Abstract
Background
Because alcohol (ALC) delays signs of pubertal development, we assessed the time course of events associated with the synthesis of critical hypothalamic peptides that regulate secretion of luteinizing hormone-releasing hormone (LHRH), the peptide that drives the pubertal process.
Methods
Immature female rats were administered either lab chow or Bio-Serve isocaloric control or ALC liquid diets from 27 through 33 days of age. On days 28, 29, 31 and 33 animals were killed by decapitation and tissue blocks containing the medial basal hypothalamus (MBH) and the rostral hypothalamic area (RHA) were isolated and stored frozen until assessed by Western blot analysis.
Results
Synthesis of dynorphin (DYN), a prepubertal inhibitor of LHRH secretion, was increased (p<0.05) in the MBH of ALC-treated animals by day 29. DYN was further elevated (p<0.01) on day 33 and was associated with an increase (p<0.01) in DYN receptor expression. ALC did not affect synthesis of neurokinin B (NKB), a prepubertal stimulator of LHRH; however, it did suppress (p<0.05) NKB receptor expression in the MBH by day 31. The most potent stimulator of prepubertal LHRH secretion, kisspeptin (Kp), was also decreased (p<0.05) in the MBH as early as day 29, with continued suppression (p<0.01) through day 33. Similar timely suppressions of mammalian target of rapamycin (mTOR), an immediate upstream regulator of Kp, were also noted. These decreases in mTOR and Kp were consistent with ALC stimulating (p<0.05) the p-AMP-activated protein kinase/raptor inhibitory pathway to mTOR on day 29, then later suppressing (p<0.001) an Akt mediated induction pathway to mTOR by day 31. In the RHA, ALC affected the pathways regulating Kp in a manner similar to that described in the MBH; however, these effects were not noted until day 33.
Conclusion
ALC acts within the MBH as early as 29 days to induce inhibitor and repressor inputs to LHRH, while depressing stimulatory inputs to the peptide. Collectively, these events lead to delayed signs of pubertal development.
Keywords: dynorphin, neurokinin B, kisspeptin, puberty, alcohol
INTRODUCTION
The onset of puberty occurs due to a complex series of neuronal and glial mediated acts within the hypothalamus that promotes the increased pulsatile secretion of luteinizing hormone-releasing hormone (LHRH), the peptide responsible for promoting pituitary and ovarian hormone secretions throughout the pubertal process. This timely increase in the release of LHRH is due to a gradual removal of prepubertal inhibitory influences, as well as the enhanced developmental responsiveness to excitatory stimuli. The prepubertal inhibition of LHRH secretion is caused by gamma aminobutyric acid, as well as the opioid peptides, β-endorphin and dynorphin (DYN; Faletti et al., 1999; Terasawa and Fernandez, 2001; Navarro et al., 2009; Lehman et al., 2010), which are synthesized by neurons within the arcuate nucleus of the medial basal hypothalamus (MBH). Several stimulatory peptides such as insulin-like growth factor-1 (IGF-1; Hiney et al., 1996; Wilson, 1998), leptin (Dearth et al., 2000; Lebrethon et al., 2000), transforming growth factor α (Ojeda et al., 1990) and glutamate (Gay and Plant, 1987; Urbanski and Ojeda, 1990; Claypool et al., 2000) have been known for generating excitatory influences resulting in enhanced prepubertal LHRH release. More recently, however, kisspeptin (Kp; Navarro et al., 2004a and 2004b; Thompson et al., 2004; Shahab et al., 2005) and neurokinin B (NKB; Navarro et al., 2009; Ramaswamy et al., 2010; Garcia et al., 2017) have emerged as important excitatory regulators of LHRH secretion and necessary for the initiation of puberty. Importantly, mutations in the Kp receptor (de Roux et al., 2003; Seminara et al., 2003), as well as mutations in the genes encoding NKB and its receptor (Topaloglu et al., 2009; Guran et al., 2009) have been shown to block pubertal development. In the rat brain, Kp is synthesized by neurons within both the rostral hypothalamic area (RHA) and the MBH, but NKB, like DYN, is mainly produced in the MBH. Since DYN, Kp and NKB all participate in the control of LHRH secretion at the time of puberty, it is likely that any substance that can either enhance the prepubertal inhibitory tone provided by DYN, or suppress the excitatory drive generated by Kp and NKB could cause a delay in the timing of pubertal development.
Chronic alcohol (ALC) exposure is known to suppress LHRH secretion and delay typical signs associated with the onset of female puberty in rats (Dees and Skelley, 1990, Emanuele et al., 2002), rhesus monkeys (Dees et al., 2000; Dissen et al., 2004) and humans (Peck et al., 2011; Richards and Oinonen, 2011). Specifically, these studies showed that ALC delayed the time of vaginal opening (VO) in rats, delayed development of a regular monthly pattern of menstruation in monkeys, and delayed breast development as well as menarche in humans. In recent years, we have made an effort to discern the effects of ALC on specific puberty-related inhibitory and stimulatory peptides in order to determine how it causes delayed puberty. While the effects of prepubertal ALC administration on hypothalamic NKB have not yet been studied, information regarding the drug’s effect on both DYN and Kp have been revealing. Six days of prepubertal ALC administration markedly increased DYN protein expression in the MBH of 33 day-old prepubertal female rats, and furthermore, ALC was shown to stimulate release of the inhibitory DYN peptide from the MBH incubated in vitro (Srivastava et al., 2015). Conversely, ALC suppressed Kp protein expression in the MBH (Srivastava et al., 2015) and recently, it was demonstrated that ALC suppressed the hypothalamic release of this excitatory peptide in vitro (Hiney et al., 2018). These observations are important, since for normal puberty to occur the inhibitory influences must decline, and the synthesis of the excitatory peptide inputs must keep pace with their release in order to stimulate, and thus drive the LHRH system throughout the pubertal process; hence, the opposite effects appear to be the case following prepubertal ALC exposure.
While ALC is capable of differentially affecting specific inhibitory and excitatory influences within the prepubertal hypothalamus, more work is required to determine when and how these ALC-induced influences occur. Therefore, this study was conducted to determine the time course of alterations in the respective synthesis of DYN, Kp and NKB across the daily prepubertal ALC exposure period, and to identify sites and mechanisms of ALC actions that ultimately relate to suppressed LHRH secretion and delayed puberty.
MATERIALS AND METHODS
Animals and Surgery
Eighteen-day pregnant female rats of the Sprague-Dawley line were purchased from Charles River (Boston, MA) and allowed to deliver pups normally in the Texas A&M University lab animal facility. Female pups were weaned at twenty-one days of age and housed three per cage under controlled conditions of light (lights on, 0600h; lights off, 1800h) and temperature (23 C), with ad libitum access to food and water. All procedures performed on the animals were approved by the University Animal Care and Use Committee and in accordance with the NAS-NRC Guidelines for the Care and Use of Laboratory Animals. Each animal was anesthetized at 23 days of age with 2.5% tribromoethanol (0.5ml/60g body weight) and was surgically implanted with a permanent intragastric cannula by a procedure that has been described previously (Dees et al., 1984). All animals recovered from surgery for 4 days prior to the beginning of the experiments.
Experimental design
When the rats were 26 days old, they were weighed and divided into three groups. Group 1 received a 5% ALC-liquid diet, and group 2 received the companion isocaloric control liquid diet (Bioserve, Inc., Frenchtown, NJ). Group 3 served as an additional set of control animals that received lab chow and water, ad libitum throughout the experiment. Each group received their respective diet by a regimen described previously (Dees and Skelley, 1990; Srivastava et al., 1995) and modified only slightly (Srivastava et al., 2009). Briefly, on day 27, the liquid-diets were administered in such a manner that 6 ml of the respective diet was injected via the intragastric cannula (four injections of 1.5 ml each) equally dispersed over the lights-on period, and then 30 mls of diet was available ad libitum (bottle) during the lights-off period. To provide an adequate food supply for these immature growing animals, beginning on day 28 and again on days 30 and 32, the amount of diet injected via the cannula was increased by 0.5 ml per injection. Also, at these same times, the amount of diet made available each night in the bottle was increased to 35, 40, and 50 ml, respectively. Thus, by day 33, each animal was receiving the maximum of four intragastric injections (3.0 ml each) of diet during the lights-on period and 50 ml of diet, ad libitum, during the lights-off period. The animals received their respective diets for either two days (days 27-29), four days (days 27-31), or six days (day 27-33).
Tissue Collection
On the mornings of days 28, 29, 31 and 33, animals were weighed and killed by decapitation 1.5 hours after their last gastric infusion. Animals were confirmed to be in the late juvenile stage of pubertal development by showing closed vaginae, small uteri, and no intraluminal fluid. Serum was stored at −80 C until assayed for blood alcohol concentrations (BAC) utilizing the EnzyChrom ™ Ethanol Assay Kit purchased from BioAssay Systems, Hayward CA. The brains were removed and the MBH and RHA were isolated as described previously (Hiney et al., 2009) and frozen on dry ice at −80 C until analyzed by Western blot analysis. These two regions of the hypothalamus were chosen because neurons within these areas synthesize neurohormones that are critical for the pubertal process. Specifically, the MBH contains a sub-population of neurons within the arcuate nucleus (AN) that co-synthesize Kp, NKB and DYN. The RHA also contains neurons that synthesize Kp; but they do not co-express DYN and NKB (Navarro et al. 2009). While the latter two proteins are present in this brain region, they are produced in low amounts by a different population of neurons (Burke et al. 2006).
Western blot analysis
Brain tissues were homogenized in 1% Igepal CA-630, 20 mM Tris-Cl, pH (8.0), 137 mM NaCl, 2 mM EDTA, 10% glycerol, 10 mM sodium pyruvate, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 0.25% protease inhibitor cocktail (Sigma Aldrich) at 4 C. The homogenates were incubated on ice for 30 min and centrifuged at 12,000×g for 15 min. The concentration of total protein in the resulting supernatant was determined by the Pierce 660nm Protein assay kit (Thermo Scientific, Rockford, IL) using bovine serum albumin as standard. Immunoblot analysis was performed by solubilizing the proteins (100 μg) in a sample buffer containing 62.5 mM Tris-Cl, pH 6.8, 2% SDS, 5% ß-mercaptoethanol, 10% glycerol and 0.02% bromophenol blue and electrophoresed through 4-20% SDS-PAGE for DYN, dynorphin kappa receptor (KOR-1), Kp, Insulin-like growth factor-1 receptor (IGF-1R), Akt, tuberous sclerosis 2 (TSC2), ras homologue enriched in brain (Rheb), mammalian target of rapamycin (mTOR), NKB, Neurokinin B receptor (NK3R), AMP-activated protein kinase α (AMPKα) and raptor under reducing conditions. The separated proteins were electrophoretically transblotted onto polyvinylidene difluoride membranes. Following transfer, membranes were blocked with 5% nonfat dried milk.1% Tween 20 in PBS (pH 7.4) for three hours. Membranes were incubated at 4 C overnight with the appropriate primary antibody: rabbit anti-total or anti p-AMPKα (1:1000; Cell Signaling Tech., Danvers, MA), rabbit anti-total (1:1000) or anti p-raptor (1:750; Cell Signaling Tech., Danvers, MA), goat anti-DYN (1:250; Santa Cruz Biotech., Santa Cruz, CA), mouse anti-KOR1 (1:250; Santa Cruz Biotech., Santa Cruz, CA), rabbit anti-total (1:1000) or anti-p-Akt (1:3000; Cell Signaling Tech., Danvers, MA), rabbit anti-total (1:2000) or anti-p-TSC-2 (1:250; Cell Signaling Tech., Danvers, MA), rabbit anti-Rheb (1μg/ml; Abcam Inc., Cambridge, MA), rabbit anti-total or anti-p-mTOR (1:1000; Cell Signaling Tech., Danvers, MA), rabbit anti-NKB (1:500; Novus Biologicals, Littlton, CO), rabbit anti-NK3R (1:1000; Novus Biologicals, Littlton, CO) and rabbit anti-Kp (3μg/ml; Novus Biologicals, Littlton, CO). After the incubation, membranes were washed in PBS/0.1%Tween-20 and then incubated with horseradish peroxidase-labeled secondary antibodies (1: 50,000; Santa Cruz Biotech., Santa Cruz, CA) for 2 hour at room temperature. Following washing, the specific signals were detected with the enhanced chemiluminescence (Western Lightning Plus-ECL, PerkinElmer, Shelton, CT) and quantified with NIH Image J software version 1.43 (National Institutes of Health, MD). Subsequently, all membranes were also stripped using Re-Blot Plus kit (EMD Millipore, Temecula, CA) and reprobed with mouse monoclonal antibody to ß-actin and goat anti-mouse secondary antibody, to normalize for the amount of sample loading when appropriate. Following washing, the detection and quantitation of ß-actin was conducted as described above.
RESULTS
Short-term ALC administration from 27 through 33 days did not cause differences between the chow-fed and liquid diet-fed control animals with regard to body weights or any of the other assessments conducted throughout the duration of this study. This is in agreement with similar studies using this feeding regimen administered to prepubertal, growing animals (Dees and Skelley, 1990; Dees et al., 1990; Srivastava et al., 1995; 2009). As before, results from these controls were combined and presented together in the following figures in order to simplify comparative descriptions. Blood samples drawn 1.5 hours after the morning gastric infusions produced a mean ±SEM BAC of 174±10 mg/dl across the 6 days of the study.
Actions of ALC in the MBH
Effects on DYN and KOR-1
No changes occurred in DYN protein expression in the MBH between control and ALC-treated animals on day 28 (not shown). However, expression of the DYN protein was increased (p<0.05) in the ALC-treated animals by day 29 (Fig. 1 A, B). This increase (p<0.05) in DYN expression was detected again on day 31 (CON: 0.55±0.07 vs ALC: 0.87±0.13) and was more marked (p<0.01) on day 33 (CON: 0.67±.01 vs ALC: 1. 07±0.05). This upregulation in DYN protein synthesis was associated with an increase (p<0.01) in the expression of the DYN receptor, KOR-1, that was observed on day 33 (Fig. 2), but not on days 29 and 31.
Figure 1.
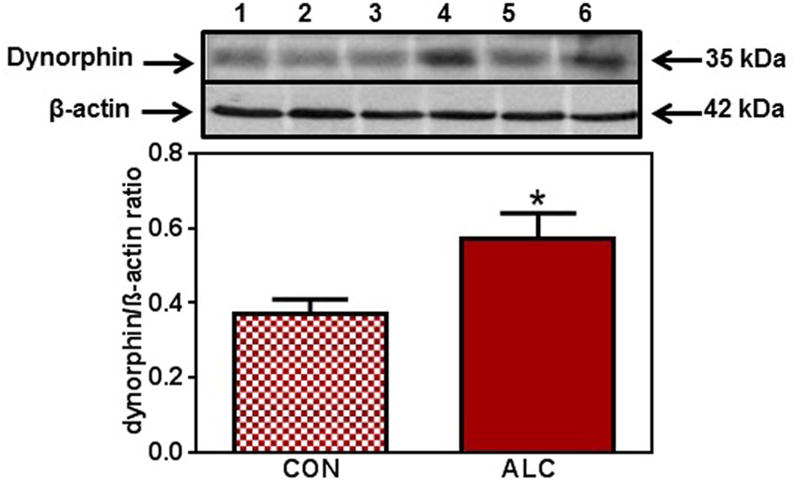
Effect of chronic ALC exposure on basal dynorphin (DYN) expression in medial basal hypothalamus (MBH) of prepubertal female rats (A) Representative Western immunoblot of DYN and β-actin proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing DYN protein expression in the MBH. These data were normalized to the internal control, β -actin protein, and the densitometric units represent the DYN/β-actin ratio. Note that DYN protein levels increased in the MBH of the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 6 in each group. *p<0.05 versus CON.
Figure 2.
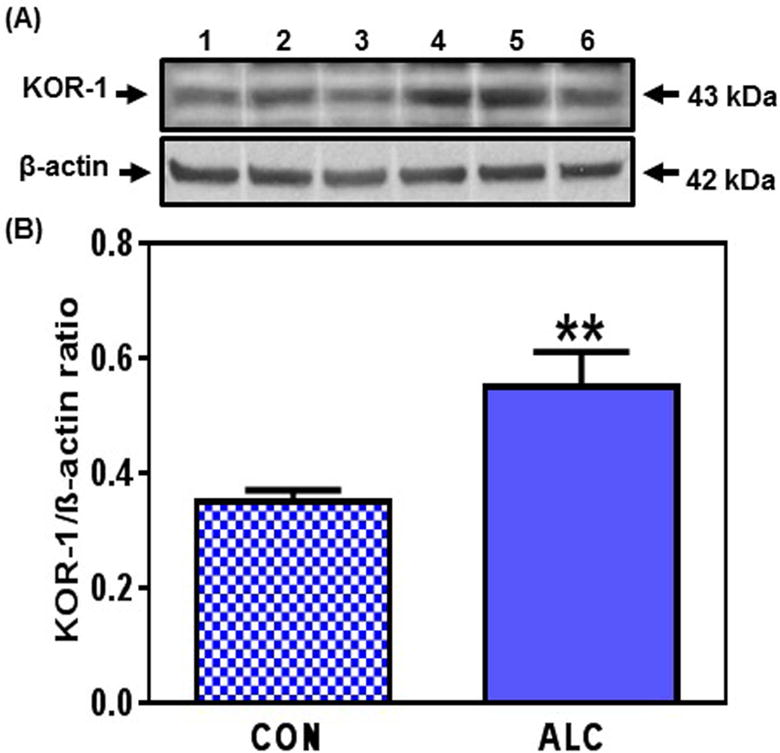
Effect of chronic ALC exposure on basal dynorphin kappa receptor (KOR-1) expression in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of KOR-1 and β-actin proteins in the MBH isolated from 33 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing KOR-1 protein expression in the MBH. These data were normalized to the internal control, β-actin protein, and the densitometric units represent the KOR-1/β-actin ratio. Note that KOR-1 protein levels increased in the MBH of the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 7 in each group. **p<0.01 versus CON.
Effects on NKB and NK3R
There were no differences in the synthesis of NKB protein in the MBH between control and ALC-treated animals throughout this study (not shown); however, the ALC did affect synthesis of the NKB receptor, NK3R. In this regard, while protein expression of the NK3R in the MBH was not altered on day 29 (not shown), it was suppressed (p<0.05) in the ALC-treated animals on day 31 (Fig.3). Furthermore, this suppression (p<0.05) in the synthesis of the NK3R continued to be observed in the MBH on day 33 (CON: 1.6±0.17 vs ALC: 1.1±0.14).
Figure 3.
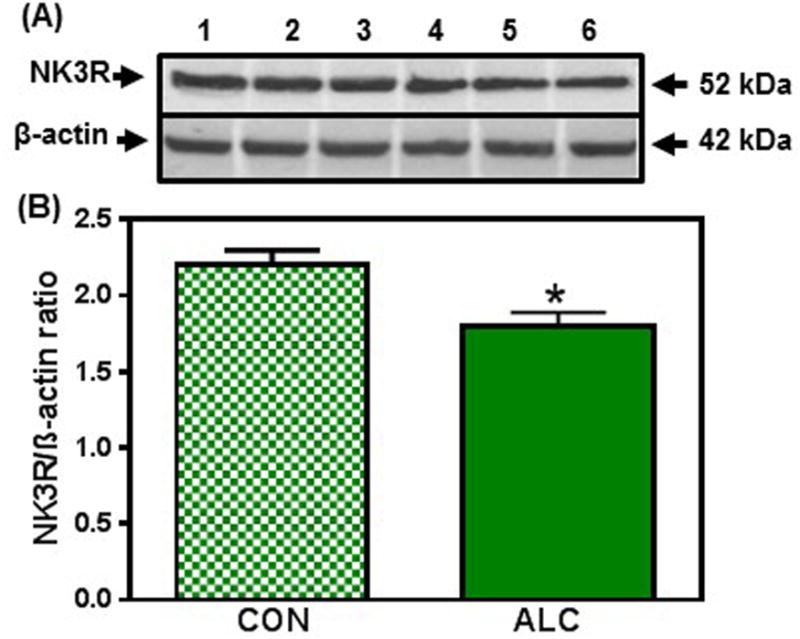
Effect of chronic ALC exposure on neurokinin B receptor (NK3R) expression in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of NK3R and β-actin proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing NK3R protein levels in the MBH. (C&D) show representative Western immunoblots and densitometric quantitations from 31 day-old rats. These data were normalized to the internal control, β-actin protein, and the densitometric units represent the NK3R/β-actin ratio. Note that NK3R protein levels decreased in the MBH of the ALC-treated animals at 31days of age. The respective bars illustrate the mean (±SEM) of an N of 5-8 in each group. *p<0.05 versus CON.
Effects on Kp
In the MBH, there were no changes detected in the expression of Kp protein between control and ALC-treated animals on day 28 (not shown). A decrease (p<0.05) in Kp protein expression, however, occurred in the MBH of ALC-treated animals on day 29 (Fig. 4 A, B). By day 31 (Fig. 4 C, D) the ALC caused a more pronounced decrease (p<0.01) in Kp protein expression, and this suppression (p<0.01) of Kp synthesis remained in effect on day 33 (CON: 0.82±0.08 vs ALC: 0.53±0.04). Since mTOR has been identified as an important immediate upstream regulator of Kp, we assessed whether ALC affects the phosphorylation of this protein. We demonstrate here that the ALC-treated animals also exhibited a modest decrease (p<0.05) in p-mTOR protein expression in the MBH as early as day 29 (Fig. 5 A, B) and like Kp, p-mTOR protein expression was also further decreased (p<0.01) by the ALC on day 31 (Fig. 5 C, D). Additionally, p-mTOR synthesis continued to be suppressed (p<0.05) on day 33 (CON: 0.81±0.09 vs ALC: 0.55±0.06).
Figure 4.
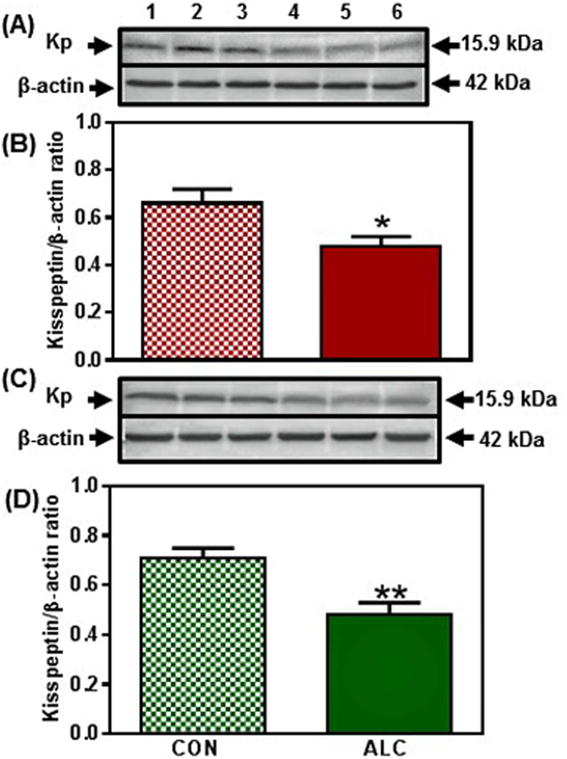
Effect of chronic ALC exposure on basal kisspeptin (Kp) expression in medial basal hypothalamus (MBH)of prepubertal female rats. (A) Representative Western immunoblot of Kp and β-actin proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing Kp protein expression in the MBH. (C&D) show representative Western immunoblots (CON, lanes 1-3; ALC; lanes 4-6) and densitometric quantitations from 31 day-old rats, respectively. These data were normalized to the internal control, β-actin protein, and the densitometric units represent the Kp/β-actin ratio. Note that Kp protein levels were decreased in the MBH of the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 6-8 in each group. *p<0.05; **p<0.01 versus CON.
Figure 5.
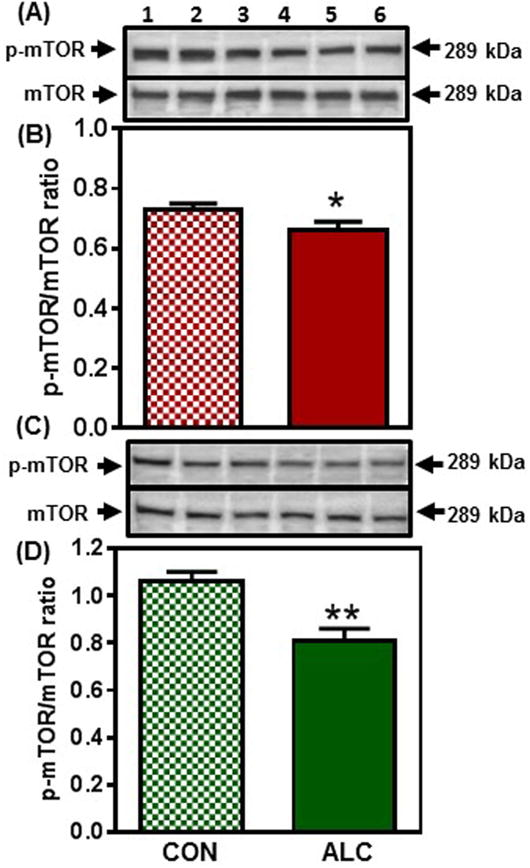
Effect of chronic ALC exposure on the phosphorylation (p) of mammalian target of rapamycin (mTOR) in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of p-mTOR (Ser 2448) and total mTOR proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing p-mTOR levels normalized to total mTOR protein expression in the MBH. (C&D) show representative Western immunoblots (CON, lanes 1-3; ALC; lanes 4-6) and densitometric quantitations from 31 day-old rats. Note that p-mTOR protein levels were diminished in the MBH of the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 8-11 in each group. *p<0.05; **p<0.01 versus CON.
Evaluation of potential effects of ALC upstream to mTOR began by assessing an Akt mediated pathway (Inoki et al., 2002, Long et al., 2005). No differences were observed in the protein levels of Akt, p-TSC2 or Rheb between control and ALC-treated animals at 28 and 29 days of age. However, compared to controls, the ALC-treated animals showed a decreased (p<0.001) expression of p-Akt on day 31 (Fig. 6). This decrease in p-Akt resulted in an increase (p<0.001) in the expression of p-TSC2 (Fig. 7A, B); an action that caused the concomitant suppression (p<0.05) of Rheb (Fig. 7C, D), the immediate upstream regulator of mTOR in this pathway (Inoki et al., 2002; Long et al., 2005). Importantly, since mTOR and Kp were suppressed earlier at 29 days, we assessed in the same tissues, an alternate pathway regulating mTOR and Kp synthesis to determine if it was affected prior to the effect on the Akt pathway. In this regard, the ALC-treated animals showed an activation (p<0.05) of AMPKα on day 29 (Fig. 8 A, B), an action resulting in increased (p<0.01) p-raptor (Fig. 8 C, D), a known negative control of mTOR (Gwinn et al., 2008). Therefore, this ALC-induced activation of the AMPK/raptor pathway on day 29 is consistent with the early suppressions in protein synthesis shown above for both mTOR and Kp.
Figure 6.
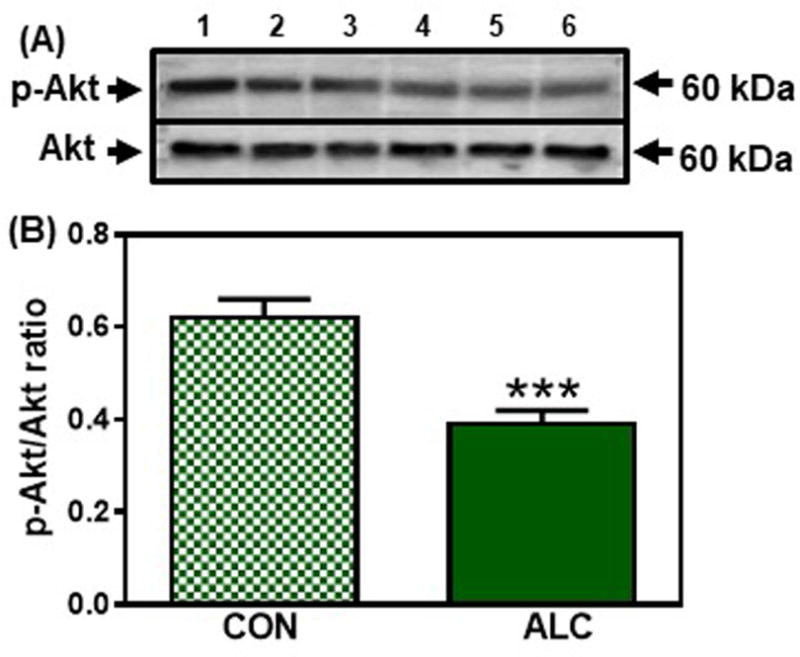
Effect of chronic ALC exposure on phosphorylation (p) of Akt in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of p-Akt (Ser473) and total Akt proteins in the MBH isolated from 31 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing p-Akt levels normalized to total Akt protein expression in the MBH. Note that p-Akt protein expression decreased in the MBH of the 31 day-old ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 8 in each group. ***p<0.001 versus CON.
Figure 7.
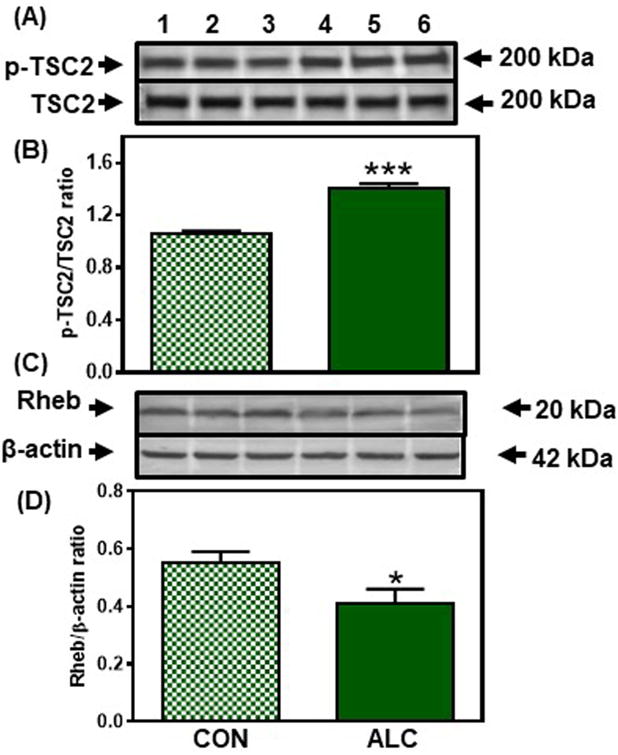
Effect of chronic ALC exposure on tuberous sclerosis 2 (TSC2) phosphorylation (p) and ras homolg enriched in brain (Rheb) expression in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of p-TSC2 (Ser1387) and total TSC2 proteins in the MBH isolated from 31 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing p-TSC2 levels normalized to total TSC2 protein expression in the MBH. Note that p-TSC2 protein expression increased in the MBH of the ALC-treated animals at 31 days of age. (C) Representative Western immunoblot of Rheb and β-actin proteins in the MBH isolated from 31 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (D) Densitometric quantitation of all of the bands from two blots assessing Rheb protein expression in the MBH. These data were normalized to the internal control, β-actin protein, and the densitometric units represent the Rheb/β-actin ratio. Note that Rheb protein expression decreased in the MBH of the ALC-treated animals at 31 days of age. The respective bars illustrate the mean (±SEM) of an N of 8 in each group. *p<0.05; ***p<0.001 versus CON.
Figure 8.
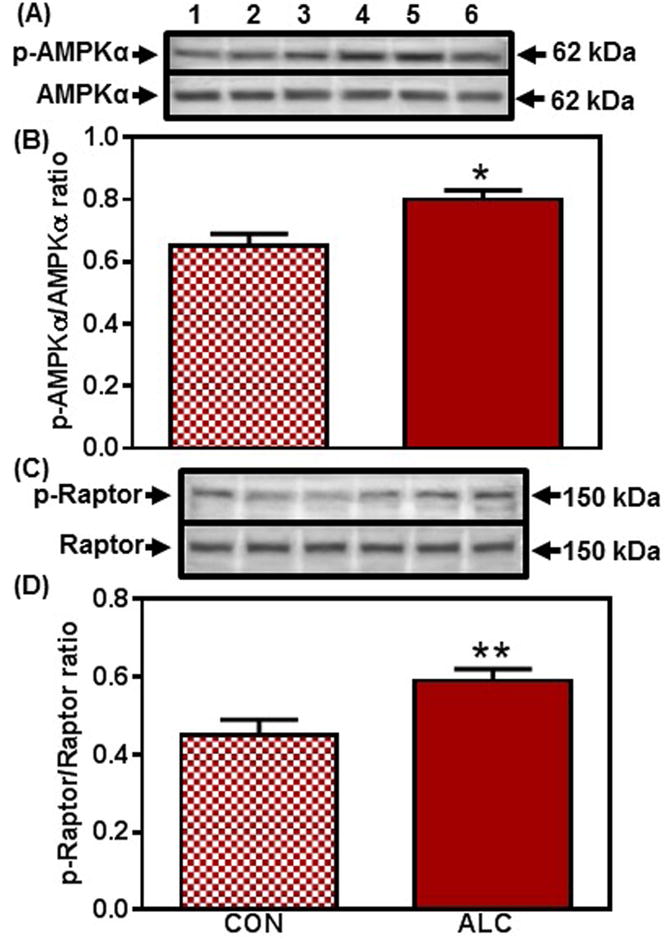
Effect of chronic ALC exposure on the phosphorylation (p) of AMP-protein kinase (AMPK)α and Raptor in medial basal hypothalamus (MBH) of prepubertal female rats. (A) Representative Western immunoblot of p-AMPKα (Thr172) and total AMPKα proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing p-AMPKα levels normalized to total AMPKα protein expression in the MBH. Note that p-AMPKα protein expression increased in the MBH at 29 days of age in the ALC-treated animals. (C) Representative Western immunoblot of p-Raptor (Ser792) and total Raptor proteins in the MBH isolated from 29 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (D) Densitometric quantitation of all of the bands from two blots assessing p-Raptor normalized to total Raptor protein expression in the MBH. Note that p-Raptor protein expression increased in association with the increased AMPKα at 29 days of age in the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 8-11 in each group. *p<0.05; **p<0.01 versus CON.
Actions of ALC in the RHA
As expected, protein levels of DYN and NKB were low in this brain region, and furthermore, no differences in expression levels were detected in either of these peptides between control and ALC-treated animals (not shown). Also, IGF-1 is considered an upstream regulator of Kp synthesis in the RHA, an action that is not applicable to the MBH (Hiney et al., 2009; Srivastava et al., 2016). Therefore, since this IGF-1 action in the RHA has been associated with activation of Akt (Srivastava et al., 2016), we also assessed the effects of prepubertal ALC administration on expression of the IGF-1R, as well as the components of the Akt-mediated pathway to Kp in this brain region. In this regard, ALC did not affect expression of the IGF-1R nor any of the components of the Akt pathway to Kp within the RHA from 28 through 31 days of age (not shown). However, the ALC-treated animals showed suppressed (p<0.05) p-IGF-1R expression on day 33 (Fig. 9), an action associated with decreased (p< 0.05) protein expression of p-Akt (CON: 0.57±0.07 vs ALC: 0.35±0.04). Downstream from Akt, the results at 33 days were similar to those shown above in the MBH. Specifically, ALC caused an increase (p<0.05) in the phosphorylation of TSC2 (CON: 0.76±0.09 vs ALC: 1.05±0.06) resulting in the suppressed (p<0.05) expression of Rheb (CON: 1.18±0.10 vs ALC: 0.81±0.10). Finally, the effect of ALC to suppress Rheb caused a decrease (p<0.05) in p-mTOR (CON: 0.73±0.08 vs ALC: 0.45±0.05) and subsequently, a marked decline (p<0.01) in the expression of Kp (CON: 0.58±0.03 vs ALC: 0.37±0.04).
Figure 9.
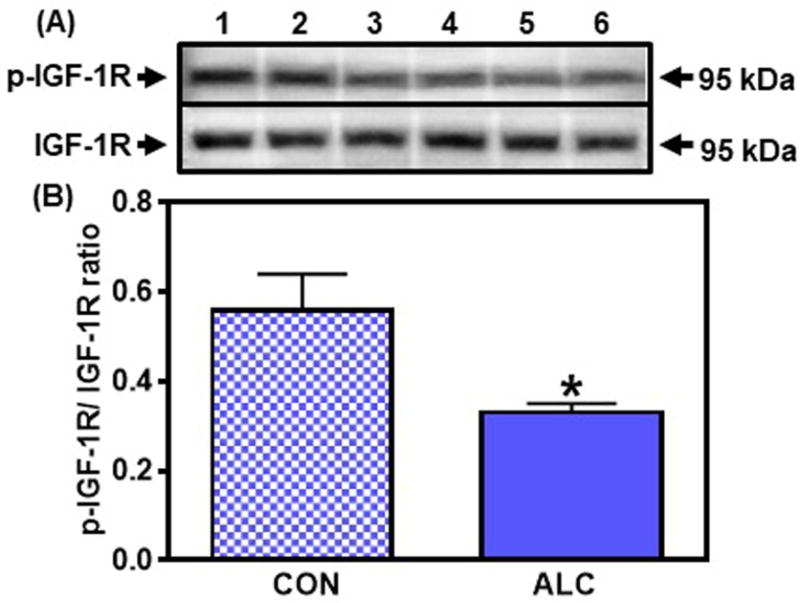
Effect of chronic ALC exposure on phosphorylation (p) of insulin-like growth factor 1R (IGF-1R) in the rostral hypothalamic area (RHA) of prepubertal female rats. A) Representative Western immunoblot of p-IGF-1R and IGF-1R proteins in the RHA isolated from 33 day-old control (CON; lanes 1-3) and ALC-treated (ALC; lanes 4-6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing p-IGF-1R levels normalized to total IGF-1R protein expression in the RHA. Note that IGF-1R protein levels decreased in the RHA in the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 6 in each group. *p<0.05 versus CON.
DISCUSSION
Initiation of puberty occurs as the result of a decline in the actions of inhibitory influences that have provided the prepubertal brake on LHRH secretion, along with the developing increased responsiveness to stimulatory influences that drive release of the peptide until first ovulation and maturity. DYN is known to inhibit prepubertal LHRH release (Navarro et al., 2009; Lehman et al., 2010), whereas NKB and Kp are potent stimulators of this peptide at the onset of puberty (Thompson et al., 2004; Navarro et al., 2009; Ramaswamy et al., 2010; Garcia et al., 2017). Since ALC can delay signs of pubertal development in rats (Dees and Skelley, 1990), monkeys (Dees et al., 2000; Dissen et al., 2004) and humans (Peck et al. 2011; Richards and Oinonen, 2011), we assessed its effects on these specific hypothalamic neuropeptides that play key roles during the maturation process. The present results have demonstrated the ontogeny of the respective differential changes in the synthesis of these peptides in the hypothalamus due to prepubertal ALC exposure. Below we discuss the important changes in these inhibitory and excitatory peptides observed within their respective brain regions in terms of mechanisms associated with ALC-induced delayed puberty.
Actions of ALC on DYN and KOR-1
Prior to the onset of puberty, DYN is already active within the MBH since it, along with other opioid peptides (Sirinathsinghji et al., 1985; Faletti et al., 1999; Terasawa and Fernandez, 2001; Navarro et al., 2009; Lehman et al., 2010), contribute to the brake on the pubertal process by inhibiting LHRH secretion. Previously, ALC was shown to increase DYN gene expression within the adult hypothalamus (Chang et al., 2007), and now we have demonstrated that prepubertal ALC administration, beginning the evening of day 27, was able to induce a greater expression of DYN protein by day 29 than that observed in the control animals. This increased expression persisted through days 31 and 33. The increased expression noted on day 33 is in agreement with our previous report (Srivastava et al., 2015). Compared to controls, the ALC-treated animals also showed an increase in expression of KOR-1, however, this effect was not noted until day 33. This increase was likely due to a combination of the continued prepubertal stimulation of KOR-1 by DYN in the animals receiving ALC, as well as the expected downregulation of the receptor in the normally maturing control animals due to the decline in the inhibitory tone beginning around this time of development. Importantly, we have already shown that ALC can induce DYN secretion from the prepubertal MBH (Srivastava et al., 2015). Thus, taken together it appears that the ALC-induced increase in the synthesis and release of DYN contributes to the prolonging of the brake on puberty by continuing to provide the inhibitory influence over LHRH release.
Actions of ALC on NKB and NK3R
NKB synthesis is known to increase in the MBH during late prepubertal development (Gill et al., 2012). This event is critical for the onset of puberty (Topaloglu et al., 2009) since this peptide, along with Kp, plays a facilitative role in developing the pulsatile secretion pattern of LHRH (Ramaswamy et al., 2010; Garcia et al., 2017; Goodman et al., 2014; Navarro et al., 2009). In this study, we first assessed the synthesis of NKB protein and observed that its expression was the same in both control and ALC-treated animals throughout the course of the study; however, protein expression of the NK3R, was decreased at 31 days, and continued to be suppressed for the remainder of the study. This is important because NK3R is also known to increase throughout late pubertal development (Gill et al., 2012). Thus, our research suggests that ALC interferes with the normal action of NKB to facilitate prepubertal LHRH/LH secretion by suppressing NK3R synthesis.
Actions of ALC on Kp
KiSS-1 is a metastasis suppressor gene that is responsible for the synthesis of Kp. The expression of KiSS-1 in the hypothalamus increases during pubertal development in primates (Shahab et al., 2005) and rats (Navarro et al., 2004a). In the MBH, neurons expressing KiSS-1 are localized within the AN, whereas in the RHA, the KiSS-1 neurons are localized within the anteroventral periventricular nucleus. These two populations of neurons appear have different physiological roles with regard to controlling LHRH secretion at puberty. The Kp neurons in the AN are proposed to be involved in controlling the pulsatile secretion of LHRH (Lehman et al., 2010; Li et al., 2009), whereas the Kp neurons in the RHA participate in the activation of LHRH neurons leading up to the first preovulatory surge at puberty (Clarkson et al., 2010). Below the MBH and the RHA are considered separately because of their different functions at puberty, and since the two regions were differentially affected by the ALC.
In the MBH, we initially showed that ALC suppressed Kp protein expression at 29 days, two days after ALC administration began. Also at 29 days, we observed a decrease in the protein expression of p-mTOR, an upstream regulator of KiSS-1/Kp (Roa et al., 2009). This decline in p-mTOR protein levels was also observed on days 31 and 33. To determine the site(s) of ALC actions, we initially assessed in this brain region a recently described Akt mediated upstream signaling pathway to mTOR (Inoki et al., 2002; Manning et al., 2002; Long et al., 2005) and subsequently, Kp (Srivastava et al., 2016). Importantly, the constituents of this Akt pathway were not altered by the ALC on day 29, but were first affected on day 31. In this regard, ALC markedly inhibited phosphorylation of Akt on days 31 and 33. This action caused a downstream increase in TSC2, which resulted in the inactivation of Rheb, and thereby decreasing the synthesis of mTOR and Kp. Interestingly, since mTOR and Kp were already depressed at 29 days, there must have been an another pathway to mTOR that was affected by the ALC prior to the action of the Akt pathway. In this regard, we assessed AMPKα, a known inhibitor of mTOR activation via raptor phosphorylation (Gwinn et al. 2008). Our results demonstrated that ALC induced the phosphorylation of AMPK at 29 days, which in turn induced the phosphorylation of raptor and hence, caused the suppression of mTOR and subsequently, Kp. Thus, the present study supports the observations that mTOR activation is influenced by different signaling pathways (Gwinn et al. 2008; Inoki et al. 2002), and clearly shows that ALC activates the hypothalamic AMPKα signaling pathway to inhibit mTOR prior to its ability to suppress the Akt mediated pathway. However, it is clear that the actions of ALC on both pathways contribute to the suppressed synthesis of Kp within the MBH.
It is important to note that the Kp producing neurons of the MBH are involved in regulating LHRH secretion by acting not only in the MBH itself, but also by projecting some of their nerve fibers rostrally into the RHA and POA (Yeo and Herbison, 2011). In the MBH, Kp has been shown to regulate LHRH secretion in vivo (Popa et a., 2008; Li et al., 2009; Lehman et al., 2010), and to stimulate release of the LHRH peptide from its nerve terminals in the median eminence (ME; d’Anglemont de Tassigny et al., 2008; Smith et al., 2011). Interestingly, the mechanism by which Kp stimulates LHRH release from the ME is not yet understood, since the Kp containing fibers from the AN do not appear to project into the external layer of the ME. However, several investigators have proposed that the Kp is released within the internal layer of the ME and subsequently, acts through volume transmission to evoke the release of LHRH in the external layer. In the RHA and POA, nerve fibers arising from the AN impinge upon and influence LHRH neurons that express Kp receptors in these areas (Han et al., 2005; Messager et al., 2005). Thus, the ALC-induced decrease in Kp synthesis by neurons in the AN beginning at 29 days indicates a diminished excitatory influence of Kp on the LHRH system early in the pubertal process.
In the RHA, ALC again suppressed mTOR and Kp proteins; however, this effect was not noted until day 33, four days after the effects on these proteins were noted in the MBH. To determine the upstream site by which ALC acts to suppress Kp, we first assessed insulin-like growth factor 1 receptor (IGF-1R) protein expression, since the IGF-1 peptide has been shown to be an upstream regulator of Kp synthesis in this brain region, but not in the MBH (Hiney et al., 2009; Srivastava et al., 2016). Furthermore, IGF-1 is critical for the onset of puberty in rats, monkeys and humans (Hiney et al., 1996; Wilson, 1998; Juul et al., 1994), and activation of the IGF-1R by the peptide causes phosphorylation of the Akt mediated pathway to mTOR and Kp, an action blocked by acute ALC administration (Hiney et. al., 2018). Importantly, results of the present study showed that chronic ALC administration first suppressed IGF-1R phosphorylation on day 33. This action was likely due to the down regulation of the receptor resulting from an ALC-induced decrease in IGF-1 mRNA synthesis in the liver (Srivastava et al., 1995; Long et al., 2009) resulting in lower circulating levels of IGF-1 crossing the blood brain barrier and entering the hypothalamus (Srivastava et al., 1995). The ability of ALC to suppress activity of the IGF-1R in this brain region resulted in subsequent decreases in protein expression of each of the downstream components of the Akt-mTOR mediated pathway to Kp described above, but again, not before day 33. Additionally, these ALC-induced suppressions on the IGF-1 and mTOR signaling pathways are consistent with results reported in other brain regions (Tong et al., 2015) as well as in skeletal (Lang et al. 2009; Simon et al., 2018) and cardiac (Vary et al, 2008; Zhang et al., 2010) muscle.
The ALC-induced suppression in Kp synthesis by neurons in the RHA occurs later in development than the effects observed in Kp neurons within the MBH. This is not unexpected since the principal action of Kp synthesized in the RHA during normal development is to increase the synthesis and release of LHRH to drive the pubertal process to its preovulatory surge during first proestrus (Clarkson and Herbison, 2009; Clarkson et al., 2010; Popa et al., 2008). However, we demonstrated here that compared to control animals, the synthesis of Kp in the ALC-treated animals was suppressed in the RHA at 33 days, a time when its synthesis would be in demand and increasing toward proestrus; thus, further showing evidence of delayed development. Importantly, the Kp synthesizing neurons in the RHA normally respond to the increased serum levels of estradiol (E2; Lehman et al. 2010; Clarkson et al., 2010) and IGF-1 (Hiney et al. 1996; Hiney et al., 2009; Srivastava et al., 2016) that enter the brain as maturation progresses toward first ovulation. It is clear that ALC causes depressed serum levels of both of these puberty-related hormones, and therefore, their availability to the hypothalamus (Dees et al., 2000; Srivastava et al., 2009). This is relevant since during routine development IGF-1 can stimulate the Kp producing neurons in the RHA at first proestrus, when E2 levels are rising (Hiney et al., 2018). It is critical to note that these RHA neurons send some of their nerve processes caudally to the MBH, but also project processes to adjacent LHRH synthesizing neurons in other areas of the RHA and in the POA, that, as stated above, express Kp receptors. Current evidence indicates that once Kp is released within the RHA (Hiney et al., 2018) it stimulates LHRH synthesis and secretion by a direct action on these LHRH neurons (Han et al., 2005; Messager et al., 2005; Clarkson et al., 2006; Smith et al., 2008; Decourt et al., 2008). Importantly, a recent study has revealed that ALC suppresses IGF-1 induced synthesis and release of Kp within the RHA at first proestrus (Hiney et al, 2018), supporting a previous report indicating ALC can inhibit the preovulatory surge of LHRH/LH and ovulation (Ogilvie and Rivier, 1997).
We have assessed the effects of prepubertal ALC administration, beginning when the animals were 27 days old, on three specific inhibitory or excitatory neuropeptide regulators of LHRH that reside within the hypothalamus. By day 29, ALC caused an increase in the synthesis of DYN, a known inhibitor of prepubertal LHRH within the MBH. At this same time, the ALC caused a decrease in the synthesis of Kp, a potent stimulator of LHRH. Also, while the synthesis of another excitatory peptide, NKB, was unaffected by ALC, the synthesis of the NK3R was suppressed by day 31; thus, also suggesting a depression in the stimulatory effects of NKB within the MBH. The fact that ALC caused a reversal in the availability of these critical puberty-related peptides is consistent with delayed maturation. In this regard, research indicates that DYN, Kp and NKB may normally operate within the MBH in an integrative fashion (Lehman et al., 2010; Li et al., 2009) to develop the pulsatile pattern of prepubertal LHRH secretion; therefore, we suggest that ALC exposure alters the balance of these peptides by a mechanism which decreases the pulsatile release of prepubertal LHRH/LH (Hiney et al., 1998; Hiney et al., 2003).
In the RHA, we observed an ALC-induced suppression in Kp synthesis by day 33. This is important since increased Kp synthesis and release in this brain region is normally responsible for activating the nearby LHRH neurons to drive the pubertal process into first proestrus and initiate the LHRH preovulatory surge; hence, this reversal is also indicative of delayed maturation, since Kp should have been increasing within the RHA at this time. Finally, we have also described the sites of action and differential effects by which ALC alters the respective Kp synthesis pathways in the MBH and RHA. This research, as summarized in Figure 10, clearly shows how and when ALC differentially effects the timely synthesis of critical inhibitory and excitatory hypothalamic neuropeptides to negatively influence the pubertal process and cause delayed maturation.
Figure 10.
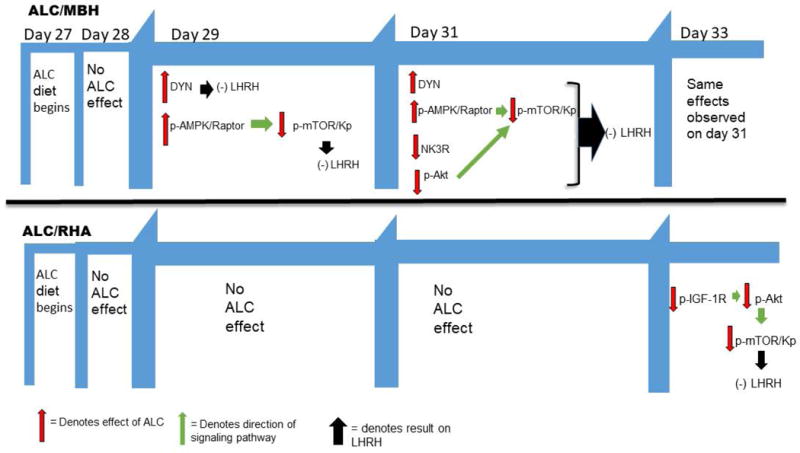
Schematic showing the timing of the effects of ALC resulting in delayed pubertal development. In the medial basal hypothalamus (MBH), ALC stimulated dynorphin (DYN), as well as the p-AMPKα/p-Raptor pathway by day 29, both known to cause suppressed LHRH secretion. These effects persisted on day 31 when ALC additionally caused suppression of the neurokinin B receptor (NK3R), as well as suppression of the p-Akt pathway; hence, inducing further inhibition of LHRH. In the rostral hypothalamic area (RHA), ALC did not have an effect until day 33, at which time it suppressed regulation of the insulin-like growth factor 1 receptor (IGF-1R), resulting in the inhibition of the p-Akt pathway to Kp and thus, delaying the well-known increase in LHRH needed to enter first proestrus.
Acknowledgments
Funding: Supported by the National Institutes of Health Grant AA-007216 (to W.L.D.).
Footnotes
DR. JILL K. HINEY (Orcid ID: 0000-0001-9286-9229)
Declaration of interest: The authors have no conflict of interest.
References
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function in within the arcuate nucleus. J Comp Neurology. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Chang G, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: Relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiologoical mechanism underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinizing hormone surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl-D,L-aspartate induces the release of LHRH in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3943. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Dees WL. Leptin acts centrally to induce the prepubertal secretion of luteinizing hormone in the female rat. Peptides. 2000;21:387–392. doi: 10.1016/s0196-9781(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36:131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. doi: 10.1210/endo.141.4.7413. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW. Effects of ethanol during the onset of female puberty. Neuroendocrinology. 1990;51:64–69. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW, Hiney JK, Johnston CA. Actions of ethanol on hypothalamic and pituitary hormones in prepubertal female rats. Alcohol. 1990;7:21–25. doi: 10.1016/0741-8329(90)90055-h. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW, Kozlowski GP. Intragastric cannulation as a method of ethanol administration for neuroendocrine studies. Alcohol. 1984;1:177–180. doi: 10.1016/0741-8329(84)90094-6. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J, Matsuda F, Chaussin J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GRP54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Ojeda SR, Dearth RK, Scott H, Dees WL. Alcohol alters luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004;145:4558–4564. doi: 10.1210/en.2004-0517. [DOI] [PubMed] [Google Scholar]
- Emanuele N, Ren J, LaPaglia N, Steiner J. Ethanol disrupts female mammalian puberty: age and dependence. Endocrine. 2002;18:247–254. doi: 10.1385/ENDO:18:3:247. [DOI] [PubMed] [Google Scholar]
- Faletti AG, Mastonardi CA, Lomniczi A, Seilicovich A, Gimeno M, McCann SM, Rettori V. ß-endorpin blocks luteinizing hormone-releasing hormone release by inhibiting the nitricoxidergic pathyway controlling its release. Proc Natl Acad Sci USA. 1999;96:1722–1726. doi: 10.1073/pnas.96.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. 2017;158:3269–3280. doi: 10.1210/en.2017-00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay VL, Plant TM. N-methyl-DL-Aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca Mulatta) Endocrinology. 1987;120:2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, Clifton DK, Carroll RS, Steiner RA, Kaiser UB. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than KiSS-1. Endocrinology. 2012;153:4883–4893. doi: 10.1210/en.2012-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99:1–15. doi: 10.1159/000355285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay Z, Canan H, Serin A, O’Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol and Metab. 2009;94:3633–3639. doi: 10.1210/jc.2009-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiney JK, Dearth RK, Srivastava V, Rettori V, Dees WL. Actions of ethanol on epidermal growth factor receptor activated luteinizing hormone secretion. J Stud Alcohol. 2003;64:809–816. doi: 10.15288/jsa.2003.64.809. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Lara F, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998;62:301–308. doi: 10.1016/s0024-3205(97)01111-9. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF-1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3727. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Vaden Anderson DN, Hartzoge NL, Dees WL. Regulation of kisspeptin synthesis and release in the preoptic/anterior hypothalamic region of prepubertal female rats: Actions of IGF-1 and alcohol. Alcoholism: Clin Exp Research. 2018;42:61–68. doi: 10.1111/acer.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by akt and suppresses mTOR signaling. Nature Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-1 in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol and Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutrition & Metabolism. 2009;6:4. doi: 10.1186/1743-7075-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, Gérard A, Parent AS, Junien JL, Bourguignon JP. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology. 2000;141:1464–1469. doi: 10.1210/endo.141.4.7432. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Kinsey-Jones JS, Cheng Y, Knox AMI, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signaling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE. 2009;4(12):e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinistide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci. 2005;102:1761–1776. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated mRNA expression of KiSS-1 and its putitive receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Effect of alcohol on the proestrous surge of luteinizing hormone (LH and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J Neurosci. 1997;17:2595–2604. doi: 10.1523/JNEUROSCI.17-07-02595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JD, Peck BM, Skaggs VJ, Fukushima M, Kaplan HB. Socio-environmental factors associated with pubertal development in female adolescents: The role of tobacco and alcohol use. J Adolesc Health. 2011;48:241–246. doi: 10.1016/j.jadohealth.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Ann Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca Mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Oinonen KA. Age at menarche is associated with divergent alcohol use patterns in early adolescence and early adulthood. J Adolesc. 2011;34:1065–1076. doi: 10.1016/j.adolescence.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, Gaytan F, Dieguez C, Pinilla L, Tena-sempere M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatizidaki E, Thresher A, Acierno J, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt S, Gusella J, O’Rahilly S, Carlton M, Crowley W, Aparicio S, Colledge W. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Jolley SE, Molina PE. Alcoholic Myopathy: Pathophysiologic mechanisms and clinical implications. Alcohol Research: Current Reviews. 2018;38:207–217. [PMC free article] [PubMed] [Google Scholar]
- Sirinathsinghji DJS, Motta M, Martini L. Induction of precocious puberty in the female rat after chronic naloxone administration during neonatal period: the opiate “brake” on pubertal gonadotrophin secretion. J Endocrinol. 1985;104:299–307. doi: 10.1677/joe.0.1040299. [DOI] [PubMed] [Google Scholar]
- Smith JT, Collen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) espression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar GP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- Srivastava V, Hiney JK, Nyberg CL, Dees WL. Effect of ethanol on the synthesis of insulin-like growth factor-1 (IGF-1) and the IGF-1 receptor in late prepubertal female rats: a correlation with serum IGF-1. Alcoholism: Clin Exp Res. 1995;19:1467–1473. doi: 10.1111/j.1530-0277.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Short-term alcohol administration alters KiSS-1 gene expression in reproductive hypothalamus of prepubertal female rats. Alcoholism: Clin Exp Res. 2009;33:1605–1614. doi: 10.1111/j.1530-0277.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Stevener K, Dees WL. Differential effects of alcohol on excitatory and inhibitory puberty-related peptides in the basal hypothalamus of the female rat. Alcoholism: Clin Exp Res. 2015;39:2386–2393. doi: 10.1111/acer.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Manganese stimulated kisspeptin is mediated by the IGF-1/akt/mammalian target of rapamycin pathway in the prepubertal female rat. Endocrinology. 2016;157:3233–3241. doi: 10.1210/en.2016-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological-mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Tong M, Yu R, Deochand C, de la Monte SM. Differential contributions of alcohol and the nicotine-derived nitrosamine ketone (NNK) to insulin and insulin-like growth factor resistance in the adolescent rat brain. Alcohol Alcoholism. 2015;50:670–679. doi: 10.1093/alcalc/agv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Vary TC, Deiter G, Lantry R. Chronic alcohol feeding impairs mTOR(ser2448) phosphorylation in rat hearts. Alcoholism: Clin Exp Res. 2008;32:43–51. doi: 10.1111/j.1530-0277.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-1 advances first ovulation in monkeys. J Endocrinology. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- Yeo SH, Herbison AE. Proliferations of arcuate nucleus and rostral periventricular kisspeptin neurons in adult female mouse brain. Endocrinology. 2011;152:2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, Ren J. Cardiac overexpression of insulin-like growth factor 1 attentuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: Roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 2010;49:1238–1253. doi: 10.1016/j.freeradbiomed.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


