eur J Heart Fail. 2018 doi: 10.1002/ejhf.1109. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Wang NC, Piccini JP, Konstam MA, Maggioni AP, Traver B, Swedberg K, Udelson JE, Zannad F, Cook T, O’Connor CM, Miller AB, Grinfeld L, Gheorghiade M. Implantable cardioverter-defibrillators in patients hospitalized for heart failure with chronically reduced left ventricular ejection fraction. Am J Ther. 2010;17:e78–87. doi: 10.1097/MJT.0b013e3181e70a65. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 17.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 doi: 10.1161/CIR.0000000000000549. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Bajaj NS, Claggett B, Lewis EF, Desai AS, Fang JC, O’Meara E, Shah SJ, Sweitzer NK, Fleg JL, Pitt B, Rouleau JL, Finn P, Pfeffer MA, Solomon SD. Influence of ejection fraction on cause-specific mortality in heart failure with preserved ejection fraction. Eur J Heart Fail. 2018 doi: 10.1002/ejhf.1040. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlstrom U, Sartipy U, Maggioni A, Swedberg K, O’Conner C, Levy WC. Seattle Heart Failure and Proportional Risk Models Predict Benefit From Implantable Cardioverter-Defibrillators. J Am Coll Cardiol. 2017;69:2606–2618. doi: 10.1016/j.jacc.2017.03.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chugh SS. Disrupting the Approach to Sudden Cardiac Death: From Vulnerable Ejection Fraction to Vulnerable Patient. Circulation. 2018;137:7–9. doi: 10.1161/CIRCULATIONAHA.117.029932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]


