Abstract
Background
Choline, an essential nutrient, serves as a methyl-group donor for DNA methylation and is a constituent of the neurotransmitter acetylcholine and a precursor to major components of cell membranes. Findings from animal studies suggest that choline supplementation during pregnancy may mitigate adverse effects of prenatal alcohol exposure on growth and neurocognitive function. We conducted a randomized, double-blind exploratory trial to examine feasibility and acceptability of a choline supplementation intervention during pregnancy.
Methods
70 heavy drinkers, recruited in mid-pregnancy, were randomly assigned to receive a daily oral dose of 2g of choline or a placebo from time of enrollment until delivery. Each dose consisted of an individually wrapped packet of powder that, when mixed with water, produced a sweet tasting grape-flavored drink. Adherence was assessed by collecting used and unused drink packets on a monthly basis and tabulating the number used. Side effects were assessed in monthly interviews. Blood samples obtained at enrollment and at 4 and 12 weeks after randomization were assayed for plasma choline concentration.
Results
Adherence was good-to-excellent (median doses taken=74.0%; interquartile range=53.9–88.7%) and was not related to a range of sociodemographic characteristics or to alcohol consumption ascertained using a timeline follow-back interview. By 4 weeks, plasma choline concentrations were significantly higher in the choline supplementation than the placebo arm, and this group difference continued to be evident at 12 weeks. The only side effect was a small increase in nausea/dyspepsia. No effects were seen for diarrhea, vomiting, muscle stiffness, blood pressure, or body odor changes.
Conclusions
This study demonstrated that a choline supplementation program with very heavy drinkers during pregnancy is feasible even among highly disadvantaged, poorly educated women. The broad acceptability of this intervention is indicated by our finding that adherence was not related to maternal education, intellectual function, depression, nutritional status, or alcohol use.
Keywords: fetal alcohol spectrum disorders, prenatal alcohol exposure, fetal alcohol syndrome, maternal choline supplementation, feasibility, adherence
Introduction
Descriptive studies spanning four decades have documented poor fetal growth, distinctive craniofacial dysmorphology, and a broad range of cognitive and behavioral deficits in infants and children with prenatal alcohol exposure (PAE; e.g., Mattson et al., 2011; Streissguth et al., 1994; Coles et al., 1997; Day et al., 2002; Kable and Coles, 2004; Jacobson et al., 1994, 2004; Jacobson et al., 2011; Carter et al., 2016). Although the adverse effects associated with alcohol exposure are well known and numerous psychosocial interventions have been attempted, alcohol consumption during pregnancy continues to pose a major health risk. There is, therefore, a growing interest in new approaches, such as pharmacological and nutritional interventions, that may be more effective. Among these, maternal choline supplementation during pregnancy seems particularly promising.
Choline is an essential nutrient that is a constituent of the neurotransmitter acetylcholine and a precursor to phosphatidylcholine and sphingomyelin, which are major components of cell membranes and play an important role in cell membrane integrity, trans-membrane signaling, and triglyceride turnover from the liver and blood (Zeisel and Niculescu, 2006). In addition, it serves as a methyl-group donor needed for homocysteine metabolism and DNA methylation, a critical mechanism in epigenetic processes that have been implicated in alcohol teratogenesis.
Choline is derived from dietary intake, principally eggs, liver, wheat germ, and milk. In addition, it can be derived from endogenous synthesis, catalyzed by the enzyme phosphatidylethanolamine-N-methyltransferase (PEMT; Resseguie et al., 2007). PEMT is induced by estrogen and is thus a significant source of choline only in premenopausal women. Estrogen induction of PEMT helps women meet some of the increased choline demands during pregnancy when transport of choline to the fetus depletes maternal stores (McMahon and Farrell, 1985, Zeisel et al., 1995). Presence of a common SNP variant (rs12325817) in the promoter region of the PEMT gene confers markedly greater risk of choline deficiency (Kohlmeier et al., 2005, da Costa et al., 2006). In one study of premenopausal women with a low choline diet, 80% of women with two variant alleles and 43% of those with one allele developed choline-deficiency-induced organ dysfunction, compared with 13% of women without the SNP variant (Fischer et al., 2010). Thus, in women of child-bearing age, this variant prevents estrogen induction of the PEMT gene, reducing the ability to synthesize choline needed to sustain normal fetal development.
Findings from animal studies suggest that supplementation with choline during pregnancy may mitigate the effects of PAE on growth and development. Choline supplementation in rats during the equivalent of the 3rd trimester in humans reduces the impact of fetal alcohol exposure on brain and body weight at birth (Thomas et al., 2009) and a range of cognitive and behavioral outcomes, including eyeblink conditioning (Thomas and Tran, 2012), hyperactivity (Thomas et al., 2004a, 2007; Idrus et al., 2017), spatial learning (Thomas et al., 2007), working memory (Thomas et al., 2000), reversal learning (Thomas et al., 2004a), and trace fear conditioning (Wagner and Hunt, 2006), although not motor balance and coordination (Thomas et al., 2004b; but cf. mouse study by Bearer et al. 2015). These changes persisted even after choline treatment was completed, indicating long-lasting effects on central nervous system organization and development. Although choline was administered in most of these studies during the equivalent of the 3rd trimester of pregnancy, treatment with this micronutrient is even more effective when administered earlier in pregnancy (Meck et al., 1989; Ryan et al., 2008; Thomas et al., 2009).
We conducted a randomized, double-blind, placebo-controlled exploratory trial to assess the feasibility, acceptability, and efficacy of maternal choline supplementation in a sample of socioeconomically disadvantaged heavy drinking pregnant women in Cape Town, South Africa. This intervention was based on the premise that infants would show improvement if supplementation were initiated early and at high doses during pregnancy. In this paper, we present findings relating to the feasibility and acceptability of the trial; findings relating to efficacy are presented in a second paper. The analyses presented in this paper were performed (1) to determine the degree to which women in this community would adhere to the supplementation protocol; (2) to examine the degree to which adherence would be related to level of maternal alcohol consumption, socioeconomic status (SES), intellectual function, and other socio-demographic characteristics; (3) to determine whether choline supplementation at this dose and frequency would lead to an increase in maternal plasma choline levels; (4) to identify any adverse side effects that may be associated with choline supplementation; and (5) to determine the prevalence in this community of two factors believed to enhance the efficacy of a choline supplementation intervention—inadequate choline intake in the diet and the presence of the PEMT SNP rs12325817.
Methods
Trial Design
Heavy drinking pregnant women initiating antenatal care by the 23rd week of gestation were randomly assigned to either choline supplementation or placebo using a randomization list (variable blocks of 2 and 4 subjects with 1:1 allocation ratio) by a biostatistician not otherwise involved in the trial, using a computer-generated schema (see Fig. 1). All participants, investigators, and research staff remained blind to the treatment group assignment throughout the trial. The randomization list was accessible only by R. Anzaldi, RPh, a senior research pharmacist at Boston Children’s Hospital who coordinated the randomization and labeling of the treatment course regimens, and W. Smythe, PhD, BPharm, Division of Clinical Pharmacology, University of Cape Town (UCT).
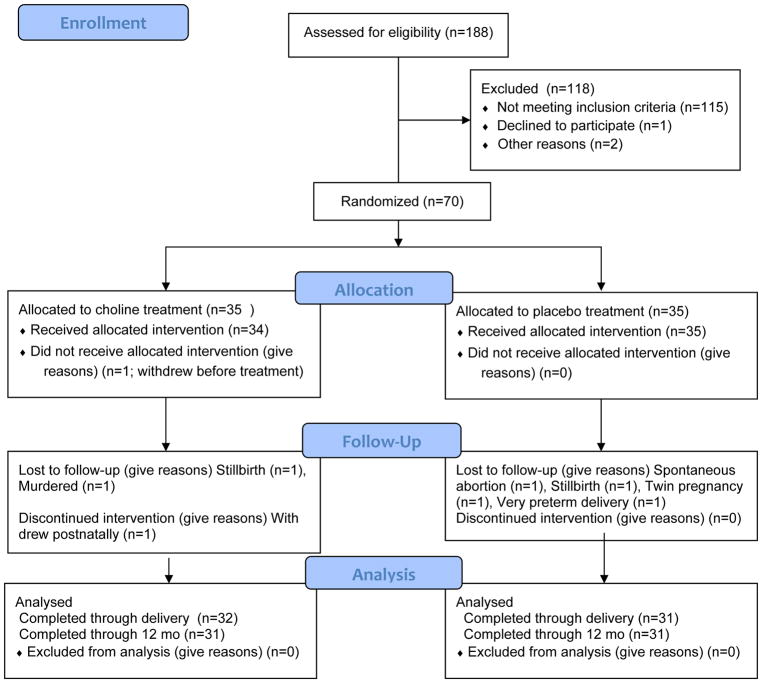
Figure 1.
Flow diagram of the progression of participants through the trial
Participants
Heavy drinking pregnant women were recruited from the Cape Coloured (mixed ancestry) community in Cape Town, South Africa. This population, descendants of white European, Malaysian, Khoi-San, and black African ancestors, historically comprised the large majority of workers in the wine-producing region of the Western Cape, South Africa. The high prevalence of heavy drinking during pregnancy and FAS in this community (Croxford and Viljoen, 1999; May et al., 2013) is a consequence of poor psychosocial circumstances and the traditional dop system, in which farm laborers were paid, in part, with wine. Although the dop system has been outlawed and despite widely-disseminated public health advisories at antenatal clinics and psychosocial community-based interventions, heavy alcohol consumption and weekend binge drinking persist in urban and rural Cape Coloured communities (May et al., 2013).
Human subjects approval was obtained from the Wayne State University, UCT Faculty of Health Sciences, and Columbia University Medical Center Institutional Review Boards and the South African Medicines Control Council. Informed consent was obtained from each mother and the father, if available. An independent data safety monitoring board, comprised of a developmental psychologist, obstetrician, neonatologist, and statistician, met with SWJ, CDM, RCC, and JLJ via telephone prior to initiation of the trial to review the research plan and every 9 months thereafter to review the progress of the trial and any study-related adverse events.
Alcohol Ascertainment and Study Recruitment
Participants were screened initially by one of our research nurses at two neighborhood midwife obstetrics units (MOUs) that serve economically disadvantaged, primarily Cape Coloured neighborhoods. Our staff driver subsequently transported each prospective participant from her home to our UCT research laboratory, where she was interviewed regarding her alcohol consumption using a timeline follow-back (TLFB) interview (Jacobson et al., 2002). We adapted this interview to include information about the type of beverage consumed, container size (including pictures of different containers, bottles, cans, glass size) and sharing (size of container divided by number of women drinking together) to reflect how pregnant women in this community frequently drink (Jacobson et al., 2008, 2017). The estimates of alcohol consumption during pregnancy derived from this interview have been validated in relation to fatty acid ethyl esters, biologically stable metabolites of alcohol that are deposited in meconium (Bearer et al., 2003), and infant (Jacobson et al., 2002) and child outcomes (e.g., Lewis et al., 2015; Lindinger et al., 2016; Carter et al., 2016; Meintjes et al., 2014). All interviews were conducted by CDM in Afrikaans or English, depending on the mother’s preference.
In the initial TLFB interview, each woman was asked about her drinking on a day-by-day basis during a typical 2-week period at time of conception. She was then asked whether her drinking had changed since conception; if so, when the change occurred and how much she drank on a day-by-day basis during the past 2 weeks. Volume was recorded for each type of beverage consumed each day and converted to oz absolute alcohol (AA; 1 oz AA ≈ 2 standard drinks) using the following weights that reflect potency of AA in Cape Town: liquor—0.4, beer—0.05, wine—0.12, cider—0.06. Heavy drinking was defined as an average of at least 1.0 oz AA/day or at least one incident of binge drinking (4 or more standard drinks/occasion). Heavy drinkers were invited to participate in the trial and randomly assigned to either the choline supplementation (n = 35) or placebo arm (n = 35). At time of recruitment and at each antenatal visit to our laboratory, CDM advised the mother that stopping or reducing her drinking would be beneficial for her and her baby and offered referral for treatment.
Maternal exclusionary criteria were age<18 years; HIV infection; multiple gestation pregnancy; pharmacologic treatment for a serious pre-existing medical condition (e.g., diabetes, hypertension, epilepsy, or cardiac problems); use of methamphetamine, a popular drug at the time of recruitment; or plans to move from the area before study completion. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, seizures, very low birth weight (<1500 g), and gestational age (GA) <32 weeks.
Treatment Regimen
Participants received a daily oral dose of either 2g of choline or a placebo from time of enrollment until delivery. The choline dose was chosen in consultation with SZ to maximize potential benefit, based on findings from pregnancy studies (e.g., Cheatham et al., 2012; Gossell-Williams et al., 2005), while being well within the parameters for safety determined by the Institute of Medicine (IOM) Food and Nutrition Board (FNB; IOM, 2006). The FNB has identified the lowest observed-adverse effect level (LOAEL) for choline as 7.5g/day based on data reporting “slight hypotension” at that dose (Boyd et al., 1977). To derive the tolerable upper intake level of choline (UL), the FNB applied an uncertainty factor of 2 to the LOAEL to obtain a UL of 3.75g/day, which was rounded down to 3.5g/day. With respect to side effects, subjects treated with very high doses of choline (10–16g/day) exhibited “fishy body odor, vomiting, salivation, sweating and gastrointestinal effects” (LSRO/FASED, 1981). Mild hypotension, the only side effect seen in patients receiving 7.5g/day, was not seen at 4g/day (Boyd et al., 1977).
To assess dietary choline intake, we developed a semi-quantitative, choline-indicated food frequency questionnaire (QFFQ), which focuses on choline-rich foods in the South African diet (Carter et al., under review), and administered it to a pilot sample of 24 women of child-bearing age from the Cape Coloured community. For a large majority (83.3%), average choline intake was below the 0.73–1.04g/day average range reported in the U.S., and none consumed >1.5g/day. To determine the choline dose for the trial, we subtracted the highest level of daily dietary choline intake seen in this pilot sample (1040mg/day) from the UL (3.5 minus 1.04g) and rounded this dose (2.46g) down to 2g to ensure safety. To ensure that with supplementation and regular dietary intake no participant would exceed the UL of 3.5g/day, we administered the QFFQ to each participant prior to initiation of and twice during the trial. Each woman was instructed to take 2 daily doses (1 in the morning, 1 in the evening) from time of enrollment until delivery. Each choline supplement dose consisted of 1.25g choline bitartrate, which contained 1g of bioavailable choline cation (Balchem, New Hampton, NY). Each dose consisted of an individually wrapped packet of powder that, when mixed with 8 oz of water, resulted in a sweet tasting, slightly fizzy, grape-flavored drink. The choline supplement and placebo were indistinguishable in terms of taste, smell, and appearance. When the participant was given the packets, CDM cautioned her that taking the drink mix would not make it safe to drink alcohol during pregnancy.
Stability
Choline content in the packets was analyzed on an YSI Biochemistry analyzer (https://www.ysi.com/ysi-2950-biochemistry-analyzer), using choline oxidase as the immobilized enzyme (Fig. 2). On average, choline content was within 2.3% of target (Median=1.1%; range=0.0–6.0%) and remained stable over the course of the study duration. Percent deviation from target dose was unrelated to time since supplement manufacture, r=−0.11.
Figure 2.
Stability of choline cation in choline-fortified grape beverage sachet/packet
Randomization and Treatment Phase Monitoring
At the initiation of the trial, CDM assigned the mother an ID number from the randomization list that determined whether she was in the treatment or placebo arm. She was given a box with a 1-month supply of drink packets and asked to return each of the used empty packets to the box. Our research nurse instructed her on how to mix the powder with water and visited her at home within 1–2 days to check how she was doing with taking the supplement and again 1 week later to interview her about possible side effects. At the end of each month, the nurse collected the box containing all the used and unused packets, replacing it with a new 1-month supply. Of the 329 boxes that were distributed, 8 (2.4%) were lost by the study subject. To assess adherence to the regimen, number of drink packets used during each month was tabulated in our laboratory by the nurse and independently by NML. Adherence was assessed as the percentage of drink packets distributed to the participant during the course of the trial that were empty when the participant’s boxes were returned to our staff. At each monthly visit, the nurse also discussed the participant’s adherence to treatment, identified potential barriers to adherence, recommended solutions to these problems, and recorded possible adverse events. Although the women liked the taste of the fizzy grape drink, a few reported nausea or discomfort and found that it was better tolerated when not consumed on an empty stomach. We, therefore, provided each participant with a monthly supply of crackers to eat with the grape drink.
Maternal Assessments during the Trial
Participants were transported in our research van to our UCT laboratory for two additional prenatal visits—at 4 and 12 weeks after randomization. Mothers were given breakfast and a snack during the morning at all laboratory visits. At the end of each visit, the mother received a small monetary compensation.
The TLFB was re-administered at the 4- and 12-week visits, and data from the three interviews were averaged to provide three continuous measures of drinking during pregnancy: average oz AA consumed/day, oz AA/drinking day (dose/occasion) and frequency (days/week). Alcohol abuse and/or dependence was diagnosed based on DSM-IV criteria using the Structured Clinical Interview for DSM-IV (First et al., 2002). Demographic and background data were also collected, including weeks gestation at randomization, maternal age, parity, gravidity, SES (Hollingshead, 2011), education (years), marital status (married/unmarried), verbal (Peabody Picture Vocabulary Test) and nonverbal (Raven Progressive Matrices) intellectual function, depression (Beck Depression Inventory), and stressful life events (Holmes and Rahe, 1967; Yumoto et al., 2008).
Mothers were also asked about their drug use during pregnancy at each prenatal visit. Marijuana (“dagga”), cocaine, heroin, methaqualone (“mandrax”), and methamphetamine (“tik”) were measured in days/week; smoking as cigarettes/day. To assess the validity of the maternal reports of drug use, urine samples were collected from the last 30 women enrolled in the trial. Samples were tested using the AccuTest™ 6+2 drugs of abuse panel test (DTA Pty Ltd, Cape Town, South Africa), an immunochemical assay that detects metabolites of drugs commonly used in this community (amphetamines, cocaine, methaqualone, methamphetamine, opiates, and marijuana (THC)), as well as pH and creatinine to test for sample adulteration. No woman refused urine drug testing.
Maternal weight during pregnancy was measured on a digital scale with 100g precision; height, with a stadiometer. GA was assessed based on ultrasound for 85.7% of the participants; for the others, it was based on last menstrual period. Dietary choline intake was assessed using our QFFQ prior to initiation and twice during the trial (Carter et al., under review). In addition, a registered dietician or a research assistant with extensive training in dietary interviewing administered a multiple-pass 24-hr dietary recall interview to assess nutritional status (Carter et al., 2017). The 24-hr recall interview was quantified by L Bechard, a research dietician at Boston Children’s Hospital, using FoodFinder3®, a dietary analysis software program developed by the South African Medical Research Council (Tygerberg, South Africa) to assess energy and nutrient content in the South African diet.
The participant was interviewed monthly either at her home or our UCT laboratory regarding any side effects, including diarrhea, nausea, vomiting, or muscle stiffness. She was also asked about any changes in body odor, since a fishy body odor has been reported when high doses are ingested (Zeisel et al., 1989; Wozniak et al., 2015). Blood pressure was measured at enrollment and at weeks 4 and 12 after randomization by trained research staff using an automated digital oscillometric meter on the subject’s left arm. Systolic (SBP) and diastolic (DBP) blood pressures were recorded, and mean arterial blood pressure (MAP) was calculated as (SBP + 2xDBP)/3.
Blood samples were collected at enrollment and at 4 and 12 weeks after randomization. Whole blood was centrifuged immediately for the collection of plasma, which was then aliquoted and stored at −80°C until shipment on dry ice to SZ’s laboratory at University of North Carolina. Targeted measurement of choline and choline-related metabolites (betaine, sphingomyelin, and phosphatidylcholine) was conducted using liquid chromatography multiple-reaction monitoring mass spectrometry coupled to stable isotope dilution (Koc et al., 2002; Zhao et al., 2015). Maternal blood samples from the initial visit were also assessed for the PEMT SNP rs12325817, using real-time PCR performed on an Eppendorf Realplex 4.0 (Eppendorf North America, Westbury, NY, USA).
Standard of care for pregnant women in Cape Town
All enrolled women received standard obstetric prenatal care at their MOU throughout the trial. Pregnant women are examined monthly until 32 weeks gestation, twice monthly until 36 weeks, and weekly until delivery. At each visit, a nurse midwife takes an obstetrical history and conducts a physical examination. Standard of care includes evaluation of fetal well-being, including fundal height growth, fetal movement, heart rate, presentation, lie, position, and maternal weight, height, blood pressure, occurrence of symptoms, such as fever, cough, diarrhea, body rash, and change in appetite in the preceding month, and urinalysis. Screening labs routinely include HIV antibody, complete blood count, and glucose. Folate and iron supplements are provided by the clinic. A glucose tolerance test is performed if there is a history of diabetes or an elevated random glucose. Women are strongly encouraged to limit or abstain from drinking during pregnancy to promote the health of the baby.
Data Analysis
Statistical analyses were performed using SPSS software (v.22; IBM, Armonk, NY, USA), except for mixed model repeated measures regression, which was performed using SAS v.9.3 software (SAS Institute Inc., Cary, NC). All variables were checked for normality of distribution. Success of the randomization was assessed by comparing the choline treatment and placebo groups using t tests and χ2 analysis on alcohol and drug use during pregnancy and a range of sociodemographic variables. T-tests were used to compare adherence (% empty drink packets returned) between groups, and Pearson r was used to examine the relation of adherence to the alcohol consumption and background measures. The validity of our assessments of adherence based on packet counts was assessed in relation to change in maternal plasma choline level during the course of the trial using mixed model repeated measures regression. T-tests and χ2 were used to compare the two groups in terms of maternal reported side effects and other medical problems that developed during the trial. χ2 was used to compare the groups on prevalence of inadequate dietary choline intake and of the PEMT polymorphism rs12325817.
Results
Participants
Recruitment and random assignment
Recruitment occurred between April 2012 and September 2014; the last infant was born in January 2015. Sample attrition is detailed in Figure 1. Seventy participants were randomly assigned to condition—35 to the choline treatment group, 35 to the placebo control group. Among the 70 that were randomized, there were 4 non-study-related fetal deaths: 1 spontaneous abortion (placebo), 2 stillbirths (1 choline, 1 placebo), and 1 fetus whose mother was murdered during pregnancy (choline group). In addition, 2 participants who met a priori exclusionary criteria were removed from the sample: 1 twin pregnancy identified after randomization (placebo) and 1 very preterm delivery (placebo<29wk gestation). Voluntary attrition was exceptionally low. One woman (choline group) withdrew immediately after randomization but prior to initiating treatment, and a second participant (choline group) withdrew after delivery but prior to the 6.5-month infant assessments. Data from the woman who withdrew before initiating treatment are not included in this report, leaving a total of 34 participants in the choline group; 35 in the placebo group. Median GA at randomization was 20.1 weeks, range=8.6 to 26.0.
There were no group differences for weeks GA at time of enrollment for antenatal care, initial maternal screening, or randomization (Table 1). The sample came from an economically disadvantaged, poorly educated population; only five mothers in the choline and four in the placebo arm had completed high school. The groups did not differ on a broad range of sociodemographic characteristics, including maternal age, SES, education, verbal and nonverbal intellectual competence, stress, and depression. Dietary caloric (energy) intake based on the 24-hr recall interview did not differ between groups, nor did rate of weight gain during pregnancy. Average dietary choline intake on the QFFQ did not differ between groups at randomization or post-treatment and was lower than the 450 mg/d recommended for pregnant women (IOM, 2006). There were also no differences on the 24-hr recall interview for the other methyl donor-related nutrients (folate, B12, methionine, lutein) that can impact dietary choline needs, after adjustment for caloric intake, all ps>0.20.
Table 1.
Maternal sociodemographic characteristics and pregnancy alcohol and drug use by treatment arm
| Treatment arm | t or χ2 | ||
|---|---|---|---|
|
| |||
| Choline (n=34) |
Placebo (n=35) |
||
| Maternal characteristics | |||
| Weeks gestation at 1st antenatal visit | 16.1 (4.3) |
14.7 (4.9) |
1.21 |
| Weeks gestation at initial screening | 17.7 (4.5) |
17.0 (4.6) |
0.63 |
| Weeks gestation at randomization | 19.6 (3.8) |
19.5 (3.9) |
0.12 |
| Maternal age at deliverya | 26.4 (5.7) |
26.9 (5.3) |
0.35 |
| Parity | 1.7 (1.8) |
1.4 (1.0) |
0.81 |
| Gravidity | 2.9 (1.9) |
2.7 (1.4) |
0.46 |
| Socioeconomic statusb | 20.9 (8.6) |
21.0 (7.3) |
0.08 |
| Education (years) | 9.2 (1.9) |
9.3 (1.6) |
0.39 |
| Marital status (% married) | 26.5 | 37.1 | 0.90 |
| Peabody Picture Vocabulary Test IQ | 47.9 (10.1) |
46.7 (9.4) |
0.45 |
| Raven Progressive Matricesc | 20.3 (11.5) |
22.6 (12.2) |
0.79 |
| Beck Depression Inventoryd | 8.6 (8.9) |
10.7 (9.6) |
0.94 |
| Life Events (number)d | 7.4 (3.3) |
8.4 (4.5) |
1.05 |
| Perceived Life Stresse | 28.5 (17.8) |
29.9 (24.5) |
0.27 |
| Nutritional indicators | |||
| Dietary caloric intake (kJ/day)f | 9334.7 (2962.0) |
10,528.1 (3952.1) |
1.42 |
| Rate of gestational weight gain (kg/wk) | 0.4 (0.3) |
0.4 (0.3) |
0.86 |
| Dietary choline intake (mg/day)g | |||
| Baseline | 360.4 (215.8) |
368.6 (253.1) |
0.14 |
| Post-treatment (average) | 253.8 (128.7) |
295.2 (112.0) |
1.40 |
| Alcohol during pregnancy | |||
| Absolute alcohol (AA)/day at conception | 1.8 (1.8) |
1.5 (0.9) |
0.93 |
| Drinks/occasion at conception | 4.6 (2.9) |
5.0 (2.7) |
0.61 |
| Frequency of drinking at conception (days/week) | 2.6 (1.4) |
2.1 (1.0) |
1.54 |
| AA/day across pregnancy | 0.9 (0.9) |
0.8 (0.7) |
0.09 |
| Drinks/occasion across pregnancy | 4.1 (2.2) |
4.2 (2.3) |
0.11 |
| Frequency of drinking across pregnancy (days/week) | 1.4 (1.0) |
1.3 (0.9) |
0.19 |
| History of alcohol abuse and/or dependence (%)h | 19.0 (57.6) |
17.0 (51.5) |
0.24 |
| Cigarettes/day during pregnancyi | 7.0 (4.4) |
5.9 (3.1) |
1.06 |
| Marijuana during pregnancy (days/month)i | 9.8 (10.4) |
6.2 (7.6) |
0.70 |
| Methamphetamine during pregnancyi (2 in each group) | 4.4 (3.3) |
0.1 (0.01) |
1.84 |
Values are mean (standard deviation).
Missing for 2 mothers in choline group (1 stillbirth, 1 mother/fetus killed) and 3 mothers in placebo group who were removed from study (1 early spontaneous abortion, 1 stillbirth, 1 twin pregnancy)
Hollingshead (2011) Scale
Missing for 3 choline, 1 placebo
Holmes and Rahe (1967) Missing for 1 placebo
Yumoto et al. (2008) Missing for 1 placebo
Based on multiple pass 24-hr dietary recall interviews, quantified using FoodFinder3®. 4.18 kJ = 1 kcal
Based on choline-indicated food frequency questionnaire (QFFQ; Carter et al. under review), adjusted for daily caloric intake
Based on Structured Clinical Interview for DSM-IV (First et al., 2002). Missing for 2 choline, 2 placebo
Users only (cigarettes: 34 choline, 25 placebo; marijuana: 11 choline, 5 placebo; methamphetamine: 2 choline, 2 placebo)
There were no group differences in alcohol consumption across pregnancy, which was very heavy for both groups at time of conception (≈10.0 standard drinks/occasion on an average of 2–3 days/wk). The women in both groups continued to drink heavily across pregnancy (≈8–9 drinks/occasion) but reduced their frequency to about 1–2 days/wk. They reported concentrating their drinking on the weekends, and binge drinking was common, with 92.6% averaging at least 4 standard drinks/occasion. A majority of the women in both groups met DSM-IV criteria for a diagnosis of alcohol abuse and dependence.
Cigarette smoking and illicit drug use during pregnancy did not differ by group. Although smoking was common, number of cigarettes smoked per day was generally light, with 81.4% of smokers reporting <0.5 pack/day, and only 3.4% reporting at least 1.0 pack (20 cigarettes)/day. None of the women reported using cocaine, heroin, or methaqualone. Marijuana use was reported by 23.5% of the women. Although methamphetamine users were not invited to participate in the study, 4 women recruited as alcohol users later reported also using methamphetamine—2 in the placebo group on a single occasion and 2 in the choline group (1, twice/month; the other, 1–2 days/week). Of the 30 women for whom urine drug tests were available, results were consistent with maternal reports for 25 (83.3%) for marijuana, cocaine, opiates, and methamphetamine. Four of the women reporting marijuana use did not test positive for THC on their urine drug screen. One woman denied all drug use but tested positive for methaqualone. Consistent with the maternal reports, no urine tests were positive for cocaine or opiates.
Adherence
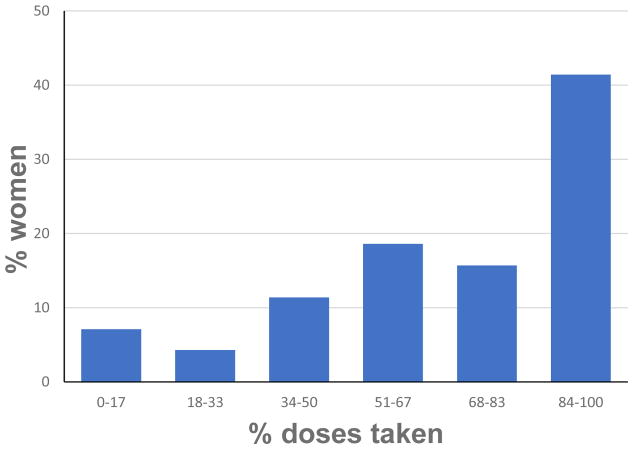
As seen in Figure 3, adherence (measured as percentage of drink packets used) averaged 68.4% across pregnancy (median=74.0%; interquartile range=53.9–88.7), and poor adherence (≤33.0%) was relatively rare (11.3% of participants). Adherence was excellent (≥84%) for 42.0% of the participants and good-to-excellent (≥68%) for 58.0%. Mean adherence was slightly, although not significantly, higher in the placebo group, M=73.5%, compared with 63.0% in the choline group, t=1.69, p=0.095. Although older and higher parity women were more adherent, adherence was not related to SES, maternal education, intellectual function, depression, stressful life events, or weight gain during pregnancy (Table 2). Heavier maternal drinking, smoking, and marijuana use during pregnancy were also not associated with poorer adherence, and adherence was good-to-excellent (≥68%) for 53.8% of the women with a history of alcohol abuse and dependence, compared with 57.1% of those who did not meet criteria for a DSM-IV diagnosis, χ2(1)=0.07, p>0.789. The negative association with methamphetamine use was due primarily to poorer adherence by the two frequent methamphetamine users in the sample.
Figure 3.
Participant adherence to the study protocol
Table 2.
Relation of alcohol and drug use and sociodemographic characteristics to protocol adherence (N = 69)
| Protocol adherence | |
|---|---|
| Sociodemographic characteristics | |
| Weeks gestation at time of randomization | −0.17 |
| Mother’s age at delivery | 0.25* |
| Parity | 0.31** |
| Socioeconomic status | 0.10 |
| Mother’s education (years) | −0.06 |
| Marital status (married/unmarried) | 0.22† |
| Peabody Picture Vocabulary Test IQ | 0.06 |
| Raven Progressive Matrices score | 0.10 |
| Beck Depression Inventory score | 0.13 |
| Stressful Life Events (number of events) | 0.16 |
| Perceived stress | 0.07 |
| Daily caloric intake (kJ/day) | −0.07 |
| Rate of pregnancy weight gain (kg/wk) | −0.02 |
| History of alcohol abuse and/or dependence | 0.01 |
| Alcohol and drug use during pregnancy | |
| Absolute alcohol (AA)/day at conception | 0.16 |
| Drinks/occasion at conception | 0.20† |
| Frequency of drinking at conception (days/week) | 0.08 |
| AA/day across pregnancy | 0.07 |
| Drinks/occasion across pregnancy | 0.11 |
| Frequency of drinking across pregnancy (days/week) | −0.02 |
| Cigarettes/day during pregnancy | 0.01 |
| Marijuana during pregnancy (days/month) | −0.15 |
| Methamphetamine during pregnancy (days/month) | −0.30* |
Values are Pearson r.
p < 0.10
p < 0.05
p < 0.01
Maternal plasma choline concentration
Group means for plasma choline concentrations collected at randomization and 4 and 12 weeks thereafter are shown in Table 3. The groups did not differ on any of the plasma metabolite concentrations prior to treatment, all ps>0.20. In a repeated-measures ANOVA, there was a significant interaction for group X treatment phase for choline but not for the three other metabolites. By 4 weeks, plasma choline concentrations were already significantly higher in the choline supplementation than the placebo group, and this group difference continued to be evident at 12 weeks (Fig. 4).
Table 3.
Choline and choline-related metabolite concentrations by treatment arm
| Choline | Placebo | |||
|---|---|---|---|---|
|
|
|
|||
| n | M ± SD | n | M ± SD | |
| Choline (μM)a | ||||
| Pre-treatment | 29 | 8.7 ± 2.5 | 30 | 8.5 ± 2.3 |
| 4 weeks | 31 | 10.7 ± 4.2 | 28 | 8.3 ± 1.9 |
| 12 weeks | 29 | 14.4 ± 11.5 | 25 | 10.6 ± 2.6 |
| Betaine (μM)b | ||||
| Pre-treatment | 29 | 20.3 ± 6.2 | 30 | 18.1 ± 7.3 |
| 4 weeks | 31 | 21.8 ± 8.8 | 28 | 14.5 ± 4.1 |
| 12 weeks | 29 | 20.1 ± 9.4 | 25 | 17.0 ± 6.1 |
| Phosphatidylcholine (μM)c | ||||
| Pre-treatment | 29 | 2178.2 ± 295.8 | 30 | 2251.2 ± 413.4 |
| 4 weeks | 31 | 2319.5 ± 327.0 | 28 | 2405.6 ± 348.5 |
| 12 weeks | 29 | 2452.3 ± 301.0 | 25 | 2508.8 ± 480.9 |
| Sphingomyelin (μM)d | ||||
| Pre-treatment | 29 | 608.9 ± 102.7 | 30 | 617.9 ± 96.0 |
| 4 weeks | 31 | 600.0 ± 99.8 | 28 | 625.6 ± 99.7 |
| 12 weeks | 29 | 664.4 ± 138.4 | 25 | 673.5 ± 149.4 |
Blood samples missing for 10 women at baseline (1 spontaneous abortion, 1 insufficient blood, 8 sample handling problems); 10, at 4 weeks (1 spontaneous abortion, 1 mother murdered, 1 multiple birth, 6 insufficient blood, 1 sample handling); and 15, at 12 weeks (1 spontaneous abortion, 1 mother murdered, 1 multiple birth, 1 very preterm birth, 3 insufficient blood, 3 sample handling, 5 delivered prior to final laboratory visit).
- Group (choline/placebo): t(66) = 2.78, p = 0.007
- Session (pre-treatment/post-treatment), t(105) = 5.10, p < 0.0001
- Group by session interaction, t(105) = 3.36, p = 0.001
- Group (choline/placebo): t(66) = 3.83, p = 0.0003
- Session (pre-treatment/post-treatment), t(105) = 0.08, p = 0.938
- Group by session interaction, t(105) = 0.37, p = 0.712
- Group (choline/placebo): t(66) = 0.96, p = .342
- Session (pre-treatment/post-treatment), t(105) = 2.53, p = 0.013
- Group by session interaction, t(105) = 0.68, p = 0.495
- Group (choline/placebo): t(66) = 0.50, p = .621
- Session (pre-treatment/post-treatment), t(105) = 1.13, p = 0.261
- Group by session interaction, t(105) = 0.65, p = 0.519
Figure 4.
Changes in plasma choline concentration across the first 12 weeks of the trial by treatment group (error bars = SE)
Side effects
Table 4 presents physical symptoms reported at randomization and aggregated from monthly interviews conducted after treatment began. There were no significant group differences at baseline; twice as many women in the placebo group reported vomiting during the pre-treatment period, but this difference was not statistically significant at baseline or during the treatment period. More women randomized to the choline group reported nausea/dyspepsia during the treatment period than in the placebo group (≈2 vs.1 d/wk). As indicated earlier, we provided each participant with a monthly supply of crackers to eat with the grape drink because several women did not enjoy drinking it on an empty stomach. Although MAP was slightly lower in the choline group both pre- (t(67)=1.85; p=0.069) and during treatment (t(66)=1.84; p=0.071), arterial pressure did not change significantly during the course of the trial in either group (choline arm: t(33)=0.28, p=0.783; placebo: t(33)=0.44; p=0.664). The groups did not differ on any of the other side effects, and there were no reported study-related serious adverse events in either group. None of the women in the choline group reported a fishy body odor.
Table 4.
Adverse events reported at baseline and during the course of the study by treatment arm
| Baseline (Pre-treatment) | During study | |||||
|---|---|---|---|---|---|---|
| Choline | Placebo | t or χ2 | Choline | Placebo | t or χ2 | |
| Incidence (n (%)) | ||||||
| Diarrhea | 2 (5.9) | 2 (6.1) | 0.001 | 3 (8.8) | 5 (15.2) | 0.64 |
| Vomiting | 7 (20.6) | 14 (40.0) | 3.07† | 12 (35.3) | 8 (22.9) | 1.30 |
| Nausea/dyspepsia | 15 (44.1) | 20 (57.1) | 1.17 | 23 (67.6) | 10 (28.6) | 10.55** |
| Muscle stiffness | 7 (20.6) | 13 (37.1) | 2.30 | 10 (29.4) | 17 (48.6) | 2.66 |
| Frequency (d/wk) | ||||||
| Diarrhea | 0.04 (0.1) | 0.1 (0.4) | 0.69 | 0.1 (0.3) | 0.2 (0.7) | 0.92 |
| Vomiting | 0.8 (2.1) | 1.5 (2.5) | 1.24 | 0.3 (0.6) | 0.2 (0.6) | 0.38 |
| Nausea/dyspepsia | 1.8 (2.8) | 2.4 (3.0) | 0.82 | 0.3 (0.5) | 0.1 (0.2) | 2.33* |
| Muscle stiffness | 0.5 (1.4) | 1.4 (2.5) | 1.70† | 0.03 (0.1) | 0.1 (0.1) | 1.37 |
| MAP (mmHg) | 80.6 (9.4) | 84.8 (9.4) | 1.85† | 81.0 (8.9) | 85.1 (0.1) | 1.84† |
MAP = mean arterial pressure
p < 0.10
p < 0.05
A small number of women developed medical problems during pregnancy: 10.1% developed hypertension, 7.2% preeclampsia, and 7.2% syphilis. There were no group differences regarding hypertension (choline 3, placebo 4), pre-eclampsia (choline 2, placebo 3), or syphilis (choline 3, placebo 2), but 4 women in the choline group developed gestational diabetes compared to none in the placebo group, χ2(1)=4.37, p=0.037.
Choline Dietary Intake and Prevalence of the PEMT Variant
Data from the QFFQs confirmed that none of the women exceeded the UL for choline intake (3.5g/day). The highest level of dietary choline intake reported in any individual interview was 1.3g/day; 1.1 and 0.9g/day were reported in two other interviews; and all others were <0.8g/day. By contrast, 50 (72.5%) women had inadequate dietary choline intake (<450mg/day) at the pre-treatment visit; 59 (85.5%), at the 12-week visit. There was no group difference in number of women with inadequate non-supplement dietary choline intake averaged across the three visits: 31 women (91.2%) in the choline vs. 31 (88.6%) in the placebo arm, χ2(1)=0.128, p>0.20.
Blood samples from 55 women analyzed for the PEMT polymorphism rs12325817 showed that 12 (21.8%) had at least one allele, including 1 (1.8%) who was homozygous for this variant, with no difference between the choline (5 GC, 1 CC) and placebo (6 GC, 0 CC) groups, χ2(1)=0.005, p>0.20.
DISCUSSION
This study demonstrated the feasibility and acceptability of a maternal choline supplementation regimen in a sample highly disadvantaged, heavy drinking pregnant women in Cape Town, South Africa. Although 8 participants were lost to follow-up due to fetal death or protocol exclusions, only two withdrew voluntarily—one immediately after randomization; the other after the birth of her infant—indicating that the protocol was widely acceptable. The success of the random assignment was confirmed by a lack of group differences on any of 18 sociodemographic characteristics.
Adherence was somewhat lower (median=74%) in this socioeconomically disadvantaged sample than in the two U.S. choline supplementation studies of children (82% and 95.7%, in the more middle-class Minnesota (Wozniak et al., 2013) and San Diego (Nguyen et al., 2016) studies, respectively). Hollingshead (2011) SES levels in the San Diego sample averaged 48.5 (small business, professional, or technical), compared with 20.0 (unskilled and semiskilled workers) in our sample, and SES in the Minnesota sample was presumably similar to San Diego, given that the Minnesota participants (children with FASD) had all been removed from the custody of their biological parents. Adherence is often better in trials with children, whose parents administer treatments, than in adults or adolescents (Ahmed and Aslani, 2013; Tebbi, 1993). In the Ukraine study, in which the assessment of adherence was based on maternal report (Kable et al., 2015), the choline-treated participants reported 100% adherence (Coles et al., 2015). However, the validity of these reports is uncertain given that maternal blood choline levels did not increase in the choline group over the course of that trial.
The high rate of adherence seen in this cohort is impressive when one considers the low SES, poor educational level, and high levels of stress and social disorganization in the communities in which the study participants live. Even for the women with 50% adherence, choline intake from the supplement was substantially higher than the 450 mg/day recommended by the IOM (2006). The validity of our packet count measure of adherence was confirmed by the significantly greater increase in plasma choline levels during the course of the trial in the choline supplemented compared with the placebo group. The increases in plasma choline levels among women in the active treatment arm across the first 12 weeks of the trial were similar in magnitude to those seen in two U.S. studies—one, a supplementation trial of pregnant women in North Carolina during the final 22 weeks of gestation and first 45 days postpartum (Fischer et al., 2010); the other, the 9-month Minnesota supplementation study of preschool-age children with FASD (Wozniak et al., 2013). The successful adherence in our trial was likely enhanced by the extensive monitoring and support provided by our two research nurses, who are from the same community as the participants and developed excellent rapport with them.
The findings that adherence was unrelated to level of alcohol consumption during pregnancy and that the prevalence of good or excellent adherence did not differ between women with or without a history of alcohol abuse and dependence demonstrate that this intervention was acceptable to even the heaviest drinking mothers, whose infants are at greatest risk for adverse effects. Good adherence was not dependent on higher levels of maternal education or intellectual function, lower levels of depression or stressful life events, or more optimal nutritional status. The only significant predictors of better adherence were higher parity and older maternal age. Adherence was poor for the two women who initiated regular methamphetamine use during the trial.
The choline dose administered in this trial (2g/day) is considerably higher than in the previous human studies (range=500–750mg/day). As detailed above, total daily intake for all participants was still well below the UL for choline (3.5g/day) established by the IOM. Low blood pressure, the side effect that provided the LOAEL on which the UL was based, was not found in either group. Fishy body odor was not reported by any participant. This odor, which was reported in a high proportion of the choline-treated children in the Wozniak et al. (2013) study, results from accumulation of trimethylamine, a compound that is formed when choline transporters in the small intestine are saturated and excess choline reaches bacteria in the gut, accumulating in sweat and urine (Zeisel et al., 1989). As recommended by SZ, we instructed the participants to split the choline dose, ingesting the contents of 1 drink packet in the morning and 1 in the evening, thereby reducing trimethylamine formation. The only side effect reported to increase in the choline-treated women during the trial was nausea/dyspepsia, which in many cases appeared to result from ingesting the fizzy grape drink on any empty stomach. This effect did not appear to be clinically significant, however, since the groups did not differ on daily caloric intake or rate of weight gain during pregnancy. Although the incidence of gestational diabetes was higher in the choline group, gestational diabetes is not a known effect of choline, and there was no sugar in the choline supplement. Future larger studies are needed to determine if this was, in fact, the result of supplementation or a Type I error in light of the large number of outcomes examined here.
Mean dietary choline intake for this sample (364.5 mg/day) was remarkably similar to that seen in a U.S. sample of pregnant women in California (392.9 mg/day; Shaw et al., 2004). The finding that the means in both these samples were substantially below the IOM (2006) 450mg/day criterion for adequate dietary intake suggests that a maternal choline supplementation intervention may be particularly efficacious in both these populations (Zeisel, 2011). By contrast, the prevalence of the rs12325817 allele of the choline-metabolizing enzyme PEMT was substantially lower (21.8%) than in the U.S. sample studied in North Carolina (72%). Silver et al. (2015) have reported that the prevalence of this SNP is also low in people of West African (The Gambia) ancestry and hypothesized that traditional diets low in choline negatively select against this polymorphism. From a statistical analysis perspective, it would be preferable to have approximately equal numbers of high and low risk participants within each group (e.g., adequate vs. inadequate dietary intake) to test the hypotheses that inadequate dietary intake and/or the presence of a PEMT allele may modify the efficacy of the maternal supplementation intervention. The very high incidence of dietary inadequacy and relatively low incidence of the PEMT allele in this sample will make it difficult to test the hypothesis that maternal choline supplementation during pregnancy is likely to be more effective in cases where insufficient choline is available to the fetus.
Limitations
Given that the sample size was small, there is a need to assess the degree to which this intervention will be scalable when administered to a larger confirmatory sample. This trial was conducted in the Cape Coloured community because the unusually high prevalence of heavy drinking during pregnancy made it possible to assess the feasibility and acceptability of this nutritional intervention in a more time-efficient and cost-effective manner than would be possible in the US. Future studies are needed to confirm its acceptability in other populations.
Conclusions
These data demonstrate that a choline supplementation program with very heavy drinkers during pregnancy is feasible even among highly disadvantaged, poorly educated women and in a cross-cultural setting. The acceptability of this intervention is indicated by impressive adherence to a twice-daily maternal nutritional supplementation protocol and our finding that adherence was not related to maternal education, intellectual function, depression, stressful life events, nutritional status, or alcohol use. One strength of this study was that all participants initiated the trial prior to end of the second trimester, with half (49.3%) initiating treatment by the middle of the second trimester. Data from the few studies that have compared the efficacy of choline supplementation in rats earlier (e.g., during the equivalent of the second trimester in humans) vs. later during development suggest that supplementation earlier in development may be more effective. The unusually high prevalence of heavy maternal drinking and FAS in Cape Town makes it an optimal setting to assess the feasibility and efficacy of a nutritional supplementation intervention for FASD.
Acknowledgments
Funding: Grants from the NIH/National Institute on Alcohol Abuse and Alcoholism R21AA020332 (to SWJ), R01AA016781 (to SWJ), and K23AA020516 (to RCC); National Institute of Diabetes and Digestive and Kidney Diseases R01DK115380 and P30DK056350 (to SHZ) and K24DK104676 and 2P30DK040561 (to CPD); and from the Lycaki-Young Fund of Michigan (to SWJ and JLJ).
We thank the members of the Data Safety Monitoring Board: Sydney Hans, Ph.D. (Chair), a developmental psychologist with expertise in assessment of long-term effects of teratogenic exposures, University of Chicago; Marylou Behnke, M.D., a neonatologist with expertise on teratogenic exposures, University of Florida; Judette Louis, M.D., M.P.H., an obstetrician with expertise in fetal alcohol-related pregnancy complications, Case Western Reserve University; and Cynthia Arfken, Ph.D., a statistician with expertise in alcohol abuse research, Wayne State University. We thank Kristine Lukasik, Balchem Corporation, New Hampton, NY, who conducted the regular quality control analyses for the choline supplement; Rocco Anzaldi, R.Ph., a senior research pharmacist at Children’s Hospital Boston, who supervised the construction of the randomization list and the labeling and shipment of the choline packets to UCT, and Wynand Smythe, Ph.D., B.Pharm., Division of Clinical Pharmacology, UCT, who retained the randomization list in case of an adverse medical event including spontaneous abortion, miscarriage or stillbirths requiring unblinding for review by the Data Safety Monitoring Review Board. We thank Lori Bechard, Ph.D., R.D., Sharmilah Booley, M.Sc., and Baheya Najaar, M.Sc., who worked on the development of the quantitative choline food frequency questionnaire with MS, as well as Monika Uys, Ph.D., Catherine Day, R.D., and Nicola Cooper, the dieticians who administered 24-hr recall interviews that were processed by Dr. Bechard. We thank our research nurses Maggie September, Patricia O’Leary, and Beverly Arendse, and our project driver Patricia Solomon for their work recruiting the cohort and conducting home visits involving adherence monitoring during the intervention. We are also extremely grateful to the mothers for their participation in this supplementation trial.
Footnotes
The authors declare no competing financial interests.
References
- Ahmed R, Aslani P. Attention-deficit/hyperactivity disorder: an update on medication adherence and persistence in children, adolescents and adults. Expert Rev Pharmacoecon Outcomes Res. 2013;13:791–815. doi: 10.1586/14737167.2013.841544. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CD, Viljoen DL, Marais AS, Chiodo LM, Cwik AS. Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143:463–469. doi: 10.1067/S0022-3476(03)00442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Wellmann KA, Tang N, He M, Mooney SM. Choline ameliorates deficits in balance caused by acute neonatal ethanol exposure. Cerebellum. 2015;14:413–420. doi: 10.1007/s12311-015-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WD, Graham-White J, Blackwood G, Glen I, McQueen J. Clinical effects of choline in Alzheimer senile dementia. Lancet. 1977;8040:711. doi: 10.1016/s0140-6736(77)90517-7. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal alcohol growth restriction and cognitive impairment. Pediatr. 2016;138:e20160775. doi: 10.1542/peds.2016-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Dodge NC, Bechard L, Meintjes EM, Molteno CD, Duggan C, Jacobson JL, Jacobson SW. Maternal alcohol use and nutrition during pregnancy: Diet and anthropometry. Alcohol Clin Exp Res. 2017;41:2114–2127. doi: 10.1111/acer.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham CL, Goldman BD, Fischer LM, da Costa KA, Reznick JS, Zeisel SH. Phosphatidylcholine supplementation in pregnant women consuming moderate choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96:1465–1472. doi: 10.3945/ajcn.112.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD CIFASD. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Maternal Child Health J. 2015;19:2605–2614. doi: 10.1007/s10995-015-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford J, Viljoen DL. Alcohol consumption by pregnant women in the Western Cape. South African Med J. 1999;89:962–965. [PubMed] [Google Scholar]
- da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp R. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometric Research; New York: 2002. [Google Scholar]
- Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr. 2010;92:336–346. doi: 10.3945/ajcn.2010.29459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J. 2005;54:355–359. doi: 10.1590/s0043-31442005000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosomatic Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Idrus NM, Breit KR, Thomas JD. Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 2017;59:43–52. doi: 10.1016/j.ntt.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Choline, in Dietary Reference Intakes. National Academies Press; Washington, DC: 2006. [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 2011;35:431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM, Carter RC, Dodge NC, Grant E, Warfield SK, Zöllei L, van der Kouwe AJW, Meintjes EM. Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res. 2017;41:965–975. doi: 10.1111/acer.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489–496. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pederson TL, Chambers CD CIFASD. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol. 2015;49:647–656. doi: 10.1016/j.alcohol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–40. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger NM, Malcolm-Smith S, Dodge NC, Molteno CD, Thomas KGF, Meintjes EM, Jacobson JL, Jacobson SW. Theory of mind in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2016;40:367–376. doi: 10.1111/acer.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LSRO/FASEB (Life Sciences Research Office/Federation of American Societies for Experimental Biology) Effects of consumption of choline and lecithin on neurological and cardiovascular systems. LSRO/FASEB; Bethesda, MD: 1981. Report # PB-82–133257. [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioural features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–30. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin. 2014;5:152–160. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. 2016;104:1683–1692. doi: 10.3945/ajcn.116.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltranferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- Silver MJ, Corbin KD, Hellenthal G, da Costa KA, Dominguez-Salas P, Moore SE, Owen J, Prentice AM, Hennig BJ, Zeisel SH. Evidence for negative selection of gene variants that increase dependence on dietary choline in a Gambian cohort. FASEB J. 2015;29:3426–35. doi: 10.1096/fj.15-271056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Carmichael Olson H, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring—a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Tebbi CK. Treatment compliance in childhood and adolescence. Cancer. 1993;15:3441–3449. doi: 10.1002/1097-0142(19930515)71:10+<3441::aid-cncr2820711751>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004a;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, O’Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004b;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res. 2013;33:897–904. doi: 10.1016/j.nutres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2015;102:1113–1125. doi: 10.3945/ajcn.114.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child Dev. 2008;79:1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol. 2011;44:185, e191. doi: 10.1007/s12035-011-8165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, da Costa KA, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr. 1989;119:800–4. doi: 10.1093/jn/119.5.800. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Mar MH, Zhou ZW, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zeisel SH, Zhang S. Rapid LC-MRM-MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N-oxide, and creatinine in human plasma and urine. Electrophoresis. 2015 doi: 10.1002/elps.201500055. [DOI] [PubMed] [Google Scholar]