Abstract
This article is a comprehensive review of diabetic gastroparesis, defined as delayed or disordered gastric emptying, including basic principles and current trends in management. This review includes sections on anatomy and physiology, diagnosis and differential diagnosis as well as management and current guidelines for treatment of diabetic gastroparesis. Diabetic gastroparesis (DGp) is a component of autonomic neuropathy resulting from long-standing poorly controlled type 1 and type 2 diabetes. The diagnostic workup of DGp first excludes obstruction and other causes including medications that may mimic delayed/disordered gastric emptying. Targeting nutrition, hydration, symptomatic relief and glycemic control are mainstays of treatment for DGp. Additionally, optimal treatment of DGp includes good glycemic management, often involving customizing insulin delivery using basal-bolus insulin and technology, including sensor-augmented pumps and continuous glucose monitoring systems. Prokinetic medications may be helpful in DGp symptoms, although only limited number of medications is currently available in the USA. Selected medication-refractory patients with DGp may benefit from gastric neuromodulation, and some from surgical interventions including pyloric therapies that can also be done endoscopically. As is true of any of the diabetic complications, prevention of DGp by early and optimal glycemic control is more cost-effective.
Funding: Hansa Medcell, India.
Keywords: Diabetes, Gastroparesis, Glucose, Insulin nausea, Type 1 diabetes, Type 2 diabetes, Vomiting
Introduction
The association between delayed gastric emptying and diabetes has been known for almost a century. Delayed gastric emptying was first noted in patients with diabetes and subsequently reported by Boas in 1925. In 1958, the term ‘Gastroparesis diabeticorum’ was coined by Kassender to describe asymptomatic gastric retention in diabetic patients [1]. Much has been learned about the symptom complex since then, including the functional, contractile, electrical and sensory dysfunction of the stomach associated with diabetes. More recently, the term diabetic gastroparesis (DGp) has been used to describe a serious complication of diabetes resulting in delayed gastric emptying with associated upper gastrointestinal (GI) symptoms in the absence of any mechanical obstruction [2]. Symptoms commonly associated with gastroparesis include postprandial fullness, nausea, vomiting, anorexia and weight loss, with or without abdominal pain. Delayed gastric emptying may result in poor glycemic control, poor nutrition and dehydration, resulting in frequent hospitalizations and poor quality of life. The diagnosis and management of DGp can be challenging, as it commonly remains undetected prior to the development of complications, and it is often refractory to therapy. Novel approaches to diagnosis and therapy represent a growing area of interest in the management of DGp [3–5]. This article is based on previously conducted studies and is not a new study with human participants or animals.
Overview of Diabetes and Its Complications
The prevalence of diabetes has been increasing exponentially, both in developing and developed nations. In 2013, the prevalence of diabetes among adults (age 20–79 years) was 382 million worldwide [6]. The most recent International Diabetes Federation (IDF) report estimates that 425 million adults worldwide (8.8% of the global population) have diabetes—a number that is projected to increase to 629 million by 2045 [6]. Diabetes is the leading cause of cardiovascular and kidney disease, and the most common preventable cause of blindness worldwide among working age adults (20–65 years). About 12% of global health care expenditure (727 billion USD) is spent on diabetes. When expanded to the age group between 18 and 99 years, the cost would total to 850 billion USD. In conjunction with the rising prevalence, the cost is expected to rise to a staggering 958 billion USD by 2045 [6–9]. Diabetes is also the leading cause of non-traumatic amputations in the USA [7].
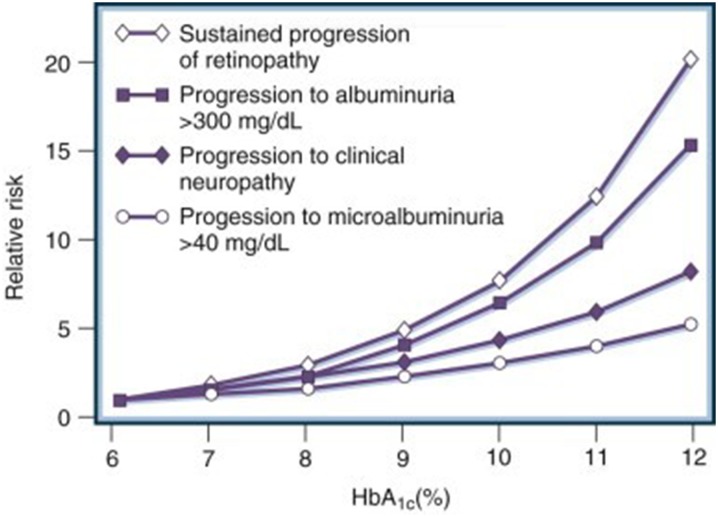
It is imperative to be familiar with current standards for screening for diabetes-related complications. Landmark studies show that early tight glycemic control slows the progression and development of diabetic autonomic neuropathy (DAN) and microvascular complications (Fig. 1) [10–14].
Fig. 1.
Relative risks for the development of diabetic complications at different mean levels of glycosylated hemoglobin (HbA1c).
Reproduced with permission from Elsevier. Skyler JS (1996) Diabetic complications: the importance of glucose control. Endocrinol Metab Clin North Am 25(2):243–254. https://www.sciencedirect.com/journal/endocrinology-and-metabolism-clinics-of-north-america
An intensive multifactorial cardiovascular risk intervention targeting glycemic, lipid and hypertension management, smoking and other lifestyle factors was shown to reduce the progression and development of cardiac autonomic neuropathy among patients with type 2 diabetes [15]. Thus, early diagnosis of diabetes and early intervention to prevent or delay complications are standards of best practice, and also economic and ethical priorities for health care providers of all specialties, including primary care.
The discussion of practice guidelines and standards of medical care for diabetes is beyond the scope of this module [16, 17].
Diabetic Autonomic Neuropathy
Neuropathy is responsible for a substantial portion of the mortality and morbidity in diabetes and can be divided into many abnormalities, including peripheral neuropathy and autonomic neuropathy (DAN). As they are thinly or un-myelinated, autonomic nerves may be especially susceptible to vascular and metabolic insult. DAN affects several organs systems, including the cardiovascular, genito-urinary, neuroendocrine and gastrointestinal systems (Table 1) [18, 19].
Table 1.
Clinical manifestations of diabetic autonomic neuropathy
| System | Clinical features |
|---|---|
| Cardiovascular |
Sinus tachycardia Postural tachycardia Bradycardia, fixed heart rate (more advanced disease) Systolic and diastolic dysfunction Decreased exercise tolerance Orthostatic hypotension with supine (nocturnal) hypertension Cardiac denervation syndrome Intraoperative and perioperative cardiovascular instability |
| Gastrointestinal |
Esophageal dysmotility Gastroparesis Diarrhea Constipation Fecal incontinence |
| Genitourinary |
Erectile dysfunction Retrograde ejaculation Neurogenic bladder and cystopathy Female sexual dysfunction (e.g., loss of vaginal lubrication) |
| Sudomotor and vasomotor |
Anhidrosis Hyperhidrosis Heat intolerance Gustatory sweating Dry skin Decreased thermoregulation Altered blood flow Impaired vasomotion Edema |
| Pupillary |
Pupillomotor function impairment (e.g. decreased diameter of dark adapted pupil) Pseudo Argyll-Robertson pupil |
| Metabolic |
Hypoglycemia unawareness Hypoglycemia unresponsiveness (delayed epinephrine secretion, reduced glucagon secretion) |
| Other |
Sleep apnea Anxiety/depression |
Reproduced with permission from Gibbons CH. Clinical features of diabetic autonomic neuropathy. In: Post TW (ed) Diabetic autonomic neuropathy. UpToDate© 2018. UpToDate, Inc., Waltham, MA. Accessed 16 Feb 2018. For more information visit www.uptodate.com
Diabetic Autonomic Neuropathy of the Gastrointestinal Tract (Gastrointestinal Neuropathies)
Diabetic autonomic neuropathy, which can have many manifestations, can be divided into groups of conditions as follows:
Esophageal dysmotility
Gastroparesis
Diabetic enteropathies including small bowel dysmotility syndromes, diabetic diarrhea and fecal incontinence [20]
Gut complications of diabetes, including diabetic diarrhea and incontinence, small intestinal bacterial overgrowth, non-alcoholic fatty liver disease and exocrine pancreatitis, have a major impact on health outcomes in individuals with long- standing poorly controlled diabetes.
Introduction to Diabetic Gastroparesis
Definition
A clear consensus regarding the definition of DGp does not exist. In the past, the terms diabetic gastropathy and gastroparesis were used interchangeably. Diabetic gastropathy was described as a neuropathy occurring in the GI system of diabetic patients. Koch et al. used the term to describe a clinical condition presenting with upper GI tract symptoms suggestive of an upper motility disturbance in patients with diabetes whether or not delayed gastric emptying was present, as some patients with this syndrome may have rapid gastric emptying [21]. A general consensus has now emerged that delayed gastric emptying occurs in the absence of mechanical obstruction in DGp [5, 22].
The American College of Gastroenterology (ACG) guidelines for the diagnosis and management of DGp state that a combination of appropriate symptoms and signs, along with delayed gastric emptying in the absence of gastric outlet obstruction or ulceration, is required to establish the diagnosis of DGp [4].
Epidemiology and Natural History of Diabetic Gastroparesis
Gastroparesis is a relatively common complication of diabetes, but often goes unrecognized.
About one-third of patients with gastroparesis have diabetes [21]. In the USA, an estimated 5 million patients suffer from some form of gastroparesis [23], and the female:male ratio is 4:1 [24, 25]. While gastroparesis has multiple etiologies, in a large single-center study of 146 gastroparesis patients, 29% were found to have diabetes, 13% developed symptoms after gastric surgery and 36% were idiopathic [25]. Nevertheless, little is known about the epidemiology of DGp, in part because the weak association between symptoms and objective studies of gastric emptying confounds diagnosis.
Diabetes affects gastric motor function more than small bowel transit, indicating an increased sensitivity of the stomach to diabetic injury. Approximately 75% of patients with diabetes have some form of GI symptoms [26] and about 18% experience upper GI symptoms [27]. In an Australian epidemiological study [27], diabetes mellitus was associated with an increased prevalence of upper and lower GI symptoms, which were linked to poor glycemic control but not to duration of diabetes or type of treatment.
DGp affects 20–50% of the diabetic population, especially those with type 1 diabetes mellitus or those with long-standing (≥ 10 years) type 2 diabetes mellitus. It is usually associated with retinopathy, neuropathy and nephropathy as well as poor early glycemic control, as noted in the DCCT-EDIC study [28]. The mean age of onset is approximately 34 years, and prevalence increases with increasing age [24]. Gastroparesis appears to be more common in patients with type 1 diabetes than in those with type 2 diabetes. Delayed gastric emptying is found in 27–65% of patients with type 1 diabetes and in up to 30% of patients with type 2 diabetes [29]. Prevalence of DGp among patients in a type 1 diabetes case registry was 5% versus 40% in tertiary care centers [30].
The increasing prevalence of type 2 diabetes has resulted in larger numbers of patients with DGp. In one case series of 146 patients with type 2 diabetes from India, the prevalence of delayed gastric emptying was 29%, and higher glycosylated hemoglobin (HbA1c) and body mass index were independent predictors of delayed gastric emptying [31]. While DGp can present as a complication of autonomic neuropathy in both type 1 and type 2 diabetes, some clinical differences do exit between these groups. In a 48-week observational study, glycemic control (HbA1c), delayed gastric emptying, hospitalization rates and stimulator placements were higher in patients with type 1 diabetes with DGp than in those with type 2 diabetics with DGp. It was also noted that patients with type 1 diabetes with DGp reported profound neuropathy, more anxiety and less reduction in symptom scores with intervention compared to those with type 2 diabetics with DGp [32]. Interestingly autoantibody (GAD 65) prevalence in both type 1(40%) and type 2 (25%) diabetes did not predict the severity of gastroparesis [33].
It is not clear whether there is an ethnic predisposition for diabetes-related gastro-enteropathies. A survey of Chinese diabetics found that 70.5% experienced GI symptoms compared to 30.8% of age- and sex-matched controls [34]. To the contrary, when diabetics in Finland were surveyed there was no difference in prevalence of GI symptoms between diabetics and non-diabetics [35].
Gender Differences in Diabetic Gastroparesis
Most studies have shown a higher prevalence of gastroparesis in women than in men [27, 34, 36], but others have noted no gender differences [31]. In a population based study from Olmstead county in Minnesota, the prevalence of gastroparesis was 24.2 per 100,000 persons for both genders, 9.6 per 100,000 for men and 37.8 per 100,000 for women [30]. The reasons for the female preponderance remain unknown. Even in diabetics without clinical gastroparesis, gastric emptying is slower in women than in men [25, 37]. Differences in neuronal nitric oxide synthase (nNOS) dimerization between females and males have been proposed as a reason for the female preponderance [38, 39].
Another factor may be a progesterone effect on gastric emptying, much like its effect on uterine contractility [40]. In fact, women of reproductive age may experience worsening of their symptoms during the luteal phase of their menstrual cycle, possibly due to higher progesterone levels [41]. On the other hand, in another study, gastric emptying was found to be slower in healthy women during the follicular phase, at which time hyperglycemia, plasma glucagon-like peptide-1 (GLP-1) and insulin levels, hunger and energy intake are less [42].
In addition, autoimmune disease, which is associated with gastroparesis, is more common in females [43]. [44].
Association of Diabetic Gastroparesis with Diabetic Complications
While some studies show a strong association among various attributes of DAN and DGp [45], others do not [46]. In the DCCT–EDIC follow-up study, delayed gastric emptying was associated with other complications of diabetes, particularly severe retinopathy, and to a lesser extent with cardiovascular vagal dysfunction and severe nephropathy [28].
Children and Adolescents
Diabetic gastroparesis is less common in children given that a longer duration of diabetes and hyperglycemia and DAN predicts DGp. However, glycemic fluctuations that may occur in adolescents may be impacted by altered gastric emptying [47].
Prognosis
Diabetic gastroparesis is associated with higher morbidity, including increased hospitalizations and emergency department and hospital visits. Hospitalizations attributed to gastroparesis rose by 138% from 1995 to 2004 [48]. Patients with type 1 or 2 diabetes mellitus with classic symptoms of gastroparesis, such as early satiety, postprandial fullness, bloating, abdominal swelling, nausea, vomiting and retching and documented delay in gastric emptying, are more likely to have cardiovascular disease, hypertension and retinopathy. Therefore, gastroparesis may be a marker of increased morbidity [49]. On the contrary, in a cohort of mostly type 1 diabetics followed in Australia over a period of approximately 25 years, DGp was not associated with a poor prognosis or with increased mortality when corrected for autonomic neuropathy and HbA1c [50].
Anatomy and Physiology of the Stomach in Health
To understand the pathophysiology of DGp, it is important to review the anatomical structure, nerve and blood supply as well as physiology of the stomach.
Anatomy of the Stomach
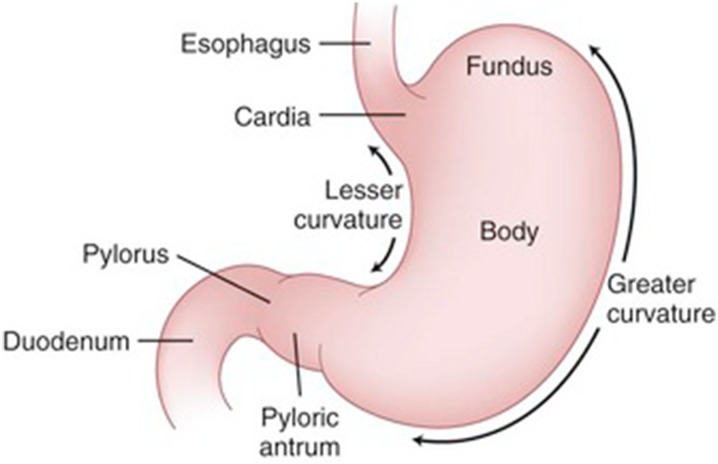
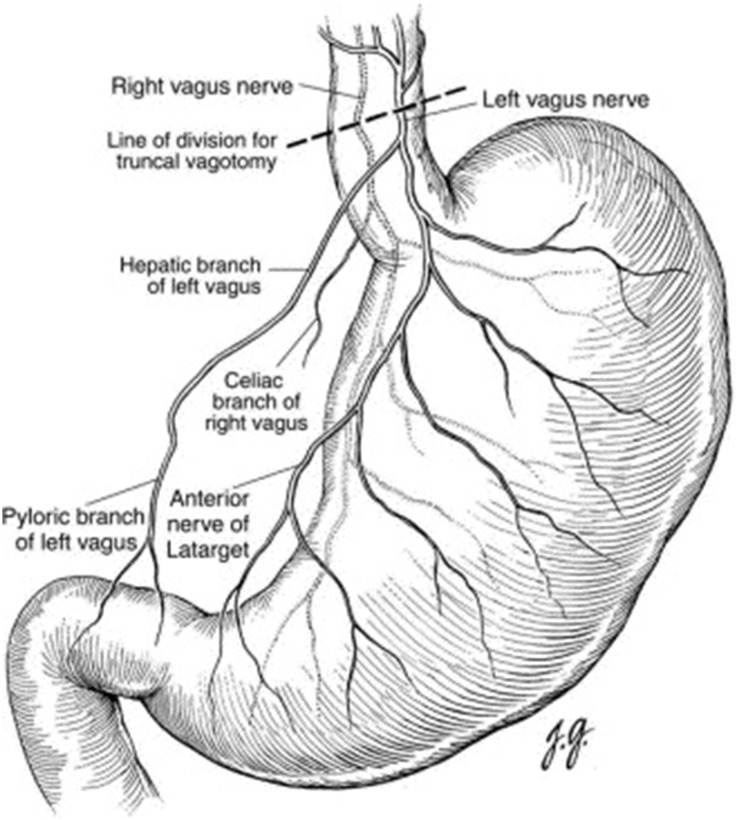
The stomach is a distensible, muscular, highly vascular bag-shaped organ located in the left upper abdominal quadrant. The anatomy of the stomach and the nerve supply to this organ are shown in Figs. 2 and 3, respectively [9].
Fig. 2.
This figure was originally published in Shackelford’s surgery of the alimentary tract, ed. 6, Philadelphia, Charles J. Yeo (2007)
Fig. 3.
Parasympathetic nerve supply of the stomach
Reprinted with permission from Elsevier (copyright 2003). Mercer DW, Liu TH (2003) Open truncal vagatomy. Oper Tech Gen Surg 5(2):80–85
Physiology of Gastric Function
The three main motile functions associated with digestion in which the stomach plays a central role include:
Acts as a reservoir for ingested food
Mixes food with gastric secretions
Empties gastric contents into the duodenum
These motile functions are accomplished by the coordinated movements of three layers of smooth muscle of the stomach—an outermost longitudinal layer, a middle circular layer and an innermost oblique layer. The longitudinal layer is present only in the distal two-thirds of the stomach, while the oblique layer is distinguishable only in the proximal half of the stomach. The circular layer is present throughout with maximum thickness in the antrum where the force of contraction is the greatest. Coordination of smooth muscle activity is dependent upon the enteric neural plexus, especially the myenteric plexus, and the intensity of contraction depends upon the sympathetic and parasympathetic efferent neural activity. The proximal stomach acts as a reservoir that accommodates to meal volume by modulating tonic contractile activity. The distal stomach generates phasic peristaltic waves of contraction for mixing, grinding and propelling contents. Neural and hormonal activity can alter the amplitude of slow waves, generation of spike potential and, therefore, the force of peristaltic contraction [51, 52].
Process of Gastric Emptying
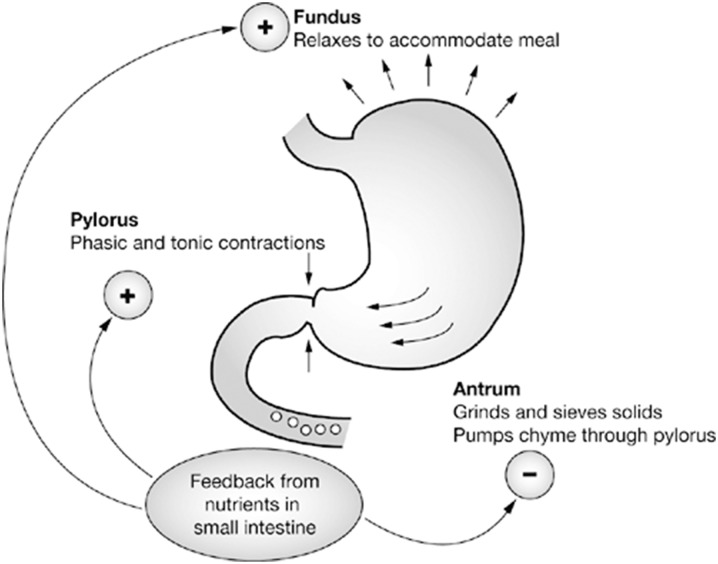
Normal gastric emptying results from the integration of tonic contractions of the fundus, phasic contractions of the antrum and the inhibitory forces of pyloric and duodenal contractions, which requires complex interactions between smooth muscle, enteric and autonomic nerves, and specialized pacemaker cells known as the interstitial cells of Cajal (ICC) (Fig. 4).
Fig. 4.
Motor events in normal gastric emptying
Reprinted with permission from M Schemann, “Gastrointestinal Motility” web tutorial. http://humanbiology.wzw.tum.de/motvid01/tutorial.pdf. Accessed 26 May 2014
The emptying of the reservoir is caused by two mechanisms: a tonic contraction of the fundus and peristaltic waves (phasic contractions) moving over the distal part of the gastric body and antrum. These two forces represent the pump of the gastric reservoir. Both the peristaltic waves and the tonic contractions of the reservoir are stimulated by cholinergic enteric neurons that are under modulatory vagal tone. In the region of the body of the stomach, peristaltic waves only produce a small circular constriction [51].
The peristaltic wave originates at the proximal stomach and propagates to the pylorus. Peristaltic waves are based on electrical waves originating in the gastric wall. A network of interstitial cells—called the ICC—exists in the wall of both the stomach and small intestine. These cells produce electrical pacesetter potentials due to oscillations in their membrane potential. The pacesetter potential of the ICC drives electrical events in smooth muscle cells where they are reflected as slow waves. Both the pacesetter potentials and slow waves start in the proximal stomach and move along the syncytium of the smooth muscle cells. The pacesetter potentials are always present but do not cause contractions by themselves. Contractions only occur when excitatory neurotransmitters, such as acetylcholine (ACH), are released. The release of ACH, and thus the stimulation of gastric motility by cephalic and gastric reflexes, is elicited by mechanoreceptors of the mouth during the ingestion of food and by mechanoreceptors and/or chemoreceptors in the stomach. In the region of the body of the stomach, the peristaltic waves are shallow, but when the peristaltic wave reaches the antrum, the circular constriction becomes deeper [51, 52].
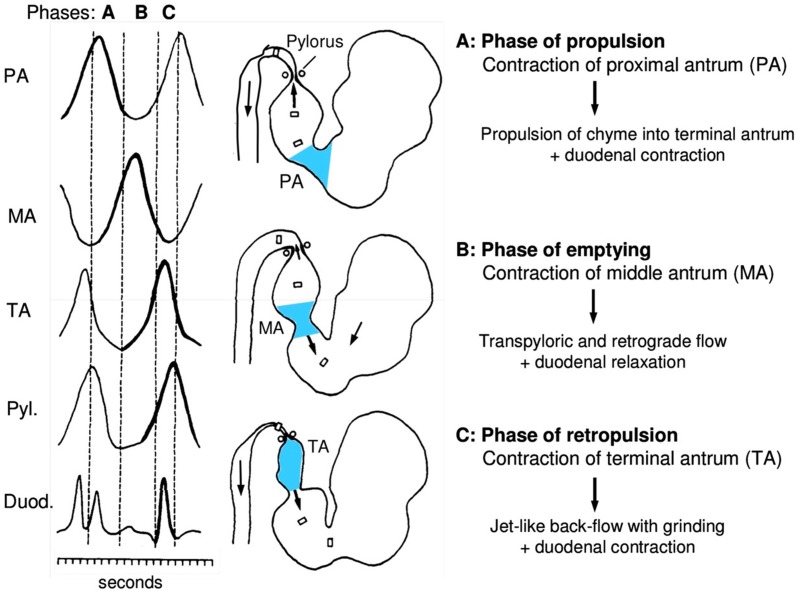
The emptying mechanism of the antral pump can be divided into three phases: (1) a phase of propulsion, (2) a phase of emptying and mixing and (3) a phase of retropulsion and grinding, as shown in Fig. 5. Due to the rhythmic pacesetter potentials, there is cyclic, coordinated pattern to the phases. When the peristaltic wave moves over the proximal antrum, the previously contracting terminal antrum relaxes, thereby allowing chyme to be propelled into the terminal antrum (phase of propulsion) [51, 52].
Fig. 5.
Function of antral pump in gastric emptying
Reprinted with permission from M Schemann, “Gastrointestinal Motility” web tutorial. http://humanbiology.wzw.tum.de/motvid01/tutorial.pdf. Accessed 26 May 2014
Once the peristaltic wave reaches the middle of the antrum, the pylorus opens and duodenal contractions are inhibited, allowing small amounts of gastric chyme to be delivered across the pylorus into the duodenum. During this phase of emptying and mixing, the peristaltic waves are far away from the pylorus. Chyme is swept into the small intestine by the peristaltic wave [51, 52].
The antral pump acts like a sieve. As liquids flow more rapidly than viscous and solid materials, liquids with small suspended particles are swept across the pylorus into the duodenum, whereas the viscous and solid mass of the chyme is retained in the stomach. The lumen of the antrum is not occluded by the peristaltic wave, and some amount of chyme flows in a retrograde manner into the relaxing proximal antrum. The phase of emptying overlaps with mixing of the gastric chyme. Simultaneously, the subsequent peristaltic wave proceeds along the gastric body, propelling chyme into the proximal antrum. Chyme of the gastric body and chyme of the middle antrum accumulate in the relaxed proximal antrum. Contraction of the terminal antrum closes the pylorus, thus stopping the transpyloric flow. The chyme present in the terminal antrum is forced retrograde across the central opening of the peristaltic wave into the relaxing middle antrum. Forceful mixing of the chyme associated with the grinding of particles occurs as a result of this jet-like retropulsion. Thus, contraction of the terminal antrum denotes the phase of retropulsion and grinding. During the emptying phase of the stomach, the duodenal contractions are inhibited and the duodenal bulb relaxes. This is known as antroduodenal coordination [51, 52].
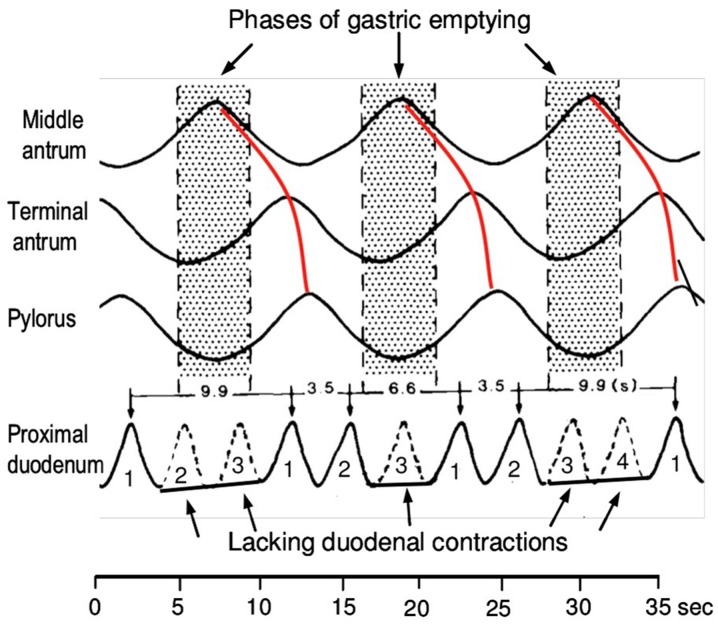
As a result of the different frequencies between the antral and duodenal contractions, the duodenum can contract three to four times during an antral wave (red lines in Fig. 6). The contractions of the proximal duodenum cease during the phases of gastric emptying. The first duodenal contraction occurs during the gastric phase of retropulsion; the second contraction occurs during the phase of propulsion [51, 52].
Fig. 6.
Antroduodenal coordination. A, B, C Phases of gastric emptying. Duod. Duodenum, Pyl. pylorus
Reprinted with permission from M Schemann, “Gastrointestinal Motility” web tutorial. http://humanbiology.wzw.tum.de/motvid01/tutorial.pdf. Accessed 26 May 2014
The complex muscular contractions of the stomach are under neuro-hormonal control, and damage to the enteric nerves, especially the ICC, can result in disruptions of the intricate mechanisms needed for normal gastric emptying to occur.
Factors Affecting Gastric Emptying
Gastric emptying depends on several factors (Table 2). The relaxation of the reservoir, the depth of the constriction of the antral waves, the degree of pyloric opening, the receptive relaxation of the duodenal bulb and the contractile pattern of the duodenum each play an important role. The motility of the stomach can also be affected by neurotransmitters, hormones or drugs (Table 4) [51, 52].
Table 2.
Physiologic factors affecting gastric emptying
| Factors that increase gastric emptying | Factors that delay gastric emptying |
|---|---|
| Stomach distension | Duodenal distension |
| Liquid content | Chyme high in H+, fat or protein |
| Smaller particles | Secretin, cholecystokinin |
| Parasympathetic stimulation |
Pain, anxiety, stress Sympathetic stimulation |
Table 4.
Drugs affecting gastric emptying
| Drugs that delay gastric emptyinga | Drugs that accelerate gastric emptying |
|---|---|
| Opioid analgesics | Metoclopramide |
| Anticholinergic agents | Erythromycin/clarithromycin |
| Tricyclic antidepressants | Cisapride |
| Calcium channel blockers | Domperidone |
| Progesterone | Tegaserod |
| Octreotide | β-Adrenergic receptor antagonists |
| Proton pump inhibitors | |
| H2-Receptor antagonists | |
| Interferon-alpha | |
| l-dopa | |
| Fiber | |
| Sucralfate | |
| Aluminum hydroxide antacids | |
| β-Adrenergic receptor agonists | |
| Glucagon | |
| Calcitonin | |
| Dexfenfluramine | |
| Diphenhydramine | |
| Alcohol | |
| Tobacco/nicotine | |
| Anti-muscarinics, e.g. atropine, glycopyrrolate |
aDrugs used for treatment of diabetes that may affect gastric emptying discussed in a different section
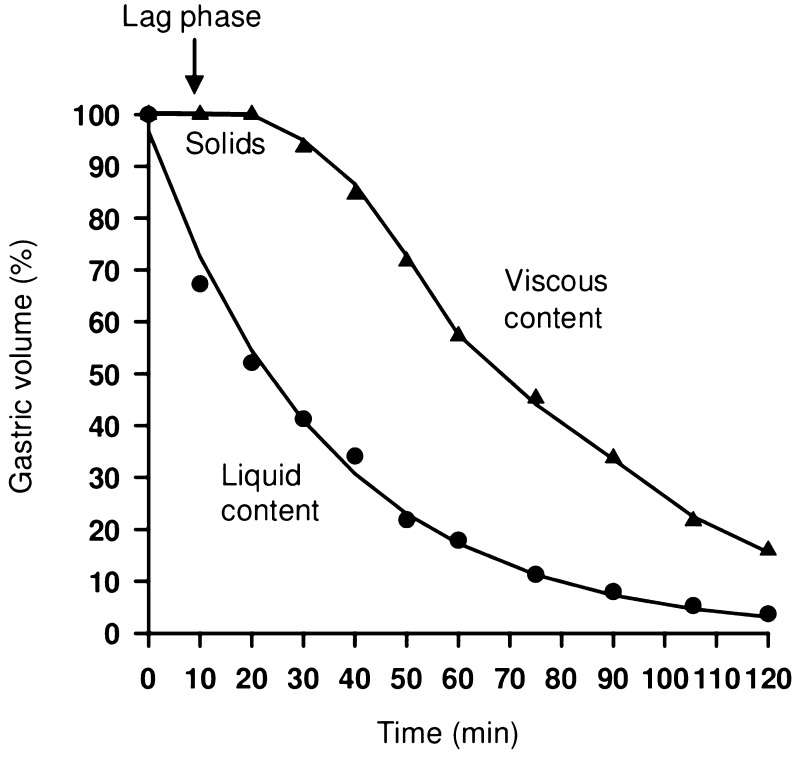
For the stomach to empty, the pressure generated by the antral pump must exceed the resistance of the pyloric sphincter. In general, emptying occurs at an exponential rate proportional to the volume of the stomach—that is, the fuller the stomach, the more rapidly it empties. This is mediated by vagal excitatory reflexes provoked by gastric distension. Stimulation of the vagus nerve with ACH as neurotransmitter increases the force and frequency of gastric contraction, whereas stimulation of sympathetic nerves inhibits gastric motility through the release of norepinephrine. Gastrin is also released in response to antral distension, and both these stimuli produce an increase in antral pump activity. The speed of emptying for liquids, or contents consisting of smaller particles, is faster than for solids (Fig. 7) [51, 52].
Fig. 7.
Velocities of emptying of solid and liquid chyme
Reprinted with permission from M Schemann, “Gastrointestinal Motility” web tutorial. http://humanbiology.wzw.tum.de/motvid01/tutorial.pdf. Accessed 26 May 2014
The emptying of liquids is exponential. In contrast, the emptying of large solid particles only begins after sufficient grinding, resulting in a lag phase followed by the emptying of the viscous chyme mainly in a linear fashion [51, 52].
The chemical composition of the chyme entering the duodenum also affects the rate of gastric emptying, and influences hormone secretion. If the chyme is too acidic, secretin is released, which slows gastric emptying, reduces the production of gastric acid and increases the secretion of alkaline pancreatic juice into the duodenum (Table 2). If the fat content of the chyme is too high, cholecystokinin (CCK) is released, which stimulates contraction of the gall bladder so that bile salts (which emulsify the fats) are secreted into the duodenum, and also reduces gastric emptying. If the content of amino acids in the chyme is too high, gastrin is released, which increases contraction of the pyloric sphincter and gastric motility and overall delays gastric emptying. Hypertonic chyme is detected by duodenal osmoreceptors and gastric emptying is slowed [51, 52].
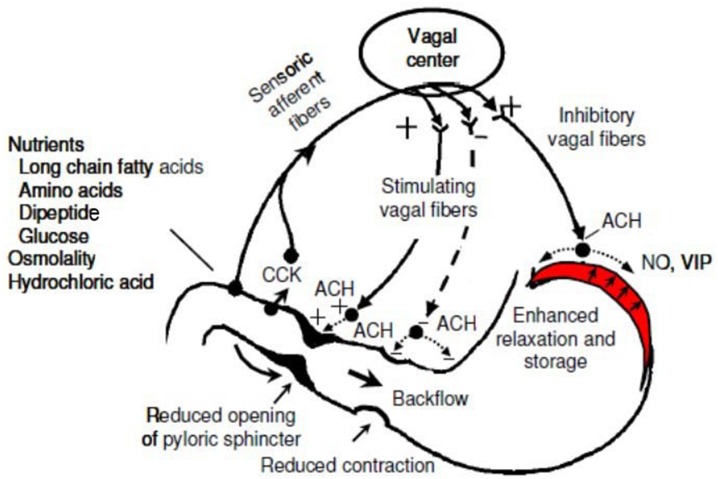
As the duodenum fills, stretch receptors are activated that inhibit the vagus nerve, which results in reduced gut tone and motility, temporarily reducing gastric emptying. As the duodenum empties, this inhibition diminishes, the tone and motility of the gut increases and gastric emptying is restored. The neural and hormonal mechanisms that originate from the duodenum and the feedback to slow gastric emptying together constitute the entero-gastric reflex. The activity of the pyloric sphincter is modulated by reflexes originating from the antrum and duodenum. A contraction of the middle antrum elicits a descending inhibitory reflex causing pyloric relaxation via the release of nitric oxide (NO) and vasoactive intestinal peptide (VIP) (Fig. 8). On the other hand, duodenal stimuli such as hydrochloric or oleic acid, induce an ascending excitatory reflex which causes frequent contractions of the pyloric sphincter associated with an increase in tone. By regulating the rate of delivery of chyme into the duodenum, the absorption of nutrients in the small intestine is maximized [51, 52].
Fig. 8.
Feedback mechanism of gastric emptying. CCK Cholecystokinin, ACH acetylcholine, VIP vasoactive intestinal peptide, NO nitric oxide
Reprinted with permission from M Schemann, “Gastrointestinal Motility” web tutorial. http://humanbiology.wzw.tum.de/motvid01/tutorial.pdf. Accessed 26 May 2014
Given the complex, precise and coordinated steps involved in the physiology of gastric emptying, factors that may affect this sequential process can impact gastric motility in diabetics. The pathophysiology of gastroparesis is heterogeneous (Fig. 8). Impaired phasic antral contractions are traditionally believed to be responsible for delayed emptying of solids in DGp, but other factors are also said to contribute. Regional defects, such as blunted antral contractions, spastic pyloric and small intestinal motility, hypersensitivity to fundic distention and impaired gastric accommodation to meals are demonstrable in diabetic patients. Type 1 diabetic patients may have impairment of smooth muscle contractility. Acute hyperglycemia is known to delay gastric emptying, disrupt antro-pyloric motility and blunt the response to prokinetic medications. Autonomic neuropathies, including vagal and sympathetic neuropathies, are likely contributors to the pathogenesis of delayed emptying in patients with long-standing diabetes.
The pathogenesis of gastroparesis as a disease involves neuronal changes resulting in an altered secretion of neuronal NO synthase (nNOS), VIP, substance P and expression of tyrosine hydroxylase. Abnormalities in the structure and function of the autonomic nervous system and smooth muscles play an active part in the pathogenesis. Abnormalities in small bowel motility might result in delayed gastric emptying of solids; gastric motor dysfunction might be associated with small bowel dysmotility caused by a common mechanism. The ICC generate an electrical signal, and gastric electric dysrhythmias or reduced power of the electrical signal in postprandial state are found in gastroparesis [53]. We will discuss proposed mechanisms in the following section.
Pathogenesis of Diabetic Gastroparesis
There are multiple mechanisms linking diabetes to gastric motor dysfunction, such as autonomic neuropathy, enteric neuropathy involving excitatory and inhibitory nerves, abnormalities of ICC [54] (Table 3), acute fluctuations in blood glucose, incretin-based medications used to normalize postprandial blood glucose (Table 5) and perhaps psychosomatic factors via autonomic mechanisms.
Table 3.
Pathophysiologic mechanisms of diabetic gastroparesis
| Etiology | Mechanism |
|---|---|
| Extrinsic denervation of stomach | Delayed gastric emptying |
| Loss of nitric oxide synthase in enteric nerves |
Impaired inhibitory input (1) Decreased gastric accommodation, and possible accelerated gastric emptying of liquids (2) Uncoordinated antral contractility resulting in delay in gastric emptying of solids (3) Pylorospasm, which in the presence of antral hypomotility, may impair gastric emptying |
| Altered function of immune cells such as type 2 macrophages | Loss of cytoprotective factors resulting in damage to ICC (cajalopathy) and smooth muscle |
| Loss of ICC (cajalopathy) | Decreased smooth muscle contractility and arrhythmias |
| Smooth muscle atrophy | ↓ IGF-1 with resultant loss of ICC |
ICC Interstitial cells of Cajal, IGF-1 insulin-like growth factor 1
Table 5.
Summary of incretin drugs
| Incretin drugs | Dose and frequency |
|---|---|
| GLP-1 receptor agonist (incretin mimetics) a | |
| Daily | |
| Exenatide | 5–10 μg SC BID within 60 min before meals and at least 6 h apart |
| Liraglutide | 0.6 mg/day SC for 1 week and then increase to 1.2 mg/day, maximum 1.8 mg/day |
| Lixisenatide | up titration to 20 mcg SC/day |
| Combination insulin analog basal/GLP1-RA | |
| Insulin glargine/lixisenatide | 15–60 units SC/day. |
| Insulin degludec/liraglutide | 100/3.6:10–50 units daily. |
| Once-Weekly | |
| Exenatide extended-release | 2 mg once-weekly |
| Albiglutide | 30 to 50 mg SC/week in a single dose pen (discontinued in 2017) |
| Dulaglutide | 0.75–1.5 mg once-weekly |
| Semaglutide | 0.5–1 mg once-weekly |
| Dipeptidyl peptidase-4 inhibitors (incretin enhancers) b | |
| Sitagliptin | 50 mg, 100 mg/day |
| Saxagliptin | 2.5 mg, 5 mg/day |
| Linagliptin | 5 mg/day |
| Alogliptin | 25 mg/day |
| Vildagliptin | 50 mg, 100 mg/day (Europe and Asia) |
| Amylinomimetic c | |
| Pramlintide | 60–120 μg SC before every major meal |
Mechanisms of Diabetic Gastroparesis
Glucose-Gut–Incretins-Islet Cross-Talk
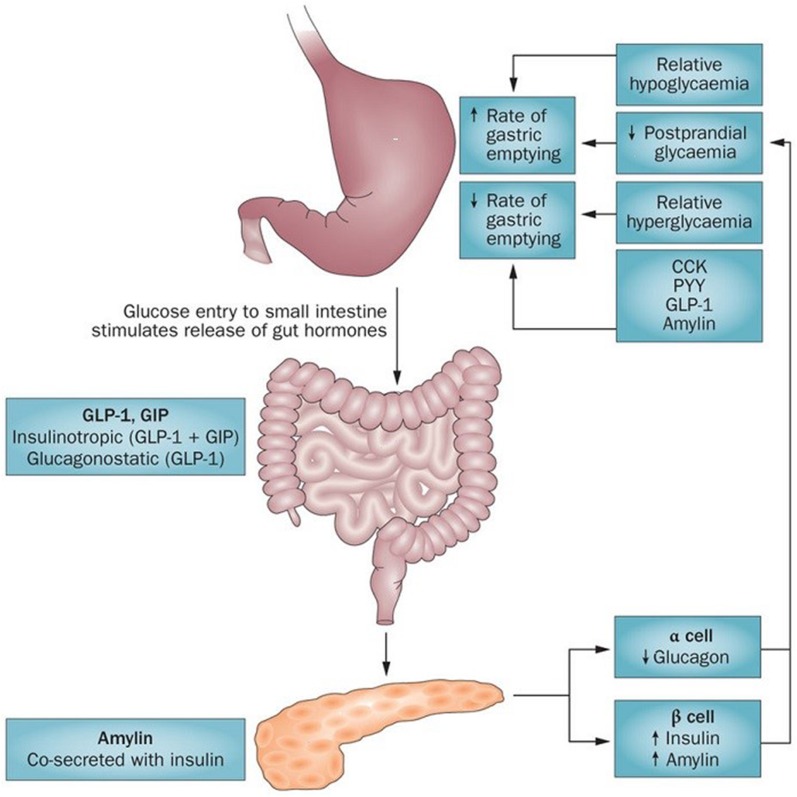
One of the more powerful factors affecting gastric emptying is glucose (from a meal and from the liver). Glucose can delay or accelerate gastric emptying and vice versa. Gut hormones and islet hormones also play an important role in maintaining gastric emptying by impacting the intragastric and intraduodenal glucose levels (Fig. 9) [55].
Fig. 9.
Glucose and gastric emptying: bidirectional relationship. The rate of gastric emptying is a critical determinant of postprandial glycemia. Glucose entry into the small intestine induces a feedback loop via CCK, peptide YY (PYY) and glucagon-like peptide 1 (GLP-1), which are secreted from the intestine in response to nutrient exposure. GLP-1 and gastric inhibitory polypeptide (GIP) induce the release of insulin, and GLP-1 inhibits glucagon secretion, which attenuates postprandial glycemic excursions. Amylin, which is co-secreted with insulin, also slows gastric emptying. At the same time, the blood glucose concentration modulates gastric emptying, such that acute elevations of blood glucose levels slow gastric emptying (effects are evident even within the physiological range) and emptying is accelerated during hypoglycemia
Reprinted with permission from Springer Nature. Phillips LK, Deane AM, Jones KL, et al. (2015) Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 11(2):112–28
A complex interplay of gut hormones called incretins (glucagon-like peptide 1 [GLP-1] and gastric inhibitory polypeptide [GIP]) secreted from K and L cells of the small intestine in response to gastric nutrients, hepatic glucose and insulin, and gastric intrinsic and extrinsic factors as described in following sections bring about the fascinating gluco-gastric equilibrium [56, 57]. The incretins lower glucose levels by stimulating insulin secretion. GLP1 has other actions, including the inhibition of glucagon secretion, appetite and gastric motility.
Enteric Neuropathy
Patients with gastroparesis often show evidence of autonomic neuropathy. Studies suggest that both the sympathetic and parasympathetic components of the autonomic nervous system are affected in DGp since abnormalities have been described in the axons and dendrites within the prevertebral sympathetic ganglia. The pancreatic polypeptide response is blunted and gastric secretion is reduced in patients with DGp when vagus nerve function is stimulated by sham feeding. Hyperglycemia may cause vagus nerve dysfunction due to demyelination [38]. After restoration of normal glycemic control and renal function with pancreas–kidney transplantation, diabetic autonomic and peripheral neuropathy can be partially reversible with improved gastric function [38].
Intrinsic Mechanisms
An increased level of oxidative stress caused by low levels of heme oxygenase-1 (HO-1) is associated with DGp in experimental models. Increasing the expression of HO-1 or improving the function of nitrergic mechanisms through experimental approaches protects against the development of gastroparesis or restores gastric emptying in diabetic mice and rats, respectively [58].
Both animal and human studies suggest that the most common gastric cellular defects in gastroparesis are the loss of expression of nNOS and the loss of ICC [4]. However, post-translational modification of nNOS may be more important than absolute nNOS levels [53].
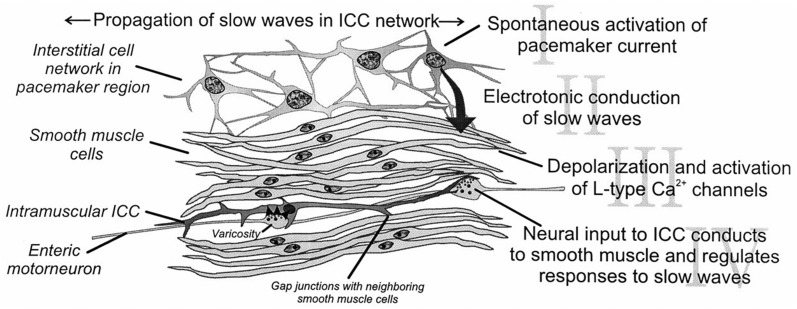
Electric pacemaker activity drives peristaltic and segmental contractions in the gastrointestinal tract, and the ICC are responsible for spontaneous pacemaker activity. Loss of ICC is the most common enteric abnormality in DGp and idiopathic gastroparesis. The stomach shows distinct regional variations in the distribution of subtypes of ICC from the cardia to pylorus, whereas the small intestine and colon both seem to retain nearly the same distribution pattern of subtypes of ICC throughout each organ. All subtypes of ICC share common ultrastructural features, such as the presence of numerous mitochondria, abundant intermediate filaments and the formation of gap junctions with the same type of cells and with smooth muscle cells. ICC are responsible for multiple functions in the GI tract. ICC generate slow waves that control smooth muscle contractility, are involved in aspects of neurotransmission, set the smooth muscle membrane potential gradient and are involved in mechanotransduction as shown in Fig. 10 [53].
Fig. 10.
Functions of the ICC. Republished with permission of Annual Reviews, Inc.; permission conveyed through Copyright Clearance Center, Inc. Horowitz B, Ward SM, Sanders KM (1999) Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61:19–43
Revisions to figure republished with permission from The American Physiological Society. Sanders KM, Ordog, T, Koh SD, Ward SM (2000) A novel pacemaker mechanism drives gastrointestinal rhythmicity. New Physiol Sci 15(6):291–298
There is continuous remodeling of the ICC, and a balance is maintained between processes that injure and repair these cells. In DGp, pathways that damage ICC by various mechanisms, such as insulinopenia, IGF-1 deficiency [59] and oxidative stress, dominate. Deficiency of ICC survival factors (insulin and IGF-1 promote the production of smooth muscle cell-produced stem cell factor, an important ICC survival factor) is detrimental to ICC [60]. Moreover, in diabetes, mechanisms that normally counteract increased oxidative stress, such as upregulation of HO-1, are impaired, leading to loss of ICC and subsequent delay in gastric emptying. Upregulation of HO-1 by hemin increases ICC and nNOS and normalizes delayed gastric emptying. The protective effects of HO-1 are said to be mediated by one of its products—carbon monoxide (CO). Therefore, the insulin/IGF-1 and the HO-1/CO pathways provide opportunities to develop therapies that are pathogenesis based. As the gut contains ICC and enteric stem cells, targeting residual stem cells or transplantation of stem cells is a new area that needs further exploration [53].
Gastric and Enteric Neuromuscular Pathology in Diabetic Gastroparesis
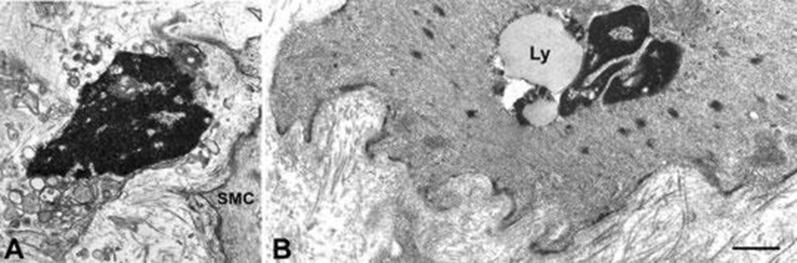
Histologic abnormalities are heterogeneous, and include absent or dysmorphic ICC, decreased nerve fibers, increased smooth muscle fibrosis, and abnormal macrophage-containing immune infiltrates [61]. Abnormal gastric slow waves, severe symptoms of gastroparesis and less improvement with gastric electrical stimulation is seen in the absence of ICC. Electron microscopy studies reveal abnormal connective tissue stroma, thick basal lamina around ICC and myocytes, and large empty nerve endings suggest more profound conduction defects [44, 62] (Fig. 11).
Fig. 11.
Altered interstitial ICC and smooth muscle in diabetic gastroparesis. a A presumed ICC with apoptotic features: clumps of compacted chromatin filling the entire nucleus, a cytoplasm containing swollen mitochondria and lysosomes. SMC smooth muscle cell. Bar 0.8 μm. b A smooth muscle cell with a large lipofuscin body (Ly) near the nucleus. Basal lamina is patchily thickened and the stroma rich in collagen fibrils. Bar 0.8 μm
Reprinted with permission from John Wiley and Sons. Faussone‐Pellegrini MS, Grover M, Pasricha PJ, et al. (2012) Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med 16(7):1573–1581
Drug-Induced and Iatrogenic Diabetic Gastroparesis
Known causes of iatrogenic gastroparesis include vagal inhibition due to vagal injury after fundoplication for gastroesophageal reflux disease and prescription medications that affect gastric emptying (Table 4). Treatment of patients with type 2 diabetes mellitus with GLP-1 receptor agonists (GLP1-RA) for type 2 diabetes mellitus and the amylin analog (pramlinitide) have been shown to delay gastric emptying (Table 5) [57]. Gastroparesis may occur in patients with diabetics following kidney and other solid organ transplantation due to treatment with calcineurin inhibitors [53].
Miscellaneous Etiologies
Native autoimmunity in gastric parietal cells has been speculated to occur in patients with type 1 diabetics with DGp [65]. Clock genes have been implicated in certain GI motility disorders, including gastroparesis, due to variations in circadian rhythm [66].
Clinical Evaluation of Patient with Suspected Diabetic Gastroparesis
Common Clinical Manifestations of Diabetic Gastroparesis
Common signs and symptoms of DGp are listed in Table 6, and some patients present with non-specific symptoms [67]. Soykan et al. reported that among 146 patients with gastroparesis, nausea was present in 92%, vomiting in 84%, abdominal bloating in 75% and early satiety in 60% [25]. While similar GI symptoms may occur with oral anti-diabetic agents, such as metformin and alpha glucosidase inhibitors (flatulence, diarrhea and pain), symptoms improve when the medication is discontinued [68]. In one study, patients with type 1 diabetes presented with worse symptoms and were more frequently hospitalized with less resolution of symptoms than those with type 2 diabetics [32]. Depending on their medical history, diabetic patients may also have other factors impacting their gastric emptying (Table 7).
Table 6.
Common symptoms of diabetic gastroparesis
| Common symptoms of diabetic gastroparesis |
|---|
| Nausea |
| Vomiting |
| Early satiety |
| Bloating |
| Postprandial fullness |
| Abdominal pain |
| Weight loss/weight gain |
| Constipation and/or diarrhea |
| Wide glycemic fluctuations |
Table 7.
Causes of gastroparesis
| General causes of gastroparesis | Etiology |
|---|---|
| Surgical causes |
Vagotomy and gastric resection/drainage Fundoplication, oesophagectomy Gastric bypass surgery Whipple procedure Heart/lung transplant |
| Infections |
Viruses: Epstein–Barr virus, varicella, parvovirus-like Chagas disease Clostridium botulinum |
| Central nervous system disorders |
Cerebrovascular accidents/trauma Tumors Labyrinthine disorders Seizures |
| Peripheral nervous system disorders |
Parkinson’s disease Guillain–Barre Multiple sclerosis Dysautonomias |
| Neuropsychiatric disorders |
Anorexia nervosa/bulimia Rumination syndrome |
| Rheumatologic disease |
Scleroderma Systemic lupus erythematosus Polymyositis/dermatomyositis |
| Endocrine and metabolism diseases |
Diabetes Hypothyroidism Electrolyte disorders Renal failure Pregnancy Neoplastic(para)-breast, small cell lung, pancreas |
| Miscellaneous neuromuscular diseases |
Amyloidosis Chronic intestinal pseudo-obstruction Myotonic dystrophy |
A careful medical history is essential. One must specifically include questions that explore the timing of symptoms with regard to meals, the typical symptom progression and the diet history. For example, early satiety or vomiting may suggest problems with gastric accommodation, while late satiation and/or vomiting may suggest abnormal gastric emptying. Also important are questions that explore diabetes control, symptoms that suggest hypothyroidism, history of previous surgery and medications (Tables 4, 5). Interestingly, in a retrospective study of 186 patients (56% type 1 diabetes mellitus) from the Netherlands, dyspeptic symptoms, with the exception of early satiety and abdominal pain, were unrelated to delayed gastric emptying [69]. In a study of patients with dyspepsia by Talley et al. [70], symptom prevalence and severity did not discriminate between those with delayed or normal gastric emptying.
On physical examination, neuropathy, abdominal distention, succussion splash, foul breath and orthostatic and postprandial hypotension may be present, but these findings are nonspecific for gastroparesis [71]. The evaluation of patients with gastroparesis is based on symptom severity. The two most commonly used scoring systems are the Gastroparesis Cardinal Symptom Index (GCSI) [72], which is a widely used quantitative scoring system, and another multidisciplinary scoring system which is qualitative.
Clinical Scoring Systems
The U.S. Food and Drug Administration (FDA) recently released guidance on symptom scoring systems for gastroparesis [73]. Although designed for pharmaceutical trials, it is useful for the documentation of symptoms and patient-reported outcomes in gastroparesis in general. There are a number of scoring systems that have and are being advocated. A popular scoring system, the GCSI, is described in detail in the following section. However, it was not derived from patient focus groups nor was it initially designed to quantify pain, which has limited its application in some settings.
GCSI Scoring System for Patient-Reported Outcomes
The GCSI is a patient-based symptom instrument in which the score is a sum of three subscale scores (each ranging from 1 to 3) for the three main symptom complexes:
Postprandial fullness/early satiety
Nausea/vomiting
Bloating
Patients are asked to rank symptoms (nausea, retching, vomiting, stomach fullness, inability to finish a normal-sized meal, feeling excessively full after meals, loss of appetite, bloating and the abdomen appearing visibly larger) using a scale of 0–5, with 0 being none and 5 being very severe. One drawback to the GCSI is that is does not measure abdominal pain.
Gastroparesis Severity Based on Severity of Illness
Another scoring system grades the severity of gastroparesis as follows [74]:
Grade 1 usually includes patients with mild intermittent symptoms that are controlled with diet modification and the avoidance of exacerbating agents.
Grade 2 patients have moderately severe symptoms but no weight loss, and require prokinetic drugs plus antiemetic agents for control.
Grade 3 patients are refractory to medication, unable to maintain oral nutrition and require frequent emergency room visits. These patients require intravenous fluids, medications, enteral or parenteral nutrition and endoscopic or surgical therapy.
Complications of Diabetic Gastroparesis
Complications of diabetes gastroparesis include [71]:
Esophagitis
Mallory–Weiss tear from chronic nausea/vomiting
Malnutrition
Volume depletion with acute renal failure
Electrolyte disturbances
Bezoar formation
Hyperglycemia emergencies including diabetic ketoacidosis and hyperosmolar hyperglycemia syndrome
In one study, patients with type 1 diabetes mellitus patients with DGp were hospitalized for diabetic ketoacidosis fourfold more often than their counterparts without DGp [75, 76].
Diagnosis of Diabetic Gastroparesis
Diabetic gastroparesis is diagnosed by the presence of upper GI symptoms suggestive of delayed gastric emptying in a diabetic patient, exclusion of mechanical obstruction that could cause upper GI symptoms and the demonstration of delayed gastric emptying. In addition to the medical history and physical examination, various diagnostic techniques can be used. Obstruction caused by an intra-abdominal mass may be excluded by diagnostic imaging. An upper endoscopy is necessary to exclude the presence of stricture, mass or ulcer. Tests that may be necessary to exclude infectious, metabolic and immunologic causes of upper GI symptoms include a complete blood count; comprehensive metabolic panel consisting of electrolytes and liver function test; urinalysis; erythrocyte sedimentation rate; and assays for thyroid-stimulating hormone, rheumatoid factor and antinuclear antibody (Table 8) [71].
Table 8.
Summary of diagnostic tools for diabetic gastroparesis
| Diagnostic tools for DGp | |
|---|---|
| Presence of symptoms | Abdominal imaging |
| Abdominal bloating | Plain radiograph |
| Abdominal pain | Computed tomography |
| Anorexia | Magnetic resonance imaging |
| Early satiety | Endoscopy |
| Nausea | Esophagoduodenostomy |
| Postprandial fullness | Gastric emptying studies |
| Vomiting | Scintigraphy |
| Weight loss | Breath tests |
| Laboratory studies | Ultrasound |
| Antinuclear antibody | Manometry |
| Complete blood count | Electrogastrography (EGG) |
| Complete metabolic panel (including renal function and anion gap to rule out ketoacidosis) | |
| Erythrocyte sedimentation rate | |
| Rheumatoid factor | |
| Thyroid-stimulating hormone | |
| Urinalysis | |
Radiographic Tests
Gastric Scintigraphy
Gastric emptying scintigraphy of a radiolabeled solid meal is the gold standard for the diagnosis of gastroparesis because it quantifies the emptying of a physiologic caloric meal and as such can assess the motor function of the stomach. Therefore, it provides a physiological, non-invasive and quantitative measure of gastric emptying. The technique involves incorporating a radioisotope tracer into a standard meal and subsequently tracking its passage through the stomach using a gamma camera. Scintigraphy is more sensitive to the measurement of the emptying of solids due to the fact that liquid emptying may remain normal despite advanced disease, but liquids can be radiolabeled as well with an additional isotope. A variety of foods, including chicken, liver, eggs, egg whites, oatmeal or pancakes are commonly used as meals. The content of the meal is important as factors as solids versus liquids, indigestible residue, fat content, calories and volume of the test meal can all influence gastric emptying time. Dual-isotope labeling of solid and liquid phases may also be performed. Emptying of solids exhibits a lag phase followed by a prolonged linear emptying phase [71].
A consensus statement from the Society of Nuclear Medicine and Molecular Imaging and the American Neurogastroenterology and Motility Society recommends the use of universally acceptable 99-m technetium sulfur-colloid-labeled low-fat, egg-white meal [77].
Indications of Scintigraphy
Measurement of gastric emptying with scintigraphy may be indicated in diabetic patients with upper GI symptoms (other than isolated heartburn or dysphagia), patients with poor glycemic control and those being considered for, or treated with hypoglycemic medications that may slow gastric emptying, including alpha glucosidase inhibitors, amylin analogs and GLP1-RAs (Table 5), and those with severe reflux symptoms unresponsive to standard therapy. 78].
Procedure
Gastric emptying scintigraphy should be performed after the exclusion of mechanical or structural causes of abnormal gastric emptying. Patients should discontinue all motility-altering medications, including prokinetics, opiates and anticholinergics for at least 2–3 days before testing, and longer if possible. GLP-1 RAs also delay gastric emptying, and it is reasonable to consider alternative therapies that do not delay gastric emptying. Long-acting GLP1 agonists should be discontinued for at least 1 week before the procedure (listed in Table 5). Patients should refrain from smoking and consuming alcohol on the test day, as both may slow gastric emptying. Significant hyperglycemia delays gastric emptying, and fasting blood glucose should be < 275 mg/dL on the day of testing [79].
After an overnight fast, the patient consumes a standardized test meal within 10 min. The most commonly used meal is a 255 kcal low-fat test meal consisting of egg beaters (120 g) labeled with 0.5 mCi technetium-99 m sulfur colloid radioisotope, two slices of bread, strawberry jam (30 g) and water (120 mL). Standard imaging of the gastric area with the patient standing is performed at baseline (after meal ingestion) and at 1, 2 and 4 h after meal ingestion. Although an alteration in body position may have marked effects on gastric emptying of radiolabeled liquids, they have only a minor effect on the intragastric meal distribution and lag-time or post-lag emptying rate for solid and liquid meals. Anterior and posterior images are obtained sequentially with a single-headed camera or a dual-headed camera tracking the passage of the meal through the stomach. Imaging should be completed over 4 h to produce a reliable estimate of half-life time. Shorter imaging protocols may complicate interpretation. The study meal should also be consumed within 10 min, and the time used for consumption should be noted as prolonged time for meal ingestion can effect the measurement of gastric emptying [78].
Interpretation of scintigraphy
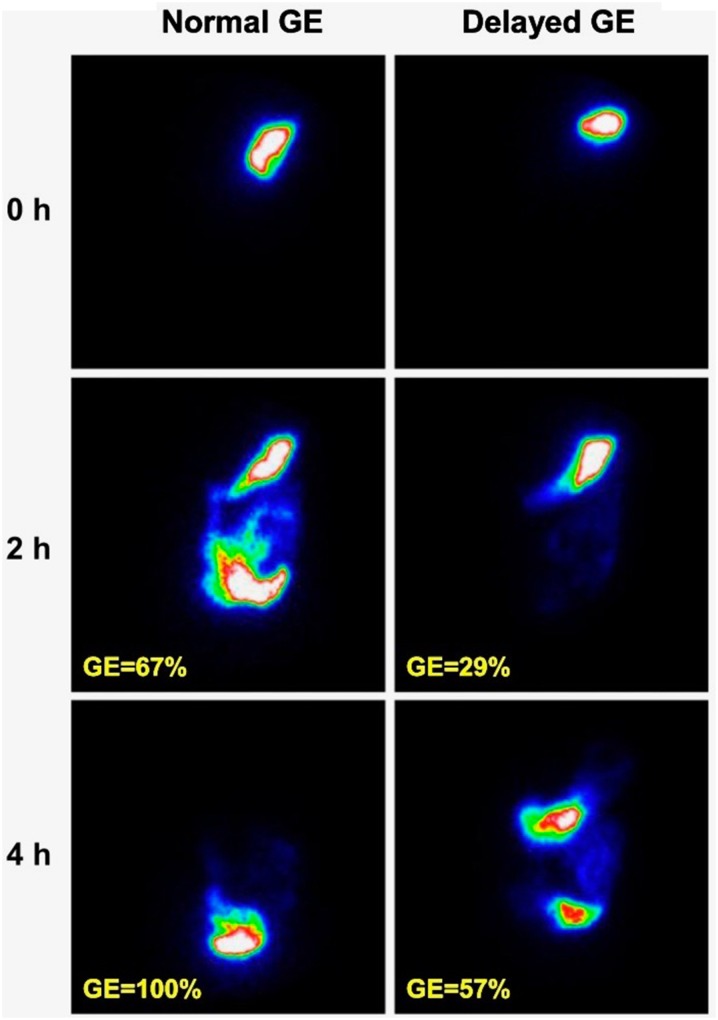
A region of interest is drawn around the stomach on both anterior and posterior images at each time point using computerized software. Geometric means of the anterior and posterior counts are calculated and corrected for tissue attenuation and isotope decay. The results are expressed as the percentage of radioactivity retained in the stomach at each time point, normalized to the baseline value. Gastruc emptyingis considered delayed if there is greater than 60% retention at 2 h or 10% retention at 4 h, as shown in Fig. 12 [78].
Fig. 12.
Gastric emptying (GE) scintigraphy showing normal and delayed GE in a patient with type 1 diabetes. The percentage shown is the percentage emptied; the current standard is to list the percentage of radioactivity retention, which would be 100% minus the percentage emptied.
Reprinted with permission of the American Diabetes Association, Inc. Copyright 2013
Radiopaque Markers
Indigestible markers, i.e. ten small pieces of nasogastric tubing, are ingested with a meal. None of the markers should remain in the stomach on an X-ray taken 6 h after their ingestion. This simple test correlates with clinical gastroparesis and is readily available and inexpensive. The drawbacks of the test include lack of standardization of the meal and size of markers and difficulty in determining if the markers are located in the stomach or in other regions that overlap with the stomach, such as the proximal small bowel and transverse colon [78].
Ultrasonography
Transabdominal ultrasound has been used to measure emptying of a liquid meal by serially evaluating cross-sectional changes in the volume remaining in the gastric antrum over time. Emptying is considered to be complete when the antral area/volume returns to the fasting baseline. Three-dimensional ultrasound is a newly developed technique that has recently been reported to be useful in determining stomach function, and duplex sonography can quantify the transpyloric flow of liquid gastric contents. These techniques are preferred over scintigraphy in certain patients, such as pregnant women and children, to minimize radiation exposure. Drawbacks of the test include operator dependence, proven reliability only for measurement of liquid emptying rates and lower reliability in obese patients or in the presence of excessive gastric air. Moreover, liquid emptying is rarely impaired in patients with severe gastroparesis [78].
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) using gadolinium can accurately measure semi-solid gastric emptying and accommodation using sequential transaxial abdominal scans. MRI provides excellent resolution with high sensitivity. It is also non-invasive and radiation free. Antral propagation waves can be observed and their velocity calculated. In gastroparesis, a significant reduction is seen in the velocity of these waves. MRI can also differentiate gastric meal volume and total gastric volume, thereby allowing gastric secretory rates to be calculated. New rapid techniques allow careful measurements of wall motion to be made in both the proximal and distal stomach during emptying, and solid markers now permit the measurement of solid meal emptying. The drawback of this test is its expense and lack of availability [78].
Single-Photon Emission computed tomography
This technique uses intravenously administered 99-Tc pertechnetate that accumulates within the gastric wall rather than the lumen and provides a three-dimensional outline of the stomach. Measurement of regional gastric volumes in real time to assess fundic accommodation and intragastric distribution can be made. The drawback of this test is the need for large radiation doses and its wide unavailability [78].
Stable-Isotope Gastric Emptying Breath Testing
The gastric emptying breath test (GEBT) using a stable isotope, i.e. 13C-labeled substrates, typically 13C-octanoic acid or 13C-Spirulina platensis (blue-green algae), is a promising alternative diagnostic modality to scintigraphy. It is a noninvasive, easy-to-perform method and does not involve radiation exposure. In the GEBT, the rate of gastric emptying of the 13C substrate incorporated in a solid meal is reflected by breath excretion of 13CO2 [78].
Indications
The indications for the GEBT is similar to those for scintigraphy; however, the former may specifically be indicated in patients in whom scintigraphy is not feasible. GEBT has an advantage over scintigraphy in that it does not require radiation exposure and may be used in pregnant women, women who are breast-feeding and children. It is also less expensive and easier to perform than gastric emptying scintigraphy. Samples can be transferred to a central laboratory, so the test can be performed anywhere [78].
Wireless Motility Capsule
The wireless motility capsule using the SmartPil has been approved by the U.S. FDA for the evaluation of gastric emptying, colonic transit time in patients with suspected slow transit constipation and for measurement of pH, temperature and pressure throughout the GI tract. It is a safe and practical alternative to scintigraphy. It consists of a 2-cm-long wireless transmitting capsule that has the ability to record and transmit data on pH, pressure and temperature to a portable receiver that may be worn around the patient’s neck. Data can be acquired continuously for up to 5 days, and significant events (e.g. meal ingestion, sleep or GI symptoms) can be recorded with a button. Gastric emptying is reflected by an abrupt change in pH as the capsule moves from the acidic environment of the stomach to the alkaline environment of the duodenum. This transit typically occurs with return of the fasting state and phase III migrating motor complex (MMC) after the emptying of liquids and triturable solids [78].
Indication
Wireless motility capsule testing is used in the evaluation of gastric emptying and whole-gut transit in patients with suspected gastroparesis.
Procedure
The procedure should begin in the morning after an overnight fast. Before testing, medications that suppress gastric acid production should be stopped, such as proton-pump inhibitors for 1 week and histamine H2 receptor antagonists for 3 days, as they may interfere with the pH-dependent measurement of gastric emptying. Similarly, medications that may affect GI motility are stopped 2–3 days before the test. The patient consumes a standardized nutrient meal on the morning of the test, followed by ingestion of the WMC with 50 mL water. The patient fasts for the next 6 h [78].
Interpretation
Sensed data are transmitted by the single-use capsule to the receiver worn by the patient, and pH values from 0.5 to 9.0 pH units, pressure activity and temperature are recorded. Gastric emptying time is defined as the time from capsule ingestion to a rise in pH from gastric baseline to 4.0 pH units, marking the passage of the capsule from the antrum to the duodenum. Normal emptying of the capsule should occur within 5 h of ingestion. If it does not occur within 6 h, a maximum gastric emptying time value of 6 h is assigned (Fig. 13).
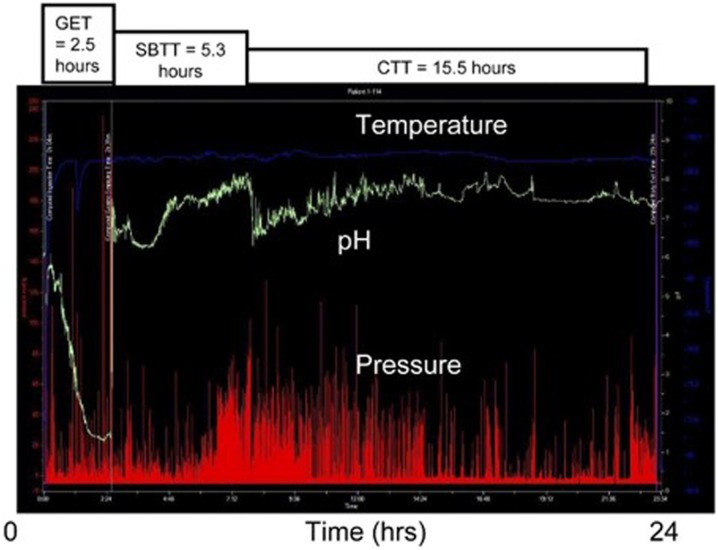
Fig. 13.
Normal gastrointestinal motility tracing using the wireless motility capsule (WMC). GET Gastric emptying time, SBTT small bowel transit time, CTT colon transit time
Reprinted with permission from Elsevier (copyright). Rao SS, Kuo B, McCallum RW, et al. (2009) Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 7(5):537–544
Limitations
Healthy subjects and patients with gastroparesis may not have a phase III MMC contraction within 6 h when the next meal is given, and capsule emptying may therefore be inhibited. Diabetic patients undergoing evaluation for gastroparesis receive a second meal at 6 h as part of the standard method and to avoid hypoglycemia in those receiving medium-duration insulin preparations. Other limitations are the possible difficulty with capsule ingestion and the potential for capsule retention or obstruction. Use of the capsule is contraindicated in children and in adult patients with a known history of esophageal stricture [78].
Electrogastrography
Electrogastrography (EGG) can be a useful adjunctive diagnostic test. EGG measures gastric slow-wave myoelectrical activity typically via cutaneous electrodes positioned along the long axis of the stomach. A pre-prandial recording is captured for approximately 45–60 min, then the patient is given a meal, followed by a 45- to 60-min postprandial recording, although shorter recording periods can be used as well. Healthy controls produce EGG recordings that exhibit uniform waveforms of three cycles per minute, which increase in amplitude after ingestion of a meal, and both the frequency and amplitude of the EGG can be important measures, as well as the propagation between channels of EGG signal. Cutaneous electrogastrography can be amplified by the use of more direct measures, such as mucosal or serosal electrograms. Electrograms are not conducted routinely, but they may offer additional sensitivity and indications of disordered gastric function in a given patient [80]. New work with high-resolution EGG systems offer the potential for more sensitive electrical measurements and possible wider utilization and acceptance.
Differential diagnosis of Diabetic Gastroparesis
The nonspecific nature of the clinical features of gastroparesis makes for a broad differential diagnosis, which includes endocrine and metabolic disorders, autoimmune and connective tissue diseases, central nervous system lesions and GI syndromes, as shown in Table 9. Careful review of clinical presentation and diagnostics is warranted since other reversible causes of nausea and emesis, may masquerade as gastroparesis [70, 81].
Table 9.
Differential diagnosis of gastroparesis
| Differential | Evaluation |
|---|---|
| Rumination syndrome | History of passive regurgitation of unpleasant tasting substances without preceding nausea |
| Cyclical vomiting syndrome | Episodic bouts of emesis with intervening asymptomatic periods |
| Pregnancy | Pregnancy testing |
| Celiac disease | Serology and endoscopy |
| Gastric outlet obstruction | Upper endoscopy or barium series |
| Complete bowel obstruction | Bowel films and other imaging |
|
Partial small-bowel obstruction Crohn’s disease with small bowel stricture |
Small bowel follow through or computed tomography enterography or enteroclysis |
| Hypothyroidism | THS testing to screen for hypothyroidism |
| Diabetes | HbA1C, or 2 h glucose tolerance test |
| Diabetic ketosis/ketoacidosis |
Acute onset, laboratory tests, including anion gap and ketone derivatives are helpful Normoglycemia does not rule out diabetic ketoacidosis |
| Functional dyspepsia | Milder symptoms: may have mild delay in gastric emptying |
| CNS disorders | Examination: cranial nerve palsies, cerebellar signs, CNS imaging |
| Addison’s (primary) or secondary adrenal insufficiency | Nausea but seldom with emesis. Clinical signs buccal pigmentation, low cortisol with elevated ACTH levels (primary). May coexist with autoimmune diseases such as type 1 diabetes mellitus, Hashimoto’s thyroiditis or Graves’ disease. Secondary (ACTH) deficiency is often from a pituitary tumor with headache and visual complaints, as well as hypogonadism |
| Medication effects | Refer to list of medications that delay gastric emptying |
| Cannabinoid hyperemesis syndrome | History of marijuana use, relief of GI symptoms with hot showers |
| Pseudo bowel obstruction | Radiograph suggestive of dilated loops with no obstruction: ANA, anti-Scl 70, fat biopsy, ANNA-1, CPK.(infiltrative diseases) |
| Eating disorders: anorexia and bulimia | Clinical presentation helpful. Re-alimentation and maintenance of body weight improves symptoms [82, 83] |
DKA Diabetic ketosis/ketoacidosisCNS central nervous system, TSH thyroid stimulating hormone, ACTH adrenocorticotropic hormone, GI gastrointestinal, ANA antinuclear antibodies, CPK creatine phosphokinase, ANNA-1 type 1 antineuronal nuclear antibodies
Gastroparesis-Like Syndrome
Patients with the symptoms of gastroparesis but with non-delayed solid emptying, have been described [29]. It is unclear if this entity of gastroparesis-like syndrome is distinct from gastroparesis.
Non-Delayed Gastric Emptying (Accelerated/Rapid Gastric Emptying)
Rapid gastric emptying of solids and/or liquids with features of dumping syndrome and diarrhea is increasingly recognized in patients with diabetes mellitus. Other conditions with rapid gastric emptying include post fundoplication and other gastric surgeries for peptic ulcer or post bariatric surgery, functional diarrhea, functional dyspepsia and autonomic dysfunction.
In contrast to delayed gastric emptying, which has been associated with long-standing complicated type 1 diabetes, rapid gastric emptying of liquids occurs with type 2 diabetes, often with early disease (Fig. 9).
Impairment of nitrergic-mediated gastric accommodation due to vagal dysfunction in diabetes mellitus predisposes to higher gastric pressures and rapid gastric emptying of liquids. Patients with rapid gastric emptying may present with poor postprandial glycemic control and postprandial upper abdominal symptoms, such as abdominal discomfort and nausea with or without vomiting, which are often indistinguishable from those of delayed gastric emptying. However, weight loss is more common among patients with delayed gastric emptying [49].
Diabetics with rapid or accelerated emptying may have similar symptoms as those with DGp. The former present with predominantly postprandial symptoms which are exacerbated by prokinetic agents. Avoiding liquids with meals and for 30 min post meals and the addition of dietary fiber (e.g. pectin, guar gum) can alleviate symptoms. GLP-1 analogs may help by slowing gastric emptying and postprandial hypoglycemia; however, randomized controlled studies are lacking in this area [84].
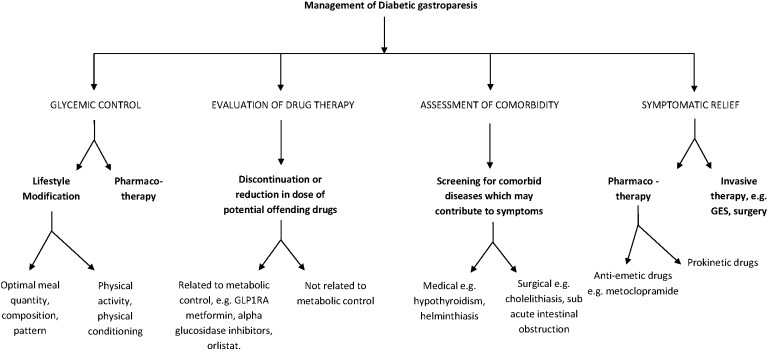
Management of Diabetic Gastroparesis
The development of gastroparesis is associated with poor glucose control [31], and the goal of optimal glycemic control needs to be emphasized. The usual treatments for DGp include nutritional assessment and dietary modifications, glycemic control, prokinetic agents and antiemetic agents, as discussed in the following sections. Although the majority of patients have mild-to-moderate disease that can be managed effectively using these measures, a small percentage of patients have severe DGp that is characterized by inadequate oral intake, malnutrition, weight loss and frequent hospitalizations. Optimal management of these patients presents a difficult challenge for the clinician, although emerging treatment options, such as gastric neurostimulation, offer a glimmer of hope. Patients with DGp often present with gastric comorbidities, including gastroesophageal reflux disease, intestinal dysmotility and fungal and bacterial infections of the GI tract [5], as well as with macro- and microvascular complications of diabetes. Therefore, effective management of patients with DGP often requires an interdisciplinary approach with the involvement of a team of specialists, including the primary care physician, gastroenterologist, endocrinologist, dietician, psychologist, interventional radiologist and surgeon.
Non-glycemic endocrine issues related to DGp include mineral and vitamin deficiency, low bone mass, hypogonadism and amenorrhea related to undernourishment in severe gastroparesis.
Vitamin and micronutrient deficiencies, such as vitamin D deficiency, may impact gastric emptying and, interestingly, some studies show a paradoxical worsening of gastric emptying with higher B12 levels. [85].
Nutritional Management
Most patients with DGp have lower-than-recommended caloric intake and extensive macro- and micronutrient deficiencies [86]. The caloric requirement can be calculated by multiplying 25 kcal by the current body weight in kilograms. The American Diabetes Association (ADA)-recommended standard low-carbohydrate and high-fiber dietary composition may not be appropriate for many of these patients.
Dietary recommendations rely on measures that promote gastric emptying or, at least theoretically, do not retard gastric emptying. At the outset, the patient should be counseled by an experienced dietician who can assess nutritional status and explore the patient’s tolerance of solids, semi-solids and liquids, as well as dietary balance, meal size and timing (Table 10). Fats and fiber tend to retard emptying, thus their intake should be minimized [87]. A step-wise approach starting with clear liquids with nutritional values, followed by soups and smoothies, and later the introduction of gastroparesis-friendly solids is another option [67]. Multiple small low-fat meals four or five times each day should be recommended. Carbonated liquids should be avoided to limit gastric distention. Patients are instructed to take fluids throughout the course of the meal and to sit or walk for 1–2 h after meals. A small particle diet may also be beneficial for symptoms and tolerance compared to a conventional diabetic diet [88]. If the above measures are ineffective, the patient may be advised to consume the bulk of their calories as liquids since liquid emptying is often preserved in patients with gastroparesis. Poor tolerance of a liquid diet is predictive of poor success with regular treatment. [5].
Table 10.
Summary of nutritional interventions for diabetic gastroparesis
Republished and modified with permission of Dove Medical Press [89]. Permission conveyed through Copyright Clearance Center, Inc.
| Hydration: if all else fails, go for liquids | On days when symptoms are worse, try taking just liquids to maintain hydration and to rest the stomach |
| Meal volume/portion size: multiply frequency and divide the portions | Eat smaller, more frequent meals |
| Meal consistency: If you can not chew, blenderize |
Chew the food thoroughly and take 20–30 min to finish the meal Try solid meals in the morning, switch to semi-liquid and liquid meals over the course of the day Any food can be blended with water, vegetable juice or broth to make a puree When symptoms worse, prefer liquid vs solid meals |
| Glycemic control: match meals with medicines | Modify meal timing, form of carbohydrate (simple, complex) according to the diabetes treatment regimen and vice versa |
| Fat: less is more | Fat in liquid is well-tolerated; maintain an intake of 20–30% of calories from fat |
| Fiber: watch for fur balls |
Identify the high-fiber foods that worsen upper GI symptoms, and individualize the sources of fiber Delaying GI transit may modulate the biome and alleviate the symptoms If bezoar formation is a concern, avoid foods causing bezoar, such as fruits with peelings, berries, coconut, legumes and fiber supplements Treat bacterial overgrowth if suspected/symptomatic |
| Address micronutrient deficiency: bones and blood |
Eat nutritious foods first before filling up on “empty calories” Replace iron, B12, vitamin D and calcium deficiency |
| Weight/body mass index: keep moving |
Check body weight twice a week, if the weight is decreasing, increase the amount of liquid supplements. Lose weight if you are overweight Physical activity may improve gastric emptying [90] (Consult your medical team) |
| Miscellaneous: do not miss the bottom line |
Avoid foods that lower esophageal sphincter pressure: pepper-mint, chocolate, fat, and caffeine Avoid caffeine, alcohol, tobacco and stress Avoid chewing gum, which increases air swallowing High-fiber foods should be avoided as they may be more difficult on the stomach and may cause bezoar formation Chew well and eat slowly (30 min meals) Do not lie down immediately after eating. Consult dental/oral health team to improve oral hygiene |
The role of a nutritionist familiar with gastroparesis nutrition needs to be underscored since hydration and nutrition are important in preventing many complications of DGp and autonomic neuropathy including diabetic ketosis/ketoacidosis, delayed wound healing and diabetic cachexia.
Lifestyle Intervention, Behavior Modification and Alternative Therapies
Patient and family education and improved awareness of the condition form an integral part of the treatment plan. The disabling chronic symptoms of gastroparesis have a profound impact on the patient’s sense of well-being and personal and social life [91]. Empathy to patient’s needs, a humanistic approach from the clinical team, and behavioral psychology counseling will help the patient cope with the disability. Patients should be informed that a number of drugs might be tried in an attempt to discover the optimal therapeutic regimen and that the aim of treatment is to control rather than cure the disorder. Addressing physical conditioning, weight and nutrition-related issues is imperative to DGp treatment [71].
Glycemic Management
It is imperative to optimize glycemic control to minimize acute symptoms of DGp and improve gastric emptying to impact overall diabetes-related outcomes. Rapid gastric emptying may cause postural hypotension, thereby precipitating falls, especially in elderly patients with DAN [92, 93]. Hyperglycemia delays gastric emptying, even in the absence of neuropathy or myopathy, which is likely to be mediated by reduced phasic antral contractility and the induction of pyloric pressure waves [94]. Hyperglycemia can inhibit the accelerating effects of prokinetic agents. Glucose levels should be maintained below 180 mg/dL to avoid inhibiting gastric myoelectric control and motility. Patient-centered interventional strategies to minimize postprandial hyperglycemia need to be devised [95].
A multidisciplinary approach with a team consisting of a certified diabetes educator, registered dietician who is familiar with nutritional assessment of gastroparesis and a behavioral psychologist is integral to implementing a strategy of individualized patient care. Also, compassionate family members/care takers who understand the dynamics and complexity of blood glucose management in patients with gut autonomic dysfunction will be effective partners in the patient care team.
Pharmacotherapy for Glucose Management in Patients with Diabetic Gastroparesis
Over the last decade, the therapeutic armamentarium for diabetes has expanded at a remarkable pace to include drugs with novel pathways and also device technology [63]. For those with type 2 diabetes, incretin mimetics and sodium glucose transporter inhibitors (SGLT-2i) have been game changers with major trials proving significant cardiovascular benefits [96]. In patients with DGp, glycemic goals and choice of pharmacotherapy should be individualized along with nutrition and lifestyle modifications [97].
Oral Agents
Oral agents are not recommended for patients with type 2 diabetics with clinically significant DGp. The pharmacodynamics/kinetics of oral agents are impacted by delayed gastric emptying and, therefore, these agents are not ideal for effective glycemic control. While biguanides (metformin) improve insulin resistance, GI intolerance often limits their use. Sulfonylureas must be used with caution given the risk of hypoglycemia. While data on the impact of dipeptidyl peptidase 4 (DPP-4) inhibitors on gastric emptying are inconsistent, absorption may be impaired depending on the rate of gastric emptying. Dehydration and euglycemic ketoacidosis are a potential risk, but the direct impact of SGLT-2i on DGp is not clear at this time [98]. Alpha-glucosidase inhibitors may be beneficial for accelerated gastric emptying, but they may also cause diarrhea and abdominal distension.
Incretins
Glucagon-like peptide-1 analogs and GLP1-RAs are well-established antidiabetic agents for patients with type 2 diabetes, with multimodal impact both on glycemic control and metabolic benefit [99] (Table 5). However, this group of agents may exacerbate symptoms in patients with delayed gastric emptying, [67]. On the other hand, there may be a role for GLP-1 analogs in those diabetics with accelerated gastric emptying [84].
Insulin Therapy
In patients with type 1 diabetes, the standard of care is insulin, either basal-bolus therapy (Table 11) or continuous subcutaneous insulin infusion (CSII).
Table 11.
Summary of available human and analog insulins and their pharmacokinetics
| Type of insulina | Onset of action | Peak | Duration of action | Frequency of dosing |
|---|---|---|---|---|
| Human insulin | ||||
| Regular | 0.5–1 h | 2–4 h | 6–8 h | Meal time (preferred in DGp, poorly controlled diabetes mellitus, enteral nutrition) |
| NPH (isophane) | 2–4 h | 4–8 h | 12–16 h | Basal insulin, given twice a day |
| U 500 regular (concentrated) | 2–4 h | 4–8 h | 12–16 h | Basal/bolus 2–3 × day or pump |
| Analog insulin | ||||
| Prandial/meal time/rapid acting | ||||
| Lispro | 5–15 min | 1 h | 2–4 h | Meal time |
| Aspart | 5–15 min | 1–3 h | 3.5–5 h | |
| Glulisine | 5–15 min | 1 h | 4–5 h | Meal time(may be administered within 20 min after a meal) |
| Aspart (fast acting) | < 15 min | 1.5–2.22 h | 5–7 h | |
| Basal/long acting analog | ||||
| Glargine (U100) | 3–4 h | Flat/12 h | 10.8–24 h |
Once or twice a day Duration dose dependent (generic available) |
| Detemir U100 | 1–4 h | Flat | 10–18 h |
Twice a day Duration dose dependent |
| Degludec (u-100) | 90 min | Flat peak | 24–42 h | Once a day |
| Concentrated insulinsb | ||||
| U-200 degludec | 90 min | Flat | 24–42 h | Basal |
| U-300 glargine | 6 h | Flat | 24 h | |
| U-500 regular | ~ 15 min | 4–8 h | ≤ 21 h |
Basal/bolus Inject 30 min before meal |
| U-200 lispro | ~ 15 min | 30–90 min | 4–5 h | Prandial |
aPremix insulins and inhaled insulins are not discussed here since their role in patients with DGp is unclear
bConcentrated insulins may be helpful in insulin-resistant patients with DGp (type 2 diabetes mellitus)
Basal insulin is long- or intermediate-acting insulin administered subcutaneously once or twice a day. Ideal basal insulin has no peak effect and maintains euglycemia independent of the prandial state. The analog basal insulins (Table 11) are closer to endogenous insulin secretion with a lack of peak effect and longer duration of action.
Basal insulin is initiated at a dose of 0.2–0.3 units/kg/day for patients with type 2 diabetics and 0.15 units/kg/day for those with type 1 diabetics. Dose titration is based on glycemic response, keeping in mind that the post-absorptive state in DGp varies widely.
Prandial insulin is generally used pre-meal to prevent postprandial glycemic excursions, but its use poses challenges given the wide postprandial glycemic variability with DGp. Prandial insulin may be administered after meals as a strategy to prevent postprandial hypoglycemia if the full meal is not consumed as planned, or there is intra-prandial emesis. Regular insulin causes less hypoglycemia post meal than does rapid-acting insulin analogs in select patients [67]. With multiple small meals, aggressive glucose monitoring and frequent small doses of rapid-acting insulins may be needed to prevent postprandial hyperglycemia.
Diabetes Technology
In patients with DGp, the variable gastric emptying poses challenges for glycemic control. Prevention of wide glucose fluctuations may be more important than maintenance of a given steady-state blood glucose level. Continuous glucose monitoring (CGM) may be helpful with predictive low glucose alerts and to ascertain the effect of certain meals on glucose levels [100] (Fig. 14). Optimal glucose control may improve antral contractility, correct gastric dysrhythmias and accelerate emptying. DGp may be an indication for insulin-pump therapy (CSII) in patients with type 1 diabetes mellitus [101]. A recent National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Gastroparesis Consortium (GpCRC)-funded open labeled pilot study of 42 diabetics with DGp (both types 1 and 2) showed improved glycemic control, less hypoglycemia and an improved DGP symptom score with the use of sensor-augmented pump or CSII and CGM) [102].
Fig. 14.
Continuous glucose monitoring system (Dexcom G4 CGM) downloaded from a patient with Type 1 diabetes with diabetic gastroparesis treated with a basal and bolus insulin regimen. The figure shows data for seven 24 hour periods (different color for each of the 7 days). Daily trends show wide glycemic fluctuations (interstitial glucose mg/dl on y-axis), mostly in postprandial state that vary from day to day. Also of note there are significant hypoglycemic events. Courtesy Dr. K. Komorovskiy
With insulin pump therapy, the patient is able to use various delivery patterns of prandial insulin. Combination and extended boluses (square wave and dual wave patterns) may eliminate the postprandial hypoglycemia that may occur with instant boluses. Combo bolus or dual wave using 10–20% with the first wave and the remainder with the second wave over 5–6 h depending on meal may be helpful [67]. A hybrid closed loop pump (CSII with CGM) which delivers interprandial insulin based on glucose trends (model 670G; Medtronic plc, Dublin, Ireland) was approved in 2016 by the FDA for those patients who need steady glycemic control [103] (Fig. 15). Further clinical trials of patients with DGp will enhance use of available technology to improve glycemic -gastric outcomes.
Fig. 15.
Data downloaded from a continuous glucose monitoring system with automated basal insulin delivery (Medtronic 670G hybrid closed loop) 4 weeks after the initiation of sensor augmented pump therapy in a patient with poorly controlled type 1 diabetes and diabetic autonomic neuropathy, including hypoglycemia unawareness, gastroparesis and status post (s/p) gastric stimulator. The report shows very few hypoglycemic events. Time in range (green) shows a significant stability in glycemic variability with the HbA1c level below 7% while on auto mode (latter controls interprandial insulin delivery based on a built-in algorithm). BG Blood glucose, SG sensor glucose
In a study of hospitalized type 1 diabetics with DGp, CSII was superior to multiple insulin injections for glycemic control, hypoglycemia prevention and length of inpatient days [104].
In the past 2 years, the U.S FDA has approved expanded indications for Dexcom G5® Mobile CGM System and Libre flash glucose monitoring systems (Abbott Laboratories, Lake Bluff, IL) to replace finger stick glucose checking in diabetic patients [105, 106]. More recently an integrated CGM (iCGM) has been approved to use with other compatible medical device platforms and electronic interfaces, including automated insulin pumps [107]. Given the available ground-breaking technology more studies are needed to evaluate the feasibility and safety of real-time glucose monitoring and predictive insulin infusion systems in patients with DGp.
Pharmacologic Treatments for Diabetic Gastroparesis
The pharmacotherapy of gastroparesis involves a stepwise, incremental and long-term treatment approach. The most commonly used drug classes include prokinetics, antiemetics and (occasionally) analgesics [108]. Several novel targeted therapies are also being studied [109].
Prokinetics
Several prokinetic drugs have been used successfully to manage the symptoms of gastroparesis. These agents include metoclopramide, domperidone, erythromycin and cisapride. Newer prokinetic agents include tegaserod, sildenafil and novel experimental motilides (e.g., ABT-229 and GM-611 [mitemcinal], synthetic ghrelin, bethanechol, levosulpiride and clonidine) [12].
Metoclopramide
Metoclopramide is one of the most commonly used agents in the management of DGP. It is both a central and a peripheral dopamine-2 (D2)-receptor antagonist with antiemetic and prokinetic actions that increases antral contractions and coordinates antral duodenal motility [110]. Restricting the total daily metoclopramide dose to 40 mg/day and using the liquid formulation to improve its pharmacokinetics provide a balance between efficacy and side effects to the central nervous system. Female gender, younger age, presence of diabetes and use of high doses are risk factors for acute dystonia.
Metoclopramide can be administered parenterally when symptoms are severe. The FDA issued a black box warning in 2009 cautioning about its use beyond 3 months [111].
An intranasal spray was found to improve symptoms compared to placebo in female but not male diabetic patients [112].
Metoclopramide increases serum prolactin levels. Gynecomastia and galactorrhea may occur in adults as well as adolescents and young children [113], and adult women may develop oligomenorrhea [114] Metoclopramide also stimulates aldosterone synthesis and may provoke uncontrolled hypertension in a subset of patients with primary hyper-aldosteronism [115]. Metoclopramide can prolong the QTc in susceptible patients. In the USA, it is recommended that metoclopramide be reserved for the most severe cases that are unresponsive to other treatment modalities [4]. A few years ago, the European Medicines Agency cautioned that the risks of extrapyramidal symptoms outweigh the benefits of metoclopramide.
Domperidone
Domperidone is a type II dopamine antagonist similar to metoclopramide, and it is equally efficacious to the latter but with less side effects to the central nervous system as it does not cross the blood–brain barrier. Domperidone has been shown to reduce GI symptoms and hospitalizations from gastroparesis and to accelerate gastric emptying at doses between 10 mg and 30 mg taken orally 30 min before meals and at bedtime. Domperidone can cause gynecomastia in men and amenorrhea and galactorrhea in women. A baseline electrocardiogram is recommended to assess corrected QT intervals, and this should be repeated as indicated. The drug is often withheld from patients with a QTc of > 470 ms in males and of > 450 ms in females, and a cardiology consultation may be indicated [116, 117]. Because of a reported association with serious cardiac arrhythmias, domperidone is restricted for use in some countries. Domperidone is available in the US through an FDA-sponsored Investigational New Drug program.
Erythromycin
Erythromycin is a macrolide antibiotic with an agonist effect on motilin receptors in the GI tract that increases gastric emptying in a dose–response fashion, with 3 mg/kg of erythromycin administered intravenously seeming to be the most effective dose. Erythromycin has been shown to stimulate gastric emptying in diabetic, idiopathic and post-vagotomy gastroparesis. Oral erythromycin administered in the dose range of 50–100 mg taken 3 times daily in combination with a low-bulk diet was found to be effective in controlling symptoms of gastroparesis in 83% patients. QTc may be prolonged by this drug, and cardiac monitoring is recommended by electrocardiogram before and with therapy [118]. In a recent interventional study using intravenous erythromycin followed by oral erythromycin in patients with type 1 diabetics with delayed gastric emptying, CGMS and [13] the GEBT with 13C-Spirulina platensis showed improved gastric emptying with a high- (3 mg/kg) but not low-dose (2 mg/kg) infusion and no change with oral administration (250 mg three times a day) [119].
Cisapride
Cisapride is a potent prokinetic drug that accelerates gastric emptying of solids and improves dyspeptic symptoms. It acts on the stomach via 5-hydroxytryptamine (5-HT4) receptors. This drug has been withdrawn from the market in many countries, including the USA, due to the risk for ventricular arrhythmias [120].
Bethanecol
Bethanecol is a muscarinic receptor agonist, usually given at a dose of 25 mg four times a day. Its reported side effects include headache, tachycardia, flushing, hypotension and urinary urgency.
Tegaserod
Tegaserod has been shown to increase gastric emptying; however, it too has been withdrawn from the market due to an association with bowel ischemia and for possible cardiovascular side effects.
Antiemetics
Nausea and vomiting are the most disabling symptoms of gastroparesis, and antiemetic agents without stimulatory activity are often used alone or in combination with prokinetic drugs to treat gastroparesis. Antiemetic medications act on a broad range of distinct receptors subtypes in the peripheral and central nervous system. Like prokinetics, the choice of antiemetic is empirical [71]. Some anti-emetics have the potential for KEG Q-Tc prolongation, as do some other drugs used for the treatment of gastroparetic symptoms.
Phenothiazines
Phenothiazines are the most commonly prescribed traditional antiemetics and include prochlorperazine and tiethyperazine. These drugs are both dopamine and cholinergic receptor antagonists that act on the area postrema in the brainstem. Side effects include sedation and extra-pyramidal effects such as drowsiness, dry mouth, constipation, skin rashes and Parkinsonian-like tardive dyskinesia.
Serotonin 5-HT3 Receptor Antagonists
These medications include ondansetron, granisetron and dolasetron, and they act on the chemoreceptor trigger zone as well as on peripheral afferent nerve fibers within the vagus nerve. They may be used in DGP when all other drugs have failed to provide symptom relief.
Antihistamines
Antihistamines act on H1 receptors to produce central antiemetic effects. Commonly prescribed antiemetics include diphenhydramine, dimenhydrinate and meclizine. These agents are most often used to treat symptoms related to motion sickness. Side effects include drowsiness, dry mouth, blurred vision, difficulty urinating, constipation, palpitations, dizziness, insomnia and tremors.
Low-Dose Tricyclic Antidepressants
Tricyclic antidepressants (TCAs) impair gastrointestinal motility through their anticholinergic activity but they have also been shown to relieve nausea, vomiting and pain in functional dyspepsia. In one study, 88% of diabetic patients with nausea and vomiting reported benefits with TCAs. Side effects associated with low-dose TCAs are uncommon, although excessive sedation and dry mouth occasionally limits use. However, a recent randomized controlled trial of nortriptyline found no benefit in idiopathic gastroparesis [121].
Pharmacotherapy in Children with Diabetic Gastroparesis
Treatment approaches differ for children and adults. Metoclopramide, domperidone and erythromycin have all been used in children with DGp [44]. However, few medications and interventions used to manage the symptoms of gastroparesis have been thoroughly studied in children.
Drugs in Development
Future Prokinetics
Motilin agonists. Motilin agonists have been explored as a treatment for gastroparesis, but no current compounds are available for investigational use [53].
- Ghrelin agonists. Ghrelin is a peptide produced predominantly by the enteroendocrine cells in the gastric mucosa. Its plasma concentration increases with fasting, and it is viewed as a ‘hunger hormone’ because it is an appetite-stimulating peptide. Ghrelin stimulates the secretion of adrenocorticotropic hormone, growth hormone and prolactin and inhibits insulin secretion. In a cross-over study, the ghrelin analog TZP-101 (80, 160, 320, or 600 μg/kg), administered intravenously, was tested in seven type 1 and three type 2 diabetics with moderate to severe gastroparesis symptoms and > 29% retention of a solid egg radiolabeled meal at 4 h after ingestion. TZP-101 reduced the half-time for gastric emptying of solids (i.e. mean acceleration of 20%) and shortened the lag time (mean reduction of 34%) relative to placebo. TZP-101 also reduced overall post-meal symptom intensity (24%) and postprandial fullness (37%) [33]. However, because of limited efficacy this drug is no longer in clinical trials. [122].
- Relamorelin. The novel pentapeptide-selective ghrelin agonist relamorelin (RM-131) has similar characteristics to native ghrelin, but with a 100-fold greater potency to reverse gastric ileus in animal models and a longer plasma half-life. RM-131 (100 μg/day, subcutaneous) accelerated gastric emptying in patients with type 1 or 2 diabetes who had upper gastrointestinal symptoms. In a phase 2, randomized, double-blind, placebo-controlled trial of 10 μg RM-131 involving 204 patients with diabetic gastroparesis (12% type 1 diabetes mellitus, 88% type 2 diabetes mellitus), with a 28-day treatment period after a 1-week, single-blinded, placebo run-in, RM-131 enhanced gastric emptying and reduced vomiting episodes and vomiting severity. In the 58.3% of patients with vomiting at baseline, all three endpoints also improved and, in addition, there was reduction in the composite score of nausea, abdominal pain, bloating and early satiety [123–126]. However, in a study of over 390 patients, 10% of whom had type 1 diabetes, although symptoms improved over a 12-week period, there was dose-dependent worsening of glycemic control in 14% of subjects [127]. The drug is in phase 3 trials.
Newer 5-HT4 agonists. New-generation 5-HT4 agonists have high selectivity for 5-HT4 receptors, with little affinity for other serotoninergic and other classes of receptors [53].
Other Therapies
Intrapyloric Botulinum Injection
Pylorospasm is thought to contribute to the development of DGP. Botulinum toxin, a potent inhibitor of neuromuscular transmission, has been reported to improve emptying and symptoms for several months in DGp and idiopathic gastroparesis in several open label studies [128].
However, several double-blind randomized placebo-controlled trials, while showing some improvement in gastric emptying demonstrated no alleviation or improvement of symptoms. Positive trials are needed before botulin toxin can be recommended for the management of pylorospasm in gastroparesis [5, 12], and the 2013 ACG recommendations on gastroparesis strongly advises against using botox for the treatment this condition [4].
Pyloroplasty
Pyloroplasty can be done either surgically or endoscopically; the latter is known as G-POEM (gastric peroral endoscopic myotomy) [129]. Renewed interest in the role of the pylorus in delayed gastric emptying has resulted in a number of ways to open the pyloric sphincter, including surgical and endoscopic approaches. While the analogy of the pyloric sphincter for the stomach to the lower esophageal sphincter for the esophagus is attractive, there have been very few controlled studies in this area, and there are currently no published guidelines for pyloric therapy [130–132].
Gastric Electrical Stimulation
For a subset of patients with severe, refractory gastroparesis that is unresponsive to medical therapy, gastric electric stimulation (GES) may be an option. GES improves nausea, vomiting, quality of life and nutritional status in patients with refractory DGp [133–136].
Three principal methods of GES have been described: gastric electrical pacing, high-frequency GES and sequential neural electrical stimulation. Based on the number of stimulation electrodes, GES can be classified into single-channel GES and multichannel GES. Gastric pacing by high-energy, low-frequency GES (long pulses) attempts to restore the regular slow wave rhythm of 3 cycles/min of normal gastric myoelectric activity and has been found to improve symptoms and gastric emptying [5]. Only high-frequency GES is approved by the U.S. FDA, and this therapy was recommended for certain drug-refractory patients, particularly those with DGp, in the 2013 ACG review [3]. A randomized trial of temporary endoscopic GES has shown the effectiveness of this strategy; it may be useful as a screening method [136].
Surgical Options in the Management of Diabetic Gastroparesis
A significant number of patients have gastroparesis that is refractory to medical management. Surgery is the last resort due to the risk for complications associated with these procedures. The main role of surgery is to palliate symptoms, decompressing the stomach, thereby providing access for enteral nutrition and enhancing gastric emptying.
Venting Gastrostomy or Jejunostomy
In patients with significant upper GI motility disorders, surgically placed venting gastrostomy, with or without a venting enterostomy, has been found to reduce hospitalizations. Therefore, these procedures may be an option, but they need further evaluation [53].
Gastrectomy
Completion or subtotal gastrectomy is performed most often for gastroparesis that followed gastric surgery for peptic ulcer disease. It has been suggested that major gastric surgery, such as Roux-en-Y reconstructions, could be helpful in palliating symptoms such as vomiting in patients with intractable gastroparesis and consequently improve the quality of life [53]. However, no controlled trials of completion gastrectomy for gastroparesis have been performed and concerns about long-term nutritional effects of gastrectomy remain.
Complimentary Alternative Therapy
Acupuncture was shown to have some benefit in a small study of 35 patients with DGp [137]. Ginger has been shown in some studies to improve symptoms in gastroparesis of varied etiology; [138] however, larger well-designed studies are needed to explore the benefits of complimentary alternative therapies.
Novel Therapeutics in Diabetic Gastroparesis
An effective, safe prokinetic is the goal for patients with gastroparesis, and medications in development, including ghrelin agonists and new generation 5-HT4 agonists, hold promise. High-frequency GES currently used in patients with severe symptoms should be considered for wider usage. In addition, the optimal conditions for entraining the electrical pacesetters that control gastric motor function are still being developed, and it is possible that advances in electrical stimulation may ultimately achieve the clinical promise that has been a goal for at least three decades. Better methods to detect the underlying electrical signal, including mucosal electrograms, may clarify the role of the electrogastrogram as well as predict response to GES. It is also pragmatic to determine if the same treatment approach can be used in idiopathic and in diabetic gastroparesis, or whether these conditions need to be treated differently. Stem cell treatment of ICC and the use of interleukin-10 are still in preliminary phase studies [57, 139]. It is important to re-emphasize that the management of patients with diabetic gastroparesis requires multidisciplinary care and co-operation. Therefore, well-designed randomized controlled trials with multidisciplinary investigators are needed to determine the optimal management of this condition.
Current Guidelines for Treatment of Diabetic Gastroparesis
Consensus guidelines for the clinical management of diabetic gastroparesis formulated by the ACG and consensus recommendations for gastric emptying scintigraphy of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine are summarized below [4, 77].
Identify the Cause. Patients with gastroparesis should be screened for the presence of diabetes mellitus, thyroid dysfunction, neurological disease, prior gastric or bariatric surgery and autoimmune disorders. Patients should undergo biochemical screening for diabetes and hypothyroidism; other tests are as indicated clinically. A prodrome suggesting a viral illness may lead to gastroparesis. Clinicians should enquire about the presence of a prior acute illness suggestive of a viral infection. Markedly uncontrolled (> 200 mg/dL, 11.1 mmol/L) glucose levels may aggravate symptoms of gastroparesis and delay gastric emptying. Optimization of glycemic control should be a target for therapy; this may improve symptoms and the delayed gastric emptying.
Diagnosis. A documented delay in gastric emptying is required for the diagnosis of gastroparesis. Scintigraphic gastric emptying of solids is the standard for the evaluation of gastric emptying and the diagnosis of gastroparesis. The most reliable method and parameter for the diagnosis of gastroparesis is gastric retention of solids at 4 h as measured by scintigraphy. Studies of shorter duration or based on a liquid challenge alone may result in decreased diagnostic sensitivity. Alternative approaches for the assessment of gastric emptying include wireless capsule motility testing and the GEBT with13C-labeled compounds such as octanoate or Spirulina incorporated into a solid meal; further validation is needed before these tests can be considered as alternates to scintigraphy for the diagnosis of gastroparesis. Medications that affect gastric emptying should be stopped at least 48 h before diagnostic testing. Patients with diabetes should have the blood glucose measured before starting the gastric emptying test; if hyperglycemia is detected, it should be treated and the test postponed until after the blood glucose is < 275 mg/dL (15.2 mmol/L).
Exclusion criteria and differential diagnosis. The presence of rumination syndrome and/or an eating disorders (including anorexia nervosa and bulimia) should be considered when evaluating a patient for gastroparesis. These disorders may be associated with delayed gastric emptying, and identification of these disorders may alter management. Cyclic vomiting syndrome (CVS), defined as recurrent episodic episodes of nausea and vomiting, should also be considered during the patient history-taking. These patients may require alternative therapy. Chronic usage of cannabinoid agents may cause a syndrome similar to CVS (Table 9).
Nutrition and enteral feeding. The first line of management for gastroparesis patients should include restoration of fluids and electrolytes and nutritional support; in patients with diabetics with gastroparesis, optimization of glycemic control must also be achieved. Oral intake is preferable for nutrition and hydration. Patients should receive counseling from a dietician regarding the consumption of frequent small-volume nutrient meals that are low in fat and soluble fiber. A high-calorie liquid nutrient component may be helpful since emptying of liquids is spared; however, a poor tolerance of liquid nutrition predicts possible oral nutrition failure. If the patient is unable to tolerate solid food, homogenized or liquid nutrient meals are recommended. Optimal glycemic control should be the goal. Since acute hyperglycemia inhibits gastric emptying, it is assumed that improved glycemic control may improve gastric emptying and reduce symptoms. Pramlintide and GLP-1 analogs may delay gastric emptying in diabetics. Cessation of these treatments and the use of alternative approaches should be considered before any therapy for gastroparesis is initiated.
Pharmacologic management. In addition to dietary therapy, prokinetic therapy should be considered to improve gastric emptying and gastroparesis symptoms, taking into account the benefits and risks of the chosen treatment. Metoclopramide has traditionally been a first-line prokinetic therapy, but this agent should be administered at the lowest effective dose and for limited periods of time due to the real risk of adverse effects. The risk of tardive dyskinesia from metoclopramide has been estimated to be < 1%. Patients should be instructed to discontinue therapy if they develop side effects, including involuntary movements. For patients unable to use metoclopramide, domperidone can be prescribed; this drug has Investigational New Drug clearance from the U.S. FDA and has been shown to be as effective as metoclopramide in reducing symptoms without the latter’s propensity for causing side effects to the central nervous system. Intravenous (IV) erythromycin should be considered when IV prokinetic therapy is needed in hospitalized patients. Oral treatment with erythromycin also improves gastric emptying. TCAs can be considered for refractory nausea and vomiting in gastroparesis but will not result in improved gastric emptying, and may potentially retard gastric emptying. Intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized controlled trials. GES may be considered for compassionate treatment in patients with refractory symptoms, particularly nausea and vomiting. Symptom severity and gastric emptying have been shown to improve in patients with DGp, but not in patients with idiopathic gastroparesis or postsurgical gastroparesis. Abdominal pain in gastroparesis may respond less well to treatment [49, 71].
Surgical management. Gastrostomy for venting and/or jejunostomy for feeding may be required for symptom relief. Completion gastrectomy could be considered in patients with postsurgical gastroparesis who remain markedly symptomatic and fail medical therapy. Surgical pyloroplasty or gastrojejunosotomy have been performed for refractory gastroparesis. However, further studies are needed before this treatment is advocated, and close nutritional monitoring is recommended before and after gastrectomy. Partial gastrectomy and pyloroplasty should be used rarely, only in carefully selected patients.
Summary
The prevalence of diabetes is increasing worldwide, with major economic and personal impact and increased morbidity and mortality. The majority of patients with diabetes develop GI symptoms during the course of their disease, and gastroparesis often goes undiagnosed. When evaluating a patient for DGp, it is important to tease out various upper, and lower GI symptoms with a detailed medical history and to exclude other common diseases with similar manifestations. A gastric emptying study should be performed after exclusion of mechanical or structural causes of abnormal gastric emptying. Effective DGp management requires consultants with expertise in the disorder. The standard of care involves a multidisciplinary team consisting of a diabetologist, a gastroenterologist with motility expertise, a certified diabetes educator, a registered dietician and a behavioral psychologist and/or a psychiatrist. The primary assessment includes risk stratification and intervention (Fig. 16). Careful nutritional assessment, hydration and electrolyte replenishment are a priority. Eliminating medications that exacerbate DGp, and life style changes, such as ceasing tobacco and alcohol use and encouraging exercise, are beneficial [140].
Fig. 16.
Algorithm for management of diabetic gastroparesis. GES Gastric electric stimulation
When choosing pharmacotherapy, the benefits need to be cautiously weighed against the adverse effect profile and cost. For drug-refractory patients who are eligible for a device, a trial of GES may be considered. Open lines of communication are essential while setting goals and expectations regarding symptom management, medications and device outcomes.
Finally, it is well known that diabetes (type 2) may be preventable [141, 142] and that its complications can be delayed or prevented by early screening and effective intervention [18, 143]. The seminal studies of glycemic control have shown that metabolic memory or legacy effect of early control can prevent or delay the development of diabetic autonomic neuropathy and other complications [10, 11, 13, 144]. Diabetic gastroparesis is a complex disease requiring a multifaceted approach, and we hope that our comprehensive review offers insight into the understanding and management of this challenging disorder.
Acknowledgments
The authors would like to thank the staff of their respective institutions. They would also like to thank Catherine McBride at the University of Louisville for help with manuscript preparation, Dr. Gregg Wendorf for assistance with illustrations, Dr. Michael Schemann for use of his figures, the reviewers and also Dr. Stephen J Winters for insightful comments.
Funding
This supplement has been sponsored by Hansa Medcell, India, who provided an unrestricted educational grant in support of the original project.
Editorial Assistance
Administrative assistance and project guidance was provided by the University of Louisville Office of Continuing Medical Education and Professional Development and Dr. Daniel Cogan, Assistant Dean.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Dr. Thomas Abell is a consultant to Theravance; an investigator for Vanda, Allergan and Theravance; a reviewer for Up To Date; an Editor of MedStudy, Neuromodulation, and Wikistim; and a founder of ADEPT-GI. Dr Sathya Krishnasamy is Investigator for Novo Nordisk, Kowa, Sanofi, Merck and Pfizer diabetes studies.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and is not a new study with human participants or animals.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6391592.
References
- 1.Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 3.Bekele G, Kabadi UM. Gastro intestinal manifestations of diabetes mellitus. Int J Diabetes Dev Countries. 1996;16:54–58. [Google Scholar]
- 4.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajumobi AB, Griffin RA. Diabetic gastroparesis: evaluation and management. Hosp Physician. 2008;44:27–35. [Google Scholar]
- 6.International Diabetes Federation (IDF). Diabetes atlas. 6th ed. Brussels, Belgium: International Diabetes Federation, 2018.
- 7.Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172–3179. doi: 10.2337/dc14-1036. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes UK. Diabetes prevalence 2016 (November 2016). Diabetes UK. 2016. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-2016. Accessed 7 June 2018.
- 9.Diabetes UK. Diabetes UK facts and stats: Diabetes UK. 2016. https://diabetes-resources-production.s3-eu-west-1.amazonaws.com/diabetes-storage/migration/pdf/DiabetesUK_Facts_Stats_Oct16.pdf. Accessed 7 June 2018.
- 10.[No authors listed]. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41:416–23. [DOI] [PMC free article] [PubMed]
- 11.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaghan BC, Little AA, Feldman EL, et al. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;cd007543. [DOI] [PMC free article] [PubMed]
- 13.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinik AI, Erbas T. Diabetic autonomic neuropathy. Handb Clin Neurol. 2013;117:279–294. doi: 10.1016/B978-0-444-53491-0.00022-5. [DOI] [PubMed] [Google Scholar]
- 15.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of Medical Care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39[Suppl 1]:S4–5. [DOI] [PubMed]
- 17.American Diabetes Association. Improving care and promoting health in populations: standards of medical care in diabetes—2018. Diabetes Care 2018;41[Suppl 1]:S7–S12. [DOI] [PubMed]
- 18.Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Comprehensive medical evaluation and assessment of comorbidities. Diabetes Care. 2018;41:S25–S32. doi: 10.2337/dc17-S006. [DOI] [PubMed] [Google Scholar]
- 20.Sellin JH, Chang EB. Therapy Insight: gastrointestinal complications of diabetes–pathophysiology and management. Nat Clin Pract Gastroenterol Hepatol. 2008;5:162–171. doi: 10.1038/ncpgasthep1054. [DOI] [PubMed] [Google Scholar]
- 21.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 22.Jones KL, Horowitz M, Wishart MJ, et al. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med. 1995;36:2220–2228. [PubMed] [Google Scholar]
- 23.Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113–133. doi: 10.1111/j.1365-2982.2009.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 25.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 26.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 27.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 28.Bharucha AE, Batey-Schaefer B, Cleary PA, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in Type 1 diabetes mellitus. Gastroenterology. 2015;149:330–339. doi: 10.1053/j.gastro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasler WL. Gastroparesis–current concepts and considerations. Medscape J Med. 2008;10:16. [PMC free article] [PubMed] [Google Scholar]
- 30.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anudeep V, Vinod KV, Pandit N, et al. Prevalence and predictors of delayed gastric emptying among Indian patients with long-standing type 2 diabetes mellitus. Indian J Gastroenterol. 2016;35:385–392. doi: 10.1007/s12664-016-0694-4. [DOI] [PubMed] [Google Scholar]
- 32.Koch KL, Hasler WL, Yates KP, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil. 2016;28:1001–1015. doi: 10.1111/nmo.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singla R, Homko C, Schey R, et al. Diabetes-related autoantibodies in diabetic gastroparesis. Dig Dis Sci. 2015;60:1733–1737. doi: 10.1007/s10620-015-3690-0. [DOI] [PubMed] [Google Scholar]
- 34.Ko GT, Chan WB, Chan JC, et al. Gastrointestinal symptoms in Chinese patients with type 2 diabetes mellitus. Diabet Med. 1999;16:670–674. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 35.Janatuinen E, Pikkarainen P, Laakso M, et al. Gastrointestinal symptoms in middle-aged diabetic patients. Scand J Gastroenterol. 1993;28:427–432. doi: 10.3109/00365529309098244. [DOI] [PubMed] [Google Scholar]
- 36.Bell RA, Jones-Vessey K, Summerson JH. Hospitalizations and outcomes for diabetic gastroparesis in North Carolina. South Med J. 2002;95:1297–1299. [PubMed] [Google Scholar]
- 37.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 38.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangula PR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–2527. doi: 10.1007/s10620-011-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu SP, DeMayo FJ. Progesterone receptor signaling in uterine myometrial physiology and preterm birth. Curr Top Dev Biol. 2017;125:171–190. doi: 10.1016/bs.ctdb.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan IM, Feltrin KL, Nair NS, et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol. 2009;297:G602–G610. doi: 10.1152/ajpgi.00051.2009. [DOI] [PubMed] [Google Scholar]
- 42.Verrengia M, Sachdeva P, Gaughan J, et al. Variation of symptoms during the menstrual cycle in female patients with gastroparesis. Neurogastroenterol Motil. 2011;23:625-e254. doi: 10.1111/j.1365-2982.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 43.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 44.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567–576. doi: 10.1016/j.cgh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darwiche G, Almer LO, Bjorgell O, et al. Delayed gastric emptying rate in Type 1 diabetics with cardiac autonomic neuropathy. J Diabetes Complic. 2001;15:128–134. doi: 10.1016/s1056-8727(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 46.Lydon A, Murray C, Cooke T, et al. Evaluation of standard haemodynamic tests of autonomic function and HbA1c as predictors of delayed gastric emptying in patients with type 1 diabetes mellitus. Eur J Anaesthesiol. 2000;17:99–104. doi: 10.1046/j.1365-2346.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 47.Perano SJ, Rayner CK, Kritas S, et al. Gastric emptying is more rapid in adolescents with Type 1 diabetes and impacts on postprandial glycemia. J Clin Endocrinol Metab. 2015;100:2248–2253. doi: 10.1210/jc.2015-1055. [DOI] [PubMed] [Google Scholar]
- 48.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313–322. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 49.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–452. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 50.Chang J, Rayner CK, Jones KL, et al. Prognosis of diabetic gastroparesis—a 25-year evaluation. Diabet Med. 2013;30:e185–e188. doi: 10.1111/dme.12147. [DOI] [PubMed] [Google Scholar]
- 51.Hellstrom PM, Gryback P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol. 2006;20:397–407. doi: 10.1016/j.bpa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Ehrlein HJ, Scheman M. Gastrointestinal motility [online tutorial]: Munich: Technical University of Munich, 2006.
- 53.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ordog T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8–18. doi: 10.1111/j.1365-2982.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 55.Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112–128. doi: 10.1038/nrendo.2014.202. [DOI] [PubMed] [Google Scholar]
- 56.Marathe CS, Horowitz M, Trahair LG, et al. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. J Clin Endocrinol Metab. 2015;100:3565–3571. doi: 10.1210/JC.2015-2482. [DOI] [PubMed] [Google Scholar]
- 57.Marathe CS, Rayner CK, Jones KL, et al. Novel insights into the effects of diabetes on gastric motility. Expert Rev Gastroenterol Hepatol. 2016;10:581–593. doi: 10.1586/17474124.2016.1129898. [DOI] [PubMed] [Google Scholar]
- 58.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horvath VJ, Izbeki F, Lengyel C, et al. Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep. 2014;14:527. doi: 10.1007/s11892-014-0527-8. [DOI] [PubMed] [Google Scholar]
- 60.Horvath VJ, Vittal H, Ordog T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–1533. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 61.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis—the missing link? Neurogastroenterol Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faussone-Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao SAN, Krishnasamy SS. Diabetes mellitus, type 2. http://5minuteconsult.com/collectioncontent/1-151583/diseases-and-conditions/diabetes-mellitus-type-2, 2017.
- 64.Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–1480. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- 65.De Block CE, De Leeuw IH, Pelckmans PA, et al. Delayed gastric emptying and gastric autoimmunity in type 1 diabetes. Diabetes Care. 2002;25:912–917. doi: 10.2337/diacare.25.5.912. [DOI] [PubMed] [Google Scholar]
- 66.Goo RH, Moore JG, Greenberg E, et al. Circadian variation in gastric emptying of meals in humans. Gastroenterology. 1987;93:515–518. doi: 10.1016/0016-5085(87)90913-9. [DOI] [PubMed] [Google Scholar]
- 67.Koch KL, Calles-Escandon J. Diabetic gastroparesis. Gastroenterol Clin North Am. 2015;44:39–57. doi: 10.1016/j.gtc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Koch CA, Uwaifo GI. Are gastrointestinal symptoms related to diabetes mellitus and glycemic control? Eur J Gastroenterol Hepatol. 2008;20:822–825. doi: 10.1097/MEG.0b013e3282f5f75e. [DOI] [PubMed] [Google Scholar]
- 69.Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 70.Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422–1428. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 71.Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456–63 (e215–6). [DOI] [PubMed]
- 73.Cutts T, Holmes S, Kedar A, et al. Twenty-five years of advocacy for patients with gastroparesis: support group therapy and patient reported outcome tool development. BMC Gastroenterol. 2016;16:107. doi: 10.1186/s12876-016-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 75.Butalia S, Johnson JA, Ghali WA, et al. Clinical and socio-demographic factors associated with diabetic ketoacidosis hospitalization in adults with Type 1 diabetes. Diabet Med. 2013;30:567–573. doi: 10.1111/dme.12127. [DOI] [PubMed] [Google Scholar]
- 76.Legaspi R, Narciso P. Euglycemic diabetic ketoacidosis due to gastroparesis, a local experience. J Ark Med Soc. 2015;112:62–63. [PubMed] [Google Scholar]
- 77.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 78.Shin AS, Camilleri M. Diagnostic assessment of diabetic gastroparesis. Diabetes. 2013;62:2667–2673. doi: 10.2337/db12-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74–101. doi: 10.1016/j.disamonth.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Parkman HP, Harris AD, Miller MA, et al. Influence of age, gender, and menstrual cycle on the normal electrogastrogram. Am J Gastroenterol. 1996;91:127–133. [PubMed] [Google Scholar]
- 81.Meneghini LF, Hogan AR, Selvaggi G. Superior mesenteric artery syndrome in type 1 diabetes masquerading as gastroparesis. Diabetes Care. 2008;31:1983–1984. doi: 10.2337/dc08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chial HJ, McAlpine DE, Camilleri M. Anorexia nervosa: manifestations and management for the gastroenterologist. Am J Gastroenterol. 2002;97:255–269. doi: 10.1111/j.1572-0241.2002.05452.x. [DOI] [PubMed] [Google Scholar]
- 83.Geliebter A, Melton PM, McCray RS, et al. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56:656–661. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- 84.Plummer MP, Jones KL, Annink CE, et al. Glucagon-like peptide 1 attenuates the acceleration of gastric emptying induced by hypoglycemia in healthy subjects. Diabetes Care. 2014;37:1509–1515. doi: 10.2337/dc13-1813. [DOI] [PubMed] [Google Scholar]
- 85.Kedar A, Nikitina Y, Henry OR, et al. Gastric dysmotility and low serum vitamin D levels in patients with gastroparesis. Horm Metab Res. 2013;45:47–53. doi: 10.1055/s-0032-1323689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141:486–498. doi: 10.1053/j.gastro.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 88.Olausson EA, Storsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109:375–385. doi: 10.1038/ajg.2013.453. [DOI] [PubMed] [Google Scholar]
- 89.Sadiya A. Nutritional therapy for the management of diabetic gastroparesis: clinical review. Diabetes Metab Syndr Obes. 2012;5:329–335. doi: 10.2147/DMSO.S31962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lipp RW, Schnedl WJ, Hammer HF, et al. Effects of postprandial walking on delayed gastric emptying and intragastric meal distribution in longstanding diabetics. Am J Gastroenterol. 2000;95:419–424. doi: 10.1111/j.1572-0241.2000.01761.x. [DOI] [PubMed] [Google Scholar]
- 91.Lacy BE, Crowell MD, Mathis C, et al. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018; 52(1):20–4. [DOI] [PubMed]
- 92.Jones KL, Tonkin A, Horowitz M, et al. Rate of gastric emptying is a determinant of postprandial hypotension in non-insulin-dependent diabetes mellitus. Clin Sci (Lond) 1998;94:65–70. doi: 10.1042/cs0940065. [DOI] [PubMed] [Google Scholar]
- 93.Jones KL, MacIntosh C, Su YC, et al. Guar gum reduces postprandial hypotension in older people. J Am Geriatr Soc. 2001;49:162–167. doi: 10.1046/j.1532-5415.2001.49037.x. [DOI] [PubMed] [Google Scholar]
- 94.Holst JJ, Gribble F, Horowitz M, et al. Roles of the gut in glucose homeostasis. Diabetes Care. 2016;39:884–892. doi: 10.2337/dc16-0351. [DOI] [PubMed] [Google Scholar]
- 95.Chaikomin R, Rayner CK, Jones KL, et al. Upper gastrointestinal function and glycemic control in diabetes mellitus. World J Gastroenterol. 2006;12:5611–5621. doi: 10.3748/wjg.v12.i35.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract. 2018;24:91–120. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- 97.American Diabetes Association. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018;41[Suppl 1]:S55–S64. [DOI] [PubMed]
- 98.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–1642. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 99.Alvarez-Villalobos NA, Trevino-Alvarez AM, Gonzalez-Gonzalez JG. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:1797–1798. doi: 10.1056/NEJMc1611289. [DOI] [PubMed] [Google Scholar]
- 100.Ramzan Z, Duffy F, Gomez J, et al. Continuous glucose monitoring in gastroparesis. Dig Dis Sci. 2011;56:2646–2655. doi: 10.1007/s10620-011-1810-z. [DOI] [PubMed] [Google Scholar]
- 101.Giugliano D, Maiorino MI, Bellastella G, et al. Comment on American Diabetes Association. Approaches to glycemic treatment. Sec. 7. In: Standards of medical care in diabetes–2016. Diabetes Care 2016;39(Suppl. 1):S52-S59. Diabetes Care 2016;39:e86-7. [DOI] [PubMed]
- 102.Calles-Escandon J, Koch KL, Hasler WL, et al. Glucose sensor-augmented continuous subcutaneous insulin infusion in patients with diabetic gastroparesis: an open-label pilot prospective study. PLoS ONE. 2018;13:e0194759. doi: 10.1371/journal.pone.0194759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with Type 1 diabetes. JAMA. 2016;316:1407–1408. doi: 10.1001/jama.2016.11708. [DOI] [PubMed] [Google Scholar]
- 104.Sharma D, Morrison G, Joseph F, et al. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia. 2011;54:2768–2770. doi: 10.1007/s00125-011-2282-6. [DOI] [PubMed] [Google Scholar]
- 105.U.S. Food and Drug Administration. FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decisions. 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534056.htm.
- 106.U.S. Food and Drug Administration. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. U.S. Food and Drug Administration; 2017.
- 107.U.S. Food and Drug Administration. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. 2018. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm602870.htm.
- 108.Thazhath SS, Jones KL, Horowitz M, et al. Diabetic gastroparesis: recent insights into pathophysiology and implications for management. Expert Rev Gastroenterol Hepatol. 2013;7:127–139. doi: 10.1586/egh.12.82. [DOI] [PubMed] [Google Scholar]
- 109.Navas CM, Patel NK, Lacy BE. Gastroparesis: medical and therapeutic advances. Dig Dis Sci. 2017;62:2231–2240. doi: 10.1007/s10620-017-4679-7. [DOI] [PubMed] [Google Scholar]
- 110.Braverman D, Bogoch A. Metoclopramide for gastroparesis diabeticorum. Diabetes Care. 1978;1:356–359. doi: 10.2337/diacare.1.6.356. [DOI] [PubMed] [Google Scholar]
- 111.Ehrenpreis ED, Deepak P, Sifuentes H, et al. The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. Am J Gastroenterol. 2013;108:866–872. doi: 10.1038/ajg.2012.300. [DOI] [PubMed] [Google Scholar]
- 112.Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26:521–528. doi: 10.1111/nmo.12296. [DOI] [PubMed] [Google Scholar]
- 113.Madani S, Tolia V. Gynecomastia with metoclopramide use in pediatric patients. J Clin Gastroenterol. 1997;24:79–81. doi: 10.1097/00004836-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 114.Srikantha M, Butterworth R. Pharmacological hyperprolactinaemia. BMJ Case Rep;2009. 10.1136/bcr.01.2009.1432. [DOI] [PMC free article] [PubMed]
- 115.Naruse M, Naruse K, Yoshimoto T, et al. Dopaminergic regulation of aldosterone secretion: its pathophysiologic significance in subsets of primary aldosteronism. Hypertens Res. 1995;18(Suppl 1):S59–S64. doi: 10.1291/hypres.18.supplementi_s59. [DOI] [PubMed] [Google Scholar]
- 116.Horowitz M, Harding PE, Chatterton BE, et al. Acute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathy. Dig Dis Sci. 1985;30:1–9. doi: 10.1007/BF01318363. [DOI] [PubMed] [Google Scholar]
- 117.Tonini M, Cipollina L, Poluzzi E, et al. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19:379–390. doi: 10.1111/j.1365-2036.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 118.Erbas T, Varoglu E, Erbas B, et al. Comparison of metoclopramide and erythromycin in the treatment of diabetic gastroparesis. Diabetes Care. 1993;16:1511–1514. doi: 10.2337/diacare.16.11.1511. [DOI] [PubMed] [Google Scholar]
- 119.Parthasarathy G, Kudva YC, Low PA, et al. Relationship between gastric emptying and diurnal glycemic control in type 1 diabetes mellitus: a randomized trial. J Clin Endocrinol Metab 2017; 102(2):398–406. [DOI] [PMC free article] [PubMed]
- 120.Evans AJ, Krentz AJ. Should cisapride be avoided in patients with diabetic gastroparesis? J Diabetes Complic. 1999;13:314–315. doi: 10.1016/s1056-8727(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 121.Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640–2649. doi: 10.1001/jama.2013.282833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasler WL. Emerging drugs for the treatment of gastroparesis. Expert Opin Emerg Drugs. 2014;19:261–279. doi: 10.1517/14728214.2014.899353. [DOI] [PubMed] [Google Scholar]
- 123.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11(1453–1459):e4. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151(87–96):e6. doi: 10.1053/j.gastro.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 125.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 127.Camilleri M, McCallum RW, Tack J, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153(1240–1250):e2. doi: 10.1053/j.gastro.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lacy BE, Crowell MD, Schettler-Duncan A, et al. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341–2347. doi: 10.2337/diacare.27.10.2341. [DOI] [PubMed] [Google Scholar]
- 129.Jacques J, Legros R, Monteil J, et al. Gastric peroral endoscopic pyloromyotomy as a salvage therapy for refractory gastroparesis: a case series of different subtypes. Neurogastroenterol Motil 2017;29. [DOI] [PubMed]
- 130.Moraveji S, Bashashati M, Elhanafi S, et al. Depleted interstitial cells of Cajal and fibrosis in the pylorus: novel features of gastroparesis. Neurogastroenterol Motil. 2016;28:1048–1054. doi: 10.1111/nmo.12806. [DOI] [PubMed] [Google Scholar]
- 131.Hibbard ML, Dunst CM, Swanstrom LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg. 2011;15:1513–1519. doi: 10.1007/s11605-011-1607-6. [DOI] [PubMed] [Google Scholar]
- 132.Khashab MA, Stein E, Clarke JO, et al. Gastric peroral endoscopic myotomy for refractory gastroparesis: first human endoscopic pyloromyotomy (with video) Gastrointest Endosc. 2013;78:764–768. doi: 10.1016/j.gie.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 133.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 134.Lin Z, Forster J, Sarosiek I, et al. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071–1076. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 135.Ringel Y. How is drug-refractory gastroparesis treated?: Medscape;2012. https://www.medscape.com/viewarticle/756815. Accessed 7 June 2018.
- 136.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74(496–503):e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L. Clinical observation on acupuncture treatment in 35 cases of diabetic gastroparesis. J Tradit Chin Med. 2004;24:163–165. [PubMed] [Google Scholar]
- 138.Gonlachanvit S, Chen YH, Hasler WL, et al. Ginger reduces hyperglycemia-evoked gastric dysrhythmias in healthy humans: possible role of endogenous prostaglandins. J Pharmacol Exp Ther. 2003;307:1098–1103. doi: 10.1124/jpet.103.053421. [DOI] [PubMed] [Google Scholar]
- 139.Choi KM, Gibbons SJ, Sha L, et al. Interleukin 10 restores gastric emptying, electrical activity, and interstitial cells of cajal networks in diabetic mice. Cell Mol Gastroenterol Hepatol. 2016;2:454–467. doi: 10.1016/j.jcmgh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neiberg RH, Rejeski JJ, Applegate WB, et al. Self-reported gastrointestinal symptoms in Type 2 diabetes improve with an intensive lifestyle intervention: results from the action for health in diabetes (Look AHEAD) Clinical Trial. Clin Diabetes. 2015;33:181–188. doi: 10.2337/diaclin.33.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crandall JP, Knowler WC, Kahn SE, et al. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382–393. doi: 10.1038/ncpendmet0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knowler WC, Fowler SE, Hamman RF, et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.American Diabetes Association. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018;41[Suppl 1]:S51–S54. [DOI] [PubMed]
- 144.Diabetes Control Complications Trial Research Group Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]