Abstract
Recent research has brought about a clear understanding that successful fracture healing is based on carefully coordinated cross-talk between inflammatory and bone forming cells. In particular, the key role that macrophages play in the recruitment and regulation of the differentiation of mesenchymal stem cells (MSCs) during bone regeneration has been brought to focus. Indeed, animal studies have comprehensively demonstrated that fractures do not heal without the direct involvement of macrophages. Yet the exact mechanisms by which macrophages contribute to bone regeneration remain to be elucidated. Macrophage–derived paracrine signaling molecules such as Oncostatin M, Prostaglandin E2 (PGE2), and Bone Morphogenetic Protein-2 (BMP2) have been shown to play critical roles; however the relative importance of inflammatory (M1) and tissue regenerative (M2) macrophages in guiding MSC differentiation along the osteogenic pathway remains poorly understood. In this review, we summarize the current understanding of the interaction of macrophages and MSCs during bone regeneration, with the emphasis on the role of macrophages in regulating bone formation. The potential implications of aging to this cellular cross-talk are reviewed. Emerging treatment options to improve facture healing by utilizing or targeting MSC-macrophage crosstalk are also discussed.
Keywords: Fracture healing, Mesenchymal stem cell, Macrophage, Oncostatin M, Prostaglandin E2, Bone Morphogenetic Protein-2
1. Introduction
Bone fractures are one of the most common injuries seen in emergency departments, with nearly 4 million fractures seen in the United States in 2013 [1]. Despite the best treatment efforts, up to 10% of bone fracture cases still report undesirable outcomes; in the USA alone, 100,000 fractures per year result in painful non-union [2]. Treatment of these non-united fractures and bone defects constitutes a major health problem with significant clinical, social, and economic implications with an average cost of over 10,000 USD per non-union [3].
There are two major pathways for bone regeneration: intra-membranous or endochondral ossification processes [4–7]. Intra-membranous ossification involves mesenchymal stem cells (MSCs) directly differentiating into osteoblasts which in turn deposit mineralized extracellular matrix. This type of healing is typically seen in rigidly fixed fractures with minimal fracture gap, and with fractures within the bone metaphysis. Fractures located in the diaphysis, with less mechanical stability, and a larger fracture gap heal via the classic stages of endochondral ossification: inflammation, soft then hard callus formation, and finally remodeling of the fracture site. In this mode of bone regeneration, the fracture hematoma is initially infiltrated by immune cells, mainly neutrophils and macrophages. Macrophages not only phagocytose necrotic cells and tissue debris at the fracture site but also initiate the recruitment of MSCs and vascular progenitor cells from the periosteum, bone marrow, and circulation [8,9]. As the inflammation subsides, MSCs and other progenitor cells proliferate, forming granulation tissue that ultimately forms cartilage callus to stabilize the fracture site [7,10]. In addition to providing mechanical stability cartilage functions as a scaffold for osteoblast-mediated bone deposition that allows for mineralization of the callus and closure of the fracture gap [11,12]. Osteoclasts then resorb immature woven bone and cartilage matrix, and with the subsequent deposition of mature lamellar bone, bone is restored to its pre-fracture structure and integrity [7,10].
The differentiation of MSCs and the subsequent formation of cartilage and bone at the fracture site is guided by several microenvironmental signals. These include growth factors released from the bone matrix as well as changes in the oxygen tension and mechanical microenvironment [7,10]. In particular, recent research has determined that both routes of fracture healing are based on carefully coordinated cross-talk between macrophages and bone forming cells.
MSCs, the precursor cells for bone and cartilage, were initially identified from human bone marrow with the ability to develop into fibroblastic colony-forming cells in vitro, and to regenerate hetero-topic bone tissue in vivo [13,14]. The exact definition of the MSC remains controversial, but the term is generally used to describe a population of stem cells that resides in the peri-vascular niche of most tissues, and with the ability to differentiate into mesodermal tissues, such as bone and cartilage [15,16]. The International Society for Cellular Therapy has defined MSCs as cells that 1) adhere to plastic in vitro cell cultures; 2) have a certain surface marker profile (CD105+, CD73+, CD90+, and CD45−, CD34−, CD14−, CD11b−, CD79−, CD19−, and HLA-DR−); and 3) have the trilineage ability to differentiate into osteoblasts, adipocytes, and chondroblasts [17]. In addition to their ability to regenerate mesenchymal tissues, MSCs have wide immunomodulatory properties making them attractive targets for tissue engineering applications.
Macrophages are cells of innate immunity that are found in nearly all tissues, where they play a key role in maintaining normal tissue homeostasis [18]. During infection and inflammation, their numbers increase greatly via homing of regional and circulating monocyte precursors to the affected area [19]. During acute inflammation, macrophages contribute to the restoration of tissue homeostasis by phagocytosing invading micro-organisms, amplifying the inflammatory reaction, and recruiting additional immune cells [20]. As the tissue insult is cleared, macrophages contribute to tissue regeneration by secreting anti-inflammatory factors, recruiting progenitor cells, and producing growth factors that regulate the differentiation of these cells including angiogenesis [18,20]. This functional plasticity of macrophages has been conceptualized as macrophage polarization; inflammatory macrophages are called classically activated or M1 macrophages, while macrophages active in tissue regeneration are known as alternatively activated or M2 macrophages [21]. Importantly macrophages can switch from one mode of function to another, making them highly attractive targets for therapeutic interventions. In humans, there is a much wider spectrum of macrophage phenotypes, corresponding to relative differences in pro- and anti-inflammatory activities.
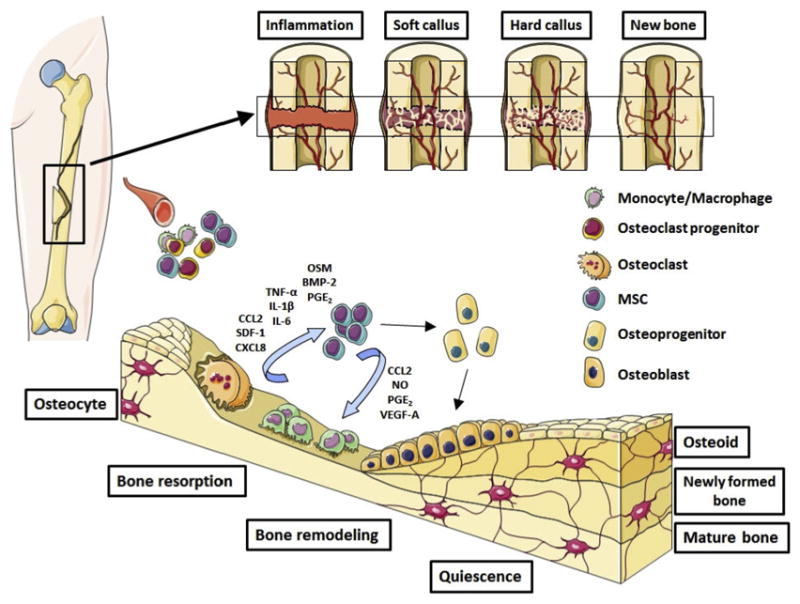
Macrophages are among the first cells to arrive to the fracture site, and have long been thought to contribute to the initial inflammation and debridement of the injury location (Fig. 1). Their key role also in the regulation of bone regeneration during both normal bone homeostasis and fracture healing has increasingly been appreciated. In addition to macrophages, closely related myeloid-lineage cells such as osteoclasts play complex roles in bone growth and regeneration [22–24], and delineating the exact roles that each of these myeloid lineage cell types play in bone regeneration remains challenging with currently available methods. Nevertheless the research has begun to identify some of the molecular mechanisms underlying the cross-talk between macrophages and bone forming cells and has led to new potential strategies to enhance bone regeneration by targeting the interaction between macrophages and MSCs.
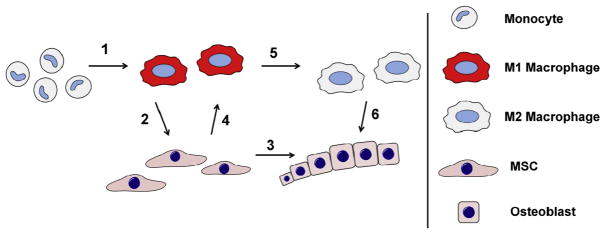
Fig. 1. Contribution of macrophages and MSCs to first three stages of fracture healing.
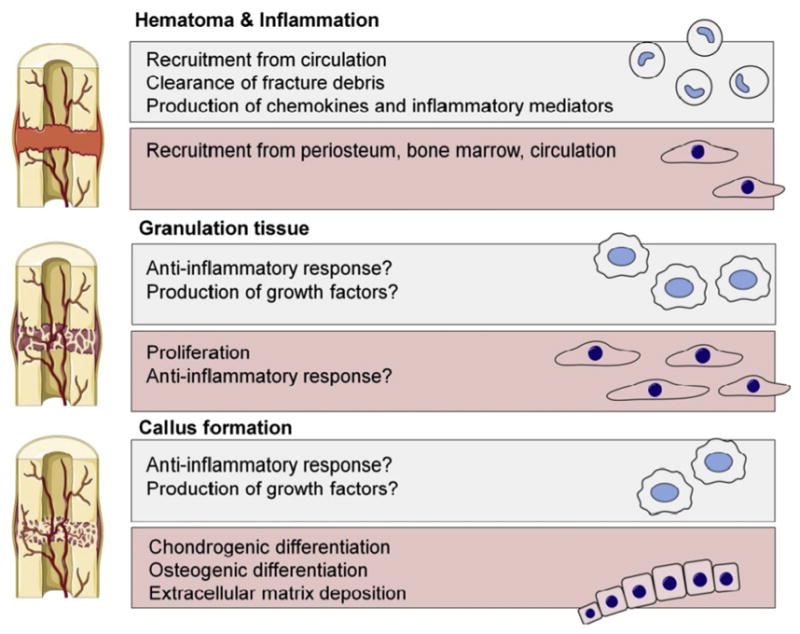
Contribution of monocyte-macrophages shown in blue and MSCs-osteoblasts in red background. Circulating monocytes arrive to the fracture site immediately following the injury. Monocyte-derived macrophages contribute to the clearance of the fracture site and amplification of the inflammatory reaction by secreting pro-inflammatory mediators and chemokines. As the fracture site is cleared of cell and extracellular matrix debris, the inflammation subsidizes. At the same time MSCs and other progenitor cells migrate to the area from periosteum, bone marrow and the circulation. MSCs proliferate and form cell-rich granulation tissue that ultimately differentiates into cartilage callus and woven bone. Macrophages are present throughout the fracture repair likely contributing to MSC differentiation by secreting growth factors but their numbers decrease as the repair progresses. The role of macrophages and MSCs in the final, remodeling phase, of the fracture healing is poorly understood but is likely dominated by signals derived from other monocyte-lineage cells, the osteoclasts.
2. Macrophages in bone regeneration
2.1. Macrophages in bone homeostasis
Bone tissue contains a resident macrophage subpopulation termed osteomacs, which is distributed among bone lining cells within both endosteum and periosteum [25]. In particular osteomacs are closely associated with areas of bone formation, forming a canopy-like structure over active cuboidal osteoblasts. When macrophages were depleted in a macrophage-fas-induced apoptosis (MAFIA) model [26], this active bone forming surface of osteoblasts was also lost, suggesting an active role for the macrophages in osteoblast mediated bone formation [25]. Indeed, Vi et al. demonstrated that the normal post-natal growth of bones was compromised in genetically modified mouse model that lacks lysozyme-M-expressing cells, mainly macrophages; interestingly, however, fetal development of the skeleton was largely unaffected in this mouse model [27]. Together these results suggest that macrophages play a role in regulating physiological bone formation and homeostasis. Subsequent studies have demonstrated that macrophages are essential for the both the intramembranous and endochondral routes of fracture healing.
2.2. Macrophages in intramembranous bone formation
In a murine model of intramembranous bone formation, macrophages were closely associated with active osteoblasts depositing woven bone at the defect site during all the stages of the fracture healing [28]. Depletion of macrophages resulted in impaired deposition of woven bone and healing of the defect; interestingly macrophage depletion using either MAFIA or clodronate liposome administration models [29] at the time of the injury was more detrimental compared to depletion of macrophages at later stages of fracture healing [28]. Similar results regarding intramembranous bone healing were reported by Sandberg et al. [30], demonstrating that macrophage depletion by clodronate liposome resulted both in inferior integration of tibial metaphyseal screws and compromised filling of a metaphyseal drill hole. Macrophage depletion prior to the injury resulted in inferior implant integration and bone healing compared to the depletion of macrophages after the injury.
2.3. Macrophages in endochondral bone formation
In the murine model of endochondral fracture healing, macrophages were closely associated with the formation of cartilage in the soft callus [31]. If macrophages were ablated using the MAFIA murine model at the early inflammatory phase, the formation of cartilagenous soft callus was completely abolished, while ablation of macrophages at later stages resulted in smaller callus; interestingly the callus size was directly proportional to the number of remaining macrophages at the callus. Vi et al. [27] further demonstrated using MAFIA model that macrophages play a role at multiple stages of endochondral fracture healing. They showed that macrophages were associated with the early stage of inflammation, chondrogenesis, and areas of woven bone deposition, but were largely absent at the remodeling state. In the same study a genetically modified mouse lacking lysozyme-M-expressing cells, mainly macrophages, demonstrated impaired formation of both soft and hard callus as well as compromised fracture union. Furthermore, similar to results of Raggatt et al. [31], induced depletion of macrophages in MAFIA mice resulted in reduced soft and hard callus formation, with earlier depletion showing greater impact than depletion later in the healing sequence. Schlundt et al. [32] also observed reduced callus size and delayed hard callus formation with delayed fracture union if macrophages were depleted by clodronate liposome injection at the initial stages of healing.
Taken together, an accumulating body of evidence indicates that macrophages are crucial for both intramembranous and endochondral fracture healing, as well as normal bone homeostasis. Interestingly, macrophages seem to play somewhat different roles in these modes of fracture healing; in intramembranous bone formation, macrophages contribute to the deposition of woven bone, while promoting soft callus formation in endochondral bone formation [25,27,28,30–32]. In both instances, depletion of macrophages is particularly detrimental if done immediately before or at the time of the injury. This suggests that macrophages have their greatest contribution on bone and cartilage formation early during the inflammatory phase of fracture healing, highlighting the role that macrophages play in the recruitment and regulation of the early differentiation of the progenitor cells.
Although the picture painted by the murine fracture models is fairly consistent, it is noteworthy that the macrophage depletion strategies used in these studies have limitations that might confound the results. Firstly, neither the MAFIA model nor clodronate liposome depletion is entirely specific to macrophages, but could also impact other closely-related cells of the myeloid lineage, importantly osteoclasts [23,33–35] As both osteoclasts and macrophages play distinct roles in bone regeneration, determining the relative importance of macrophage depletion from the potential concurrent depletion of other cells of the myeloid lineage remains difficult. For example, it was reported that myeloid-lineage osteoclasts were depleted by clodronate liposome injection [35], while osteoclast activity remained intact in the MAFIA model [36]. This difference may explain the opposite findings of the macrophage depletion effect on parathyroid hormone-mediated anabolic bone remodeling using MAFIA and clodronate liposome models [37]. Secondly, the macrophage depletion is not 100% effective; For example, 90% of macrophages in the peritoneum or in bone marrow and only 70% of macrophages in blood and spleen expressed the depleting suicide gene in the MAFIA mice [25–28,30–32]. Thirdly the depletion is not permanent and, once subsided, could results in a reactive increase in macrophage numbers with unknown consequences.
3. Mechanisms of macrophage/MSC interaction
The exact mechanisms by which macrophages contribute to bone regeneration remain unclear. It is likely that the initial inflammatory reaction and pro-inflammatory macrophage activation contributes to the recruitment of MSCs and osteoprogenitor and vascular progenitor cells to the fracture site. These signals that control progenitor cell homing include chemokines CCL2, CXCL8 and SDF-1, all of which are secreted by activated macrophages [38–42]. The general paradigm of macrophage functional polarization in tissue regeneration suggests that M1 macrophages contribute to the initial acute inflammatory stage and clearance of debris from the fracture site, while growth factors secreted by M2 macrophages support MSC-mediated bone formation in the later stages of the fracture healing [18,20]. Indeed, there is evidence that macrophage populations, at least during endochondral fracture healing, follow this general pattern of inflammatory macrophages predominating in the early stages of the healing followed by predominance of M2-like phenotype in the later stages [31,32]. However, as detailed below, the relative importance of M1 and M2 derived signals in promoting bone formation has been challenging to pinpoint, and seems to be dependent on the specifics of the experimental setup. In addition, there is compelling evidence that non-polarized macrophages can also support bone formation by osteoblast and osteoprogenitor cells. Furthermore MSCs reciprocally regulate macrophage function.
3.1. Impact of M0 non-activated monocyte/macrophages on bone formation
Chang et al. demonstrated that primary osteoblast cultures isolated from the murine calvarium contain significant numbers of macrophages, and that the bone forming ability of these cultures is impaired if the macrophages “contaminating” the cultures are depleted [25]. Furthermore, in transwell co-cultures, macrophages were shown to enhance osteoblast-mediated bone formation [25]. Similarly, Vi et al. [27] demonstrated that ablation of macrophages from murine bone marrow cell cultures diminished the bone forming ability of these cultures, while addition of conditioned media from macrophages restored the bone formation.
The first mechanistic insight delineating the contribution of macrophages to osteogenic differentiation of MSCs was provided by Champagne et al. [43], who demonstrated that conditioned media from non-activated J774A.1 murine macrophage cell-line increases alkaline phosphatase (ALP) activity in human MSCs. Experiments utilizing a neutralizing antibody further demonstrated that the effect was mediated by BMP-2. Treating J774A.1 cells with LPS to induce an inflammatory phenotype abolished the BMP-2 expression and osteogenic effect, while the effect of M2 polarization on the osteogenesis was not studied. Subsequently, Pirraco et al. [44] showed similar effects using non-activated human monocytes and human bone marrow derived MSCs (hbMSCs) in a transwell co-culture. It was found that monocytes increased MSC proliferation, ALP activity and the expression of osteocalcin and osteopontin and that these effects were partially dependent on BMP-2.
Nicolaidou et al. [45] found that non-activated human peripheral blood mononuclear cells (PBMCs) and differentiated macrophages in a direct co-culture with hbMSCs increased the bone formation in a dose dependent manner, with increasing number of macrophages in the culture leading to increased bone formation. In an elegant and comprehensive series of experiments the group demonstrated that the effect was mediated by the cytokine Oncostatin M (OSM) secreted by monocytes after direct cell-to-cell contact with MSCs.
Together, these results suggest that non-activated macrophages contribute to bone formation, perhaps representing the physiological role of tissue resident macrophages in maintenance of normal bone homeostasis.
3.2. Impact of M1 inflammatory monocyte/macrophages on bone formation
Omar et al. [46] studied the impact of human PBMCs on hbMSCs. They found that conditioned media from LPS stimulated PBMCs induced the expression of BMP-2, RUNX2 and ALP in hbMSCs while the conditioned media from non-activated or IL-4 treated PBMCs did not facilitate osteogenic differentiation. In a follow up study, the same group demonstrated that LPS activated monocytes secrete exosomes that partially explain the induction of RUNX2 and BMP-2 in hbMSCs [47]. Guihard et al. [48] demonstrated that conditioned media from human monocytes stimulated with LPS or various other Toll-like receptor (TLR) ligands enhanced bone formation by hbMSCs. It was further shown that LPS induced the production of OSM from monocytes via induction of COX-2 and PGE2 and that OSM signaling via gp130 on MSCs was mainly responsible for the enhanced osteogenesis. The enhanced osteogenesis was not observed when hbMSCs were exposed to conditioned media from IL-4 or IL-10 treated monocytes and no OSM secretion was detected in these cases. Lu et al. [49] studied the effects of M0, M1 and M2 murine bone marrow macrophages on the osteogenic differentiation of murine bone marrow MSCs in a direct co-culture system. All of the macrophage subtypes enhanced bone formation, with M1 macrophages showing the greatest positive effect. Similar to results by Nicolaidou et al. [45], the effect was dose dependent with 5:1 macrophage to MSC ratio required for the promotion of the osteogenesis. Interestingly increased levels of PGE2 were detected in the co-cultures; subsequent studies with the COX-2 inhibitor celecoxib demonstrated that PGE2 mediates the osteoinductive cross-talk between macrophages and MSCs.
Taken together, the results of these studies suggest that inflammatory M1-like monocyte/macrophages present during the early stages of the fracture repair contribute to bone formation. The observed role of COX-2/PGE2 in mediating the osteoinductive communication between inflammatory macrophages and MSCs might explain the potentially detrimental effect of nonsteroidal anti-inflammatory drugs (NSAIDs) on fracture healing.
3.3. Impact of M2 alternatively activated monocyte/macrophages on bone formation
Fernandes et al. [50] demonstrated that conditioned media from human umbilical cord blood-derived macrophages enhanced the osteogenic differentiation of adipose tissue MSCs, and the effect was dependent on OSM secretion from macrophages. In an interesting contrast to studies by Guihard et al. and Nicolaidou et al. [45,48], however, OSM production and subsequent bone formation by MSCs were observed with conditioned media from non-activated or alternatively activated M2 macrophages. Indeed, various forms of induced M1 macrophage polarization showed reduced levels of OSM and did not enhance bone formation in this experimental setting. Gong et al. [51] studied the effect of murine bone marrow macrophages on osteogenesis by murine bone marrow MSCs in indirect transwell co-culture. M1 macrophages inhibited and M2 macrophages significantly promoted MSC mediated osteogenesis in this culture setting. Significant upregulation of pro-inflammatory cytokines was observed in M1 macrophages, while upregulation of growth factors TGF-β, VEGF and IGF-1 was observed in M2 macrophages. Although the exact identity of the soluble mediator contributing to the osteogenesis was not identified, the authors speculated that the pro-inflammatory profile of M1 macrophages undermined, and the upregulation of growth factors in M2 macrophages contributed to the enhanced bone formation. Using human monocyte/macrophage cell line THP-1 and adipose tissue derived MSCs, Zhang et al. [52] studied the effect of macrophage polarization of osteogenesis in direct and indirect transwell co-cultures. Both the M1 and M2 macrophages impacted the osteogenic differentiation of MSCs but in a different way: M1 macrophages increased the early and middle stages of osteogenesis without enhancing matrix mineralization. In contrast, direct or indirect co-culture with M2 macrophages resulted in increased matrix mineralization. The authors also studied the production of osteogenic factors from polarized macrophages and from the co-cultures: OSM production was increased in M1 cultures and BMP-2 in M2 co-cultures suggesting that different factors might be responsible for driving MSC differentiation in M1 and M2 cultures.
Taken together, these studies suggest that M2 macrophages can contribute to the bone formation by MSCs, possibly reflecting the role of M2 macrophages during the later stages of the fracture healing.
3.4. Macrophages and chondrogenesis
The compromised soft callus formation observed in macrophage-depleted animal models of endochondral fracture healing [27,31,32] suggests that in addition to regulating osteogenic differentiation, macrophages also play a role in the chondrogenic differentiation of MSCs. The topic remains largely unexplored, but there are indications that macrophages can both inhibit and enhance MSC mediated chondrogenesis [53,54]. The microenviromental ques that regulate the cross-talk between MSCs and macrophages inducing the osteogenic or chondrogenic differentiation remains an intriguing topic for further studies.
3.5. Impact of MSCs on macrophages
Not only do macrophages regulate MSC function, but MSCs also regulate the function of macrophages. In particular, MSCs possess broad immunomodulatory functions in response to an inflammatory microenvironment [16,55]. Exposure to specific TLR ligands polarizes MSCs into pro-inflammatory or anti-inflammatory phenotypes, termed MSC1 and MSC2 [56]. The crosstalk between MSCs and immune cells modulates both adaptive [57] and innate [58] immune reactions via juxtacrine and paracrine signaling [59]. In a co-culture model, MSCs dramatically suppressed the LPS-induced production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and increased the secretion of IL-10 by murine macrophages [60,61]. Mechanistic studies demonstrated that MSCs mediated this immunomodulation via iNOS and a COX2 dependent pathway to enhance PGE2 production, which in turn increases IL-10 secretion in macrophages through binding with EP2 and EP4 receptors [60,61]. The ability of MSCs to suppress inflammatory macrophage activation was also demonstrated in an in vivo model in which administered MSCs protected against LPS-induced septic shock; the protective effect was eliminated by macrophage depletion or IL-10 inhibition in the murine model, indicating that macrophages were the primary target of MSC mediated immunomodulation [61]. Cho et al. demonstrated that MSCs in transwell co-culture with macrophages significantly prevented M1 macrophage polarization and induced M2 polarization [62]. Kim et al. observed similar results using human peripheral blood monocytes and MSCs showing induction of an anti-inflammatory, M2-like phenotype in macrophages co-cultured with MSCs [63]. François et al. demonstrated that human MSC exposed to IFN-g and TNF-a upregulated indoleamine 2,3-dioxygenase (IDO) expression, which was implicated in the polarization of monocytes into IL-10-secreting M2 macrophages and indirect suppression of T cell proliferation [64]. However, the roles of iNOS and the COX2 pathway in immunomodulation in human MSC were not characterized.
In addition to exerting direct immunomodulatory effects on macrophages, MSCs also regulate macrophage chemotaxis, with macrophage recruitment being crucial for the immunomodulatory process mediated by MSCs. Compared to fibroblasts, human and mouse bone marrow-derived MSCs secret high amounts of several chemokines including CCL2 and CCL4 [65,66], both of which are major chemoattractants for monocytes and macrophages [40]. Combination of INF-γ with another pro-inflammatory cytokine (TNF-α, IL-1α, or IL-1β) further activates MSCs in damaged or inflamed tissues [57]. The activated MSCs dramatically increased the secretion of several chemokines including CCL2, and mediated immunomodulation of infiltrated macrophages [57]. This MSC-mediated macrophage recruitment and modulation of macrophage phenotype could potentially enhance tissue regeneration [67] including wound healing [65], healing of spinal injury [68] and myocardial repair [42]. For example, Maggini et al. demonstrated in a murine model with subcutaneous implanted glass cylinders that local MSC injection to tissues surrounding glass cylinders increased macrophage recruitment to the peri-implant tissues, but also polarized the infiltrated macrophages into M2-like tissue regenerative macrophages [60]. Seebach et al. demonstrated that MSCs implanted into a rat bone defect site in a hydrogel induced high VEGF expression, rapid infiltration of M1 type of macrophages, and endothelial cells resulting in improved vascularization and bone regeneration at the defect site [66]. Similarly, Zhou et al. showed that MSCs partially differentiated to osteoblasts in vitro and implanted to murine cranial defect model induce recruitment of macrophages and improved defect healing [69]. In vitro, these osteogenic MSCs induced inflammatory M1-like macrophage phenotype; these effects were shown to be dependent on VEGF expression of the osteogenic MSCs by neutralizing antibody experiments.
Taken together, there is accumulating evidence that MSCs regulate the chemotaxis and function of macrophages and that in some cases, MSC-derived signals can contribute to the bone regeneration by modulating macrophage function. However, the exact role that MSCs regulation on macrophages plays in bone regeneration remains to be determined. The key signaling molecules involved to the cross-talk between MSCs and macrophages are summarized in Fig. 2 (Fig. 2) and the proposed dynamic of macrophage-MSCs interactions during fracture healing in Fig. 3 (Fig. 3).
Fig. 2. Paracrine signaling molecules involved in the cross-talk between macrophages and MSCs.
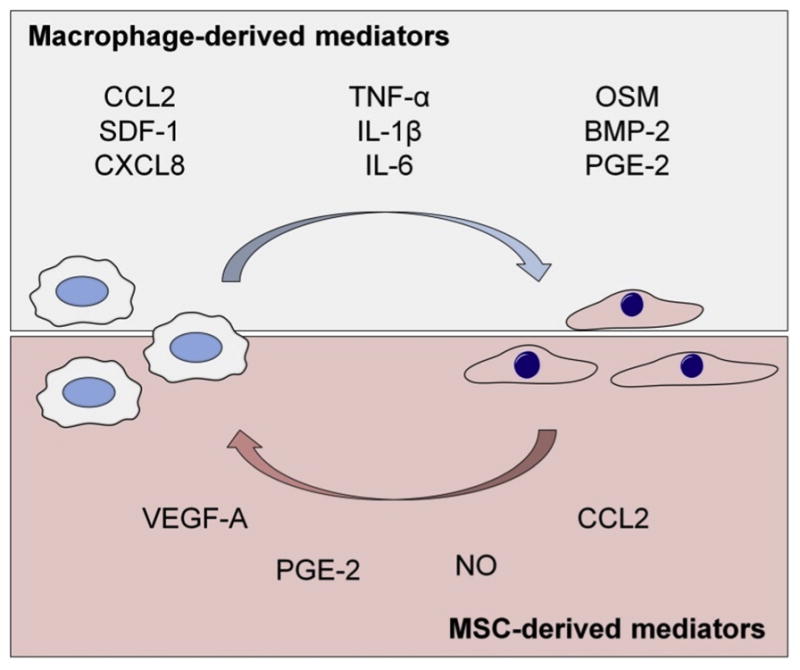
Macrophages regulate the recruitment and differentiation of the MSCs. On the upper part of the image are shown the best known macrophage derived chemokines, pro-inflammatory cytokines, and osteoinductive factors involved in the regulation of MSC functions. MSCs reciprocally regulate macrophage recruitment and function, generally having PGE2 and iNOS mediated immunosuppressive impact on macrophages.
Fig. 3. Interactions of macrophages and MSCs during fracture healing.
(1) Local damage associated molecular patterns (DAMPs) and other danger signals released from necrotic cells and damaged extracellular matrix as well as complement and coagulation system components activate recruited monocytes into inflammatory M1 macrophages. (2) Inflammatory macrophages secrete chemokines that recruit MSCs from periosteum, bone marrow, and circulation. Once recruited to the fracture site MSCs are exposed to macrophage derived inflammatory cytokines and osteoinductive factors. (3) Together with other microenviromental signals such as oxygen tension and mechanical cues these cytokines and growth factors guide the MSC differentiation along osteogenic and chondrogenic pathways. (4) MSCs reciprocally modulate the function of macrophages, possibly contributing to further monocyte/macrophage recruitment, induction of osteoinductive molecules, and eventual suppression of the inflammatory reaction. (5) As the fracture site is cleared of tissue debris and other signals maintaining the inflammatory macrophage phenotype, macrophages change their phenotype to tissue regenerative M2. (6) These tissue regenerative macrophages possibly contribute to bone formation by MSCs and osteoblasts via osteoinductive signaling molecules that might be different from M1-derived osteoinductive signals.
4. Impact of aging on the MSC/macrophage cross-talk
There is increasing evidence that aging induces profound changes in the physiology of both macrophages and MSCs. Choudhery et al. [70] harvested young and aged murine MSCs and compared their regenerative potential. Through a tube-forming assay with matrigel, the authors showed a decrease in wound healing with aged MSCs, and significant downregulation of VEGF, SDF-1 and protein kinase B. Bernet at al. [71] showed that aged resident muscle MSCs lose their self-renewal abilities via alterations in FGF receptor 1 and p38αβ MAPK signaling pathways. Impairment of aged MSCs’ abilities are explained by shorter mean telomere restriction fragment (mTRF), increased senescence associated β-galactosidase expression and genetic flaws with overexpression of p53, p21, BAX or MYC genes, among other changes [72–75]. Recently, our group demonstrated that the impaired ability of MSCs to form bone was associated to chronically elevated NF-kB activity [76]. Similarly, aging has been associated with changes both in the innate and adaptive immunity; in particular chronic low-level systemic inflammation known as “inflammaging” and has been shown to also impact macrophage polarization [77–79].
Although there are ample studies showing that aging impacts the physiology of both macrophages and MSCs, very little is currently known how aging influences the interactions of these cells. One underlying reason for the compromised fracture healing in the elderly may lie in difficulties in inter-cellular communication. Yin et al. [80] co-cultured young and aged MSCs with RAW264.7 macrophages. Interestingly young MSCs increased mRNA expression of M2-related Arg1 and IL-10 in RAW264.7 cells, whereas aged MSCs increased mRNA expression of M1-related TNF-α. In a chemotaxis migration assay, the authors showed increased migration when RAW264.7 cells were co-cultured with aged MSCs. Lee et al. [81] studied the effect of MSC engraftment to a wound in young and aged mice. The authors showed an increased efficacy of activated MSCs in aged mice and impaired macrophage function. They concluded that activated MSCs restored the regenerative process in aged mice and reversed the effect of aging on host macrophages. Naik et al. described reduced COX-2 activity and subsequently delayed callus maturation and healing in aged murine femoral fracture model [82]. Taken together, there is evidence that the altered cross-talk between MSCs and macrophages plays a role in the compromised bone regeneration in the elderly but further studies are warranted.
5. Therapeutic potential of utilizing macrophage-MSC crosstalk for bone regeneration
Current research on cell-based bone tissue engineering has largely focused on utilizing the bone regenerative potential of MSCs. [83,84] MSCs are highly attractive targets for cell-based therapies to induce bone formation at the site of the bone defect or non-union, given their multi-potency for bone, cartilage, and blood vessels, and the ease of harvesting MSCs from adult tissues and expanding them in vitro. In contrast, relatively little research has thus far focused on utilizing macrophages or targeting macrophage-MSC cross talk to enhance bone formation. However, as the crucial role of macrophages in bone regeneration becomes apparent, treatment options with a goal of enhancing fracture healing are also emerging. Based on the current literature the following two strategies to enhance bone formation by targeting macrophage-MSC cross talk can be outlined 1) modulating the number of macrophages at the fracture site and 2) modulation of local macrophage polarization to support bone formation.
A proof principle that bone regeneration can be enhanced by increasing the number of macrophages at the fracture site was provided by Alexander et al. [28] and Raggat et al. [31]. These studies demonstrated that local injection of M-CSF, the main macrophage growth factor, at the time of injury significantly increased the number of macrophages at the fracture site subsequently resulted in the improvement of both the intramembranous and endochondral bone formation. Alternatively, Slade Shantz et al. observed that decreasing macrophage recruitment to the fracture site improved callus formation in elderly mice while having no impact to fracture healing in young animals [85]. In the clinical setting, autologous bone marrow is now commonly utilized to treat fracture non-unions and osteonecrosis [86–88]. Although the primary goal in these strategies is to deliver bone marrow osteoprogenitor cells to the injury site, these preparations also unavoidably contain a larger amount of monocyte/macrophage lineage and other cells. Indeed it can be argued that most of the delivered cells are not osteoprogenitors but other mononuclear cells such as monocytes. The role that these cells play in the overall performance of the graft remains to be determined.
Several studies have postulated the possibility of enhancing bone regeneration via modulating macrophage polarization, mainly via induction of M2 polarization. Chen et al. studied the effects of the osteoconductive biomaterial β-tricalcium phosphate (β-TCP) on the interaction of the mouse macrophage cell line RAW 264.7 and human bone marrow derived MSCs [89,90]. RAW 264.7 cells stimulated with β-TCP extracts or grown on scaffolds coated with β-TCP assumed an M2-like phenotype with diminished production of pro-inflammatory mediators and increased production of BMP-2 and VEGF. Conditioned media from these β-TCP exposed macrophages increased the expression of osteogenic markers and matrix mineralization by MSCs. Schlundt et al. [32] showed that IL-4 and IL-13 soaked collagen scaffolds implanted to femoral osteotomies at the time of injury enhanced callus formation and bone regeneration, presumably by inducing M2 macrophage polarization. In addition to these collagen sponges, several biomaterial scaffolds capable of releasing IL-4 and subsequently modulating macrophage polarization towards an M2 phenotype have been developed; with increased vascularity and reduced adverse foreign body response these scaffolds show favorable properties in vivo but the performance of these scaffold in models of fracture healing remain to be demonstrated [91–93]. We recently reported the development of genetically modified MSCs that are capable of secreting IL-4 as a response to NF-kB activation; the utility of these cells in bone tissue regeneration will be assessed in future studies [94]. The current strategies of utilizing macrophages and macrophage MSCs cross talk for bone regeneration are summarized in Fig. 4 (Fig. 4a,b).
Fig. 4. Therapeutic potential of utilizing macrophage-MSC crosstalk for bone regeneration.
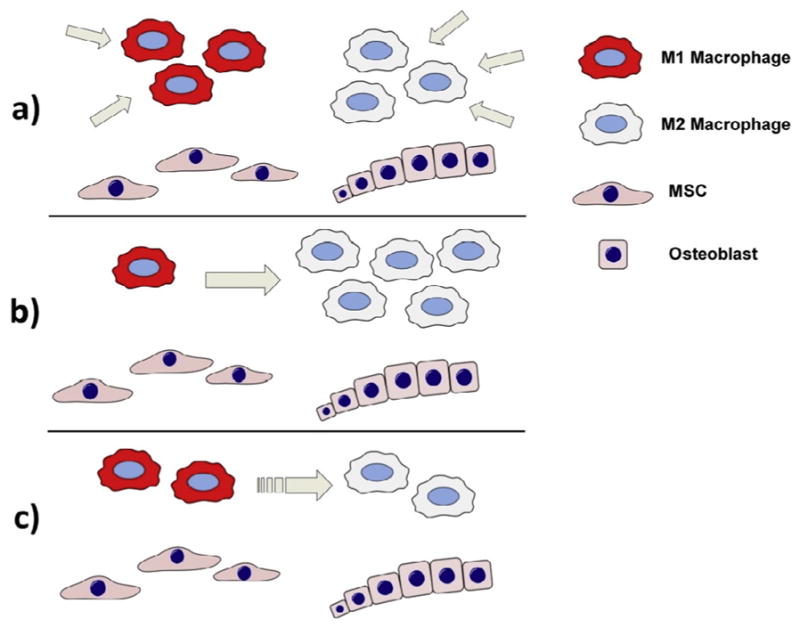
(a) The beneficial impact of macrophages on MSC-mediated bone formation can potentially be enhanced by increasing the number of macrophages at the fracture site, either delivering monocyte/macrophages directly to the fracture site or by introducing macrophage growth factors. (b) Macrophage polarization can be modulated towards an anti-inflammatory and tissue regenerative M2 phenotype either pharmacologically or by utilizing bioactive materials. This approach is likely particularly beneficial in cases of excessive or chronic M1-mediated inflammation, that is detrimental to bone healing. (c) An attractive strategy to optimize bone heling appears to be facilitating a short period (several days) of inflammation prior to modulation of macrophages to an anti-inflammatory phenotype. This approach might optimally utilize the bone regenerative properties of both M1 and M2 macrophages in a succession that mimics the physiological fracture healing.
6. Final thoughts
6.1. Macrophage polarization, duration of inflammation, and bone formation
Studies using animal models have established that macrophage/MSC cross talk is crucial for bone regeneration and that macrophages likely have the greatest impact on fracture healing during the early inflammatory phase. The mechanisms of interaction of monocyte/macrophages and bone forming cells have been studied in vitro. Although these studies have arrived at the same general conclusion that macrophages promote MSC and pre-osteoblast mediated bone formation, details of the interaction remain controversial. Indeed, many of the studies have yielded seemingly contradicting results. For example, depending on the study, M1 macrophages have been observed to either promote or inhibit bone formation. Similarly secretion of OSM, a cytokine most convincingly associated with macrophage-induced bone formation, has been described from all macrophage subtypes including M0, M1 and M2. This controversy can be partially explained by technical differences among the studies, such as different source of cells, maturity of the monocyte/macrophages, and the specific co-culture conditions.
An interesting possibility emerging from the seemingly contradicting results is that all monocyte and macrophage subtypes do, in fact, have the capacity to promote MSC mediated osteogenesis but their relevance may change during different physiological conditions: M0s might support osteogenesis during normal bone homeostasis while both M1 and M2 macrophages sequentially contribute to different stages of fracture healing. It also seems likely that the timing and duration of the M1 macrophage mediated inflammatory reaction is a critical determinant in the outcome of the subsequent bone regeneration. There is ample evidence that the initial inflammatory reaction is required for optimal fracture healing [7,8,11]. At the same time, sustained, chronic, M1-macrophage mediated inflammation is highly detrimental to bone, with the continued production of pro-inflammatory cytokines resulting in bone resorption via increased osteoclast activity and suppression of bone formation by osteoblasts [7,8,11,95]. Thus, the duration of M1-mediated inflammation seems crucial, with brief acute inflammation being beneficial and chronic inflammation highly detrimental to bone healing. This model is also supported by the current in vivo macrophage depletion studies showing that the depletion of the macrophages early on during the initial inflammatory phase has the most detrimental impact on fracture healing. Considering the immunomodulatory function of MSCs an attractive hypothesis is that MSCs first recruited to the fracture site by inflammatory macrophages, subsequently mediate the M1 to M2 phenotype switch during physiological fracture healing. Dysregulation in the switch from pro-inflammatory M1-dominated environment to the M2 dominated anti-inflammatory environment might be the underlying reason, in some instances, of failed bone regeneration. Similarly, too early suppression of the immune response e.g. by NSAIDs or glucocorticoid medications might result in suboptimal bone regeneration [96–98]. Thus, the timely modulation of macrophage polarization could be one strategic means to enhance bone healing. Indeed, Loi et al. [99] studied the impact of sequential modulation of macrophage phenotype on pre-osteoblast mediated bone formation in vitro: All of the main macrophage phenotypes M0, M1 and M2 increased MC3T3 cell mediated bone formation in direct co-culture. Interestingly however, modulation of macrophage polarization from M1 to M2 after 72 h of M1-MC3T3 co-culture further enhanced bone formation, suggesting that transient inflammatory phase followed by M2 dominated regenerative phase might lead to optimal bone formation. Similar to Guihard et al. and Zhang et al. [48,52], most OSM production was seen in M1 co-cultures, and in M1 cultures subsequently treated with IL-4. Together with the results of Zhang et al. [52], these results suggest that both pro-inflammatory and tissue-regenerative macrophage phenotypes play a role in optimizing bone formation and that timely modulation of inflammation to tissue regeneration might constitute means to improve bone regeneration (Fig. 4c). Although this model follows the general paradigm of macrophage activity during tissue regeneration and is attractive in its simplicity, it still remains speculative, and needs to be validated by further in vivo studies. In particular the assumed role of M2 macrophages in the later stages of bone regeneration remains to be proven by further research.
6.2. Mediators of macrophage/MSC interaction
Similar to the question of which macrophage activation state is most beneficial for bone formation, the exact mediators of the macrophage/MSC cross-talk remain elusive. In vitro experiments showing the beneficial effect of macrophages of various types on bone formation have mostly been conducted using either macrophage-conditioned media [27,43,46–48,50,89,90] or indirect transwell [25,44,51,52] co-culture settings. Although the beneficial effect of macrophages on bone formation in direct co-cultures with bone forming cells has also been demonstrated [25,45,49,52,99], the collective evidence derived from these studies suggests that the beneficial effect is mediated by soluble mediators, and that direct cell-to-cell contact is not absolutely required. However, it remains a possibility that the mechanisms of macrophage/MSC interaction might be different in indirect and direct culture settings. Indeed, it seems that mere direct addition of macrophages to MSC cultures is sufficient to enhance bone formation, with the activation state of the added macrophages playing a relatively minor role [27,45,49,52,99].
It also seems likely that a combination of factors, rather than a single mediator, is responsible for the macrophage mediated enhanced osteogenesis. For example this could be the case in LPS activated monocytes that secrete both OSM and exosomes; in the light of the current studies it is possible that these factors function in synergy to stimulate bone formation [46–48]. Still, OSM, PGE2, and BMP-2 have arisen as the best recognized paracrine mediators of macrophage/MSC cross-talk. In particular, OSM is one of the key cytokines produced by macrophages that enhances bone formation in vitro [45,48,50]. OSM is a pleiotropic cytokine that has been shown both to enhance bone formation and induce bone resorption in vitro and in vivo [100,101]. Guihard et al. [48] were first to describe OSM’s crucial role in mediating macrophage/MSC interaction followed closely by reports by Nicolaidou et al. [46] and Fernandes et al. [50]. A recent study expands these in vitro observations to murine model showing that OSM is indeed expressed in macrophages during intramembranous fracture healing mainly during the early inflammatory phase [102]. In macrophage depletion and OSM KO mice experiments, the formation of trabecular woven bone was compromised with reduced number of osteoblasts and reduced expression of osteoblast differentiation factors at fracture site. These observations provide further support to the conclusion that inflammatory monocyte/macrophages and their secreted factors, especially OSM, play key roles in the macrophage promotion of bone formation in the early stages of fracture healing. Interestingly it has been shown that in addition to danger signaling via TLRs, activation of both complement and coagulation cascades can induce OSM production from monocytes, providing a possible link between the early facture hematoma and OSM production by invading monocytes [103,104].
In conclusion, successful bone regeneration is based on coordinated cross-talk between MSCs and macrophages (Fig. 5). Much still remains unknown about the interaction of macrophages and MSCs during bone repair, but elucidating this cellular cross talk holds great promise to solve one of the most fundamental problems in orthopaedics, creating bone where it is deficient.
Fig. 5. Summary of the MSC-macrophage interactions during bone regeneration.
Shown the key cells and signaling molecules involved to the cross-talk between inflammatory and bone forming cells.
Acknowledgments
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko Foundation.
References
- 1.C. Control. National Hospital Ambulatory Medical Care Survey: 2013 Emergency Department Summary Tables. 2013 Available from: https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- 2.Miranda M, Moon M. Treatment strategy for nonunions and malunions. In: Stannard J, Schmidt P, editors. Surgical Treatment of Orthopaedic Trauma. Thieme; New York: 2007. pp. 77–100. [Google Scholar]
- 3.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, Simpson H, Alt V. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014 Jun;45(Suppl 2):S3–S7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002 Sep;20(5):1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 5.Uhthoff HK, Rahn BA. Healing patterns of metaphyseal fractures. Clin Orthop Relat Res. 1981 Oct;(160):295–303. [PubMed] [Google Scholar]
- 6.Aspenberg P, Sandberg O. Distal radial fractures heal by direct woven bone formation. Acta Orthop. 2013 Jun;84(3):297–300. doi: 10.3109/17453674.2013.792769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016 May;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastian O, Pillay J, Alblas J, Leenen L, Koenderman L, Blokhuis T. Systemic inflammation and fracture healing. J Leukoc Biol. 2011 May;89(5):669–673. doi: 10.1189/jlb.0810446. [DOI] [PubMed] [Google Scholar]
- 9.Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. BoneKEy Rep. 2013 Jun 26;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011 Jun;42(6):551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012 Jan 31;8(3):133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 12.Harwood PJ, Newman JB, Michael ALR. An update on fracture healing and non-union. Orthop Traumatol. 2010;24:9–23. [Google Scholar]
- 13.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966 Dec;16(3):381–390. [PubMed] [Google Scholar]
- 14.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974 Apr;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006 Nov;13(6):419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011 Oct 14;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011 Oct 10;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008 Jan 1;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 22.Schell H, Lienau J, Epari DR, Seebeck P, Exner C, Muchow S, Bragulla H, Haas NP, Duda GN. Osteoclastic activity begins early and increases over the course of bone healing. Bone. 2006 Apr;38(4):547–554. doi: 10.1016/j.bone.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Davison NL, Gamblin AL, Layrolle P, Yuan H, de Bruijn JD, Barrère-de Groot F. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials. 2014 Jun;35(19):5088–5097. doi: 10.1016/j.biomaterials.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Odgren PR, Witwicka H, Reyes-Gutierrez P. The cast of clasts: catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect Tissue Res. 2016 May;57(3):161–174. doi: 10.3109/03008207.2016.1140752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008 Jul 15;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 26.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004 Apr;75(4):612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 27.Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, Mylvaganam S, Grynpas M, Alman BA. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015 Jun;30(6):1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 28.Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011 Jul;26(7):1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 29.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth. 1994 Sep 14;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg OH, Tatting L, Bernhardsson ME, Aspenberg P. Temporal role of macrophages in cancellous bone healing. Bone. 2017 Aug;101:129–133. doi: 10.1016/j.bone.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014 Dec;184(12):3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2015 Oct 31; doi: 10.1016/j.bone.2015.10.019. pii: S8756-3282(15)00392–0. [DOI] [PubMed] [Google Scholar]
- 33.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999 Dec 20;190(12):1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Lévesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010 Dec 2;116(23):4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 35.Lin HN, O’Connor JP. Osteoclast depletion with clodronate liposomes delays fracture healing in mice. J Orthop Res. 2017;35(8):1699–1706. doi: 10.1002/jor.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho SW. Role of osteal macrophages in bone metabolism. J Pathol Trans Med. 2015 Mar;49(2):102–104. doi: 10.4132/jptm.2015.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, van Rooijen N, McCauley LK. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A. 2014 Jan 28;111(4):1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008 Jun 5;2(6):566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007 May 1;101(1):135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004 Dec;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009 Mar;60(3):813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 42.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011 Aug 1;91(3):402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002 Jan;30(1):26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 44.Pirraco RP, Reis RL, Marques AP. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med. 2013 May;7(5):392–400. doi: 10.1002/term.535. [DOI] [PubMed] [Google Scholar]
- 45.Nicolaidou V1, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, Cope AP, Horwood NJ. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLos One. 2012;7(7):e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omar OM, Granéli C, Ekström K, Karlsson C, Johansson A, Lausmaa J, Wexell CL, Thomsen P. The stimulation of an osteogenic response by classical monocyte activation. Biomaterials. 2011 Nov;32(32):8190–8204. doi: 10.1016/j.biomaterials.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 47.Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLos One. 2013 Sep 18;8(9):e75227. doi: 10.1371/journal.pone.0075227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, Richards CD, Chevalier S, Rédini F, Heymann D, Gascan H, Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cell. 2012 Apr;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 49.Lu LY, Loi F, Nathan K, Lin TH, Pajarinen J, Gibon E, Nabeshima A, Cordova L, Jamsen E, Yao Z, Goodman SB. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 2017 Nov;35(11):2378–2385. doi: 10.1002/jor.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes TJ, Hodge JM, Singh PP, Eeles DG, Collier FM, Holten I, Ebeling PR, Nicholson GC, Quinn JM. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLos One. 2013 Sep 12;8(9):e73266. doi: 10.1371/journal.pone.0073266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong L, Zhao Y, Zhang Y, Ruan Z. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016 Winter;46(1):65–71. [PubMed] [Google Scholar]
- 52.Zhang Y, Bose T, Unger RE, Jansen JA, Kirkpatrick CJ, van den Beucken JJ. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017 Aug;369(2):273–286. doi: 10.1007/s00441-017-2598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, van der Kraan PM, Murphy JM, Bastiaansen-Jenniskens YM, van Osch GJ. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014 Aug;22(8):1167–1175. doi: 10.1016/j.joca.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Sesia SB, Duhr R, Medeiros da Cunha C, Todorov A, Schaeren S, Padovan E, Spagnoli G, Martin I, Barbero A. Anti-inflammatory/tissue repair macrophages enhance the cartilage-forming capacity of human bone marrow-derived mesenchymal stromal cells. J Cell Physiol. 2015 Jun;230(6):1258–1269. doi: 10.1002/jcp.24861. [DOI] [PubMed] [Google Scholar]
- 55.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 Nov 15;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 56.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLos One. 2010 Apr 26;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008 Feb 7;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012 Apr 25;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013 Jan;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 60.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLos One. 2010 Feb 16;5(2):e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009 Jan;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014 Jan 10;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009 Dec;37(12):1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012 Jan;20(1):187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLos One. 2008 Apr 2;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014 Nov;10(11):4730–4741. doi: 10.1016/j.actbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013 Oct 3;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012 May 20;29(8):1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y, Huang R, Fan W, Prasadam I, Crawford R, Xiao Y. Mesenchymal stromal cells regulate the cell mobility and the immune response during osteogenesis through secretion of vascular endothelial growth factor A. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2327. (in press) [DOI] [PubMed]
- 70.Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012 Aug 1;36(8):747–753. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- 71.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014 Mar;20(3):265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cell. 2004;22(5):675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 73.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008 Jun;7(3):335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuehn BM. Genetic flaws found in aging stem cell lines. J Am Med Assoc. 2005 Oct 19;294(15):1883–1884. doi: 10.1001/jama.294.15.1883. [DOI] [PubMed] [Google Scholar]
- 75.Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014 Mar;16(3):201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin TH, Gibon E, Loi F, Pajarinen J, Córdova LA, Nabeshima A, Lu L, Yao Z, Goodman SB. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J Orthop Res. 2017 Feb;35(2):281–288. doi: 10.1002/jor.23270. [DOI] [PubMed] [Google Scholar]
- 77.Gibon E, Loi F, Córdova LA, Pajarinen J, Lin T, Lu L, Nabeshima A, Yao Z, Goodman SB. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regen Eng Trans Med. 2016 Jun;2(2):98–104. doi: 10.1007/s40883-016-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016 Feb;17(1):7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012 Mar;4(3):166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin Y, Wu RX, He XT, Xu XY, Wang J, Chen FM. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res Ther. 2017 Jun 24;8(1):153. doi: 10.1186/s13287-017-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S, Szilagyi E, Chen L, Premanand K, DiPietro LA, Ennis W, Bartholomew AM. Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J Surg Res. 2013 May 1;181(1):20–24. doi: 10.1016/j.jss.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 82.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O’Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009 Feb;24(2):251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patterson TE, Kumagai K, Griffith L, Muschler GF. Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am. 2008 Feb;90(Suppl 1):111–119. doi: 10.2106/JBJS.G.01572. [DOI] [PubMed] [Google Scholar]
- 84.Dawson JI, Kanczler J, Tare R, Kassem M, Oreffo RO. Concise review: bridging the gap: bone regeneration using skeletal stem cell-based strategies - where are we now? . Stem Cell. 2014 Jan;32(1):35–44. doi: 10.1002/stem.1559. [DOI] [PubMed] [Google Scholar]
- 85.Slade Shantz JA, Yu YY, Andres W, Miclau T, 3rd, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28(Suppl 1):S10–S14. doi: 10.1097/BOT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Bances I, Perez-Basterrechea M, Perez-Lopez S, Nuñez Batalla D, Fernandez Rodriguez MA, Alvarez-Viejo M, Ferrero-Gutierrez A, Menendez-Menendez Y, Garcia-Gala JM, Escudero D, Paz Aparicio J, Carnero Lopez S, Lopez Fernandez P, Gonzalez Suarez D, Otero Hernandez J. Repair of long-bone pseudoarthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy. 2013 May;15(5):571–577. doi: 10.1016/j.jcyt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005 Jul;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 88.Goodman SB, Hwang KL. Treatment of secondary osteonecrosis of the knee with local debridement and osteoprogenitor cell grafting. J Arthroplasty. 2015 Nov;30(11):1892–1896. doi: 10.1016/j.arth.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 89.Chen Z, Wu C, Gu W, Klein T, Crawford R, Xiao Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014 Feb;35(5):1507–1518. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z, Mao X, Tan L, Friis T, Wu C, Crawford R, Xiao Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with beta-tricalcium phosphate. Biomaterials. 2014 Oct;35(30):8553–8565. doi: 10.1016/j.biomaterials.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 91.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015 Jan;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017 Jan;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minardi S, Corradetti B, Taraballi F, Byun JH, Cabrera F, Liu X, Ferrari M, Weiner BK, Tasciotti E. IL-4 release from a biomimetic Scaffold for the temporally controlled modulation of macrophage response. Ann Biomed Eng. 2016 Jun;44(6):2008–2019. doi: 10.1007/s10439-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 94.Lin T, Pajarinen J, Nabeshima A, Lu L, Nathan K, Yao Z, Goodman SB. Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy. 2017 Sep;19(9):1025–1034. doi: 10.1016/j.jcyt.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013 Oct;101(10):3033–3045. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandberg O, Aspenberg P. Different effects of indomethacin on healing of shaft and metaphyseal fractures. Acta Orthop. 2015 Apr;86(2):243–247. doi: 10.3109/17453674.2014.973328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandberg OH, Aspenberg P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Joint Res. 2015 Oct;4(10):170–175. doi: 10.1302/2046-3758.410.2000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bissinger O, Kreutzer K, Gotz C, Hapfelmeier A, Pautke C, Vogt S, Wexel G, Wolff KD, Tischer T, Prodinger PM. A biomechanical, microcomputertomographic and histological analysis of the influence of diclofenac and prednisolone on fracture healing in vivo. BMC Muscoskel Disord. 2016 Sep 5;17(1):383. doi: 10.1186/s12891-016-1241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loi F, Córdova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016 Jan 22;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song HY, Jeon ES, Kim JI, Jung JS, Kim JH. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101(5):1238–1251. doi: 10.1002/jcb.21245. [DOI] [PubMed] [Google Scholar]
- 101.Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG, Nicola NA, Gillespie MT, Martin TJ, Sims NA. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest. 2010;120(2):582–592. doi: 10.1172/JCI40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guihard P, Boutet MA, Brounais-Le Royer B, Gamblin AL, Amiaud J, Renaud A, Berreur M, Rédini F, Heymann D, Layrolle P, Blanchard F. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am J Pathol. 2015 Mar;185(3):765–775. doi: 10.1016/j.ajpath.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Kastl SP, Speidl WS, Katsaros KM, Kaun C, Rega G, Assadian A, Hagmueller GW, Hoeth M, de Martin R, Ma Y, Maurer G, Huber K, Wojta J. Thrombin induces the expression of oncostatin M via AP-1 activation in human macrophages: a link between coagulation and inflammation. Blood. 2009 Sep 24;114(13):2812–2818. doi: 10.1182/blood-2009-01-200915. [DOI] [PubMed] [Google Scholar]
- 104.Kastl SP, Speidl WS, Kaun C, Katsaros KM, Rega G, Afonyushkin T, Bochkov VN, Valent P, Assadian A, Hagmueller GW, Hoeth M, de Martin R, Ma Y, Maurer G, Huber K, Wojta J. In human macrophages the complement component C5a induces the expression of oncostatin M via AP-1 activation. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):498–503. doi: 10.1161/ATVBAHA.107.160580. [DOI] [PubMed] [Google Scholar]