With rising life expectancy of the population worldwide, the prevalence of open-angle glaucoma and systemic hypertension will increase in the coming decades.(1, 2) Both conditions are chronic and age-related. Moreover, lowering blood pressure (BP) is a proven strategy to prevent death from cardiovascular causes,(3) and lowering intraocular pressure (IOP) is a proven strategy for preventing visual impairment.(4) Given the frequent coexistence of these two conditions,(5) which disproportionately affect at-risk populations, and their complex underlying mechanisms – including treatment interactions – there has been growing interest in better understanding their relationship.
Because the relationship between glaucoma and systemic hypertension is multifaceted and often puzzling, it is important to first understand some basic features of these conditions and how they correlate with one another. First, there is a positive, significant (though weak) relationship between systemic BP and IOP. In a longitudinal population-based study, there was a 0.21 and 0.43 mm Hg increase in IOP for each 10 mm Hg increase in systolic and diastolic BP, respectively. More importantly, longitudinally measured decreases in systolic or diastolic BP by more than 10 mm Hg over 5 years were significantly associated with decreased IOP.(6) However, the pathophysiology underlying this relationship remains unclear. Second, there is a significant relationship between the prevalence rates of open-angle glaucoma and systemic hypertension. In the British General Practitioner Research Database (GPRD), 13% of patients diagnosed with glaucoma had concomitant systemic hypertension and glaucoma patients were 23 to 36% more likely to suffer from systemic hypertension than non-glaucomatous controls.(5) Nonetheless, when evaluating the diastolic perfusion pressure (diastolic BP – IOP), four population-based studies (7–10) have shown that this relationship is non-linear. In fact, these studies suggest that this relationship follows a non-linear trend, with highest glaucoma prevalence among patients with lowest diastolic perfusion pressure (Figure 1). Finally, treatment for systemic hypertension is a potential modifier of the relationship between systemic BP and glaucoma. For instance, in the Thessoloniki Eye Study, although the effect of antihypertensive treatment on the prevalence of open-angle was not statistically significant, diastolic ocular perfusion pressure was significantly associated with glaucoma in subjects on antihypertensive treatment (28% higher risk per 10 mm Hg lower diastolic perfusion pressure).(11) Note that this finding does not imply that treating systemic hypertension increases the risk of glaucoma, but rather that among those treated for this condition having a lower ocular perfusion pressure increases that risk. This will be the focus of our discussion, in particular the left-tail of this non-linear relationship between perfusion pressure and glaucoma and how lower perfusion pressure can influence the outcomes of glaucoma, particularly among patients treated for systemic hypertension.
Figure 1.
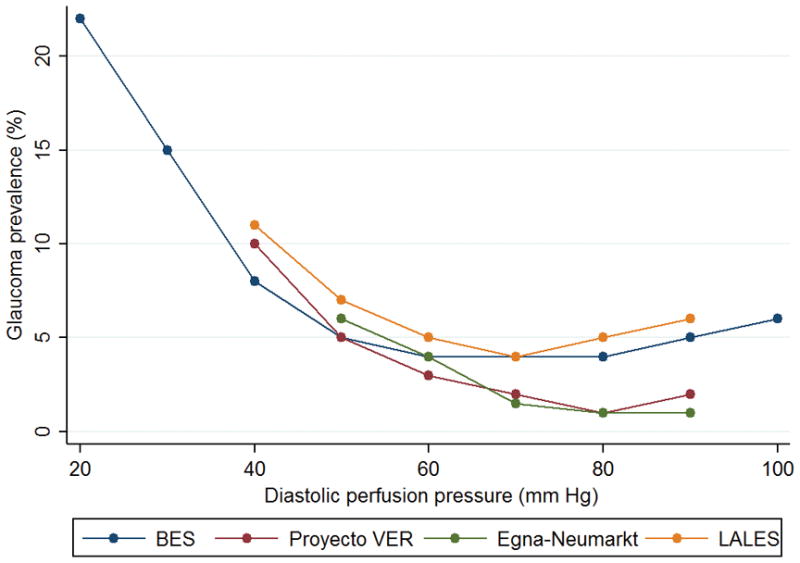
Composite curves depicting the relationship between diastolic ocular perfusion pressure (diastolic blood pressure - intraocular pressure) and glaucoma prevalence in 4 population-based studies. BES: Baltimore Eye Survey; LALES: Los Angeles Latino Eye Survey.(7–9)
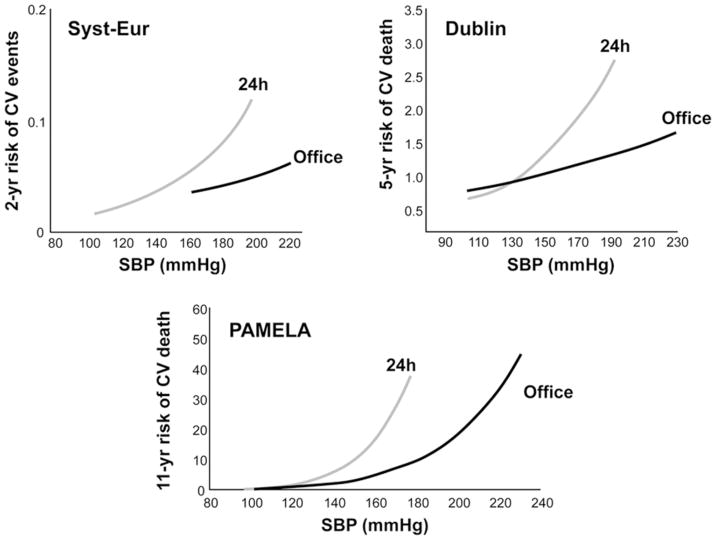
Population-based studies,(11–13) prospective longitudinal studies,(14–16) and randomized clinical trials(4, 17) have provided compelling evidence that lower systemic BP and lower ocular perfusion pressure are associated with greater glaucoma prevalence and a higher rate of glaucoma progression. Nonetheless, there is little agreement regarding the parameters that can best explain this relationship. For instance, the Early Manifest Glaucoma trial (EMGT) reported that single, daytime lower ocular systolic perfusion pressure (defined as systolic BP minus intraocular pressure IOP) was a significant predictor of visual field progression in patients followed for a median of 8 years.(4) When analyzing a subset of patients with low baseline IOP, however, lower systolic BP also was a significant predictor for progression. In the Low-pressure Glaucoma Treatment Study, daytime mean ocular perfusion pressure (defined as mean arterial pressure minus IOP) measured every 4 months during follow-up was independently associated with visual field progression. As systemic blood pressure has a circadian rhythm, a notable limitation of both aforementioned trials is that ambulatory BP monitoring was not performed. There is strong evidence that BP measurements obtained from ambulatory monitoring hold a steeper relationship with cardiovascular events and death than those obtained during office-hour (Figure 2).(18) Therefore, it is reasonable to suggest that this approach should also be extended to studies that aim to assess the relationship between systemic BP and glaucoma progression.
Figure 2.
Relationship between office blood pressure or 24-hour average systolic blood pressure with cardiovascular events or mortality in 3 studies: the Systolic Hypertension in Europe (SYST-EUR) study on patients with isolated systolic hypertension, the Dublin study, and the Pressioni arteriose monitorate e loro associazioni (PAMELA) population study.(18)
Following that recommendation, Costa et al.(19) compared the 24 hour IOP, BP, and perfusion pressure of primary open-angle glaucoma patients with healthy individuals and found that, despite higher systolic perfusion pressure measurements among POAG patients during the morning, they had lower diastolic perfusion pressure during the night. When employing 48-hour ambulatory BP monitoring, Charlson et al.(14) found that the duration and magnitude of the nocturnal fall of mean arterial BP relative to daytime average were the best predictors of progressive visual field loss. Similarly, in the Maracaibo Aging Study, extreme falls in nighttime – but not daytime – systolic and diastolic blood pressure were significantly associated with a higher prevalence of glaucomatous optic neuropathy in a population followed with 24-hour BP monitoring. More recently, a meta-analysis (with the highest level of evidence) showed that there was no difference in mean systolic or diastolic diurnal and nocturnal BP between patients with or without progressive visual field loss.(20) Notwithstanding this finding, nocturnal reductions >10% in systolic or diastolic blood pressure were associated with progressive visual field loss in glaucoma.
Despite these different definitions of BP parameters and how they correlate with glaucoma progression, there is converging evidence that this relationship is a causative one. As previously mentioned, there is also increasing evidence that medical treatment (or perhaps, overtreatment) of systemic hypertension is a significant modifier of this relationship.(11, 14, 17, 20) Therefore, a combined approach that includes glaucoma specialists and primary care physicians is warranted for patients with coexisting glaucoma and medically treated systemic hypertension. In addition, the highest-level of evidence recommends that “anti-hypertensive therapy in glaucomatous patients should be controlled with ambulatory blood pressure monitoring”.(20)
A new challenge to our current management of coexisting glaucoma and medically treated systemic hypertension has developed with the publication of the Systolic Blood Pressure Intervention Trial (SPRINT).(3) SPRINT is a multicenter, randomized clinical trial of 9,361 patients with systemic hypertension (but without diabetes). The investigators assessed whether achieving a target systolic BP of 120 mm Hg compared to the usual target of 140 mm Hg reduced a combined primary cardiovascular disease endpoint that included myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes. Importantly, the intervention was stopped early after a median follow-up of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group than in the standard-treatment group (1.65% per year vs. 2.19% per year; hazard ratio with intensive treatment, 0.75; 95% confidence interval [CI], 0.64 to 0.89; P<0.001). Given that the ultimate goal of medical therapy is to increase longevity and mitigate comorbidities, the results of SPRINT have had a meaningful impact on the medical community. In addition, the 2017 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines now identifies the overall goal of treatment as a reduction in BP to a systolic BP target of less than 130 mm Hg and a diastolic BP target of less than 80 mm Hg.(21) This is a remarkable change from the 2003 Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, which recommended a goal of less than 140/90 mm Hg (or 130/80 mm Hg for patients with diabetes or chronic kidney disease).(22) Based upon this new definition, about 46% of U.S. adults will have a diagnosis of systemic hypertension, as compared with about 32% under the previous definition.(23) As suggested in a recent perspective article on the topic, “the unintended consequence may be that many people, now labeled as patients with hypertension, receive pharmacologic therapy that is unlikely to provide benefit given their low absolute risk, and they may therefore experience unnecessary adverse events.”(23)
Although lowering BP on a population basis enhances longevity and decreases morbidity from cardiovascular events, personalized medicine dictates that these tenets be applied to the appropriate individuals. Of note, in SPRINT the number needed to treat (NNT) with a strategy of intensive blood-pressure control to prevent one primary outcome event was 61, and the number needed to treat to prevent one death from any cause was 90. In addition, rates of serious adverse events of hypotension, syncope, electrolyte abnormalities, and acute kidney injury or failure were higher in the intensive-treatment group than in the standard-treatment group.(3) Given that kidney function is the most widely applied surrogate measure for tissue perfusion, many reports addressing these adverse events have stressed their clinical importance.(24–26) In SPRINT, 3.8% of participants suffered acute renal injury events in the intensive arm and 2.3% in the standard arm (HR, 1.64; 95% CI, 1.30–2.10; P<0.001).(24) The National Health and Nutrition Examination Survey (NHANES) produced a projection of estimated deaths prevented and excess serious adverse events incurred if the SPRINT intensive systolic BP treatment goal were implemented in all eligible U.S. adults. Even though intensive BP control was projected to prevent 46,100 (95% CI, 41,800–50,400) cases of heart failure annually, it would give rise to 56,100 (95% CI, 50,800–61,400) episodes of hypotension, 34,400 (95% CI, 31,200–37,600) episodes of syncope, 43,400 (95% CI, 39,400–47,500) serious electrolyte disorders, and 88,700 (95% CI, 80,400–97,000) cases of acute kidney injury per year.(26) Importantly, SPRINT did not include routine assessment for glaucoma or its progression.
Based upon estimates of the prevalence rates of open-angle glaucoma around 2.5% in the U.S. population age 40 and older [https://nei.nih.gov/eyedata/glaucoma] and that of systemic hypertension of 40% [https://www.cdc.gov/nchs/fastats/hypertension.htm], we estimate that the prevalence of open-angle glaucoma among patients with systemic hypertension is around 0.8% in that age group. Therefore, there are approximately 494,000 patients with coexisting glaucoma and treated systemic hypertension in the U.S. This number may increase to 880,000 and 1.3 million in 2030 and 2050, respectively, assuming the proportion of patients with systemic hypertension among open-angle glaucoma remains constant. Blood pressure dipping has been defined by a working group of the European Society of Hypertension as a fall of greater than 10% for systolic or diastolic blood pressure compared to their respective daytime values.(27) Based upon 2009 data, the percentage nocturnal fall in mean arterial pressure was 11.5±7.0% in the controlled hypertensive patients,(28) which translates to 41% of patients experiencing greater than 10% dipping. With the newer and stricter SPRINT guidelines, this number may increase significantly. Extrapolating these numbers to the 2010, 2030, and 2050 data, there may be 202,000, 360,000, and 530,000 significant dippers among patients with coexisting glaucoma and treated systemic hypertension. Given the new guidelines for BP targets with their potential increase in rates of nocturnal BP falls and an odds ratio of 3.0 for deteriorating visual fields over 2 years with nocturnal falls greater than 10%,(20) it is plausible that there will be hundreds of thousands of new, unexpected, glaucoma progression events in the next decades.
Assuming that optic nerve hypoperfusion occurs when impaired auto-regulation is superimposed with intense BP lowering, and given the large body of evidence on the (causative) association between low BP and glaucoma progression, an extrapolation of the SPRINT findings and the new guidelines for treatment of systemic hypertension may have detrimental effects for glaucoma management in the coming years. Specifically, ophthalmologists and glaucoma specialists may be confronted more often by patients with coexisting glaucoma and systemic hypertension experiencing glaucoma progression despite seemingly reasonable IOP and target IOP control. For instance, for a patient whose IOP is 14 mmHg and whose BP is controlled according to the older guidelines (140/90 mmHg), the mean ocular perfusion pressure is approximately 57 mmHg. With a new target of 130/80, this would be down to 50 mmHg. Unfortunately, no other similar clinical trial of systemic hypertension included ophthalmologic evaluation to test this hypothesis. At this point, although speculative in nature, the potential burden caused by the leading cause of irreversible visual impairment and blindness in the world makes this hypothesis one of compelling public health relevance. This is particularly relevant among patients of African-American descent; they not only have an average earlier age of disease onset, but also are at increased risk of both systemic hypertension and glaucoma. They also tend to have worse outcomes from both conditions.(29, 30)
Even if our assumptions are proven correct, we do not suggest they outweigh the importance of preventing death by setting lower BP targets. However, we recommend closer surveillance of patients with these coexisting conditions. Until the availability of results from randomized trials that test whether systemic BP is a modifiable risk factor for glaucoma progression, we recommend increased surveillance of patients with coexisting glaucoma and medically-treated systemic hypertension. Specifically, this should be achieved by (i) closer communication between ophthalmologists and primary care physicians, (ii) ambulatory BP monitoring at regular intervals (e.g.: every year, or after changes in the antihypertensive regimen, or progression is suspected despite low IOP), and more frequent visual field and imaging examinations. Nonetheless, if significantly low BP is documented, particularly nocturnal falls relative to daytime averages,(13, 14, 20) there is no consensus as to the next steps of glaucoma management.(31)
Because IOP is currently the only proven modifiable risk factor for glaucoma progression,(4) it may be possible that further IOP lowering may be needed to counter-balance a decrease in ocular perfusion. Nevertheless, based upon the mean ocular perfusion pressure estimates described above, it would be problematic, for example, to lower the IOP by 7 mmHg (from 14 to 7 mmHg) to balance such meaningful change due to new BP targets. Despite the lack of evidence, one may consider mitigating nocturnal hypotension by either modifying systemic and ocular medications with vasoactive or cardiac effects which could be causing nocturnal BP falls beyond physiologic levels. Salt-loading or pharmacologic damping of nocturnal BP falls are approaches for which no evidence is yet available. More importantly, increased nocturnal BP or a non-dipping profile are associated with cardiovascular organ damage;(32) hence, clinicians should be particular cautious with interventions aimed at elevating nocturnal BP.
With the strong evidence for the detrimental effects of low systemic BP on the optic nerve, clinical trials that provide clear evidence to link these two conditions are urgently needed given the recent changes in systemic hypertension treatment guidelines. In addition, clinicians, industry, and funding agencies should consider systemic BP as a new modifiable risk factor for glaucoma and glaucoma progression and seek ways to better understand the relationship between these two highly prevalent and inter-related conditions.
Acknowledgments
Supported by: Unrestricted departmental grant from Research to Prevent Blindness, New York, NY (Department of Ophthalmology, Columbia University Medical Center, New York, NY; Department of Ophthalmology, University of California at San Diego, La Jolla, CA); NIH/NEI grant: EY025253 (CGDM): NIH/NEI grant EY029058 (RNW).
Footnotes
Financial disclosures: The authors have no disclosures relevant to the content of this manuscript.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Bloch MJ. Worldwide prevalence of hypertension exceeds 1.3 billion. Journal of the American Society of Hypertension: JASH. 2016;10(10):753–4. doi: 10.1016/j.jash.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Langman MJ, Lancashire RJ, Cheng KK, Stewart PM. Systemic hypertension and glaucoma: mechanisms in common and co-occurrence. The British journal of ophthalmology. 2005;89(8):960–3. doi: 10.1136/bjo.2004.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein BE, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. The British journal of ophthalmology. 2005;89(3):284–7. doi: 10.1136/bjo.2004.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Archives of ophthalmology (Chicago, Ill: 1960) 2001;119(12):1819–26. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 8.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107(7):1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 9.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Archives of ophthalmology (Chicago, Ill: 1960) 1995;113(2):216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 10.Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Investigative ophthalmology & visual science. 2010;51(6):2872–7. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topouzis F, Wilson MR, Harris A, Founti P, Yu F, Anastasopoulos E, et al. Association of open-angle glaucoma with perfusion pressure status in the Thessaloniki Eye Study. American journal of ophthalmology. 2013;155(5):843–51. doi: 10.1016/j.ajo.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Melgarejo JD, Lee JH, Petitto M, Yepez JB, Murati FA, Jin Z, et al. Glaucomatous Optic Neuropathy Associated with Nocturnal Dip in Blood Pressure: Findings from the Maracaibo Aging Study. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, de Moraes CG, Link A, Wells MT, Harmon G, Peterson JC, et al. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology. 2014;121(10):2004–12. doi: 10.1016/j.ophtha.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon J, Lee J, Choi J, Jeong D, Kook MS. Association Between Nocturnal Blood Pressure Dips and Optic Disc Hemorrhage in Patients With Normal-Tension Glaucoma. American journal of ophthalmology. 2017;176:87–101. doi: 10.1016/j.ajo.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Moore NA, Harris A, Wentz S, Verticchio Vercellin AC, Parekh P, Gross J, et al. Baseline retrobulbar blood flow is associated with both functional and structural glaucomatous progression after 4 years. The British journal of ophthalmology. 2017;101(3):305–8. doi: 10.1136/bjophthalmol-2016-308460. [DOI] [PubMed] [Google Scholar]
- 17.De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the low-pressure glaucoma treatment study. American journal of ophthalmology. 2012;154(4):702–11. doi: 10.1016/j.ajo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circulation research. 2015;116(6):1034–45. doi: 10.1161/CIRCRESAHA.116.303755. [DOI] [PubMed] [Google Scholar]
- 19.Costa VP, Jimenez-Roman J, Carrasco FG, Lupinacci A, Harris A. Twenty-four-hour ocular perfusion pressure in primary open-angle glaucoma. The British journal of ophthalmology. 2010;94(10):1291–4. doi: 10.1136/bjo.2009.167569. [DOI] [PubMed] [Google Scholar]
- 20.Bowe A, Grunig M, Schubert J, Demir M, Hoffmann V, Kutting F, et al. Circadian Variation in Arterial Blood Pressure and Glaucomatous Optic Neuropathy--A Systematic Review and Meta-Analysis. American journal of hypertension. 2015;28(9):1077–82. doi: 10.1093/ajh/hpv016. [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex: 1979) 2017 doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Bakris G, Sorrentino M. Redefining Hypertension - Assessing the New Blood-Pressure Guidelines. The New England journal of medicine. 2018;378(6):497–9. doi: 10.1056/NEJMp1716193. [DOI] [PubMed] [Google Scholar]
- 24.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT) American journal of kidney diseases: the official journal of the National Kidney Foundation. 2017 doi: 10.1053/j.ajkd.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, et al. Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial. Annals of internal medicine. 2017;167(6):375–83. doi: 10.7326/M16-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, et al. Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey) Circulation. 2017;135(17):1617–28. doi: 10.1161/CIRCULATIONAHA.116.025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. Journal of hypertension. 2014;32(7):1359–66. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 28.Friedman O, Logan AG. Nocturnal blood pressure profiles among normotensive, controlled hypertensive and refractory hypertensive subjects. The Canadian journal of cardiology. 2009;25(9):e312–6. doi: 10.1016/s0828-282x(09)70142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. Survey of ophthalmology. 2003;48(3):295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 30.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136(21):e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 31.Caprioli J, Coleman AL. Blood pressure, perfusion pressure, and glaucoma. American journal of ophthalmology. 2010;149(5):704–12. doi: 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Tsioufis C, Andrikou I, Thomopoulos C, Syrseloudis D, Stergiou G, Stefanadis C. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. Journal of human hypertension. 2011;25(5):281–93. doi: 10.1038/jhh.2010.113. [DOI] [PubMed] [Google Scholar]