Abstract
The ‘cognitive map’ hypothesis proposes that brain builds a unified representation of the spatial environment to support memory and guide future action. Forty years of electrophysiological research in rodents suggests that cognitive maps are neurally instantiated by place, grid, border, and head direction cells in the hippocampal formation and related structures. Here we review recent work that suggests a similar functional organization in the human brain and reveals novel insights into how cognitive maps are used during spatial navigation. Specifically, these studies indicate that: (i) the human hippocampus and entorhinal cortex support map-like spatial codes; (ii) posterior brain regions such as parahippocampal and retrosplenial cortices provide critical inputs that allow cognitive maps to be anchored to fixed environmental landmarks; (iii) hippocampal and entorhinal spatial codes are used in conjunction with frontal lobe mechanisms to plan routes during navigation. We also discuss how these three basic elements of cognitive map based navigation spatial coding, landmark anchoring, and route planning might be applied to non-spatial domains to provide the building blocks for many core elements of human thought.
Introduction
The idea of a cognitive map was originally proposed by Tolman, in an effort to explain navigational behaviors in rodents that could not be logically reduced to associations between specific stimuli and rewarded behavioral responses1. Tolman observed, for example, that rats who had learned a roundabout route to a goal would quickly switch to a more direct path if the familiar route was blocked. He concluded that the animals must have access to spatial knowledge about the environment, akin to the spatial knowledge obtainable from a map, that could be used to guide behavior in a flexible manner.
This idea received neurobiological support from O’Keefe and Dostrovsky’s discovery of place cells in the rodent hippocampus, which fire as a function of the spatial position of the animal2. Building on these results, O’Keefe and Nadel3 proposed that the hippocampus provided the neural instantiation of a spatial map, and they further hypothesized that this map took the form of a Euclidean coordinate system that allowed landmarks and goals to be encoded in terms of their allocentric locations. Although the precise nature of the hippocampal code remains hotly debated4, 5, subsequent discoveries have fleshed out the cognitive map hypothesis by revealing additional components of a putative spatial navigation system6, including: (i) grid cells in medial entorhinal cortex, which fire in a regular hexagonal lattice of locations tiling the floor of the environment; (ii) head direction (HD) cells in several cortical and subcortical structures, which fire based on the orientation of the head in the navigational plane; (iii) border cells in entorhinal cortex and boundary cells in subiculum, which fire when the animal is at set distances from navigational boundaries at specific directions. Grid cells are thought to support coding of metric distances as the animal moves through the world7, HD cells are implicated in the tracking of heading direction8, and border cells are believed to help relate the firing fields of place and grid cells to the fixed features of the environment9. Cells in the hippocampal system have also been discovered that encode other navigationally-relevant quantities, such as distance and direction to navigational goals10.
The spatial positioning system supported by these cells is often taken to be a model system for understanding how the brain processes high-level cognitive information. A key unresolved question, however, is whether a similar navigational system is implemented in humans. The fact that anatomical structures the hippocampal formation and Papez circuit are conserved across mammalian species11 argues in favor of functional homologies between humans and rodents. However, there are numerous differences between the species, including the fact that rats have less complex visual systems and are nocturnal rather than diurnal. Moreover, damage to navigation-related structures in humans (for example, in the famous patient Henry Molaison) typically leads to broad memory deficits that are not limited to the spatial domain. It has been challenging to resolve this issue, in part because noninvasive neuroimaging methods used in humans do not interrogate the level of neuronal information processing revealed by single-cell recording studies. However, recent advanced neuroimaging analysis methods have allowed researchers to mitigate this limitation to some degree (Box 1). Here we review studies on cognitive-map based navigation, with an emphasis on connecting this recent human neuroimaging work to the rodent neurophysiology literature.
BOX 1. USING FMRI SIGNALS TO INTERROGATE NEURAL CODES.
fMRI data are acquired in spatially discrete units, called voxels. A typical voxel of 3x3x3 mm contains roughly 600,000 neurons. Given the coarseness of the signal, one might think it impossible to use fMRI to ask questions about neural representations implemented at the single-unit or columnar level. However, researchers have developed several methods that allow fMRI signals to be related to a representational code.
fMRI adaptation
fMRI adaptation (also known as fMRI repetition suppression) occurs when repeated presentation of the same stimulus leads to a reduction in the fMRI signal. Adaptation across two different stimuli provides evidence for a common neural representation, while an absence of adaptation (or “recovery from adaptation”) is evidence that the two stimuli are representationally distinct.
Multivariate pattern analysis (MVPA)
MVPA involves analysis of patterns of fMRI activity across multiple voxels and testing the information that can be decoded from these patterns. Popular decoding methods include correlation-based classification and support vector machines. A common extension of MVPA is representational similarity analysis (RSA), in which the similarities between fMRI activation patterns are taken as a proxy for the similarities between the corresponding neural representations.
Encoding models
Here one models fMRI responses by describing stimuli in terms of simpler features that are hypothesized to be represented at the neuronal level. A training dataset is used to estimate the extent to which each voxel’s response is modulated by each feature. The model is then evaluated based on how well it predicts fMRI responses to independent test stimuli. If the predictions are accurate, then the model is deemed to contain an accurate description of the neural representations within each voxel.
Representing space: Maps, Grids, and Contexts
Participants in functional magnetic resonance imaging (fMRI) experiments must remain stationary in the scanner bore, so it is not possible to use fMRI to monitor blood oxygenation level-dependent responses (a proxy for neural activity) while people perambulate about the world. Consequently, fMRI studies often resort to examining activity during virtual navigation, imagined navigation, spatial memory recall, or viewing of navigationally-relevant stimuli. Although vestibular and proprioceptive inputs are absent in these studies, memory/planning systems are engaged, and visual inputs are often present. The earliest neuroimaging navigation studies using these approaches, performed in the late 1990s12–14, revealed a network of brain regions that were more active during navigation compared to perceptually-matched control conditions (Fig. 1). Contemporaneous work found that a subset of these regions, including the posterior parahippocampal cortex and the retrosplenial/medial parietal region, responded strongly during mere passive viewing of buildings, landscapes, cityscapes, and rooms15, implicating them in the visual processing of navigation-related stimuli. Other brain regions in the “navigation network”, such as frontal lobe regions, have been shown to respond primarily during active navigation, consistent with the view that their role in navigation relates to planning16,17.
Figure 1. Neuroimaging studies reveal a network of brain regions involved in spatial navigation.
Neurosynth149 was used to perform an automated meta-analysis of 64 studies of human navigation (www.neurosynth.org), revealing common activation across these studies in the hippocampus (Hipp), as well as parahippocampal, retrosplenial, and entorhinal cortices, among other regions (Map thresholded at p<0.01, FDR-corrected). This navigational network overlaps with three regions (OPA, RSC, OPA) that response strongly during viewing of scenes and buildings, which were defined in a large group of participants (n=42) using standard methods150. Only the right hemisphere inflated cortical surface is shown, though similar regions are also found in the left hemisphere.
In rodents, the hippocampus and entorhinal cortex are believed to be central for cognitive map-based navigation. In human fMRI studies, the hippocampus responds when people use a cognitive-map-based strategy during virtual navigation, as evidenced by the use of short-cuts or the planning of efficient novel routes18–20, and activity in the hippocampus also predicts accuracy of navigation when using such strategies21. In contrast, use of a response-based strategy, in which a familiar route is followed by implementing a sequence of actions associated with specific visual cues, is associated with activity in the caudate19, 20. London taxi drivers, who spend years learning an extensive “map” of London streets, have larger right posterior hippocampi as a result of their training22, and the size of this part of the hippocampus has also been shown to predict learning of the allocentric spatial relationships between buildings on a college campus23 and the allocentric topography of an artificial landscape24. Thus, activity in the human hippocampus is associated with cognitive map based navigation, and the size of the hippocampus may predict the ability to acquire a cognitive map.
Recently, fMRI researchers have taken these results a step further, by showing that the hippocampus in humans supports map-like spatial codes. A key feature of a map is that it preserves distance relationships: entities that are closer together (vs. farther apart) in the real world are closer together (vs. farther apart) on the map. One of the first studies to examine such distance relationships in the hippocampus used the technique of fMRI adaptation (Fig. 2A)25. Participants were college students, who viewed images of familiar campus buildings, shown one at a time. fMRI activity in the hippocampus in response to each building scaled with the distance between that building and the building shown on the immediately preceding trial. This pattern of “recovery from adaptation” indicated that the hippocampus considered closer buildings to be representationally similar and distant buildings to be representationally dissimilar.
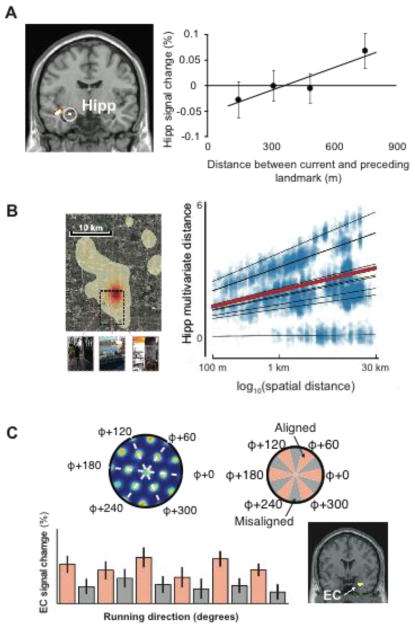
Figure 2. Map- and grid-like coding of navigable space in humans.
A) Evidence from fMRI adaptation. When viewing images of landmarks from a familiar college campus, fMRI activity in the left hippocampus scales with the real-world distance between the landmark shown on each trial and the landmark shown on the immediately preceding trial (adapted from ref. 25). B) Evidence from multi-voxel pattern analysis (MVPA). Voxelwise activity patterns in the hippocampus reflect distances between events intermittently logged by a camera worn by participants in the 30 days prior to the scan (aerial map of navigated territory shown on the left, as well as example pictures; adapted from ref. 28). C) Evidence from an encoding model. Participants performed a virtual reality navigation task. Grid cells in an individual rat all have the same orientation (φ; top row), and thus it was predicted that movements aligned with the grid orientation should result in more fMRI activity than movements misaligned with the grid. The expected pattern of results was observed in human entorhinal cortex (EC, bottom row; adapted from ref. 29)
Map-like codes in the hippocampus have also been identified using multi-voxel pattern analysis (MVPA) of spatially distributed fMRI responses. Hassabis and colleagues26 examined activation while participants navigated through a virtual environment consisting of two connected square rooms. Activation patterns in the hippocampus distinguished between the corners of each room, while activation patterns in parahippocampal cortex distinguished between the rooms. Subsequent work with larger environments indicated that similarities in the hippocampal patterns reflected distances in both time and space27. In a particularly striking example, the locations and times of real-world events were recorded by participants wearing a life-logging device around their necks for 1 month as they went about their daily lives (Fig. 2B). When subjects were subsequently scanned while recalling these events in response to photographs taken by the device, activity patterns in the left anterior hippocampus reflected both temporal and spatial proximities28.
Remarkably, researchers have also been able to use fMRI to identify grid-like codes in entorhinal cortex (Fig. 2C). This work uses an encoding model approach, in which the fMRI response is predicted based on the expected responses in the underlying neurons. Doeller and colleagues observed that in rodents, the preferred heading direction of conjunctive (location x direction) grid cells tend to be aligned with their grids29. Because the orientation of all EC conjunctive grid cells in an individual tend to be aligned to each other, they predicted that the average neural response should be greater for movements that align with the grid than for movements that are misaligned. Indeed, this predicted effect was observed in the form of a 60° periodic modulation of fMRI response by movement direction while human participants navigated through a virtual environment. Subsequent work using the same approach found that grid representations in EC were also active during imagined movements30.
The neural reality of these map-like and grid-like representations have been confirmed by intracranial recording studies performed on presurgical epilepsy patients. When participants played a “taxi driver” game that required them to pick up passengers and navigate to a destination, a quarter of the recorded neurons in the hippocampus were classified as place cells based on firing that was selective for location but independent of the facing direction31. Other cells in the target regions (which included hippocampus, parahippocampal cortex, amygdala, and the frontal lobes) encoded specific views (usually views of buildings) or the identity of the current goal (also buildings). Grid cell-like activity has also been identified in entorhinal cortex using similar methods32, as have cells that code the direction of movement around a closed loop33.
Beyond distinguishing between locations and representing the distances between them, another key characteristic of the rodent hippocampus is that it can store multiple maps, thus allowing it to represent multiple environments, or multiple states of the same environment35. This ability to distinguish between different contexts is indexed by global remapping and rate remapping36. In the former case, the set of place cells that fire in one context is different from the set of place cells that fire in another, whereas in the latter case, the same place cells fire in the same locations, but with reliably different maximal firing rates. During learning, the rodent hippocampus may fail to distinguish between similar contexts for some time, but then suddenly exhibit unique representation for each37. At retrieval, the hippocampus will then show an “all-or-nothing” response characteristic of attractor networks whereby either one or the other context is represented, even when the cues are intermediate between them38. Multivoxel patterns in human hippocampus show similar attractor-like effects under conditions of environmental ambiguity39. These results may be related to a general hippocampal function of pattern separation40, whereby different environments41, routes42, and behavioral contexts43 are orthogonalized from each other, thus allowing them to be distinguished even when they share overlapping features.
Finally, neuroimaging and neuropsychological studies indicate that the hippocampus and EC are not the only regions that mediate long-term spatial memories. Pre-morbidly learned cognitive maps remain intact after medial temporal lobe damage44, although they seem to take a somewhat schematized form45. Thus, some spatial knowledge may be encoded in the cortex, but the hippocampus might still be needed for retrieval of fine spatial details46. fMRI studies suggest that the retrosplenial/medial parietal region might be a particularly important neocortical locus for the processing or storage of long-term spatial knowledge47–50. An important question for future research will be to understand how the hippocampal formation and cortical regions interact to support different kinds of spatial knowledge.
Anchoring cognitive maps to the world
For a cognitive map to be useful, the organism must have a mechanism for connecting map coordinates to fixed aspects of the environment that can be identified by perceptual systems. These might include discrete objects such as buildings, statues, or mailboxes, or more distributed entities such as the shape of a room or the topography of a landscape51. We use the term landmark to refer to items that are stably related to specific locations or bearings on the map, including both object-like landmarks and environmental boundaries. In this section we discuss how landmarks are represented, and how they are used to anchor the cognitive map.
It is first worth noting that it is possible to navigate without using landmarks. Many navigation episodes start from a familiar “home” or “base”. In such cases, self-motion cues (e.g., vestibular and proprioceptive signals, motor efference copies, optic flow) can be used to keep track of displacement from the starting point. This strategy, known as path integration or dead reckoning, is used by many animals, including mammals, birds and insects52,53. In rodents, path integration is believed to involve the use of HD cells and grid cells to calculate a displacement vector7, and in humans path integration accuracy correlates with activity in the hippocampus, medial prefrontal cortex, and other regions54, 55. A limitation of this strategy is that error inevitably accumulates over time. When this happens, landmarks can be used to recalibrate position and heading. One can also navigate exclusively by using landmarks, without any path integration at all, a strategy known as landmark-based piloting53.
Landmark control of cognitive maps
Landmark anchoring involves the use of environmental cues to determine the orientation and displacement of the cognitive map that is, the angle and position of the putative coordinate axes56. Relevant to understanding this function is 40 years of research in rodents that has explored how the firing fields of place, grid, and HD cells are controlled by these cues57. We will not attempt to summarize this literature here; however, one consistent result is that objects at the extremities of the navigable environment are strong controllers of the orientation of the cognitive map, at least in animals who have maintained an internal sense of direction and are primarily using landmarks to correct errors in path integration. When distal, extra-maze cues, or cue cards along the chamber wall, are rotated around the center of the chamber, place and grid field locations rotate with the cues, as do HD tuning curves (Fig. 3A)57, 58. In addition, recent work suggests that environmental geometry may also play some role in setting cognitive map orientation59, as evidenced by reports that grid fields rotate with chamber boundaries even when fixed distal cues are visible60, and that grid fields exhibit consistent alignments and distortions that are related to chamber geometry60,61.
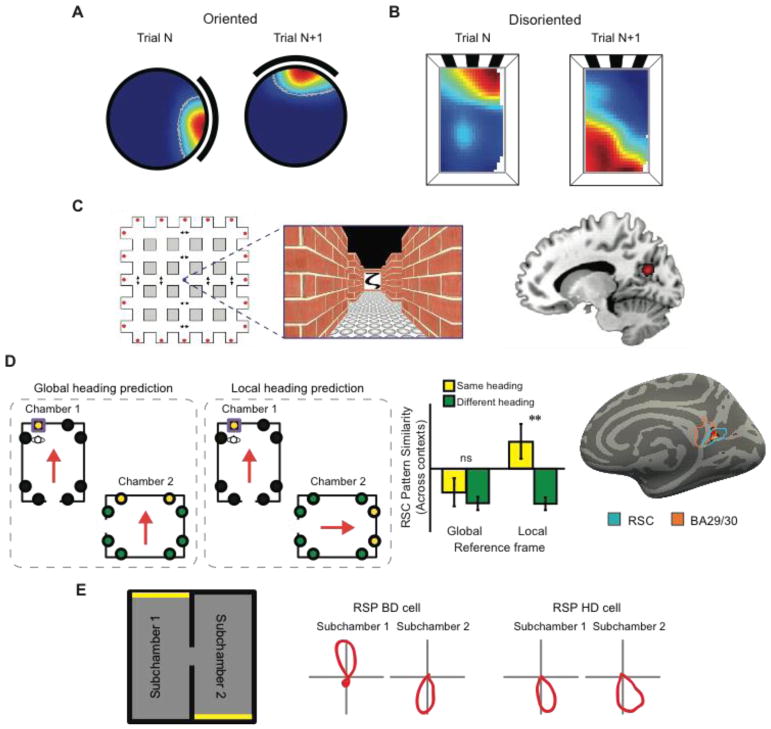
Figure 3. Anchoring the cognitive map to the world.
A) In oriented rats, from trial-to-trial, the orientation of the hippocampal map is set by featural cues on the walls of the chamber, rotating in concert with rotation of those cues. B) Following disorientation, the hippocampal map is anchored primarily by the geometric shape of the chamber rather than featural cues. For this example place cell, from trial-to-trial, two place fields were observed relative to chamber geometry, one being 180° rotation of the other, mirroring the chamber’s geometric symmetry (adapted from ref. 64). C) fMRI evidence that human retrosplenial/medial parietal region represents heading direction (adapted from ref. 87). During scanning, participants were shown pictures associated with different facing directions learned in a virtual-reality arena (left). fMRI adaptation was found in medial parietal cortex (BA 31) when the same facing direction was elicited on successive trials (right). D) fMRI evidence that the retrosplenial complex (RSC) represents heading in a local reference frame (adapted from ref. 85). During training before scanning, participants learned the locations of objects (denoted by circles) inside virtual reality museums. During scanning, participants performed a task that required them to imagine facing each object encountered during training. Multivoxel activity patterns in RSC were similar for facing directions across the two museums defined in a local, but not global, reference frame. E) In rodents, retrosplenial cortex (RSP) contains both “bidirectional” (BD) cells that represent heading in a local reference frame and head direction (HD) cells that represent heading in a global reference frame (adapted from ref. 95).
Environmental boundaries act as the primary cue for determining the orientation of the cognitive map under one circumstance: when animals have lost their bearings that is, when they have become confused about which direction they are facing. In such circumstances, rodents, birds, fish, mammals, and human infants rely heavily on the shape of the local environment to recover their sense of direction62. In geometrically symmetric environments such as rectangular chambers, they will make "geometric errors" whereby they search for goals in locations that are in directions 180 degrees offset from the correct locations, even in the presence of non-geometric cues that could potentially be used to resolve the geometric ambiguities63. Consistent with these behavioral results, the hippocampal place field map in mice64, and HD cells in rats65 are oriented primarily by chamber geometry after disorientation (Fig. 3B), and the resulting alignment predicts the navigational behavior of the animal64. Boundaries may be important for reorientation because they are typically fixed to the terrestrial surface (or even form a part of it), and thus they are inherently spatially stable53. Punctate objects, on the other hand, may change their location, although a navigator may come to learn that certain objects are stably related to certain positions or bearings66,67, and hippocampal and HD cells may become anchored to objects in reflection of this knowledge68. Moreover, punctate objects within the environment are only useful as orientational references if the location of the animal is known69, or if they have distinguishable facades, whereas environmental geometry can define an orientational axis based on its own intrinsic shape.
The displacement of the cognitive map is also strongly controlled by environmental boundaries. The locations of individual place cell firing fields within the oriented coordinate frame is primarily determined by distances to chamber walls70 and grid fields distort when these walls are displaced71. Border and boundary cells are likely crucial for mediating these effects. In humans, hippocampal activity during scene imagination relates to the number of boundaries in an environment72 and hippocampal activity during navigation predicts learning of object locations relative to boundaries73. Effects of boundary displacement can also be observed on spatial memory in humans navigating to hidden locations within a virtual room74 and rats navigating to a hidden platform in the Morris Water Maze75. In both cases, search locations translate with local environmental boundaries when these boundaries are displaced.
Perceiving and Using Landmarks
For landmarks to have an effect on the cognitive map, they must first be processed by perceptual systems. There are three regions of the human brain that have been implicated in this function based on their strong fMRI response during viewing of stimuli that might be broadly classified as landmarks76, 77: (i) the parahippocampal place area (PPA), located in the collateral sulcus near the posterior parahippocampal/anterior lingual boundary; (ii) the retrosplenial complex (RSC), located in the parietal-occipital sulcus (POS), posterior to and partially overlapping with BA29/30; (iii) the occipital place area (OPA), located in the dorsal occipital lobe near the transverse occipital sulcus. Although these regions were initially studied primarily in terms of their strong activation to visual scenes (e.g. landscapes, cityscapes, rooms), more recent work suggests that they might be involved in processing both scene-like and object-like landmarks51. When single objects are viewed in isolation, decontextualized from the surrounding scene, response in these regions is greater for objects that are physically larger, more distant, and more spatially stable compared to objects that are physically smaller, closer and spatially more movable (see ref. [78] for review). Response is also greater for objects that are associated with navigational decision points compared to objects that are associated with less navigationally relevant locations79. Thus, these “scene” regions respond not only to scenes, but also to objects that make potential landmarks, either in virtue of their physical properties (e.g. size, stability), or in virtue of their location in the world. Scene-responsive regions corresponding to the PPA, RSC, and OPA have also been observed in macaque monkeys77, 80, but the existence of similar regions in rodents is unclear.
Of the three landmark-sensitive regions, RSC appears to play a particularly important role in using environmental cues to anchor the cognitive map. fMRI response to scenes in RSC is significantly increased when subjects attempt to recover the location or implied heading of the scene within the broader spatial environment that is, when they use the scene to orient or localize themselves49, 76. Moreover, although PPA, RSC, and OPA all respond more strongly to stable vs. unstable objects78, retrosplenial cortex (BA 29/30) shows an additional response enhancement that is specific to the most permanent objects67, 81. Relatedly, although both PPA and RSC are active when participants make spatial judgments relative to fixed environmental elements82, only RSC has been shown to exhibit activity that scales with the size of viewpoint changes in the environmental frame83.
Insight into a possible RSC anchoring mechanism comes from several studies that have examined adaptation or multivoxel patterns in this region during spatial memory retrieval. Typically, participants in these studies are prompted by scene, object, or word cues to imagine themselves facing specific directions at specific locations within a familiar campus84 or a recently-learned virtual environment85–87. These studies have revealed evidence for coding of the recovered facing direction (and also location) in several parts of RSC, including POS84,85 and BA29/3086 (Fig. 3C). Notably, one MVPA study found that heading codes were anchored to local geometry in POS, as evidenced by generalization of equivalent local headings across different enclosed subspaces that had similar geometries (Fig. 3D)85. Such local heading codes might be crucial for aligning the cognitive map: if a navigator can determine its heading relative to local geometry, and knows the orientation of the local geometry relative to the rest of the world, then it can calculate its heading in the global environment. Complementing this local heading code in POS, a recent adaptation study found that heading in BA29/30 was represented in a more global manner that extended across multiple connected local environments86. Results from other studies indicate that RSC exhibits considerable flexibility of spatial scope, distinguishing between local environments in some experiments88 but generalizing across them in others84, 85. Such a flexible mechanism would allow RSC to mediate between the local egocentric scene and the broader allocentric map8, 89, 90.
Recording studies in rodents and monkeys support this view of RSC. Rodent retrosplenial cortex contains a variety of cells whose firing would facilitate the transformation between local and global reference frames. In the open field, these include HD cells91 and direction-dependent place cells92, and in constrained paths, these include cells that code combinations of turn direction, path position, and world position92. In monkey medial parietal cortex, neurons have been observed that represent turn directions at specific path positions during virtual navigation94. In a recent study on rodents, Jacob and colleagues examined directional responses in retrosplenial cells while animals explored an environment consisting of two connected rectangular subchambers that were polarized in 180 degree opposite directions by cue cards at the end of each subchamber (Fig. 3E)95. Intermixed with classical HD cells, which exhibited directional preferences that were consistent across the entire environment, they observed a new class of “bidirectional” cells that fired facing one direction in one subchamber, and the opposite direction in the other subchamber. This striking result suggests that these cells encode heading in a reference frame that is determined by the orientation of the local environment (in this case, the polarization of each subchamber), echoing human fMRI results85. Interactions between bidirectional cells and classical HD cells might be used for aligning the HD system to the local reference frame, or (conversely) for determining the stability of potential landmarks.
With regards to the perceptual processing of landmarks, an extensive literature has explored the PPA’s response to many kinds of information that can be used to determine the identity of scenes and landmarks, including local spatial layout, object category, textures, and ensemble statistics (see [51, 96] for review). These results may be reflective of a more general PPA function of representing co-located perceptual items97, 98 that can be used to identify the local place or context99. OPA has been somewhat less investigated, but recent work suggests that it is especially important for processing spatial aspects of scenes that are essential for navigation100, including environmental boundaries101 and local navigational affordances102. The division of labor among the three landmark-sensitive regions, whereby PPA and OPA are primarily involved in the perceptual analysis and visual recognition of landmarks, while RSC uses landmarks to anchor the cognitive map, is also supported by neuropsychological studies76,103, 104. A key question for future work will be understanding in detail the transformations by which perceptual information about landmarks are used to select, align, and position cognitive maps58, 105.
Using Cognitive Maps to Navigate
A second requirement for a cognitive map to be useful is that it must include a mechanism for planning a route to one’s destination. At a minimum, this involves calculating the distance and direction to the goal. Moreover, in many environments, routes cannot be direct because of obstacles in the terrain. The capacity to take efficient detours around these obstacles and to identify useful shortcuts is the crux of what a cognitive map provides1. Recent fMRI research has provided new insights into how the brain represents distance and direction to goal locations, supports route planning, and solves detour problems.
Coding the distance and direction to the goal
A number of recent models have explored how grid and place codes might be combined to support navigation106–108. According to these models, the entorhinal grid cell network computes a vector consisting of the Euclidean distance to the goal independent of any barriers and the direction relative to an environmental axis (e.g. 42 degrees north west). The hippocampus then operates in conjunction with the entorhinal cortex to derive the optimal path around obstacles, and the posterior parietal cortex calculates the direction to turn the body to orient along the path9. A number of rodent electrophysiology studies have provided evidence for a hippocampal role in route planning, by showing that CA1 activity traces out the future trajectory of paths109 and distance along the path to the goal110.
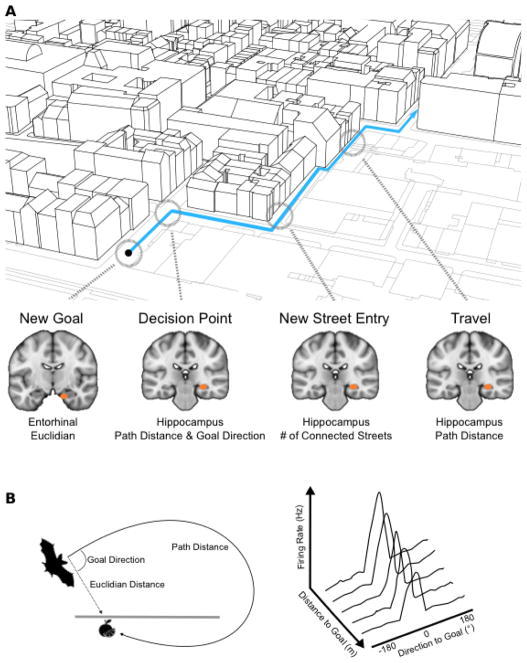
Mirroring this theoretical and recording work, several fMRI studies have reported hippocampal or entorhinal activity correlated with the distance to the goal during navigation50, 55, 111–113. In two studies where it was possible to distinguish path distance from Euclidean distance, activity the entorhinal region was more strongly related to Euclidean distance112, 114. For example, Howard et al (2014) had participants learn a region of London’s (UK) Soho street network and subsequently navigate a film simulation of the city streets during fMRI. Entorhinal activity tracked changes in Euclidean distance when new goals were presented, while posterior hippocampal activity tracked the path distance to the goal at various stages of the journey (Fig. 4A). Moreover, at decision points, activity in the posterior hippocampus was greater when the goal was close and directly ahead. Consistent with this last result, a recent study identified cells in the dorsal hippocampus of flying bats that code the distance and direction to specific goals, with more cells selective for close distances than far distances, and more cells selective for direct headings than for oblique headings10 (Fig. 4B).
Figure 4. Hippocampus codes metrics of the environment along a journey.
A) Map showing an example street journey in London’s Soho that was used in Howard et al. (2014) and Javadi et al. (2017)112, 116. At various points in the journey, entorhinal cortex codes the Euclidean distance to the goal, while the right posterior hippocampus codes path distance, an interaction between goal direction and path distance, as well as a more complex aspects of environment, such as how many other streets a given street is connected with (degree centrality). Right anterior hippocampus (not shown) activity increases when entering streets with high global connections (closeness centrality). B) Left: Path distance and goal direction coding has also been found in the hippocampus of bats while they freely fly towards a target location. Activity increases as the goal is closer and more directly ahead (adapted from ref. 10).
Knowing how far to travel is important for navigation, but arguably more critical is knowing the direction to the goal. While numerous recording studies have reported head direction cells that code allocentric facing direction8, there have been no reports of neurons that code allocentric goal direction. This is despite computational model predictions of such a code in the entorhinal circuit107, 108. To explore this issue, Chadwick et al., (2015) had fMRI participants judge the direction to goal locations in a virtual environment115. Consistent with other results84, activity patterns in the entorhinal region contained information about both allocentric facing direction and allocentric goal direction. Notably, activation patterns were similar for trial pairs in which the facing direction in one trial (e.g. North) matched the goal direction in the other (e.g. North). One possible explanation is that these activity patterns reflect the firing of HD cells, which may briefly switch from the current facing direction to the anticipated facing direction as subjects imagine travelling in the direction of the goal108, 115. In order to move in the direction of the goal an allocentric direction code needs to be converted and processed as an egocentric code, e.g. ‘45 degrees to the left’. Chadwick et al (2015) and several other studies112, 114, 115 have reported evidence for such a code in the posterior parietal cortex, consistent with computational models8. An important question for future research is how distance and direction are processed in highly familiar environments, where the hippocampus is not as needed for navigation44, 45, 48, 50.
Paths & Planning
In real-world situations, such as navigating a city, there may be more than one route to a destination. The more options to consider, the greater demands placed on the brain regions needed to retrieve the network of possible paths and select the optimal route. A recent study by Javadi et al. (2017)116 explored this issue by relating fMRI activity collected during virtual navigation112 to graph-theoretic measures of the topological connections of the streets. Upon entry to a street, activity in the posterior hippocampus increased if the street offers many more paths to choose from for future travel. By contrast, anterior hippocampal activity increased when entering a street with greater global connectivity to rest of the street network116. These results dovetail with recent evidence of topological coding of navigable spaces by place cells117, 118; for example, Wu and Foster’s observation that hippocampal “re-play” of place cells on a set of connected tracks preserved the topological structure of the tracks118. It is unclear at this point how this topological coding of space relates to a possible Euclidean spatial code.
While the hippocampus supports the retrieval of path options, evaluation of these paths appears to be the province of prefrontal cortex. Further analysis by Javadi et al. (2017) revealed that, when forced to re-plan a route, lateral prefrontal cortex activity scales with the demands of a breadth-first-search through the street network (Fig. 5). Other recent studies have demonstrated increased activity in rostrodorsal medial prefrontal cortex when participants are engaged in hierarchical spatial planning113, and increased coupling between a similar region and the hippocampus when sequential decisions must be made in order to plan the shortest path to a goal119 (Fig. 5). These results agree with an extensive literature on the involvement of prefrontal cortex in classical planning tasks that require inhibition of actions and resolution of goal-sub-goal conflicts17, 120. Recent research has also sought to link neural activity during navigation to parameters from reinforcement learning models121, 122, which may prove a useful way to dissect the neural systems that support route planning.
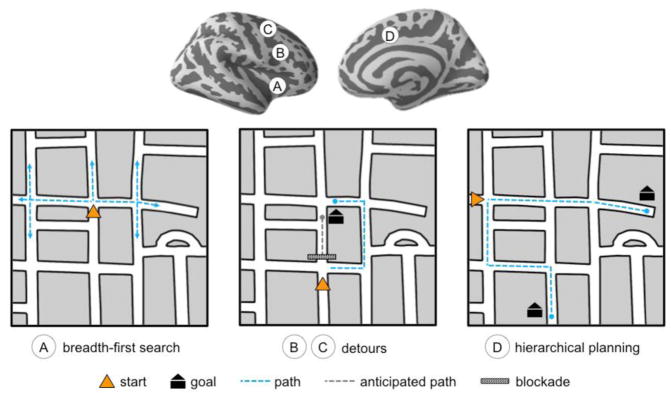
Figure 5. Frontal areas involved in planning during navigation.
A number of prefrontal areas have been identified that support navigation in humans. Inferior lateral prefrontal activity has been shown to correlate with the number of possible paths available at a choice point (A), while lateral PFC and superior frontal gyrus activations have been found when participants encounter a detour and need to find an alternative way (B&C). Hierarchical planning involves dorsal-medial frontal areas, independent of distance to the goal. In the example shown in D, the two routes the goals are equal in length, but one involves multiple turns and street segments, and intersections where decisions need to be made thus requiring a hierarchical route plan.
Maps and navigation beyond physical space
Humans live in complex worlds, and though locomotion is a large aspect of our lives, we spend a considerable amount of time navigating interpersonal relationships and abstract concepts. Some of the most exciting recent work in navigation has begun to explore how the mechanisms discussed above spatial coding, landmark anchoring, route planning might apply to non-physical “spaces”. This work has the potential to resolve longstanding controversies over the function of the hippocampus and other regions4,5. Although it has long been hypothesized that cognitive maps might be applied broadly to many cognitive domains1, 3, 123, recent work takes this idea beyond a general metaphor, by showing concretely how this application might work.
Social and conceptual spaces
Considerable evidence suggests that the hippocampus and entorhinal cortex represent nonspatial information. In rodents, cells have been identified that code for odors124, timepoints125 and sound frequencies126 when these are the central elements of a behavioral task. In humans, “concept cells” fire when participants think about famous people or buildings, independent of the particular stimulus used to evoke those thoughts34. Recent work has expanded on these findings by showing that these non-spatial codes can be organized into “maps” of social and conceptual spaces.
For example, Tavares and colleagues127 examined the coding of a social space defined by affiliation and hierarchy. Participants had to “navigate” the social space by interacting with 6 characters in a role-playing game. The social position of each character relative to the participant was tracked. fMRI response in the hippocampus scaled with the angle of the vector from the participant’s position to the character’s position in the social space, with greatest response to characters with higher power and high affiliation. fMRI response in the posterior cingulate, on the other hand, scaled with the magnitude of the vector, with greatest response to more socially distant characters. These results were interpreted as evidence that humans represent their social standing relative to others in map-like space that is coded in the hippocampus and posterior cingulate. An important question for future research is whether this social map is inherently centered on the participant (i.e. egocentric), or whether it might also represent social relationships between other people (i.e. allocentric).
Further evidence for coding of abstract spaces in this case, in entorhinal cortex comes from a recent study by Constantinescu and colleagues128. Using the same fMRI methods as Doeller et al. (2010; see section 1 on human grid cells), these authors tested for a grid-like coding of an abstract “space” consisting of morphed stimuli (birds with their neck or legs, or both, changing). They found that when participants viewed sequences of these morphed stimuli, response in entorhinal cortex was greater for sequences that were aligned vs. misaligned to the six-fold rotational symmetry of the putative grid representation. This effect was also found in the ventromedial prefrontal cortex, with performance on a task that indirectly tapped spatial knowledge being related to the amount of grid-like signal in this area. Other contemporary work suggests that the hippocampal-entorhinal system can encode “spaces” that are not inherently continuous, but defined based on transitions between discrete items129, 130.
Contexts and orientation in abstract spaces
How are abstract spaces anchored to the world? At present, it is not entirely clear how to apply ideas such as landmark, boundary, or local geometry to non-physical domains. To our knowledge, for example, there have been no reports of cells that fire to the “boundary” of a concept or a social milieu. Some progress has been made in the temporal domain131, where episodic memories have been shown to be affected by transitions between behavioral contexts delimited by temporal boundaries132, similar to the way that they are affected by transitions between spatial regions delimited by physical boundaries133. Although it may not turn out to be the case that all cognitive maps are supported by the same mechanistic rules, we believe that there are a few basic principles that might operate across domains.
Most notably, the distinction between context retrieval and orientation might be broadly applicable. In the spatial domain, context retrieval refers to recovery of a map that is appropriate for a specific environment, whereas orientation refers to determination of one's specific coordinates and heading direction on the map. In rodents, these two functions can be dissociated based on different behavioral responses to geometric vs. non-geometric cues during spatial reorientation134 and differential sensitivity of hippocampal place cells to metric vs. non-metric cues135. Although the precise manner in which these functions are applied to non-spatial domains has not been established, we speculate that in the social domain, context retrieval might involve bringing up the appropriate map of a social space (e.g. “the people I work with”) and orientation might involve aligning the current situation to salient dimensions such as affiliation and social hierarchy. Similarly, in the semantic domain, context retrieval might involve bringing up knowledge related to a given topic (e.g. “living creatures”) and orientation might involve alignment to salient prototypes and axes in the corresponding semantic similarity space.
We have previously speculated that context retrieval in humans relies primarily on inputs from the PPA to the hippocampus, whereas orientation relies primarily on computations performed in RSC49. Several researchers have explored the idea that the PPA and RSC might be sensitive to nonspatial cues that define a context136 and it is notable that RSC is commonly activated in semantic memory tasks137. In a recent review, Ranganath and Ritchey138 proposed that the PPA and RSC form part of a posterior-medial input system to the medial temporal lobe, which they characterize as supporting models of places, contexts, and situations, in contrast to the anterior-temporal system, which supports identification and evaluation of individual entities. Recent work suggests that the human hippocampus encodes non-spatial contexts139; for example, parallel storylines within a movie140. Understanding how the navigational system supports context retrieval and orientation in non-physical spaces seems likely to be a fruitful area for future research.
Navigating the past and the future
Finally, what is the equivalent of route planning in non-physical space? In abstract terms, route planning involves imagining a sequence of possible future states. Both humans and animals do this. For example, when a rat reaches an intersection in a maze, it pauses and looks left and right, as if considering which path to take. As it does so, place cells fire corresponding to positions along the possible paths, thus providing neural evidence that the animal is ‘thinking’ about locations that would be encountered if it travelled down each route141, 142. This principle that route planning involves considering the future using representations that were laid down in the past can be applied more broadly, to explain the involvement of the navigational system in other core cognitive functions such as episodic memory and prospective thinking.
Many authors have considered variants of this idea. Under one theory, the key cognitive process is scene construction: the ability to set up a spatial framework, populate it with meaningful content, and imagine what the resulting scene would look like from different points of view143. Other researchers have focused on the importance of being able to construct a sequence of related states that might form an episodic narrative4, 144, 145, which can then be used to evaluate the consequences of possible behaviors146. Route planning might also apply to the social and conceptual domains, as a mechanism for creating meaningful sequences of thought. Indeed, the idea that thinking is like navigation is an old one William James famously described the stream of thought as “like a bird’s life…made of an alternation of flights and perchings”.
We will not attempt to survey this literature here, which has been extensively discussed in earlier reviews4, 143, 147, 148. We simply note our belief that a deeper understanding these abilities will likely come from application of insights obtained from the spatial navigation literature, where the computational mechanisms can be defined precisely in terms of concrete quantities such as distance, angle, and path complexity.
Conclusion
It has now been 70 years since Tolman first proposed the idea of the cognitive map and 40 years since O’Keefe and Nadel outlined the data linking it to the hippocampus. For a long time, the evidence for cognitive maps, both behavioral and neurological, was primarily derived from rodents. In this review, we have outlined recent work suggesting that the concept might be equally well applied to humans. We have focused in particular on the important question of how cognitive maps are used during spatial navigation for example, how they are anchored to the environment and deployed to plan a route and we have described new data that suggests that cognitive maps might apply to both physical and non-physical spaces. We expect that future studies, perhaps using new methods, will allow researchers to draw even tighter connections between navigational behavior, neural responses, and cognitive processes, thus fulfilling Tolman’s vision of a map in the brain.
Acknowledgments
We thank Kate Jeffery for comments on the manuscript. We apologize to the many authors whose work was not cited because of length restrictions on the reference list. This work was supported by the U.S. National Institutes of Health (EY022350 and EY027047 to RAE), National Science Foundation (GRFP to JBJ), JSMF (to HJS) and Wellcome Trust (094850/Z/10/Z to HJS).
Bibliography
- 1.Tolman EC. Cognitive maps in rats and men. American Psychological Association; 1948. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 4.Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NN this issue
- 6.Grieves RM, Jeffery KJ. The representation of space in the brain. Behavioural Processes. 2017;135:113–131. doi: 10.1016/j.beproc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 7.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the'cognitive map'. Nature Reviews Neuroscience. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 8.Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 9.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychological review. 2007;114:340. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarel A, Finkelstein A, Las L, Ulanovsky N. Vectorial representation of spatial goals in the hippocampus of bats. Science. 2017;355:176–180. doi: 10.1126/science.aak9589. [DOI] [PubMed] [Google Scholar]
- 11.Clark RE, Squire LR. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proceedings of the National Academy of Sciences. 2013;110:10365–10370. doi: 10.1073/pnas.1301225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cerebral cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 13.Ghaem O, et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8:739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- 14.Maguire EA, et al. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 15.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 16.Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Frontiers in human neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 19.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. Journal of Neuroscience. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchette SA, Bakker A, Shelton AL. Cognitive mappers to creatures of habit: differential engagement of place and response learning mechanisms predicts human navigational behavior. Journal of Neuroscience. 2011;31:15264–15268. doi: 10.1523/JNEUROSCI.3634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. Journal of Neuroscience. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woollett K, Maguire EA. Acquiring "the Knowledge" of London's layout drives structural brain changes. Current Biology. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23:515–528. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartley T, Harlow R. An association between human hippocampal volume and topographical memory in healthy young adults. Frontiers in human neuroscience. 2012;6:338. doi: 10.3389/fnhum.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan LK, MacEvoy SP, Aguirre GK, Epstein RA. Distances between real-world locations are represented in the human hippocampus. Journal of Neuroscience. 2011;31:1238–1245. doi: 10.1523/JNEUROSCI.4667-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassabis D, et al. Decoding neuronal ensembles in the human hippocampus. Current Biology. 2009;19:546–554. doi: 10.1016/j.cub.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deuker L, Bellmund JL, Schröder TN, Doeller CF. An event map of memory space in the hippocampus. eLife. 2016;5:e16534. doi: 10.7554/eLife.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielson DM, Smith TA, Sreekumar V, Dennis S, Sederberg PB. Human hippocampus represents space and time during retrieval of real-world memories. Proceedings of the National Academy of Sciences. 2015;112:11078–11083. doi: 10.1073/pnas.1507104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horner AJ, Bisby JA, Zotow E, Bush D, Burgess N. Grid-like processing of imagined navigation. Current Biology. 2016;26:842–847. doi: 10.1016/j.cub.2016.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs J, et al. Direct recordings of grid-like neuronal activity in human spatial navigation. Nature neuroscience. 2013;16:1188–1190. doi: 10.1038/nn.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JF, Fried I, Suthana N, Jacobs J. Repeating spatial activations in human entorhinal cortex. Current Biology. 2015;25:1080–1085. doi: 10.1016/j.cub.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neuroscience & Biobehavioral Reviews. 2004;28:201–218. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends in neurosciences. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- 38.Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steemers B, et al. Hippocampal Attractor Dynamics Predict Memory-Based Decision Making. Current Biology. 2016;26:1750–1757. doi: 10.1016/j.cub.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 40.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyle CT, Stokes JD, Lieberman JS, Hassan AS, Ekstrom AD. Successful retrieval of competing spatial environments in humans involves hippocampal pattern separation mechanisms. eLife. 2015;4:e10499. doi: 10.7554/eLife.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanales AJ, Oza A, Favila SE, Kuhl BA. Overlap among Spatial Memories Triggers Repulsion of Hippocampal Representations. Current Biology. 2017 doi: 10.1016/j.cub.2017.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenzie S, et al. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- 45.Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- 46.Kolarik BS, et al. Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia. 2016;80:90–101. doi: 10.1016/j.neuropsychologia.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolbers T, Büchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. Journal of Neuroscience. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. "I have often walked down this street before": fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- 49.Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. Journal of Neuroscience. 2007;27:6141–6149. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patai EZ, et al. Long-term consolidation switches goal proximity coding from hippocampus to retrosplenial cortex. bioRxiv. 2017 [Google Scholar]
- 51.Epstein RA, Vass LK. Neural systems for landmark-based wayfinding in humans. Phil Trans R Soc B. 2014;369:20120533. doi: 10.1098/rstb.2012.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- 53.Gallistel CR. The organization of learning. The MIT Press; 1990. [Google Scholar]
- 54.Wolbers T, Wiener JM, Mallot HA, Büchel C. Differential recruitment of the hippocampus, medial prefrontal cortex, and the human motion complex during path integration in humans. Journal of Neuroscience. 2007;27:9408–9416. doi: 10.1523/JNEUROSCI.2146-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherrill KR, et al. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. Journal of Neuroscience. 2013;33:19304–19313. doi: 10.1523/JNEUROSCI.1825-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sholl MJ. Cognitive maps as orienting schemata. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:615. doi: 10.1037//0278-7393.13.4.615. [DOI] [PubMed] [Google Scholar]
- 57.Knierim JJ, Hamilton DA. Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiological reviews. 2011;91:1245–1279. doi: 10.1152/physrev.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoder RM, Clark BJ, Taube JS. Origins of landmark encoding in the brain. Trends in neurosciences. 2011;34:561–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shelton AL, McNamara TP. Systems of spatial reference in human memory. Cognitive psychology. 2001;43:274–310. doi: 10.1006/cogp.2001.0758. [DOI] [PubMed] [Google Scholar]
- 60.Krupic J, Bauza M, Burton S, Barry C, O'Keefe J. Grid cell symmetry is shaped by environmental geometry. Nature. 2015;518:232–235. doi: 10.1038/nature14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stensola T, Stensola H, Moser MB, Moser EI. Shearing-induced asymmetry in entorhinal grid cells. Nature. 2015;518:207–212. doi: 10.1038/nature14151. [DOI] [PubMed] [Google Scholar]
- 62.Cheng K, Huttenlocher J, Newcombe NS. 25 years of research on the use of geometry in spatial reorientation: a current theoretical perspective. Psychonomic Bulletin & Review. 2013;20:1033–1054. doi: 10.3758/s13423-013-0416-1. [DOI] [PubMed] [Google Scholar]
- 63.Cheng K. A purely geometric module in the rat's spatial representation. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 64.Keinath AT, Julian JB, Epstein RA, Muzzio IA. Environmental Geometry Aligns the Hippocampal Map during Spatial Reorientation. Current Biology. 2017 doi: 10.1016/j.cub.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knight R, Hayman R, Ginzberg LL, Jeffery K. Geometric cues influence head direction cells only weakly in nondisoriented rats. Journal of Neuroscience. 2011;31:15681–15692. doi: 10.1523/JNEUROSCI.2257-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biegler R, Morris R. Landmark stability is a prerequisite for spatial but not discrimination learning. Nature. 1993;361:631. doi: 10.1038/361631a0. [DOI] [PubMed] [Google Scholar]
- 67.Auger SD, Zeidman P, Maguire EA. A central role for the retrosplenial cortex in de novo environmental learning. eLife. 2015;4:e09031. doi: 10.7554/eLife.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. Journal of Neuroscience. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bicanski A, Burgess N. Environmental anchoring of head direction in a computational model of retrosplenial cortex. Journal of Neuroscience. 2016;36:11601–11618. doi: 10.1523/JNEUROSCI.0516-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 71.Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nature neuroscience. 2007;10:682–684. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- 72.Bird CM, Capponi C, King JA, Doeller CF, Burgess N. Establishing the boundaries: the hippocampal contribution to imagining scenes. Journal of Neuroscience. 2010;30:11688–11695. doi: 10.1523/JNEUROSCI.0723-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartley T, Trinkler I, Burgess N. Geometric determinants of human spatial memory. Cognition. 2004;94:39–75. doi: 10.1016/j.cognition.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:100. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- 76.Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in cognitive sciences. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nasr S, et al. Scene-selective cortical regions in human and nonhuman primates. Journal of Neuroscience. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Troiani V, Stigliani A, Smith ME, Epstein RA. Multiple object properties drive scene-selective regions. Cerebral cortex. 2012:bhs364. doi: 10.1093/cercor/bhs364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janzen G, Van Turennout M. Selective neural representation of objects relevant for navigation. Nature neuroscience. 2004;7:673–677. doi: 10.1038/nn1257. [DOI] [PubMed] [Google Scholar]
- 80.Kornblith S, Cheng X, Ohayon S, Tsao DY. A network for scene processing in the macaque temporal lobe. Neuron. 2013;79:766–781. doi: 10.1016/j.neuron.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Auger SD, Mullally SL, Maguire EA. Retrosplenial cortex codes for permanent landmarks. PloS one. 2012;7:e43620. doi: 10.1371/journal.pone.0043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Committeri G, et al. Reference frames for spatial cognition: different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. Journal of Cognitive Neuroscience. 2004;16:1517–1535. doi: 10.1162/0898929042568550. [DOI] [PubMed] [Google Scholar]
- 83.Sulpizio V, Committeri G, Lambrey S, Berthoz A, Galati G. Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behavioural brain research. 2013;242:62–75. doi: 10.1016/j.bbr.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 84.Vass LK, Epstein RA. Common neural representations for visually guided reorientation and spatial imagery. Cerebral cortex. 2017;27:1457–1471. doi: 10.1093/cercor/bhv343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marchette SA, Vass LK, Ryan J, Epstein RA. Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nature neuroscience. 2014;17:1598–1606. doi: 10.1038/nn.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shine JP, Valdés-Herrera JP, Hegarty M, Wolbers T. The human retrosplenial cortex and thalamus code head direction in a global reference frame. Journal of Neuroscience. 2016;36:6371–6381. doi: 10.1523/JNEUROSCI.1268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumann O, Mattingley JB. Medial parietal cortex encodes perceived heading direction in humans. Journal of Neuroscience. 2010;30:12897–12901. doi: 10.1523/JNEUROSCI.3077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vass LK, Epstein RA. Abstract representations of location and facing direction in the human brain. Journal of Neuroscience. 2013;33:6133–6142. doi: 10.1523/JNEUROSCI.3873-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meilinger T. The network of reference frames theory: A synthesis of graphs and cognitive maps. Spatial cognition VI. Learning, reasoning, and talking about space. 2008:344–360. [Google Scholar]
- 90.Marchette SA, Ryan J, Epstein RA. Schematic representations of local environmental space guide goal-directed navigation. Cognition. 2017;158:68–80. doi: 10.1016/j.cognition.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. Experimental Brain Research. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 92.Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral neuroscience. 2001;115:3. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- 93.Alexander AS, Nitz DA. Retrosplenial cortex maps the conjunction of internal and external spaces. Nature neuroscience. 2015;18:1143–1151. doi: 10.1038/nn.4058. [DOI] [PubMed] [Google Scholar]
- 94.Sato N, Sakata H, Tanaka YL, Taira M. Navigation-associated medial parietal neurons in monkeys. Proceedings of the National Academy of Sciences. 2006;103:17001–17006. doi: 10.1073/pnas.0604277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacob P-Y, et al. An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nature neuroscience. 2016 doi: 10.1038/nn.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Epstein RA. Neural systems for visual scene recognition. In: Bar M, Kveraga K, editors. Scene vision. 2014. pp. 105–134. [Google Scholar]
- 97.Marchette SA, Vass LK, Ryan J, Epstein RA. Outside looking in: Landmark generalization in the human navigational system. Journal of Neuroscience. 2015;35:14896–14908. doi: 10.1523/JNEUROSCI.2270-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. Journal of Neuroscience. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends in cognitive sciences. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamps FS, Lall V, Dilks DD. The occipital place area represents first-person perspective motion information through scenes. Cortex. 2016;83:17–26. doi: 10.1016/j.cortex.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Julian JB, Ryan J, Hamilton RH, Epstein RA. The occipital place area is causally involved in representing environmental boundaries during navigation. Current Biology. 2016;26:1104–1109. doi: 10.1016/j.cub.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonner MF, Epstein RA. Coding of navigational affordances in the human visual system. Proceedings of the National Academy of Sciences. 2017;114:4793–4798. doi: 10.1073/pnas.1618228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aguirre GK, D'Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 104.Maguire E. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scandinavian journal of psychology. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 105.Wilber AA, Clark BJ, Forster TC, Tatsuno M, McNaughton BL. Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. Journal of Neuroscience. 2014;34:5431–5446. doi: 10.1523/JNEUROSCI.0511-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kubie JL, Fenton AA. Linear look-ahead in conjunctive cells: an entorhinal mechanism for vector-based navigation. Frontiers in neural circuits. 2012;6:20. doi: 10.3389/fncir.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bush D, Barry C, Manson D, Burgess N. Using grid cells for navigation. Neuron. 2015;87:507–520. doi: 10.1016/j.neuron.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Erdem UM, Milford MJ, Hasselmo ME. A hierarchical model of goal directed navigation selects trajectories in a visual environment. Neurobiology of learning and memory. 2015;117:109–121. doi: 10.1016/j.nlm.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wikenheiser AM, Redish AD. Hippocampal theta sequences reflect current goals. Nature neuroscience. 2015;18:289–294. doi: 10.1038/nn.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Viard A, Doeller CF, Hartley T, Bird CM, Burgess N. Anterior hippocampus and goal-directed spatial decision making. Journal of Neuroscience. 2011;31:4613–4621. doi: 10.1523/JNEUROSCI.4640-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howard LR, et al. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Current Biology. 2014;24:1331–1340. doi: 10.1016/j.cub.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balaguer J, Spiers H, Hassabis D, Summerfield C. Neural mechanisms of hierarchical planning in a virtual subway network. Neuron. 2016;90:893–903. doi: 10.1016/j.neuron.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spiers HJ, Maguire EA. A navigational guidance system in the human brain. Hippocampus. 2007;17:618–626. doi: 10.1002/hipo.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chadwick MJ, Jolly AE, Amos DP, Hassabis D, Spiers HJ. A goal direction signal in the human entorhinal/subicular region. Current Biology. 2015;25:87–92. doi: 10.1016/j.cub.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Javadi AH, et al. Hippocampal and prefrontal processing of network topology to simulate the future. Nature Communications. 2017;8:14652. doi: 10.1038/ncomms14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dabaghian Y, Brandt VL, Frank LM. Reconceiving the hippocampal map as a topological template. eLife. 2014;3:e03476. doi: 10.7554/eLife.03476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X, Foster DJ. Hippocampal replay captures the unique topological structure of a novel environment. Journal of Neuroscience. 2014;34:6459–6469. doi: 10.1523/JNEUROSCI.3414-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaplan R, et al. The Neural Representation of Prospective Choice during Spatial Planning and Decisions. PLOS Biology. 2017;15:e1002588. doi: 10.1371/journal.pbio.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 121.Simon DA, Daw ND. Neural correlates of forward planning in a spatial decision task in humans. Journal of Neuroscience. 2011;31:5526–5539. doi: 10.1523/JNEUROSCI.4647-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribas-Fernandes JJ, et al. A neural signature of hierarchical reinforcement learning. Neuron. 2011;71:370–379. doi: 10.1016/j.neuron.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schiller D, et al. Memory and space: towards an understanding of the cognitive map. Journal of Neuroscience. 2015;35:13904–13911. doi: 10.1523/JNEUROSCI.2618-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 125.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aronov D, Nevers R, Tank DW. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature. 2017;543:719–722. doi: 10.1038/nature21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tavares RM, et al. A map for social navigation in the human brain. Neuron. 2015;87:231–243. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Constantinescu AO, O'Reilly JX, Behrens TE. Organizing conceptual knowledge in humans with a gridlike code. Science. 2016;352:1464–1468. doi: 10.1126/science.aaf0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schapiro AC, Turk-Browne NB, Norman KA, Botvinick MM. Statistical learning of temporal community structure in the hippocampus. Hippocampus. 2016;26:3–8. doi: 10.1002/hipo.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garvert MM, Dolan RJ, Behrens TE. A map of abstract relational knowledge in the human hippocampal-entorhinal cortex. eLife. 2017;6:e17086. doi: 10.7554/eLife.17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ekstrom AD, Ranganath C. Space, Time and Episodic Memory: the Hippocampus is all over the Cognitive Map. Hippocampus. 2017 doi: 10.1002/hipo.22750. [DOI] [PubMed] [Google Scholar]
- 132.Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horner AJ, Bisby JA, Wang A, Bogus K, Burgess N. The role of spatial boundaries in shaping long-term event representations. Cognition. 2016;154:151–164. doi: 10.1016/j.cognition.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Julian JB, Keinath AT, Muzzio IA, Epstein RA. Place recognition and heading retrieval are mediated by dissociable cognitive systems in mice. Proceedings of the National Academy of Sciences. 2015;112:6503–6508. doi: 10.1073/pnas.1424194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. Journal of Neuroscience. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 137.Fairhall SL, Caramazza A. Brain regions that represent amodal conceptual knowledge. Journal of Neuroscience. 2013;33:10552–10558. doi: 10.1523/JNEUROSCI.0051-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 139.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. Journal of Neuroscience. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Milivojevic B, Varadinov M, Grabovetsky AV, Collin SH, Doeller CF. Coding of Event Nodes and Narrative Context in the Hippocampus. Journal of Neuroscience. 2016;36:12412–12424. doi: 10.1523/JNEUROSCI.2889-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. Journal of Neuroscience. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brown TI, et al. Prospective representation of navigational goals in the human hippocampus. Science. 2016;352:1323–1326. doi: 10.1126/science.aaf0784. [DOI] [PubMed] [Google Scholar]
- 143.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in cognitive sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Redish AD. Beyond the cognitive map: from place cells to episodic memory. MIT Press; 1999. [Google Scholar]
- 145.Hasselmo ME. How we remember: brain mechanisms of episodic memory. MIT press; 2011. [Google Scholar]
- 146.Miller KJ, Botvinick MM, Brody CD. Dorsal hippocampus contributes to model-based planning. Nat Neurosci. 2017 doi: 10.1038/nn.4613. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buckner RL. The role of the hippocampus in prediction and imagination. Annual review of psychology. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 148.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage. 2012;60:2357–2364. doi: 10.1016/j.neuroimage.2012.02.055. [DOI] [PubMed] [Google Scholar]