Abstract
Background
Alcohol use disorders are characterized by a complex behavioral symptomatology, which includes the loss of control over alcohol consumption and the emergence of a negative affective state when alcohol is not consumed. Some of these symptoms can be recapitulated in rodent models, for instance following chronic intermittent exposure to ethanol vapor inhalation (CIE). However, the detection of negative affect in mice withdrawn from CIE has proven challenging and variable between strains. The present study aimed to detect reliable indices of negative emotionality in CIE-exposed C57BL/6J (C57) and DBA/2J (DBA) mice. Males were used because they are known to escalate their voluntary ethanol consumption upon CIE exposure, which is hypothesized to be driven by negative reinforcement (relief from negative affect).
Methods
Adult male mice were exposed to 4-6 weeks of CIE and were evaluated 3-10 days into withdrawal in the social approach, novelty-suppressed feeding, digging, marble burying, and bottle brush tests.
Results
Withdrawal from CIE decreased sociability in DBA mice but not in C57 mice. Conversely, hyponeophagia was exacerbated by CIE in C57 mice but not in DBA mice. Withdrawal from CIE robustly increased digging activity in both strains, even in the absence of marbles. Aggressive responses to bottle brush attacks were elevated in both C57 and DBA mice following CIE exposure, but CIE had an opposite effect on defensive responses in the two strains (increase in C57 versus decrease in DBA).
Conclusions
Our results indicate that withdrawal from CIE elicits negative emotionality in both C57 and DBA mice but different tests need to be used to measure the anxiogenic-like effects of withdrawal in each strain. Increased digging activity and irritability-like behavior represent novel indices of affective dysfunction associated with withdrawal from CIE in both mouse strains. Our findings enrich the characterization of the affective symptomatology of protracted withdrawal from CIE in mice.
Keywords: Alcoholism, dependence, anxiety, depression, anger
Introduction
Alcohol use disorders are characterized by pathological patterns of alcohol consumption, in which attempts to cut down on drinking can be complicated by the experience of a negative emotional state (e.g., depressed mood, anxiety, anger) during abstinence. Negative affect is hypothesized to be a major drive in the transition to alcohol addiction, as alcohol drinking becomes progressively motivated by the relief from negative affect (i.e. negative reinforcement) rather than by the positively reinforcing properties of alcohol (Koob, 2017). Rat models have been extensively used to understand the neurobiological basis of this phenomenon. Notably, rats chronically exposed to ethanol via passive vapor inhalation exhibit a time-dependent elevation in intracranial self-stimulation thresholds during withdrawal, indicating reduced brain reward (Schulteis et al., 1995). Numerous studies have also reported that rats undergoing acute or protracted withdrawal from chronic ethanol exposure exhibit increased anxiety-like behavior (Baldwin et al., 1991; Knapp et al., 1998; Lal et al., 1991; Overstreet et al., 2004; Rasmussen et al., 2001; Somkuwar et al., 2017; Valdez et al., 2002; Zhao et al., 2007). Similar measures of negative affect have been frustratingly difficult to obtain in mice, although recent studies have started to expand the repertoire of withdrawal-associated behavioral alterations in mice (see Holleran and Winder, 2017 for review). Furthermore, a report indicated between-strain variability in the manifestation of negative affect in this species, whereby ethanol-withdrawn DBA/2J (DBA) mice, but not C57Bl/6J (C57) mice, exhibited increased anxiety-like behavior in the light-dark transition test compared to their ethanol-naïve counterparts (McCool and Chappell, 2015). Interestingly, both strains escalate their voluntary ethanol consumption when made dependent to ethanol, as long as the taste aversion of DBA mice for ethanol is circumvented (Cunningham et al., 2013; Fidler et al., 2012; McCool and Chappell, 2015; but see Lopez et al., 2017). We reasoned that if ethanol intake escalation is indeed driven by negative reinforcement, it should be possible to obtain measures of negative affect in both strains, albeit behavioral tests tapping into different dimensions of negative affect may be needed in each strain.
In the present study, we used four assays to characterize the affective behaviors of adult male C57 and DBA mice following 4 to 6 weeks of chronic intermittent ethanol inhalation (CIE) and 3-10 days of withdrawal, a time frame when CIE-exposed mice display escalated ethanol drinking and other behavioral alterations (Becker and Lopez, 2004; Jury et al., 2017; Kroener et al., 2012; McCool and Chappell, 2015; Rose et al., 2016). We only tested males because evidence for robust ethanol intake escalation following dependence induction has only been obtained for this sex (Jury et al., 2017). We first used the social approach test (SAT), which measures the propensity of mice to interact with a unfamiliar conspecific (sociability), as well as their preference for social novelty (Moy et al., 2004). Reduced social interaction can be interpreted as evidence of social anxiety (Toth and Neumann, 2013). We also used the novelty-suppressed feeding (NSF) test, which measures the unconditioned inhibition of feeding due to novelty (see Dulawa and Hen, 2005 for review). In this test, the latency of food-deprived mice to initiate feeding in a novel environment provides an index of anxiety-like behavior, although changes in appetite and impulsivity-like behavior may also influence this variable (Dulawa and Hen, 2005; Holleran and Winder, 2017). Digging was measured both in the absence or presence of marbles to clarify whether previous reports of increased marble burying during ethanol withdrawal reflected anxiety-like behavior or a general increase in digging activity (Deacon, 2006b). Finally, we implemented the bottle brush test (BBT), in which aggressive and defensive responses to repeated attacks with a mechanical stimulus provide an index of irritability-like and avoidance-like behavior, respectively (Lagerspetz and Portin, 1968; Riittinen et al., 1986). We found that withdrawal from CIE affected all these measures, albeit in a mouse strain-dependent manner.
Materials and Methods
Animals
Male C57BL/6J (C57) mice were obtained from The Scripps Research Institute Rodent Breeding Colony. Male DBA/2J (DBA) mice were purchased from The Jackson Laboratories (Sacramento, CA). The mice were at least 10 weeks old when ethanol exposure started. Mice were maintained on a 12 h/12 h light/dark cycle and all behavioral testing was performed during the dark phase. Food (Teklad LM-485, Envigo) and acidified or reverse osmosis purified water were available ad libitum except for a 24-h period of food deprivation before the novelty-suppressed feeding test. Sani-Chips (Envigo) were used for bedding substrate. The mice were group-housed except for irritability testing. For all behavioral tests, mice were transferred to the experimental room at least 1 h prior to start of testing. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Scripps Research Institute Institutional Animal Care and Use Committee.
Ethanol vapor exposure
Mice were subjected to chronic intermittent ethanol (CIE) exposure in inhalation chambers (La Jolla Alcohol Research Inc., La Jolla, CA), as previously described (Becker and Hale, 1993; Becker and Lopez, 2004; Contet et al., 2011) and according to the standard operating procedure used by The Scripps Research Institute Alcohol Research Center. During each CIE week, mice were subjected to four 16-h periods of intoxication (ethanol vapor inhalation) separated by 8-h periods of withdrawal (air inhalation). Before each 16-h ethanol vapor inhalation period, mice received an intraperitoneal injection of ethanol and pyrazole to stabilize serum ethanol concentrations across the 16-h period (target level = 200 mg/dL). C57 mice received 1.5 g/kg ethanol and 68.1 mg/kg pyrazole, while DBA mice received half of these doses during their first week of CIE, and three quarters of these doses during subsequent weeks. We had to adjust the dose used for DBA mice because they showed signs of overintoxication when the full dose was used. Control mice received injections of pyrazole only. Tail vein blood samples were collected periodically (at least once a week) at the end of intoxication periods using heparinized capillary tubes and centrifuged for 5 min at 13000 rpm. The supernatant was processed in a GM7 analyzer (Analox Instruments, London, UK). Average serum ethanol concentrations in each cohort are shown in Table 1. There was no significant difference between the serum ethanol concentrations of C57 and DBA mice tested in the same behavioral assay (see Table 2). Mice were exposed to 4-6 weeks of CIE and tested 3-10 days after their last ethanol vapor exposure (see Table 1 for details).
Table 1. Cohort information.
This table indicates the number of mice per treatment in each cohort, serum ethanol concentrations (SEC) measured in CIE-exposed mice and the time-point of behavioral testing.
| Strain | Cohort | Group sizes | SEC (mg/dL) | Behavioral tests |
|---|---|---|---|---|
| C57 | Cohort 1 | Air, n=10 CIE, n=10 |
211.2 ± 25.0 | SAT 6-7 days withdrawal from CIE4 (Fig. 1A-D) DMB 10 days withdrawal from CIE4 (Fig. 3A-C) |
| Cohort 2 | Air, n=12 CIE, n=7 |
206.2 ± 17.5 | NSF 5 days withdrawal from CIE5 (Fig. 2A-C) BBT 3-7 days withdrawal from CIE6 (Fig. 4A-B) |
|
| Cohort 3 | Air, n=4* CIE, n=5 |
217.5 ± 22.6 | NSF 5 days withdrawal from CIE5 (Fig. 2A-C) BBT 3-7 days withdrawal from CIE6 (Fig. 4A-B) |
|
| DBA | Cohort 1 | Air, n=7 CIE, n=6 |
253.3 ± 16.0 | SAT 6-7 days withdrawal from CIE4 (Fig. 1E-H) |
| Cohort 2 | Air, n=5 CIE, n=5 |
179.5 ± 22.3 | NSF 5 days withdrawal from CIE4 (Fig. 2D-F) BBT 3-7 days withdrawal from CIE6 (Fig. 4C-D) |
|
| Cohort 3 | Air, n=7 CIE, n=5 |
169.5 ± 19.9 | NSF 5 days withdrawal from CIE4 (Fig. 2D-F) DMB 10 days withdrawal from CIE4 (Fig. 3D-F) |
CIEn, week n of CIE exposure; SAT, social approach test; NSF, novelty-suppressed feeding; BBT, bottle brush test; DMB, digging and marble burying.
one Air male from C57 cohort 3 was excluded from the NSF test because it kept trying to escape from the arena and home cage
Table 2. Serum ethanol concentrations.
This table indicates the average serum ethanol concentrations measured in CIE-exposed C57 and DBA mice tested in a given behavioral assay. There was no significant difference between strains in any of the assays, as evaluated by unpaired two-tailed t-test.
| Behavioral assay | C57 mice | DBA mice | t-test |
|---|---|---|---|
| Social approach test | 211.2 ± 25.0 | 253.3 ± 16.0 | t54 = −1.26, n.s. |
| Novelty-suppressed feeding | 208.8 ± 14.3 | 173.3 ± 14.7 | t55 = 1.72, n.s. |
| Digging and marble burying | 211.2 ± 25.0 | 169.5 ± 19.9 | t26 = 1.32, n.s. |
| Bottle brush test | 208.8 ± 14.3 | 179.5 ± 22.3 | t39 = 1.03, n.s. |
Social approach test
The social approach test (SAT) was performed using a three-chamber paradigm as described previously (Kaidanovich-Beilin et al., 2011; Moy et al., 2004; Sidhu et al., 2014). The apparatus was a rectangular box constructed from clear Plexiglas and containing three adjacent chambers 19 cm × 45 cm each, with 30 cm high walls. The three chambers were separated by dividing walls made from clear Plexiglas with openings between the central chamber and each side chamber. Removable doors over these openings permitted chamber isolation or free access to all chambers. Testing was conducted under red lights and comprised three consecutive phases: habituation, sociability (session 1) and preference for social novelty (session 2). During habituation, the test mouse was placed in the central chamber with free access to the side chambers and allowed to explore for 5 min before testing began. In session 1, an unfamiliar mouse (stranger 1) was placed under a small wire cup (Galaxy cup, Spectrum Diversified) in one of the side chambers. The other side chamber contained an empty cup. The test mouse was allowed to explore all three chambers for 10 min. The time spent in each chamber (all four paws) and the time the test mouse spent interacting with stranger 1 were recorded. In session 2, a novel unfamiliar mouse (stranger 2) was placed under the empty wire cup in the second side chamber, while stranger 1, the already-investigated unfamiliar mouse, remained in place. The test mouse was allowed to explore all three chambers for another 10 min and the same parameters were recorded. Placement of stranger 1 in the left or right side chambers was randomly alternated between test mice. Both stranger mice were males and strain-matched to the test mouse. The floor of the chamber was cleaned with 2-3% acetic acid, 70% ethanol, and water between tests to eliminate odor trails.
Novelty-suppressed feeding
Novelty-suppressed feeding (NSF), also known as hyponeophagia or novelty-induced hypophagia (Deacon, 2011; Dulawa and Hen, 2005), was measured by presenting regular chow to food-deprived mice, as described by Samuels and Hen (Samuels and Hen, 2011). Mice were transferred to a new, clean home cage without food approximately 24 h before testing. Right before testing, mice were transferred to new, clean holding cages to clear home cages. Testing consisted of two consecutive phases: feeding in arena and feeding in home cage. The experimental arena consisted of a brightly lit (400 lux) Taconic Transit Cage (56-cm long × 40-cm wide × 18-cm deep) whose bottom was lined with 2 cm of fresh bedding. One food pellet was secured onto a platform (see Samuels and Hen, 2011 for details) located in the center of the arena. The test mouse was placed in a corner of the arena and the latency to eat the food pellet was recorded, with a cutoff time of 10 min. The mouse was removed from the arena as soon as it started eating the food pellet and was immediately transferred to its home cage with a single food pellet of known weight (no lid on the cage, same lighting condition as the arena). The latency to eat this pellet was recorded. After 5 min the mouse and the pellet were weighed in order to determine body weight loss and amount of food consumed in home cage. The mouse was then transferred into a new, clean home cage with free access to food and water.
Digging and marble burying
Digging and marble burying (DMB) were measured as described by Deacon (Deacon, 2006b). Testing was conducted under dim lighting (20 lux). The mouse was placed in a new, clean cage with a bedding thickness of 5 cm and no lid, and allowed to freely dig for 3 min. The number of digging bouts and total digging duration were recorded (phase 1). The mouse was then removed from the cage, the bedding flattened and 12 marbles arranged in a 4 × 3 array on top of the bedding. The mouse was reintroduced into the cage and allowed to bury the marbles for 30 min with a lid covering the cage. The number of marbles that were buried (two-thirds or more) was counted at the end of the test (phase 2).
Bottle brush test
The bottle brush test (BBT) was conducted as described by Riittinen and colleagues (Riittinen et al., 1986). The BBT measures defensive and aggressive responses to an “attack” by a mechanical stimulus (a moving bottle brush, Lagerspetz and Portin, 1968). This test was recently used to reveal irritability-like behavior during ethanol withdrawal in CIE-exposed Wistar rats (Kimbrough et al., 2017; Somkuwar et al., 2017). Testing was conducted under red lights on five consecutive days. The mice were single-housed for three days before testing started and throughout the testing period. The mouse was “attacked” by moving a bottle brush (14-cm long × 5-cm wide cylindrical brush, 33-cm total length with handle) toward it. The attacks were made in the home cage with the lid and food tray removed. On each testing day, the mouse was attacked 10 times and the attacks were carried out consecutively with 10-15 s intervals. Each attack consisted of five stages as follows:
Brush (in rotation) approaching the mouse from the opposite end of the cage (starting position).
Brush (in rotation) touching the whiskers of the mouse.
Brush (in rotation) returning to the starting position in the opposite end of the cage.
Brush (in rotation) at the starting position.
Brush (no rotation) at the starting position.
Each stage of the attack lasted 1.5 s, except for stage 5 that was prolonged, if necessary, until the mouse returned to its end of the cage or 5 s had elapsed. Responses to the attacks were observed. The total number of occurrences of each behavior across all phases of all 10 attacks was recorded. These behaviors were further classified as aggressive (smelling/exploring the brush, biting the brush, boxing the brush, following the brush, and tail rattling) or defensive behaviors (escaping from the brush, digging, jumping, climbing/rearing, defecation, vocalization and grooming).
Experimental cohorts
Data were generated from three independent cohorts of C57 mice and three independent cohorts of DBA mice tested by the same experimenter (HS) over a total span of 3 years. The group sizes, serum ethanol concentrations during CIE and testing timeline are presented in Table 1 for each of these cohorts. NSF and BBT data from C57 cohorts 2 and 3, and NSF data of DBA cohorts 2 and 3 were pooled.
Statistical analysis
Data from C57 and DBA mice were analyzed separately. In the SAT, the time spent in each chamber of the apparatus was analyzed by two-way analysis of variance (ANOVA), with treatment as between-subject variable and chamber as within-subject variable. The time spent interacting with stranger mice was analyzed by unpaired t-test for session 1 and one-way ANOVA (treatment as between-subject variable and stranger mouse as within-subject variable) for session 2. In the NSF, latencies to eat the pellet in the arena and home cage were analyzed by two-way ANOVA (treatment as between-subject variable and environment as within-subject variable), and amount eaten and body weight lost were analyzed by unpaired t-tests. DMB data were analyzed by unpaired t-tests. BBT data were analyzed using two-way ANOVA with treatment as between-subject variable and day as within-subject variable. When ANOVA interactions were significant, posthoc pairwise comparisons were conducted using the Bonferroni correction. All t-tests were two-tailed. Data are expressed as mean ± standard error of the mean (s.e.m.).
Results
Withdrawal from CIE reduces social interaction in DBA but not in C57 mice
Results obtained during SAT session 1 in C57 mice are shown in Fig. 1A-B. Overall, C57 mice spent differential amounts of time in the chamber containing the stranger mouse (S1), the central compartment (C) and the chamber containing the empty cup (E) (main effect of chamber, F2,18=124.0, p<0.001). They spent more time in the S1 chamber than in the C and E chambers, reflecting normal sociability (Fig. 1A). There was no significant main effect of CIE on the time spent in each chamber of the SAT apparatus (F1,18=1.0, n.s.) nor an interaction between treatment and chamber (F2,36=0.5, n.s.). In addition, CIE mice spent the same amount of time directly interacting with the stranger mouse as Air mice, indicating that CIE did not alter sociability in C57 mice (t18=−0.8, n.s., Fig. 1B).
Figure 1. Withdrawal from CIE reduces social interaction in DBA but not in C57 mice.
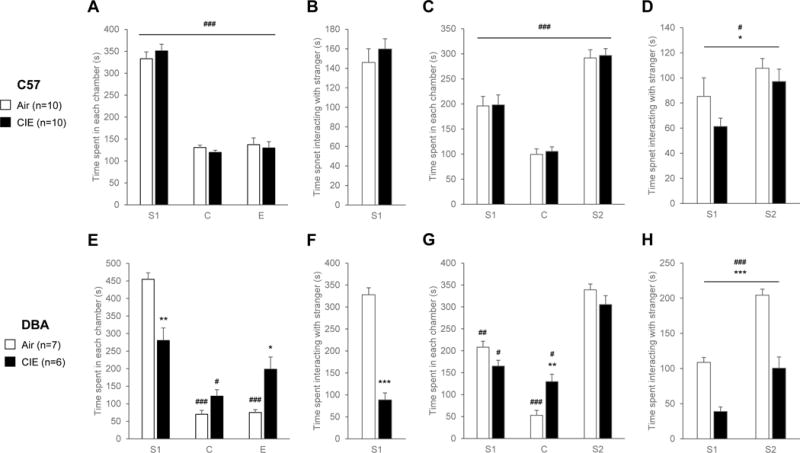
C57 (A-D; Air, n=10; CIE, n=10) and DBA (E-H; Air, n=7; CIE, n=6) mice were tested in the social approach test (SAT) 6-7 days into withdrawal from 4 weeks of chronic intermittent ethanol (CIE) inhalation. Sociability was first evaluated by assessing the behavior of the mouse in the presence of a stranger mouse (S1) in one of the three chambers of the SAT apparatus (A-B, E-F). Preference for social novelty was then evaluated by introducing another stranger mouse (S2) in the opposite chamber. A, C, E, G: Time spent in each chamber (S1, chamber containing first stranger mouse under a wire cup; C, central chamber; E, chamber containing empty wire cup; S2, chamber containing novel stranger mouse under a wire cup). B, D, F, H: Time spent directly interacting with the stranger mice. *, effect of treatment. #, effect of chamber (A, C, E, G) or stranger novelty (D, H). One symbol, p<0.05; two symbols, p<0.01; three symbols, p<0.001.
Results obtained during SAT session 2 in C57 mice are shown in Fig. 1C-D. There was again a significant main effect of chamber (F2,18=52.9, p<0.001), no main effect of treatment (F1,18=1.5, n.s.) and no interaction between the two variables (F2,36=0.005, n.s.). Both Air and CIE mice spent more time in the S2 compartment than in the S1 compartment, reflecting normal preference for social novelty (Fig. 1C). This preference was less pronounced when examining the time spent directly interacting with each stranger mouse (effect of stranger, F1,18=5.5, p<0.05, Fig. 1D). In contrast to session 1, CIE reduced the time spent directly interacting with stranger mice, but the effect was modest (effect of treatment, F1,18=5.3, p<0.05) and there was no significant interaction between variables (F1,18=0.3, n.s., Fig. 1D).
Results obtained during SAT session 1 in DBA mice are shown in Fig. 1E-F. Overall, DBA mice also spent differential amounts of time in the S1, C and E chambers (main effect of chamber, F2,11=56.4, p<0.001). Although there was no significant main effect of CIE on the time spent in each chamber of the SAT apparatus (F1,11=3.7, p=0.08), there was a significant interaction between treatment and chamber (F2,22=15.9, p<0.001). Posthoc analysis revealed that CIE-exposed DBA mice spent less time in the S1 chamber (p<0.01) and more time in the E chamber (p<0.05) compared to their air-exposed counterparts (Fig. 1E). As expected, Air mice spent more time in the S1 chamber compared to both the C and E chambers (Fig. 1E, p<0.001 for both). In contrast, CIE mice spent an equivalent amount of time in the S1 and E chambers, indicating reduced sociability (Fig. 1E). Furthermore, they spent less time directly interacting with the stranger mouse compared to Air mice (t11=10.6, p<0.001, Fig. 1F). Results obtained during SAT session 2 in DBA mice are shown in Fig. 1G-H. There was again a significant main effect of chamber (F2,11=81.4, p<0.001), no main effect of treatment (F1,11=1.0, n.s.) and a significant interaction between the two variables (F2,22=6.7, p<0.01, Fig. 1G). Posthoc analysis revealed that both air- and CIE-exposed DBA mice preferred the compartment containing the novel stranger mouse (S2) to the S1 (p<0.01 for Air mice, p<0.05 for CIE mice) and C (p<0.001 for Air mice, p<0.05 for CIE mice) chambers (Fig. 1G). However, CIE mice spent more time in the C chamber than Air mice (Fig. 1G, p<0.01). In addition, they spent less time directly interacting with both stranger mice, as reflected by a main effect of treatment (F1,22=73.0, p<0.001, Fig. 1H). Overall, DBA mice exhibited preference for social novelty, i.e. they spent more time interacting with the novel stranger (S2) than with the already-investigated stranger (S1) (effect of stranger, F1,11=65.2, p<0.001) and there was no significant interaction between variables (F1,11=3.0, n.s., Fig. 1H).
Withdrawal from CIE increases hyponeophagia in C57 but not in DBA mice
Results obtained in the NSF test in C57 mice are shown in Fig. 2A-C. One-way ANOVA revealed a main effect of environment on the latency to eat the pellet (F1,25=95.1, p<0.001), consistent with hyponeophagia (i.e., feeding inhibition in the anxiogenic environment of the arena; Fig. 2A). There was also a main effect of treatment (F1,25=11.4, p<0.01) and an interaction between treatment and environment (F1,25=23.0, p<0.001). Posthoc analysis indicated that although both Air and CIE mice exhibited hyponeophagia (arena vs. home cage, p<0.001 for both), CIE mice had a longer latency to eat the pellet in the arena (p<0.001), albeit not in the home cage, compared to Air mice (Fig. 2A). There was no effect of treatment on the amount of food consumed in the home cage, indicating that the effect of CIE on feeding latency in the arena did not result from reduced appetite (t25=−0.3, n.s.; Fig. 2B). CIE also did not affect the extent of body weight loss during the 24-h food deprivation (t25=−0.3, n.s.; Fig. 2C).
Figure 2. Withdrawal from CIE increases hyponeophagia in C57 but not in DBA mice.

C57 (A-C; Air, n=15; CIE, n=12) and DBA (D-F; Air, n=12; CIE, n=10) mice were tested in the novelty-suppressed feeding test 5 days into withdrawal from 4-5 weeks of chronic intermittent ethanol (CIE) inhalation. A, D: Latency to initiate feeding in the experimental arena and in the home cage. B, E: Amount of food eaten during first 5 min in the home cage. C, F: Body weight lost during 24-h food deprivation. *, effect of treatment. #, effect of environment. Three symbols, p<0.001.
Results obtained in the NSF test in DBA mice are shown in Fig. 2D-F. In this strain, there was also a main effect of environment on the latency to eat the pellet (F1,20=61.1, p<0.001), reflecting hyponeophagia (Fig. 2D). However, in contrast to C57 mice, there was no effect of treatment (F1,20=0.5, n.s.) nor an interaction between variables (F1,20=0.3, n.s.). As in C57 mice, there was no effect of CIE on appetite (t20=1.6, n.s.; Fig. 2E) and body weight loss (t20=−1.2, n.s.; Fig. 2F).
Withdrawal from CIE increases digging activity in both C57 and DBA mice
Results obtained for C57 mice in the DMB test are shown in Fig. 3A-C. CIE increased the number of digging bouts (t18=−5.7, p<0.001, Fig. 3A) and the total duration of digging (t18=−3.8, p<0.01, Fig. 3B) during the first phase of the test, and the number of marbles buried (t18=−3.6, p<0.01, Fig. 3C) during the second phase of the test.
Figure 3. Withdrawal from CIE increases digging in both C57 and DBA mice.
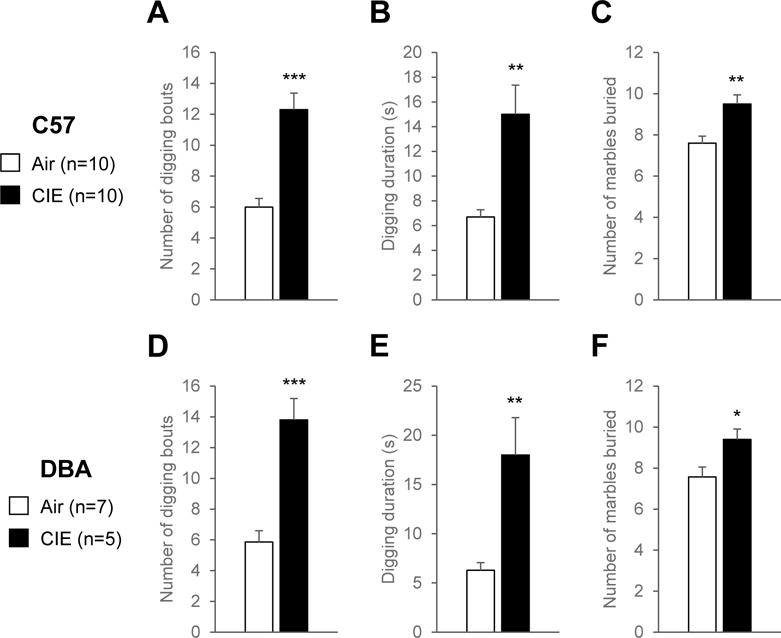
C57 (A-C; Air, n=10; CIE, n=10) and DBA (D-F; Air, n=7; CIE, n=5) mice were tested in the digging and marble burying test 10 days into withdrawal from 4 weeks of chronic intermittent ethanol (CIE) inhalation. A, D: Number of digging bouts. B, E: Total duration of digging. C, F: Number of marbles buried. *, effect of treatment. One star, p<0.05; two stars, p<0.01; three stars, p<0.001.
Results obtained for DBA mice in the DMB test are shown in Fig. 3D-F. CIE also increased the number of digging bouts (t10=−5.5, p<0.001, Fig. 3D), the total duration of digging (t10=−3.6, p<0.01, Fig. 3E) and the number of marbles buried (t10=−2.6, p<0.05, Fig. 3F) in this strain.
Withdrawal from CIE increases irritability-like behavior in both C57 and DBA mice
Results obtained in the BBT for C57 mice are shown in Fig. 4A-B. For aggressive responses, repeated-measures ANOVA revealed significant effects of treatment (F1,26=73.4, p<0.001) and time (F4,104=3.9, p<0.01), as well as a significant interaction between time and treatment (F4,104=5.0, p<0.01). These effects reflected a gradual increase in aggressive responses that peaked 6 days into withdrawal in CIE mice (day 4: p<0.01; days 5-7: p<0.001), while there was no effect of repeated testing in Air mice (Fig. 4A). CIE mice also exhibited more defensive responses than Air mice, as indicated by a significant effect of treatment (F1,26=5.7, p<0.05). There was a main effect of time (F4,104=14.6, p<0.001) but no time × treatment interaction (F4,104=0.4, n.s.), as both Air and CIE mice gradually reduced their defensive responses over time (Fig. 3B). Results obtained in the BBT in DBA mice are shown in Fig. 4C-D. There was a significant main effect of treatment on aggressive responses (F1,8=34.5, p<0.001), with CIE mice also being more aggressive than Air mice in this strain (Fig. 4C). The effect of time (F4,32=49.3, p<0.001) and the time × treatment interaction (F4,32=17.2, p<0.001) were both significant, reflecting the steep decrease in aggressive responses of CIE mice after their first testing day (3 days into withdrawal), compared to subsequent days (Fig. 4C). Posthoc analysis revealed a significant difference between Air and CIE mice on withdrawal days 3 (p<0.01), 4, 6 and 7 (p<0.05). The effect of treatment almost reached significance for defensive responses (F1,8=5.0, p= 0.056), with CIE mice tending to exhibit less defensive responses than Air mice 5-7 days into withdrawal (Fig. 4D). There was a significant effect of time (F4,32=4.0, p<0.01) but no interaction between time and treatment (F4,32=0.9, n.s.).
Figure 4. Withdrawal from CIE increases irritability-like behavior in both C57 and DBA mice.
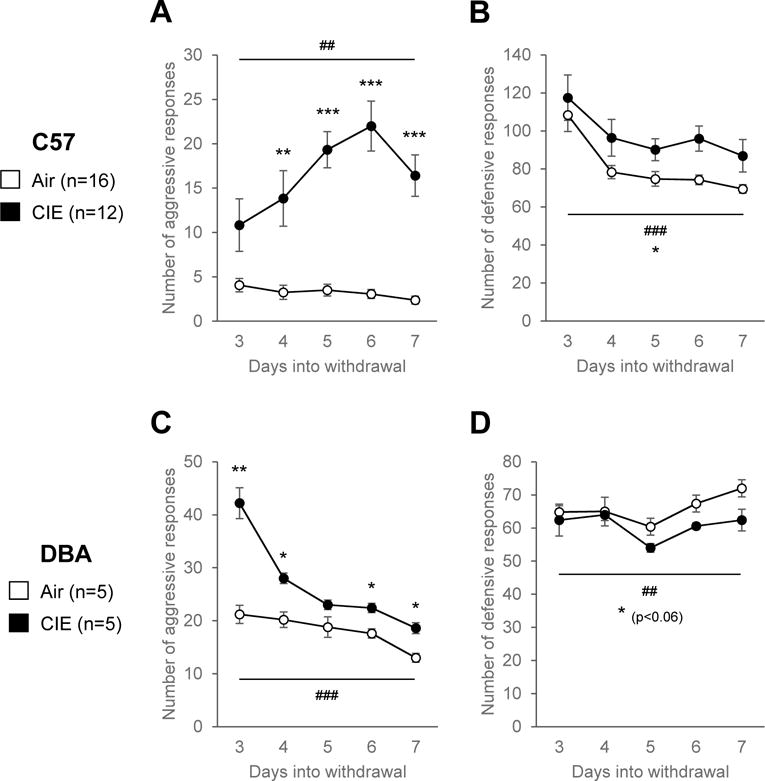
C57 (A-B; Air, n=16; CIE, n=12) and DBA (C-D; Air, n=5; CIE, n=5) mice were tested in the bottle brush test 3-7 days into withdrawal from 6 weeks of chronic intermittent ethanol (CIE) inhalation. A, C: Number of aggressive responses. B, F: Number of defensive responses. *, effect of treatment. #, effect of time. One symbol, p<0.05; two symbols, p<0.01; three symbols, p<0.001.
Discussion
Our study shows that the behavioral symptomatology of ethanol withdrawal in mice, as evaluated 3-10 days after 4-6 weeks of chronic intermittent ethanol vapor inhalation, reflects negative affect but differs between C57 and DBA mouse strains. Withdrawal from CIE reduced social interaction in DBA mice, but not in C57 mice. It did not alter preference for social novelty in either strain. In contrast, withdrawal from CIE increased hyponeophagia in C57 mice, but not in DBA mice. The effect of CIE in C57 mice did not result from differential body weight loss or appetite. Withdrawal from CIE was associated with a robust increase in digging activity in both strains, as reflected both by direct measures of digging behavior and by marble burying. Furthermore, withdrawal from CIE increased aggressive responses to bottle brush attacks in both strains, albeit with a different time-course, with the effect of withdrawal peaking earlier in DBA mice (3 days) than in C57 mice (6 days). Withdrawal from CIE also increased defensive responses in C57 mice, but tended to decrease them in DBA mice. These results corroborate previous findings and enrich the characterization of affective disturbances elicited by ethanol withdrawal in mice. Importantly, different behavioral assays are needed to capture anxiety-like behavior in C57 and DBA mice withdrawn from CIE.
In accordance with our findings, recent studies showed that chronic ethanol exposure via inhalation (CIE) or drinking (continuous two-bottle choice) exacerbates hyponeophagia in C57 mice experiencing 3 days or 2-5 weeks, respectively, of abstinence (Holleran et al., 2016; Jury et al., 2017; Pang et al., 2013) and that CIE increases marble burying in C57 mice 2-6 days into withdrawal (Jury et al., 2017; Pleil et al., 2015; Rose et al., 2016). Other studies are consistent with the mouse strain specificity we observed in the SAT and NSF assays. For instance, CIE reduced social interaction in DBA mice 24 h and 7 days into withdrawal (Lowery-Gionta et al., 2015; Marcinkiewcz et al., 2015), while C57 mice fed an ethanol liquid diet for 12 days and tested during early withdrawal (5-6 h) exhibited unaltered sociability (Moy et al., 2013). Conversely, anxiety-like behavior could be detected in DBA mice but not C57 mice using the light/dark box assay 3-10 days into withdrawal from CIE (Bray et al., 2017; McCool and Chappell, 2015). Importantly, each of these behavioral assays tap into different aspects of anxiety, a complex, multifaceted affective state, by using different incentives (food, social interaction, environment exploration) to establish an approach-avoidance conflict. It appears that genetic variations between mouse strains produce differential sensitivity of each of these aspects to the anxiogenic-like effects of CIE withdrawal. The discrepant withdrawal symptomatology of C57 and DBA mice may result from differential effects of CIE and abstinence on neuronal activity and gene expression in the two strains. For instance, c-fos induction in the amygdala, hippocampus, prelimbic cortex and lateral septum is much stronger and differential gene expression in the hippocampus is more extensive in DBA mice than in C57 mice following 72 h of ethanol vapor inhalation and 7 h withdrawal, suggesting that similar strain differences may exist in mice withdrawn from several weeks of CIE (Chen et al., 2009; Daniels and Buck, 2002).
The present study focused on affective responses exhibited by CIE-exposed males. An effect of sex on the behavioral symptomatology of withdrawal from CIE was previously reported in C57 mice, with females not exhibiting the increased ethanol consumption, hyponeophagia and marble burying that were observed in males (Jury et al., 2017). In the same way that C57 and DBA males manifest negative affect differentially across a battery of behavioral tests, our study suggests that alternative assays may be needed to detect negative affect in CIE-exposed C57 females.
Our study implemented two assays that had not been previously used to characterize the symptomatology of ethanol withdrawal in mice: the BBT and digging in the absence of marbles. Two recent studies used the BBT to measure irritability-like behavior in Wistar rats subjected to operant ethanol oral self-administration combined with passive ethanol vapor inhalation (CIE). In both studies, CIE increased aggressive responses during withdrawal from ethanol vapor (from 8 h onwards and up to 2 weeks), but did not affect defensive responses at any time point (Kimbrough et al., 2017; Somkuwar et al., 2017). Consistent with the rat phenotype, C57 and DBA mice also showed a significant increase in aggressive responses during their first week of withdrawal. CIE also affected defensive responses in mice, although it had a differential effect in C57 and DBA mice (significant increase and trend for reduction, respectively). An earlier study that characterized the effect of social isolation and overstimulation on BBT responses in male mice (mixed Swiss/NMRI background) identified a first factor underlying data variance for responses that were categorized as aggressive in the present study (following, boxing, biting), which was interpreted as irritability, while responses categorized here as defensive (escape, digging, jumping, climbing) mostly loaded onto a second factor that was hypothesized to reflect avoidance or fear (Riittinen et al., 1986). Accordingly, our results together with previous rat studies indicate that withdrawal from CIE consistently increases irritability-like behavior in rodents, while the effect on avoidance-like behavior is species- and strain-dependent. This observation corroborates the factor analysis of Riittinen and colleagues, which showed that the two types of responses were not sharing a common source of variance (i.e., were not correlated). Furthermore, the fact that C57 mice showed an increase in both aggressive and defensive responses indicates that these two coping strategies are not mutually exclusive. The consistent effect of withdrawal from CIE on irritability-like behavior across rodent species and mouse strains has clinical relevance. Heavy drinkers and alcohol-dependent subjects can experience frustration or exhibit aggressive behavior during abstinence (Cardoso et al., 2006; Miczek et al., 2015; Winward et al., 2014). Interestingly, aggression and frustration sensitivity were stronger predictors of relapse than anxiety, depression or impulsivity in a sample of abstinent male alcoholics (Baars et al., 2013).
Several studies had previously reported that CIE-exposed male C57 mice bury more marbles when tested 2, 3 or 6 days into withdrawal from vapor and interpreted this observation as an indication of increased anxiety-like behavior (Jury et al., 2017; Pleil et al., 2015; Rose et al., 2016). However, the validity of marble burying as a measure of anxiety-like behavior has been repeatedly called into question (Deacon, 2006b; Gyertyan, 1995; Njung’e and Handley, 1991; Thomas et al., 2009). Lack of habituation and lack of avoidance of the marbles indicated that marble burying is a by-product of spontaneous digging behavior, rather than an aversive response to the presence of marbles (Gyertyan, 1995; Njung’e and Handley, 1991; Thomas et al., 2009). Our observation that CIE increased digging activity even in the absence of marbles corroborates this interpretation. The effect of CIE on digging activity was more pronounced than on marble burying in both C57 and DBA mice, in accordance with the observation of Gyertyan that “digging time” is a more sensitive measure than “marble buried” (Gyertyan, 1995). Digging is an instinctive behavior that many species of wild rodents use to make burrows for habitat and predator protection. In the laboratory setting, digging shows some features of compulsivity (stereotypy, repetitiveness and resistance to extinction, Dalley et al., 2011; Pitman, 1989; see also De Boer and Koolhaas, 2003; Gyertyan, 1995 for discussion), and the fact that chronic ethanol exposure increases its frequency may reflect a worsening of compulsivity-like tendencies, as proposed in earlier studies (Perez and De Biasi, 2015; Umathe et al., 2008). Nevertheless, our observation that withdrawal from CIE increases digging/burying activity in both C57 and DBA mice does not provide an indication of the exact nature of the affective state associated with excessive digging, which could possibly include anxiety, compulsivity and/or irritability.
Recent efforts to identify emotional disturbances in mice withdrawn from chronic ethanol exposure turned to the forced swim test (FST), an assay that can predict antidepressant efficacy and is therefore hypothesized to provide a measure of depression-like behavior. Several studies reported that prolonged abstinence (2 weeks) from chronic voluntary ethanol administration increases immobility in the FST (see Holleran and Winder, 2017 for review). Immobility in the FST was not affected (or even decreased) in mice exposed to 4 weeks of CIE and tested 8 h to 12 days after last vapor exposure (Bray et al., 2017; Maldonado-Devincci et al., 2016), but additional studies are needed to determine whether immobility may increase after longer periods of abstinence post-CIE, as previously observed in rats (Walker et al., 2010). Another study reported reduced nest building during the first 24 h of withdrawal in mice exposed to 3 days of CIE (Greenberg et al., 2016). Nest building is a species-typical behavior of many small rodents that serves a critical role in the wild for heat conservation, sheltering and reproduction, and is considered a good ethological indicator of mouse welfare in the laboratory setting (Deacon, 2006a; Gaskill et al., 2013). In the future, it would be interesting to determine whether nest building could be used as another indicator of affective disruption during protracted abstinence from long-term exposure to ethanol.
Overall, our study provides evidence that exposure of mice to CIE elicits a negative emotional state during the first week of withdrawal, which can be detected in various assays evaluating anxiety-like, compulsive-like and irritability-like behaviors. The new measures introduced in this paper (digging and irritability) can be used to refine the experimental design of studies investigating the effects of chronic ethanol exposure in mouse models, as they provide easily accessible behavioral endpoints that can be correlated with molecular and cellular variables. Altogether, our findings enrich the characterization of the affective symptomatology of protracted withdrawal from CIE in mice and emphasize that multiple behavioral assays are needed to capture complex affective phenotypes.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: R21 AA024198, F32 AA024952, P60 AA006420 and R01 AA021491. The authors have no conflict of interest to declare.
Footnotes
DR. CANDICE CONTET (Orcid ID : 0000-0002-4459-9540)
Contributions
HS and CC designed experiments, HS and MK conducted experiments, HS and CC analyzed data and wrote the manuscript.
References
- Baars MY, Muller MJ, Gallhofer B, Netter P. Relapse (number of detoxifications) in abstinent male alcohol-dependent patients as related to personality traits and types of tolerance to frustration. Neuropsychobiology. 2013;67:241–248. doi: 10.1159/000350483. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bray JG, Roberts AJ, Gruol DL. Transgenic mice with increased astrocyte expression of CCL2 show altered behavioral effects of alcohol. Neuroscience. 2017;354:88–100. doi: 10.1016/j.neuroscience.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JM, Barbosa A, Ismail F, Pombo S. NETER alcoholic typology (NAT) Alcohol Alcohol. 2006;41:133–139. doi: 10.1093/alcalc/agh247. [DOI] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43:411–420. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Gardon O, Filliol D, Becker JA, Koob GF, Kieffer BL. Identification of genes regulated in the mouse extended amygdala by excessive ethanol drinking associated with dependence. Addict Biol. 2011;16:615–619. doi: 10.1111/j.1369-1600.2010.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Murphy KV, Mulgrew JA, Smitasin PJ. Time-dependent negative reinforcement of ethanol intake by alleviation of acute withdrawal. Biol Psychiatry. 2013;73:249–255. doi: 10.1016/j.biopsych.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Buck KJ. Expression profiling identifies strain-specific changes associated with ethanol withdrawal in mice. Genes Brain Behav. 2002;1:35–45. doi: 10.1046/j.1601-1848.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat Protoc. 2006a;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006b;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Hyponeophagia: a measure of anxiety in the mouse. J Vis Exp. 2011 doi: 10.3791/2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P, Cunningham CL. Dependence induced increases in intragastric alcohol consumption in mice. Addict Biol. 2012;17:13–32. doi: 10.1111/j.1369-1600.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp. 2013;51012 doi: 10.3791/51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Huang LC, Spence SE, Schlumbohm JP, Metten P, Ozburn AR, Crabbe JC. Nest building is a novel method for indexing severity of alcohol withdrawal in mice. Behav Brain Res. 2016;302:182–190. doi: 10.1016/j.bbr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyan I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995;6:24–31. [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LE, Patel S, Winder DG. Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology. 2016;41:2062–2071. doi: 10.1038/npp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Winder DG. Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav. 2017;16:8–14. doi: 10.1111/gbb.12338. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011 doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, de Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, George O. CRF1 Receptor-Dependent Increases in Irritability-Like Behavior During Abstinence from Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res. 2017;41:1886–1895. doi: 10.1111/acer.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Koob GF. Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology (Berl) 2017;234:1315–1332. doi: 10.1007/s00213-016-4484-6. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerspetz K, Portin R. Simulation of cues eliciting aggressive responses in mice at two age levels. J Genet Psychol. 1968;113:53–63. doi: 10.1080/00221325.1968.10533808. [DOI] [PubMed] [Google Scholar]
- Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: reversal by buspirone. Alcohol. 1991;8:467–471. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, Becker HC. Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol. 2017;58:73–82. doi: 10.1016/j.alcohol.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Kampov-Polevoi A, McKinley RE, Morrow DH, O’Buckley TK, Morrow AL. Chronic Intermittent Ethanol Exposure Alters Stress Effects on (3alpha,5alpha)-3-hydroxy-pregnan-20-one (3alpha,5alpha-THP) Immunolabeling of Amygdala Neurons in C57BL/6J Mice. Front Cell Neurosci. 2016;10:40. doi: 10.3389/fncel.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL. Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology. 2015;89:157–167. doi: 10.1016/j.neuropharm.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol. 2015;49:111–120. doi: 10.1016/j.alcohol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, DeBold JF, Hwa LS, Newman EL, de Almeida RM. Alcohol and violence: neuropeptidergic modulation of monoamine systems. Ann N Y Acad Sci. 2015;1349:96–118. doi: 10.1111/nyas.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Shafer GO, Nikolova VD, Riddick NV, Agster KL, Baker LK, Knapp DJ. Disruption of social approach by MK-801, amphetamine, and fluoxetine in adolescent C57BL/6J mice. Neurotoxicol Teratol. 2013;36:36–46. doi: 10.1016/j.ntt.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, Hannan AJ. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci. 2013;37:1803–1810. doi: 10.1111/ejn.12195. [DOI] [PubMed] [Google Scholar]
- Perez EE, De Biasi M. Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol. 2015;49:237–243. doi: 10.1016/j.alcohol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. Animal models of compulsive behavior. Biol Psychiatry. 1989;26:189–198. doi: 10.1016/0006-3223(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Riittinen ML, Lindroos F, Kimanen A, Pieninkeroinen E, Pieninkeroinen I, Sippola J, Veilahti J, Bergstrom M, Johansson G. Impoverished rearing conditions increase stress-induced irritability in mice. Dev Psychobiol. 1986;19:105–111. doi: 10.1002/dev.420190203. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Novelty-Suppressed Feeding in the Mouse. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. 2011. pp. 107–121. [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci. 2014;34:9867–9879. doi: 10.1523/JNEUROSCI.1162-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Umathe S, Bhutada P, Dixit P, Shende V. Increased marble-burying behavior in ethanol-withdrawal state: modulation by gonadotropin-releasing hormone agonist. Eur J Pharmacol. 2008;587:175–180. doi: 10.1016/j.ejphar.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res. 2014;38:1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]


