Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with significant morbidity and increased mortality. As body mass index (BMI) is increasingly recognized as an important risk factor for the development of AF, we tested the hypothesis that BMI modulates symptomatic AF burden. Cross-sectional data collected from 1382 patients in the Vanderbilt AF Registry was analyzed. AF severity was assessed using the Toronto atrial fibrillation severity scale (AFSS). BMI was categorized according to WHO guidelines and patients were grouped according to their current AF treatment regimen: no treatment (n=185), rate control therapy with atrioventricular (AV) nodal blocking agents (n=351), rhythm control with antiarrhythmic drugs (AAD; n=636) and prior AF ablation (n=210). Patients with BMI >35 kg/m2 had higher AFSS scores than those with BMI<30 kg/m2 in the rate control (43.57 vs 38.21: P=0.0057), rhythm control (46.61 vs. 41.08: P=1.6 × 10−4) and ablation (44.01 vs. 39.02: P=0.047) groups. In univariate linear models, BMI was associated with an increase in the AFSS score in the rate control (0.27, 95% confidence interval [CI] 0.05–0.5, P=0.02), rhythm control (0.38, 95% CI 0.21–0.56, P=2.49 × 10−5) and ablation (0.38, 95% CI 0.03–0.73, P=0.03) groups. The association remained significant in the rhythm control groups after adjusting for age, gender, race and comorbidities (0.29, 95% CI 0.11–0.49, P=0.002). In conclusion, increasing BMI was directly associated with patient reported measures of AF symptom severity, burden and quality of life. This was most significant in patients treated with rhythm-control strategies.
Keywords: atrial fibrillation, AF severity scale, body mass index
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice and leads to an increased risk of developing heart failure, myocardial infarction (MI), stroke, dementia and kidney failure.1–3 With the aging population in the US, the incidence of AF is expected to surge to 2.6 million with the prevalence reaching 12.1 million by year 2030.4 The rate of obesity has also been rising in the US, with significant increases since 1970. It is estimated that 65 million more Americans will be affected with obesity by 2030.5 With BMI being increasingly recognized as an important risk factor for the development of AF, the rising incidence of the arrhythmia may in part be explained by the obesity epidemic. Fifty percent of patients with AF are symptomatic and require rhythm control therapy either with antiarrhythmic drugs (AADs) or catheter ablation. Obese patients with AF are at a higher risk for complications associated with catheter ablation than non-obese patients, and AADs can be associated with significant adverse effects.6,7 However, the role of BMI in modulating AF symptoms has not been defined. Here, we tested the hypothesis that BMI modulates symptomatic AF burden.
Methods
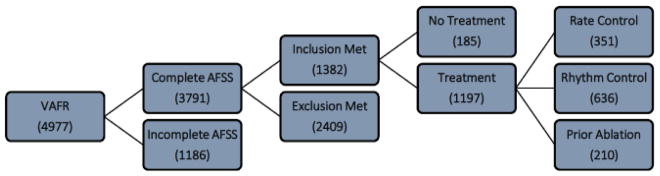
The Vanderbilt AF Registry (VAFR) is a clinical and genetic database established in 2003 with Institutional Review Board (IRB) approval for patients with ECG verified AF.8,9 Data was drawn retrospectively from the time VAFR was established to 2015. At enrollment into the registry, data was collected from a detailed medical history and physical examination with all patients asked to complete a symptom questionnaire pertaining to non-valvular AF in a clinic setting. Multiple questionnaires were recorded from many patients but not all. We therefore analyzed only the baseline enrollment questionnaire from identified patients. Patients were eligible for inclusion if they were over the age of 18 years and had a confirmed diagnosis of AF. There was 3791 patients with a complete atrial fibrillation severity scale (AFSS) of which 46% required manual calculation of the symptom, burden and/or total scores. We excluded 64% of patients from the analysis due to missing data fields for non-treatment or treatment groups and BMI categories (Figure 1).
Figure 1. A flow diagram showing the number of participants in our study at each stage.
A cohort formation diagram that represents the number of participants that we analyzed after filtering out missing data points during the stratification design. AFSS = atrial fibrillation severity scale.
The AFSS is a tool to assess the severity of AF. It is composed of three sub-scores that factor into the total AFSS score. The symptom score was calculated by taking the sum of ratings for seven symptom categories. The burden score was defined as the sum of ratings for event frequency, duration and the mean of the severities of the most recent event and the first event. The way we calculated AF burden has been adapted and modified from the University of Toronto AFSS to take into consideration the first AF event where classically that is excluded from the calculation.10,11 We prospectively defined a quality of life score that takes into account a current life assessment, lifetime cardioversions, emergency visits, hospitalizations and clinic visits with a specialist regarding their AF. The total AFSS score was calculated by adding the symptom score, burden score and quality of life score to give a global estimate for patient reported AF severity (Table 1).
Table 1.
Modified Atrial Fibrillation Severity Scale questionnaire that takes into account symptom score, burden score, and quality of life to provide a total score for Atrial Fibrillation severity.
| Variables | Scoring Criteria | |||||
|---|---|---|---|---|---|---|
| Atrial Fibrillation Symptom Score (0–35) | Palpitations (0–5) | Chest Pain (0–5) | Dyspnea (0–5) | Exercise Intolerance (0–5) | Fatigue (0–5) | Dizziness (0–5) |
| Atrial Fibrillation Quality of Life Score (0–127) | Life Assessment (0–10) | Cardioversions (0–99) | Emergency Visits (0–6) | Admissions (0–6) | Specialist Visits (0–6) | |
| Atrial Fibrillation Burden Score (0–40) | Frequency (0–10) | Duration (0–10) | First Episode Severity (0–10) | Last Episode Severity (0–10) | ||
Height and weight was recorded at the time of enrollment. BMI was calculated by dividing weight in kilograms by the square of height in meters. BMI was defined according to World Health Organization (WHO) guidelines with BMI < 18.5 kg/m2 categorized as underweight; 18.5–25 kg/m2 categorized as normal weight; 25–30 kg/m2 categorized as overweight; 30–35 kg/m2 categorized as obese class 1; 35–40 kg/m2 categorized as obese class 2; and BMI > 40 kg/m2 categorized as obese class 3.12
Patients were stratified according to the type of AF treatment they received. Those not receiving any therapy at the time of their AFSS were classified as a no treatment class. Patients taking atrioventricular (AV) nodal blocking agents, defined as beta-blockers, non-dihydropyridine calcium channel blockers, and digoxin, were categorized in the rate-control class. Patients taking class I, III or IV antiarrhythmic medications at the time of AFSS were classified as the AAD treatment class. Patients with a history of an ablation procedure for AF prior to completing their AFSS survey, excluding ablation for atrial flutter and surgical AF-ablation procedures were categorized in the ablation class. Treatment classes were structured as a step-up approach in order to fairly classify patients that fall into more than one treatment category at the time of their AFSS. For example, patients taking both an AV nodal blocker and AAD were classified in the latter group, and patients on an AAD and having undergone prior AF ablation were classified in the AF ablation group.
To compare total AFSS scores with BMI categories across treatment classes, median and interquartile range (IQR) data was calculated. For the primary analysis, each treatment class was divided into non-obese (BMI <30 kg/m2) and obese (BMI >30 kg/m2). For the secondary analysis, three BMI categories (<30 kg/m2, 30–35 kg/m2 and >35 kg/m2) were used. Comparisons of total AFSS scores between BMI categories using the 3-level division were performed with ANOVA using the F-test. Comparisons of different BMI groups across treatment classes and between BMI categories within each treatment class were performed using T-tests. A p-value of less than 0.05 was used to determine significance in all analyses. The univariate and multivariate regression models were constructed within each treatment class to assess the association between AFSS scores and BMI. Multivariate models included age, sex, race, AF type (paroxysmal vs non-paroxysmal), history of MI, congestive heart failure (CHF) and hypertension, and diagnosis of obstructive sleep apnea as covariates along with BMI. Stepwise parametric modeling was used in the multivariate regression to identify covariates accounting for the greatest variance in the model. Regression models were run using multiple outcome variables (AFSS total score as well as the symptom, burden and quality of life sub-scores) and the resultant coefficients for BMI were compared across the different models.
Results
There were 1382 patients completed a baseline AFSS with BMI data. These data were then analyzed and categorized into a treatment class based on therapies they were receiving at the time of their AFSS. Baseline characteristics of these patients were similar to one another within each treatment group, but not between the non-treatment versus treatment groups (Table 2). When compared to the treatment groups, patients in the no treatment group were more likely to be younger (53.9±15 vs 59.2±12 years), male (74.1% vs 65.3%), and to have a BMI <30 kg/m2 (58.9% vs 45.5%). The three groups receiving AF treatment (AV nodal blocker, AAD and prior ablation) had similar distribution of age (59.0±12, 59.6±12, 58.4±12 years), male sex (66.1%, 64.6%, 66.2%) and patients with a BMI >30 kg/m2 (57.8%, 53.5%, 51.9%).
Table 2.
Baseline characteristics, Atrial Fibrillation Severity Scale scores, Body Mass Index parameters and covariates from the study population.
| Variables | Overall | Body Mass Index (Kg/m2) | |||
|---|---|---|---|---|---|
| n = 1382 | <30 n = 654 |
30–34 n = 376 |
35–39 n = 216 |
>39 n = 136 |
|
| No Treatment | 185 | 109 (17%) | 39 (10%) | 22 (10%) | 15 (11%) |
| Rate Control | 351 | 148 (23%) | 111 (30%) | 59 (27%) | 33 (24%) |
| Rhythm Control | 636 | 296 (45%) | 172 (46%) | 99 (46%) | 69 (51%) |
| Ablation | 210 | 101 (15%) | 54 (14%) | 36 (17%) | 19 (14%) |
| Men | 920 | 420 (64%) | 264 (70%) | 151 (70%) | 85 (62%) |
| Women | 462 | 234 (36%) | 112 (30%) | 65 (30%) | 51 (38%) |
| White | 1321 | 633 (97%) | 357 (95%) | 203 (94%) | 128 (94%) |
| Black | 49 | 17 (3%) | 12 (3%) | 12 (6%) | 8 (6%) |
| Hispanic | 6 | 0 (0%) | 5 (1%) | 1 (0%) | 0 (0%) |
| Other | 6 | 4 (1%) | 2 (1%) | 0 (0%) | 0 (0%) |
| Atrial Fibrillation Type (Paroxysmal) | 702 | 370 (57%) | 188 (50%) | 99 (46%) | 45 (33%) |
| Atrial Fibrillation Type (Persistent) | 85 | 34 (5%) | 22 (6%) | 17 (8%) | 12 (9%) |
| Atrial Fibrillation Type (Permanent) | 592 | 247 (38%) | 166 (44%) | 100 (46%) | 79 (58%) |
| Myocardial Infarction | 124 | 50 (8%) | 31 (8%) | 29 (13%) | 14 (10%) |
| Hyperlipidemia | 788 | 320 (49%) | 235 (63%) | 151 (70%) | 82 (60%) |
| Diabetes Mellitus | 262 | 69 (11%) | 69 (18%) | 74 (34%) | 50 (37%) |
| Congestive Heart Failure | 197 | 69 (11%) | 50 (13%) | 45 (21%) | 33 (24%) |
| Obstructive Sleep Apnea | 340 | 87 (13%) | 92 (24%) | 82 (38%) | 79 (58%) |
| CHADS2 <2 Score | 999 | 534 (82%) | 279 (74%) | 115 (53%) | 71 (52%) |
| CHADS2 >1 Score | 383 | 120 (18%) | 97 (26%) | 101 (47%) | 65 (48%) |
| CHA2DS2VASc <2 Score | 608 | 330 (51%) | 170 (45%) | 69 (32%) | 39 (29%) |
| CHA2DS2VASc >1 Score | 774 | 324 (50%) | 206 (55%) | 147 (68%) | 97 (71%) |
| Atrial Fibrillation Severity Scale Symptom Score | 17.1 | 16.2 ±7.6 | 17.2 ±8.3 | 18.2 ±8.5 | 19.2 ±8.7 |
| Atrial Fibrillation Severity Scale Burden Score | 16.2 | 15.5 ±7.2 | 16.5 ±7.2 | 17.0 ±7.7 | 17.6 ±7.9 |
| Atrial Fibrillation Severity Scale Total Score | 41.4 | 39.4 ±14.5 | 41.8 ±14.9 | 43.9 ±15.5 | 46.3 ±16.2 |
| Age Atrial Fibrillation Onset (years) | 52.4 | 52.8 ±15.7 | 53.2 ±13.5 | 50.6 ±13.8 | 51.1 ±11.8 |
| Atrial Fibrillation Duration (years) | 6.0 | 6.6 ±10.6 | 5.5 ±8.0 | 5.5 ±8.0 | 5.1 ±7.3 |
| Mean Body Mass Index (Kg/m2) | 31.0 | 25.8 ±2.6 | 31.6 ±1.5 | 36.7 ±1.3 | 44.8 ±5.2 |
| Left Atrial Diameter (mm) | 41.9 | 40.0 ±7.8 | 42.4 ±6.9 | 44.0 ±7.9 | 45.8 ±7.6 |
| Left Ventricular Ejection Fraction (%) | 55.4 | 56.0 ±9.5 | 55.4 ±9.9 | 54.4 ±10.4 | 54.8 ±10.1 |
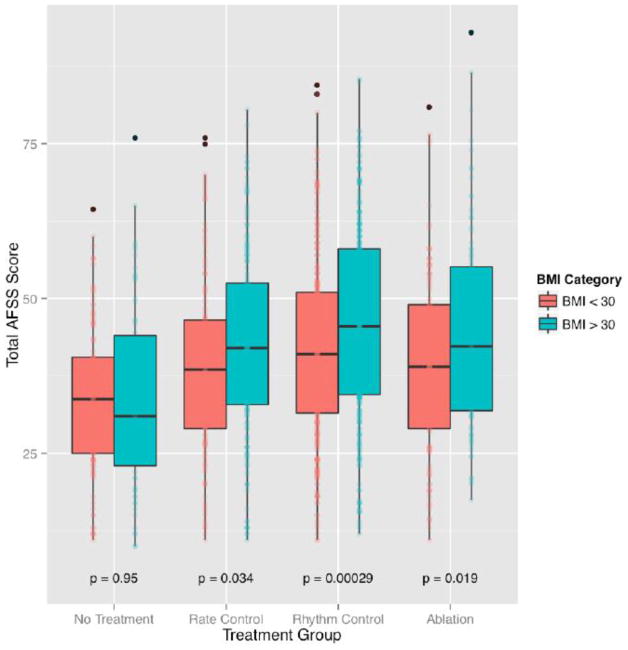
When AFSS scores were compared within treatment groups across two BMI categories, obese patients (BMI > 30 kg/m2) had higher AFSS scores than non-obese patients (BMI < 30 kg/m2). AFSS scores were found to be significantly lower in the BMI < 30 kg/m2 group for patient in the AV-nodal blockade (confidence interval [CI] = −6.38 – −0.26, P = 0.03), AAD (CI = −6.68 – −2.00, P = 2.9 × 10−4) and prior ablation (CI = −9.16 – −0.83, P = 0.02) groups. There was no significant difference between the AFSS scores for the BMI<30 kg/m2 and BMI>30 kg/m2 groups in the non-treatment category (CI = −4.34 – 4.07, P = 0.95) (Figure 2; Table 3).
Figure 2. Dichotomized Body Mass Index Model: Atrial Fibrillation Severity Scale score by Body Mass Index category in each treatment class.
Displays boxplots showing measures of AF symptom severity increasing across two BMI categories (<30 kg/m2 and >30 kg/m2) for all patients on AF treatments (0 = no treatment; 1 = rate control therapy; 2 = rhythm control therapy; and 3 = ablation therapy). AFSS = atrial fibrillation severity scale; BMI = body mass index.
Table 3.
Dichotomized Body Mass Index Model - Comparing mean Atrial Fibrillation Severity Scale scores in two Body Mass Index categories across non-treatment and all treatment classes.
| Treatment Groups | Mean AFSS (BMI < 30) | Mean AFSS (BMI > 30) | P-value |
|---|---|---|---|
| No Treatment | 33.83 | 33.96 | 0.95 |
| Atrioventricular Blockers | 39.16 | 42.48 | 0.034 |
| Antiarrhythmic Drugs | 41.64 | 45.98 | 0.00029 |
| Prior Ablations | 39.45 | 44.45 | 0.019 |
Abbreviations: AFSS: atrial fibrillation severity scale; BMI: body mass index.
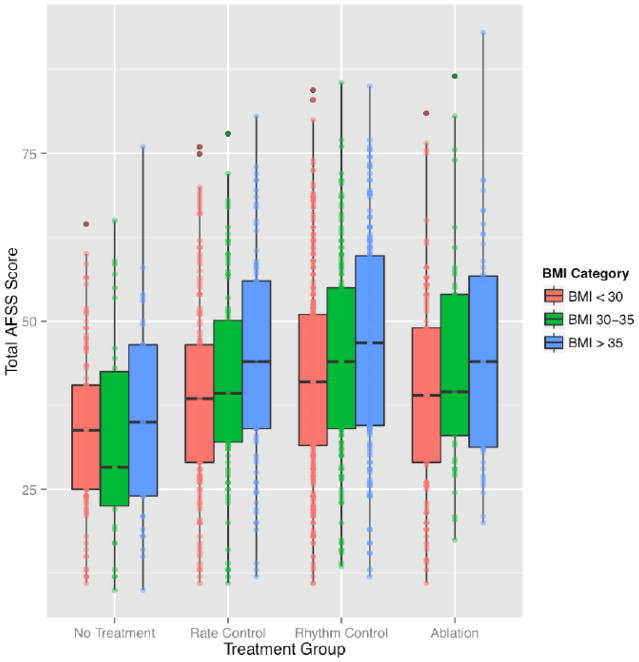
When three BMI categories (< 30, 30 – 35, or > 35 kg/m2) were used for comparison, one-way analysis of variance demonstrated significant association between BMI category on AFSS score in the AV-nodal blocker (F [N=1349] = 7.22, P = 0.0075), AAD (F [N=1,634] = 15.24, P = 1.0 × 10−4) and prior ablation (F [N=1,208] = 5.911, P = 0.02) groups. Patients with a BMI >35 kg/m2 reported more severe AF symptoms than all other BMI categories within each treatment class (Figure 3; Table 4).
Figure 3. Ordinal Body Mass Index Model: Atrial Fibrillation Severity Scale score by Body Mass Index category in each treatment class.
Displays boxplots showing measures of AF symptom severity increasing across three BMI categories (<30 kg/m2; 30–35 kg/m2; and >35 kg/m2) for all patients based on AF treatments (0 = no treatment; 1 = rate control therapy; 2 = rhythm control therapy; and 3 = ablation therapy). AFSS = atrial fibrillation severity scale; BMI = body mass index.
Table 4.
Ordinal Body Mass Index Model - Comparing mean Atrial Fibrillation Severity Scale scores in three Body Mass Index categories and p-values were adjusted accordingly for each Body Mass Index category with the Benjamini Hochberg method across non-treatment and all treatment classes.
| Treatment Groups | Mean AFSS (BMI<30) | Mean AFSS (BMI 30–35) | Mean AFSS (BMI>35) | BH-adjusted P-value (BMI <30 vs BMI 30–35) | BH-adjusted P-value (BMI 30–35 vs BMI > 35) | BH-adjusted P-value (BMI < 30 vs BMI > 35) |
|---|---|---|---|---|---|---|
| No Treatment | 33.82 | 32.79 | 35.15 | 0.69 | 0.69 | 0.69 |
| Atrioventricular Blockers | 39.16 | 40.74 | 44.46 | 0.39 | 0.12 | 0.019 |
| Antiarrhythmic Drugs | 41.64 | 44.74 | 47.16 | 0.048 | 0.16 | 0.00051 |
| Prior Ablations | 39.45 | 43.42 | 45.43 | 0.198 | 0.51 | 0.065 |
Abbreviations: AFSS: atrial fibrillation severity scale; BH: Benjamini Hochberg; BMI: body mass index.
Results of multivariate linear regression models for AFSS on continuous BMI. Each 1-unit increase in BMI was associated with increase in measures of AF symptoms of 3.00% (P = 0.02) for patients treated with AV-nodal blockers, 4.11% (P = 2.5 × 10−5) for patients receiving AADs and 4.10% (P=0.03) for patients with a prior history of AF ablation. After adjusting for age, sex, race, hypertension, MI, CHF and obstructive sleep apnea, BMI was found to be significantly associated with AF symptom severity for patients in all treatment categories (Supplemental Figure 1). The largest association was seen in the group receiving AAD therapy (0.29, 95% CI 0.11 – 0.49, P = 0.002). In this group, patients with BMI <30 kg/m2 were more likely to receive an AAD than patients with a BMI >30 kg/m2 and the same held true for the BMI 30–35 kg/m2 and BMI >35 kg/m2 groups with the most commonly prescribed AAD in our cohort being sotalol, propafenone, amiodarone and flecainide in descending order. The standardized coefficient in this multivariable regression corresponds to a 3.11% increase in AFSS score for each 1-unit increase in BMI.
The AFSS score used as a dependent variable in these analyses is composed of multiple sub-scores. We considered whether any of the component sub-scores, which measure symptoms, burden and quality of life in AF patients, would exhibit different behavior than the overall AFSS score. The univariate and multivariable linear models were constructed using overall AFSS score and each of the component sub-scores as dependent variables. One-way analysis of variance demonstrated a significant association between symptom and burden outcome variables and BMI beta coefficients in the univariate for the rhythm control category. The statistical significance remained in the multivariate analysis for the symptom outcome, but not for the burden outcome in the rhythm control category. All other categories did not show statistical value (Figure 4).
Figure 4. Association between Body Mass Index on Atrial Fibrillation Severity Scale score under various regression conditions.
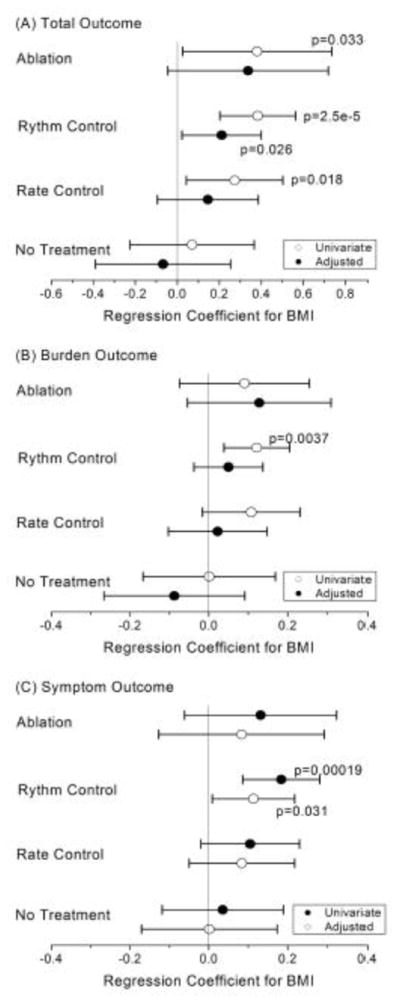
A forest plot showing regression coefficients with error bars. Regression coefficients are given for both univariate and multivariate models and displays the outcome variables within each non-treatment and treatment class; no treatment; rate control therapy; rhythm control therapy; and ablation therapy.
Discussion
In this study, we showed that obese patients are more symptomatic with their AF and there is a direct correlation between BMI and severity of AF symptoms. The strongest association between BMI and rhythm control therapy was in the group of patients who received AADs for treatment of AF. Patients with WHO types II and III obesity were more likely to be symptomatic with AF than patients with type I obesity when compared to non-obese patients. In contrast, obese patients who presented with their first episode of AF and were not on any form of treatment were less symptomatic when compared to non-obese patients on no therapies. At this time, it remains unclear if these findings are related to an overlap between subjective obesity- and AF-related symptoms or whether symptoms in obese patients are directly modulated by rhythm control therapies.
The severity of AF experienced by patients is highly variable, but AF guidelines rely heavily on the severity of the patient reported symptoms for selecting treatment strategy offered to individuals with AF. In our study, we noticed that patients with moderate to severe obesity who were treated with an AV nodal blocker had more AF symptoms when compared to patients who were not obese. However, there were no differences seen between patients with mild obesity as compared to patients with moderate to severe obesity. While the reason for this is unclear, we suspect that this may be related to differences in heart rates in these individuals. We also showed that patients reporting the highest number or most severe symptoms were being treated with AADs. While the reasons for this are not fully understood, several conclusions can be drawn from this observation. First, patients with severe AF at initial presentation are more likely to be selected for a rhythm control strategy as opposed to rate control therapy. Second, the type of AF when the baseline AFSS was completed dictated which treatment strategy patients were allotted. Third, an interaction in the obesity pathway may attenuate the efficacy of AADs and impair the response. This in practical terms renders a more aggressive approach taken by treating physicians and ultimately predisposes patients to a higher likelihood of side effects seen from these drugs. These are plausible explanations that put our findings into context but may over simplify the complexity of the results seen within the antiarrhythmic class.
While AF burden is likely to become the gold standard for assessing response to therapy, it is difficult to measure in most patients with AF. One unexpected finding was that there was no significant difference in the no treatment group with the first episode of AF when the mean AFSS scores were compared in patients with low (<30 kg/m2) versus high BMI (>30 kg/m2) (Table 3). This suggests that both groups had similar heart rates at presentation and the ventricular response was not rapid obviating the need for AV nodal blockers. Low mean AFSS scores in the two groups also provide support for this hypothesis (Table 3). It is possible that one mechanism by which BMI modulates symptomatic AF burden is directly or indirectly modulating the AV nodal response during AF. This paradox may provide important insights into potential mechanisms by which BMI modulates symptomatic AF burden.
A number of limitations should be mentioned. First, we used a modified AFSS questionnaire that has yet to be validated. Second, we did not correlate the AFSS scores directly with efficacy of therapeutic response to AV nodal blockers, AADs or ablation therapy for AF. While assessing response to AF therapies can be challenging and measuring AF burden has recently been proposed as the ‘gold’ standard, we and others have used the AFSS score as a good surrogate measure for evaluating symptomatic response to AF therapies.(10,11) Third, the interval period from time of AF diagnosis to baseline AFSS questionnaires was not determined, making it difficult to assess patient reported outcomes at different time points in a progressive disease. Four, only baseline AFSS questionnaires were analyzed and this makes it challenging to know if the reported symptoms pertain to one prior AF episode or if they refer to chronic AF symptoms. Fifth, we had a limited sample size for assessing the temporal relationship between BMI and severity of AF symptoms. However, fluctuations in BMI are unlikely to be responsible for the association with severity of AF symptoms and this was consistent with our cohort of participants. Sixth, perhaps surprisingly the number of AF ablations performed in patients did not correlate with BMI. While the precise reasons for this are unclear, it may relate to the small number of patients that underwent two or more AF ablation procedures. Seventh, the time of ablation and baseline questionnaire were not correlated with one another, impacting severity of AF symptoms.
In conclusion, we showed that obese patients are more symptomatic with their AF and there is direct correlation between BMI and severity of AF symptoms. Additional research needs to be performed to determine if this finding is due to an overlap with subjective obesity- and AF-related symptoms or whether obesity itself directly modulates response to rhythm control therapies for this common and morbid condition.
Supplementary Material
Acknowledgments
This work was supported by grants from the American Heart Association 17MCPRP33420153 (Brandon Chalazan), National Institutes of Health grants R01 HL092217 (Dawood Darbar) and K23 HL127704 (M. Benjamin Shoemaker). The project was also supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflicts of Interest: All authors: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980–1998. Am J Epidemiol. 2002;155:819–826. doi: 10.1093/aje/155.9.819. [DOI] [PubMed] [Google Scholar]
- 2.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 6.Cha YM, Friedman PA, Asirvatham SJ, Shen WK, Munger TM, Rea RF, Brady PA, Jahangir A, Monahan KH, Hodge DO, Meverden RA, Gersh BJ, Hammill SC, Packer DL. Catheter ablation for atrial fibrillation in patients with obesity. Circulation. 2008;117:2583–2590. doi: 10.1161/CIRCULATIONAHA.107.716712. [DOI] [PubMed] [Google Scholar]
- 7.Shoemaker MB, Muhammad R, Farrell M, Parvez B, White BW, Streur M, Stubblefield T, Rytlewski J, Parvathaneni S, Nagarakanti R, Roden DM, Saavedra P, Ellis C, Whalen SP, Darbar D. Relation of morbid obesity and female gender to risk of procedural complications in patients undergoing atrial fibrillation ablation. Am J Cardiol. 2013;111:368–373. doi: 10.1016/j.amjcard.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darbar D, Roden DM. Symptomatic burden as an endpoint to evaluate interventions in patients with atrial fibrillation. Heart Rhythm. 2005;2:544–549. doi: 10.1016/j.hrthm.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, George AL, Roden DM. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Cvitkovic SS, Kerr CR, Crystal E, Gillis AM, Guerra PG, Mitchell LB, Roy D, Skanes AC, Wyse DG. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS-SAF scale. Can J Cardiol. 2006;5:383–386. doi: 10.1016/s0828-282x(06)70922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: WHO; 1998. Report of a WHO Consultation on Obesity. [PubMed] [Google Scholar]
- 13.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, Bradley DJ, Bluhm CM, Haroldson JM, Packer DL. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Rienstra M, Lubitz S, Mahida S, Magnani JW, Fontes JD, Sinner MF, Van Gelder IC, Ellinor PT, Benjamin EJ. Symptoms and Functional Status of Patients With Atrial Fibrillation. Circulation. 2012;125:2933–2943. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darbar D, Roden DM. Genetic mechanisms of atrial fibrillation: impact on response to treatment. Nat Rev Cardiol. 2013;10:317–329. doi: 10.1038/nrcardio.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parvez B, Vaglio J, Rowan S, Muhammad R, Kucera G, Stubblefield T, Carter S, Roden D, Darbar D. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, Kaiser DW, Makino S, MacRae CA, Ellinor PT, Wasserman BS, Kannankeril PJ, Donahue BS, Roden DM, Darbar D. ACE I/D polymorphism associated with abnormal atrial and atrioventricular conduction in lone atrial fibrillation and structural heart disease: Implications for electrical remodeling. Heart Rhythm. 2009;6:1327–1332. doi: 10.1016/j.hrthm.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.